NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-.
ABSTRACT
Post transplantation diabetes mellitus (PTDM), also known as New Onset Diabetes After Transplantation, is a common and important complication following solid organ transplantation. PTDM may arise from both transplant-related and traditional risk factors and has variably been reported to be associated with decreased patient and graft survival and other adverse outcomes including increased cardiovascular disease risk, infection, and graft rejection. This chapter reviews the nomenclature change for post-transplant diabetes, diagnostic criteria, risk factors, incidence after solid organ transplantation, and associated adverse effects. Screening for PTDM including pretransplant evaluation and early detection in the posttransplant period, and the unique aspects of diabetes management in the context of organ transplantation are also discussed. For complete coverage of all related areas of Endocrinology, please visit our on-line FREE web-text, WWW.ENDOTEXT.ORG.
NOMENCLATURES AND DIAGNOSIS OF POSTTRANSPLANTATION DIABETES MELLITUS: HISTORICAL PERSPECTIVES
Nomenclatures
Post transplantation diabetes mellitus (PTDM) was first described in kidney transplant recipients in 1964 (1). It was subsequently recognized as a complication of kidney transplantation in the 1970s. Over the years, PTDM has undergone changes in nomenclatures including steroid diabetes, post transplantation diabetes mellitus (PTDM), new onset diabetes mellitus (NODM), transplant-associated hyperglycemia (TAH), and new onset diabetes after transplantation (NODAT) (2, 3, 4, 5, 6). In 2014, the International Expert Panel consisting of transplant nephrologists, diabetologists, and clinical scientists recommended changing the terminology NODAT back to PTDM, excluding transient post transplantation hyperglycemia (7). Utilizing the term NODAT is thought to be misleading because it seemingly excludes patients with pretransplant diabetes. Pre-existing diabetes is often undiagnosed because of the effect of chronic kidney disease on insulin metabolism and clearance, and the lack of effective pretransplant screening. The term PTDM will be utilized for the remainder of this chapter.
Diagnosis
Historically, PTDM has been variably defined as having random glucose levels greater than 200 mg/dL, fasting glucose levels greater than 140 mg/dL, or the need for insulin or oral hypoglycemic agents in the posttransplant period (8). In 2003 the International Expert panel consisting of leaders from both the transplant and diabetes fields suggested that the definition and diagnosis of diabetes and impaired glucose tolerance should be based on the definition and diagnosis described by the World Health Organizations (9). In 2011, the American Diabetes Association (ADA) incorporated hemoglobin A1C (A1C) > 6.5% as a diagnostic criterion for diabetes mellitus in the general population based on the observed association between A1C level and the risk for future development of retinopathy (10). In 2014, the International Expert Panel recommended expanding screening tests for PTDM using postprandial glucose monitoring and A1C. However, A1C test is not recommended early after transplantation (arbitrarily defined as within 45 days after transplantation) because of potential confounding factors (7). A normal A1C does not exclude the diagnosis of PTDM in the presence of early posttransplant anemia and/or dynamic kidney allograft function. In a small single-center study consisting of 30 diabetic patients with CKD stage 3 b and 4, treatment with intravenous iron and erythropoietin stimulating agent (ESA) has been shown to result in a fall in A1C independent of glycemic changes (11). It is speculated that the fall in A1C level associated with either treatment is due to the formation of new erythrocytes in the circulation (causing a change in the proportion of young to old red blood cells), and an alteration in the red-cell glycation rates. A similar study in the transplant setting is lacking and warrants further exploration because intravenous iron and ESA therapy are commonly administered in the early posttransplant period. Although not widely used in clinical practice, oral glucose tolerance (OGTT) remains the gold standard for diagnosing PTDM. It should be noted that the algorithmic approach to the screening and diagnosis of PTDM is largely based on published kidney transplantation literature. Similar studies in the settings of liver, heart, and lung transplants are lacking. However, it is speculated that the principles are relevant to all forms of solid organ transplantation (7). The 2022 ADA criteria for prediabetes and diabetes are shown in Figure 1.
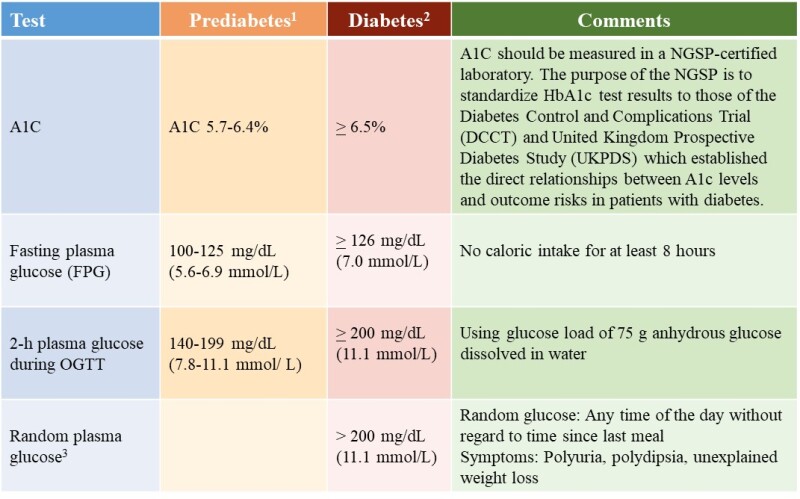
Figure 1.
The 2022 American Diabetes Association Diagnostic Criteria for Prediabetes and Diabetes.
1For A1C, FPG and 2-h OGTT, risk is continuous, extending below the lower limit of the range, becoming disproportionately greater at the higher end of the range. 2In the absence of unequivocal hyperglycemia, diagnosis of DM using A1C, FPG or 2-h OGTT requires two abnormal test results from the same sample or in two separate samples. 3Random plasma glucose is only diagnostic in patient with classic symptoms of hyperglycemia or hyperglycemic crisis (https://doi.org/10.2337/cd22-as01). OGTT, oral glucose tolerance test; A1C, hemoglobin A1C; NGSP, National Glycohemoglobin Standardization Program
INCIDENCE
PTDM has been reported to occur in 4% to 25% of kidney transplant recipients, 2.5% to 25% of liver transplant recipients, 4% to 40% of heart transplant recipients, and 30% to 35% of lung transplant recipients (9, 12-15). Higher incidences have also been reported. Variations in the reported incidence may be due in part to the prior lack of a standard definition, presence of both modifiable and nonmodifiable risks factors, duration of follow-up, type of organ transplants, and primary diagnostic indications for transplant. In one retrospective cohort study of 415 liver transplant recipients, PTDM occurred in 34.7%, 46.9%, and 56.2% of patients at 1, 3, and 5-year follow-up, respectively (15). The 33rd International Society of Heart and Lung Transplantation database demonstrated that approximately 29% of lung transplant recipients who survived 5 years post-transplantation developed PTDM, with the highest incidence occurring among those whose primary diagnosis for lung transplantation was cystic fibrosis. (16).
RISK FACTORS FOR PTDM
PTDM may arise from both transplant-related and traditional risk factors. The diabetogenic effect of various immunosuppressive agents have been well described. Corticosteroids may reduce peripheral insulin sensitivity, inhibit pancreatic production/secretion, and increase hepatic gluconeogenesis. The calcineurin inhibitors tacrolimus and cyclosporine decrease insulin secretion and synthesis. Sirolimus increases peripheral insulin resistance and impairs pancreatic beta-cell response. The antimetabolites azathioprine and mycophenolic acid derivatives (mycophenolate mofetil and mycophenolate sodium) are not diabetogenic. Belatacept is a humanized fusion protein that inhibits the costimulatory pathway. Its use in kidney transplant recipients has not been shown to increase PTDM risk. Transplant patients may have improved appetite and a more liberal diet which can lead to obesity. Risk factors for PTDM can be loosely categorized into those that are non-modifiable, potentially modifiable, and modifiable (8, 17-24).
Solid organ transplant recipients with specific end-organ diagnosis such as end-stage kidney disease due to polycystic kidney disease, end-stage lung disease due to cystic fibrosis, or end-stage liver disease due to hepatitis C infection or nonalcoholic steatohepatitis have been reported to be at increased risk for PTDM compared with those without such diagnosis (21). Donor liver steatosis has also been reported to be associated with an increased incidence of PTDM (22). Suggested risk factors for the development of PTDM are presented in Figure 2. A more extensive discussion of the studies evaluating PTDM risk factors is beyond the scope of this chapter. Interested readers are referred to reference Pham and colleagues (8).

Figure 2.
Suggested Risk factors for PTDM
1Curative therapy for chronic hepatitis C can be achieved with interferon-free direct acting antiviral-based regimen. Stable transplant recipients with HCV viremia by PCR should be referred to Hepatology for treatment. In HCV-positive kidney transplant candidate with a living donor, pretransplant treatment of HCV infection should be considered. 2Posttransplantation CMV prophylaxis is preferred over preemptive therapy after heart and lung transplant. Either prophylaxis or preemptive therapy is recommended after kidney or liver transplant recipients. However, for programs or patients who are unable to meet the stringent logistic requirements required with preemptive therapy, prophylaxis therapy is recommended. 3Persistent hypomagnesemia can occasionally be seen despite aggressive replacement therapy because of ongoing calcineurin inhibitor-induced urinary magnesium wasting. 4Manipulation of immunosuppression should be weighed against the risk of acute rejection. 5Donor liver steatosis has also been reported to be associated with increased PTDM risk. PPAR, peroxisome proliferators activated receptor; IGT, impaired glucose tolerance; IFG, impaired fasting glucose
IMPACT OF PTDM ON OUTCOMES AFTER TRANSPLANTATION
Studies evaluating the association between PTDM and morbidity and mortality have yielded mixed results (25-33).
PTDM After Kidney Transplantation
Retrospective analysis of the United States Renal Data System consisting of more than 11,000 kidney transplant recipients demonstrated that PTDM was a strong, independent predictor of mortality (p < 0.0001), graft failure (unadjusted for graft loss due to rejection) (p < 0.0001), and death-censored graft failure (p < 0.0001) (18). One single-center study consisting of more than 700 kidney transplant recipients similarly demonstrated worse 10-year actuarial patient survival among patients with PTDM compared with those without PTDM (67.1% vs. 81.9%, respectively). Five- and 10-year graft survival rates were similar among patients with PTDM and those without PTDM (25). In contrast, in a multicenter longitudinal cohort study consisting of 632 kidney transplant recipients of deceased-donor kidneys, no association between PTDM and mortality or graft failure was observed at a median follow-up of 6 years post-transplantation (n=632) (26). Subgroup analyses showed that late onset PTDM (developing beyond 1-year post-transplantation) was associated with worse graft outcomes. A retrospective analysis of the UNOS/OPTN database (n > 37,000) similarly failed to demonstrate the negative impact of PTDM on transplant survival or cardiovascular mortality during a median follow up of 548 days (27). However, the study results were considered inconclusive because of the wide confidence intervals and relatively short duration of follow-up.
PTDM After Liver Transplantation
Retrospective analysis of the UNOS/OPTN database consisting of > 13,000 liver transplant recipients demonstrated that the presence of both PTDM and acute rejection at 1-year posttransplant but not PTDM alone was associated with higher overall graft failure and mortality risk (27). However, it should be noted that UNOS database did not distinguish transient post transplantation hyperglycemia from established PTDM. A single-center retrospective cohort study (n=994) compared the incidence of major cardiovascular events (MCE) among four groups of liver transplant recipients 1) without diabetes (39%), 2) with pre-existing diabetes (24%), 3) with transient PTDM (16%), and 4) with sustained PTDM (20%). Sustained PTDM was found to be associated with a significant increase in mortality risk and a doubling of major cardiovascular events at a median follow up of 54.7 months (sub-distribution HR 1.95, 95% CI 1.20–3.18). A greater than threefold increased risk of death was observed among those who experienced MCE (sustained PTDM was defined as PTDM for at least 6 months after transplant). MCE was defined as a composite of cardiac arrest, fatal and nonfatal myocardial infarction, ischemic stroke, and symptomatic peripheral artery disease requiring a revascularization intervention) (30). In a retrospective cohort study of 415 adult liver transplant recipients, PTDM was found to be associated with higher rejection rates (31.9% vs. 21.8%, respectively; p=0.055) and a trend towards worse patient survival compared with no-PTDM at 5 year follow up (72.5% vs. 77.2%, respectively; p=0.460) (15). Although studies on the association between PTDM and patient and allograft outcomes after liver transplantation have yielded variable results, most studies demonstrated that PTDM after liver transplantation is associated with increased mortality risk (31).
PTDM After Heart Transplantation
Meta-analysis of observational studies in heart transplant recipients demonstrated that pre-existing diabetes was associated with a 37% increase in mortality risk (HR 1.37, CI 1.15-1.62) (32). Studies on the impact of PTDM on outcomes after heart transplantation are lacking. In one single-center South Korean study consisting of 391 isolated heart transplant recipients 1) without diabetes (n=257), 2) with pre-existing diabetes (n=46), and 3) with PTDM (n=88), the risk of death was found to be twofold higher among transplant recipients with pre-existing as well as post transplantation diabetes compared with their non-diabetic counterparts (33).
PTDM After Lung Transplantation
The 27th International Society for Heart and Lung Transplantation Registry consisting of more than 32,000 lung transplant recipients demonstrated that pre-existing diabetes was associated with a 21% increase in 5-year mortality risk (RR 1.21, p=0.0023) (34). Limited studies suggest that PTDM similarly adversely affects survival among lung transplant recipients. In a single-center prospective observational Australian study consisting of 210 patients who underwent their first single, bilateral, or heart-lung transplant between 2010-2013, hyperglycemia in both the early and late posttransplant periods (defined as first 4 months and beyond 4 months) was found to be associated with increased mortality risk. Of 210 patients, 80 had no DM, and 90 had persistent DM. Patients with preexisting DM (n=45) and PTDM (n=45) were classified together as “persistent DM”. In the whole cohort, each 18 mg/dL increase in mean fasting blood glucose (FBG) and random blood glucose and each 1% increase in mean A1C were associated with 18% (p=0.006), 38% (p< 0.001), and 46% (p=0.002) increase in mortality risk, respectively (median follow up of 3 years). Of interest, random blood glucose correlated with mortality in both the persistent DM and no DM groups (35%, p=0.012 and 109%, p=0.041, respectively). It was concluded that glycemic control strongly correlated with survival after lung transplant (35). The same group of investigators previously demonstrated that DM conferred a nearly fourfold increase in mortality risk compared with no DM. When patients were classified into subgroups including 1) no diabetes, 2) pre-existing DM, 3) PTDM, 4) DM diagnosed within 2 weeks of death, and 5) DM developing after transplant but death within 90 days of transplant, pre-existing DM and PTDM were associated with a 65% (p=0.003) and a 90% (p<0.001) increase in mortality risk, respectively (36).
Although studies on the impact of PTDM on outcomes after non-renal solid organ transplantation remain limited, PTDM appears to be associated with increased mortality risk regardless of the type of organ transplants (kidney, liver, heart, lung transplant) (21). Patients with PTDM may also develop many of the complications associated with diabetes similar to that observed in the general population. In a study of 4105 patients with PTDM, one or more diabetic complications arose in 58% including ketoacidosis (8%), hyperosmolarity (3%), renal complications (31%), ophthalmic complications (8%), neurological complications (16%), peripheral circulatory disorders (4%), and hypoglycemia/shock (7%). These complications occurred within a mean of 500-600 days of developing PTDM, indicating an accelerated pace for the development of complications (28). Moreover, PTDM patients had an increased rate of infections and sepsis compared with their non-diabetic counterparts.
DETECTION OF PTDM
Pretransplant Baseline Evaluation
Pretransplant Evaluation should include history of hyperglycemia, prediabetes, diabetes, and risk factors for PTDM including family history and hepatitis C virus. The 2004 International Consensus Guidelines suggest that a pretransplant baseline evaluation should include a complete medical and family history, including documentation of glucose history (37). Those with risk factors for metabolic syndrome can be screened further with laboratory testing. Patients with evidence of risk factors can be counseled of their risk for PTDM. Those with evidence of prediabetes can be counseled on lifestyle modifications including dietary modifications, thirty minutes of moderate intensity physical activity, and overall five to ten percent weight reduction (38). In HCV-positive kidney transplant candidates with a living donor, pretransplant treatment of HCV infection should be considered. With the advent of the interferon-free direct acting antiviral based regimen, treatment of hepatitis C in the posttransplant period is a reasonable alternative in selected prospective kidney transplant candidates without a living donor due to a considerably shorter waiting time for a deceased HCV-positive donor kidney (39). The choice of an immunosuppressive regimen should be tailored to each individual patient, weighing the risk of acute rejection against that for PTDM.
Early Detection of PTDM After Transplantation
New onset perioperative hyperglycemia is common and may develop in the context of high dose corticosteroid, as a consequence of posttransplant stress hyperglycemia, or both. Limited studies suggest that posttransplant stress hyperglycemia is an independent risk factor for subsequent diabetes (40). The 2014 International Consensus guidelines on PTDM screening is shown in Figure 3 (7). The expert panel suggested that patients with early post-transplant hyperglycemia (defined as hyperglycemia before 45 days after transplantation) should not be diagnosed as PTDM.

Figure 3.
The 2014 International Consensus Guidelines on Screening, Diagnosis, and Management of PTDM
1Seldom performed in clinical practice (time-consuming/cost). 2 A1C cannot be accurately interpreted within the first 3 months after transplantation because anemia and impaired graft function can directly interfere with the A1C assay. Recent blood transfusion and dapsone may alter A1C level. 3A1C alone < 365 days may underestimate PTDM and require confirmatory testing. 4Within the past several years newer injectable antidiabetic agents have increasingly been used (however, it should also be noted that evidenced-based recommendations are lacking). PTDM, post-transplantation diabetes mellitus; OGTT, oral glucose tolerance test; A1C, hemoglobin A1C
At the authors’ institution, fasting and premeal home glucose monitoring is routinely recommended for patients with new-onset post transplantation hyperglycemia particularly those requiring insulin therapy in the immediate post transplantation period. Nonetheless, it should be noted that monitoring a 2- hour postprandial blood glucose may be a better indicator of diabetes, particularly in steroid-treated patients. Clinically stable patients with persistent post transplantation hyperglycemia for > 3 months should be screened for PTDM using A1C test. Although evidence-based screening guidelines for the early detection of PTDM are lacking, obtaining baseline A1C at 3 months after transplant, then at 6 months, 9 months, 12 months, and annually thereafter seems reasonable. If screening A1C is in the prediabetic range, patients should be counseled on dietary and lifestyle modification and A1C monitored every 3 months. While OGTT remains the gold standard for diagnosing PTDM, there remains insufficient evidence to recommend OGTT for all kidney transplant recipients (7). In addition, screening all patients with OGTT may be impractical in clinical practice and should be individualized and reserved for those with multiple risk factors (opinion-based) (40,41).
PREVENTION AND MANAGEMENT OF PTDM
Non-Pharmacological Preventive and Management Strategies
Studies in the general population demonstrated that lifestyle modification promoting reduced fat/energy diet, daily moderate intensity physical activity, and modest weight loss reduce the incidence of type 2 diabetes (42). Similar studies in the context of solid organ transplantation are limited. Small single-center studies showed that post transplantation weight gain is associated with persistent PTDM (43). In a small single center study consisting of 25 kidney transplant recipients with impaired glucose tolerance, reversal to normal glucose tolerance with lifestyle modification was observed in 13 patients after a median of 9 months with only one patient progressing to PTDM (44). In contrast, a single-center, randomized controlled trial designed to Compare the benefits of Active Versus passive lifestyle Intervention in kidney Allograft Recipients (CAVIAR) showed no improvement in surrogate markers of glucose metabolism (insulin secretion, insulin sensitivity, and disposition index) among patients randomized to active lifestyle intervention (lifestyle change with the guidance of a renal dietitian, n=66) compared with their passive lifestyle intervention counterparts at 6 month follow-up (leaflet advice alone, n=64). However, clinically, active versus passive lifestyle intervention resulted in weight loss (-2.47 kg, P=0.002) and reduction in fat mass (mean difference, -1.537 kg, P=0.123). A trend towards reduction in PTDM incidence (7.6% versus 15.6%, P = 0.123) was observed in the active intervention arm (45).
Pharmacological Preventive and Management Strategies
In the immediate posttransplant period, the pancreatic β-cells are exposed to multiple hyperglycemic stressors including the transplant surgery itself, high-dose corticosteroids, and the introduction of cyclosporine or tacrolimus immunosuppression therapy. It has been suggested that early basal insulin therapy decreases PTDM through insulin-mediated protection of pancreatic beta-cells (46-47). In a randomized controlled trial, Hecking et al. demonstrated that early basal insulin therapy following detection of early post transplantation hyperglycemia (defined as < 3 weeks) reduced the subsequent odds of developing PTDM within the first year after transplantation by 73% (47). In an open-label, multicenter randomized trial comparing early post-operative basal insulin therapy vs. standard of care for the prevention of PTDM in kidney transplant recipients, early insulin therapy was similarly found to result in reduced odds for PTDM at 12 months (OR: 0.21 [95% CI, 0.07 to 0.62]) and at 24 months (OR 0.35 [95% CI, 0.14 to 0.87]) after adjustment for baseline differences including polycystic kidney disease. However, treatment resulted in significantly higher hypoglycemia rates (48). Currently, initiation of insulin therapy in the early post-transplantation period solely to prevent PTDM cannot be routinely recommended and awaits further study. The glucose threshold for starting insulin therapy remains to be defined. Insulin tapering or withdrawal and transitioning to noninsulin-based regimen can be considered after the first 1-3 month after transplant when insulin requirement is less than 15-20 units a day (opinion-based). The choice of individual agents should be based on the potential advantages and disadvantages of different classes of agents at the discretion of the clinicians (Figure 4).

Figure 4.
The potential advantages and disadvantages of various classes of antihyperglycemic agents.
1 KDIGO guidelines: Reduce dose if estimated glomerular filtration rate (eGFR) < 45 cc/min/1.73 m2. Discontinue if eGFR < 30 cc/min/1.73m2. 2 From Parekh TM, Raji M, Lin YL, et al. Hypoglycemia after antimicrobial drug prescription for older patients using sulfonylureas. JAMA Intern Med. 2014;174(10):1605-1612. 3 Contraindicated in patients with personal history or family history of medullary thyroid cancer or multiple endocrine neoplasia (MEN) type 2. 4 Sitagliptin may prolong QT interval particularly when used with cyclosporine.
Modification of Immunosuppression
Although clinical trials comparing the incidence of PTDM in cyclosporine versus tacrolimus-treated patients have yielded variable results, tacrolimus has more consistently been shown to have a greater diabetogenic effect than cyclosporine (49). Modification of immunosuppression including cyclosporine to tacrolimus conversion therapy or steroid avoidance, or withdrawal has variably been shown to improve glycemic control (8, 49-53). However, manipulation of immunosuppression is not without immunological risk. In a meta-analysis of controlled clinical trials to assess the safety and efficacy of early steroid withdrawal or avoidance, Pascual et al. showed that steroid avoidance or steroid withdrawal after a few days reduced PTDM incidence among cyclosporine but not tacrolimus-treated kidney transplant recipients (54). However, among cyclosporine-treated patients, acute rejection episodes were more frequently observed in steroid avoidance compared with conventional steroid treated groups. The same group of investigators demonstrated no significant beneficial effect of late steroid withdrawal (3 to 6 months after transplantation) on the incidence of PTDM (55). In the current era of immunosuppression, the beneficial effect of steroid avoidance or withdrawal on the incidence of PTDM has been questioned by experts in the field because rapid steroid taper and the use of lower target cyclosporine and tacrolimus levels are now common practice (7). The use of tacrolimus and mTOR inhibitor combination therapy may increase PTDM risk and should probably be avoided. Nonetheless, low dose calcineurin inhibitor (cyclosporine or tacrolimus) and mTOR inhibitor combination therapy seems justifiable in transplant recipients with a history of malignancies (such as skin cancers, renal cell carcinoma, or Kaposi sarcoma). Due to the lack of well-defined guidelines, modification of immunosuppression to alleviate the incidence of PTDM should be tailored to each individual patient. Reduction in immunosuppression should be weighed against the risk of acute rejection.
Management of Established PTDM in the Late Posttransplant Period
Although there may be differences in the pathogenesis and presentation of PTDM compared to type 2 diabetes mellitus, management of established PTDM in the late posttransplant period should follow the conventional approach and clinical guidelines as established by well-recognized organizations. The American Diabetes Association and European Association for the Study of Diabetes generally recommend an A1c target of < 7% (56). Lower A1C levels may be acceptable and even beneficial if it can be achieved safely without significant hypoglycemia or other treatment-related adverse effects. In contrast, less stringent A1C goals may be appropriate for patients with limited life expectancy or where the harms of treatment are greater than the benefits (57). Lifestyle modifications including weight reduction, dietary changes, and regular moderate cardiovascular activity should be employed. If glycemic control does not reach therapeutic targets, medical management with antidiabetic agents and ultimately insulin can be initiated.
Metformin has not been widely used in the setting of transplantation due to the concern for lactic acidosis in the presence of dynamic kidney allograft function particularly in the early post transplantation period. In contrast, the potential beneficial effects of metformin including weight neutral or weight loss, cardio protection, and lack of significant drug-drug interactions renders metformin an attractive treatment option for solid organ transplant recipients. There has been only one randomized clinical trial assessing the efficacy of metformin in the prevention of PTDM in kidney transplant recipients –The Transplantation and Diabetes (Transdiab) study (58). The Transdiab study is a single-center, open label, randomized controlled trial designed to assess the feasibility, gastrointestinal tolerability, and efficacy of metformin in patients with post transplantation impaired glucose tolerance. The latter is diagnosed using a 2-hour oral glucose tolerance test in the 4-12 weeks after transplant. Patients with eGFR < 30 mL/min/1.73 m2 were excluded from the study. Eligible patients with IFG were randomized to standard of care (n=9) or standard of care and metformin 500 mg twice daily (n=10). The efficacy of metformin was assessed by measuring fasting blood glucose and A1C at 3, 6, 9, and 12-month follow up. The study demonstrated similar tolerability and efficacy between the two groups. The former was evaluated by the gastrointestinal symptom rating scale at 3- and 12-months post randomization. At 12-month follow-up, 60% of patients in the metformin arm and 22% in the control arm returned to a normal OGGT (P=0.2). Both groups gained weight by the end of 12 months with the intervention group gaining 2.2 kg and the control group 6.7 kg (P=0.12). One patient discontinued metformin due to gastrointestinal symptoms and another patient required metformin dose reduction due to a metallic taste. One patient in the control group was started on metformin 500 mg twice daily by the treating physician 6 months after randomization due to elevated FBG and A1C. There were no episodes of lactic acidosis or serious adverse events in either arm. Although large randomized controlled trials to assess the risk and benefit ratio of metformin are needed before it can be endorsed as the oral antidiabetic agent of choice in PTDM, its use appears safe in kidney transplant recipients with mild to moderate renal impairment (eGFR 30-60 mL/min).
Experimental studies suggest that sulfonylureas are associated with β-cell apoptosis and β-cell exhaustion (59), raising theoretical concern about their use in PTDM, particularly in the early posttransplant period. In contrast, the anti-hyperglycemic dipeptidyl peptidase-4 inhibitor (DPP-4) inhibitors have been shown to preserve pancreatic beta-cell function in diabetic animal models (60-61).
Early clinical studies suggest that DPP-4 inhibitors are safe and effective in the treatment of PTDM in kidney transplant recipients (62-64). In a single-center study consisting of 71 stable kidney transplant recipients with PTDM newly diagnosed by an oral glucose tolerance test, Haidinger et al. demonstrated that patients treated with vildagliptin at baseline had significantly reduced HbA1C levels at 3, 6,12, and 18 months, whereas no improvement in glycemic control was observed among their sulfonylurea-treated counterparts (62). In a randomized controlled trial comparing vildagliptin with placebo in the treatment of PTDM, the same group of investigators demonstrated that treatment with vildagliptin significantly improved A1C levels within 3 months compared with placebo (65). In a systematic review and meta-analysis to assess the efficacy and safety of DDP-4 inhibitors in kidney transplant recipients with PTDM, DDP4-inhibitor use was found to have a favorable glycemic effect (assessed by A1C) compared with either placebo or oral anti-hyperglycemic agent (A1C= -0.993, p=0.001) at 6-month follow-up. No significant changes in eGFR or tacrolimus levels were observed in DDP-4 inhibitor-treated patients (66).
Studies evaluating the safety and efficacy of DDP-4 inhibitors in non-renal solid organ transplant recipients remain lacking. In a small retrospective study of 30 stable heart transplant recipients with type 2 diabetes, vildagliptin was found to significantly reduce A1C level compared with their control counterparts. Mean A1C in the vildagliptin-treated patients was 7.4% ± 0.7% before versus 6.8% ± 0.8% after 8 months of therapy (P = 0.002 vs baseline). Mean A1C levels at baseline and at 8-month follow up in the control group were 7.0% ± 0.7% versus 7.3% ± 1.2%, respectively (P = 0.21) (67). No statistically significant changes in body weight, total cholesterol or triglyceride levels were seen in vildagliptin-treated patients. Furthermore, no significant changes in immunosuppressive drug levels or dosages were observed in either group. Whether vildagliptin is safe and effective in the treatment of PTDM after orthotopic heart transplantation warrants further exploration. In contrast, in a multicenter, randomized, double-blind, placebo-controlled trial designed to evaluate the long-term cardiovascular efficacy and safety of saxagliptin in patients with type 2 DM at risk of cardiovascular events, saxagliptin administration was unexpectedly found to be associated with a significant 27% increase in hospitalizations for heart failure [the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus–Thrombolysis in Myocardial Infarction 53 (SAVOR-TIMI 53) trial, n =16,492) (68). However, subsequent post-hoc analyses of two large randomized placebo-controlled trials (EXAMINE and TECOS trials) showed no increase in heart failure risk in alogliptin- (69) or sitagliptin-treated patients (70) compared with their placebo-treated counterparts, suggesting that the increase in heart failure incidence observed with saxagliptin may be specific to the drug rather than a drug class effect. Nonetheless, based on early clinical study results, the FDA has issued a warning about the potential for increased risk for heart failure associated with the use of saxagliptin and alogliptin. Saxagliptin use in recipients of heart transplantation with PTDM is not recommended. Whether alogliptin is safe for use after heart transplantation awaits further studies.
GLP-1 agonists therapy may confer cardioprotective (liraglutide, dulaglutide, and semaglutide) and weight-loss benefits, counteracting the weight gain commonly seen in the setting of hyperglycemia and steroid therapy after transplantation (16, 71-72)
The novel sodium-glucose cotransporter type 2 inhibitor (SGLT2i) antidiabetic drug class inhibits glucose reabsorption in the proximal convoluted tubule resulting in glucosuria. The glucosuric effect of SGLT2i is attenuated in patients without hyperglycemia thereby lessening hypoglycemia risk. An experimental animal model of tacrolimus-induced diabetes demonstrated that empagliflozin improves hyperglycemia and suppressed the tacrolimus-induced twofold increase in the expression of SGLT2 receptors (73). Furthermore, empagliflozin was found to have a direct protective effect on tacrolimus-induced renal injury. The study findings suggest that SGLT2 inhibitor is a suitable therapeutic option for transplant recipients with tacrolimus induced PTDM.
Although initially approved for use as an antidiabetic agent, SGLT2i use was unexpectedly found to have cardio- and reno-protective effects in subjects with or without type 2 DM (74-76). The EMPEROR-Reduced randomized placebo-controlled trial designed to study the effect of empagliflozin on cardiovascular and kidney outcomes across the spectrum of kidney function demonstrated a significant reduction in cardiovascular death, heart failure hospitalization, and total heart failure hospitalization among empagliflozin-treated patients compared with their placebo-treated counterparts at a median follow up of 16 months. A reduction in the composite kidney outcome (defined as sustained profound decline in eGFR, chronic dialysis, or transplant) was also observed among patients randomized to receive empagliflozin irrespective of baseline renal function (HR for patients with vs. without CKD: 0.53 vs. 0.46, respectively, p=0.78) (77). Whether the cardiorenal benefits of SGLT2i seen in the general population can be extrapolated to the transplant population awaits further studies. Limited prospective and retrospective studies in the setting of solid organ transplantation showed that SGLT2i has a modest effect on glycemic control and a favorable effect on weight reduction (78-80). In a single-center, prospective, double-blind study consisting of 44 kidney transplant recipients with PTDM randomized to receive either empagliflozin (n=22) or placebo (n=22) for 24 weeks, a significant reduction in A1C was observed among empagliflozin-treated patients compared with their placebo-treated counterparts (-0.2% vs. 0.1%, p=0.025). A significant reduction in body weight was also observed (-2.5 kg vs. +1.0 kg, respectively p=0.014). There were no significant differences in adverse events, immunosuppressive drug levels, or eGFR between the two treatment groups (78). A small retrospective single-center observational study consisting of 97 heart transplant recipients with PTDM demonstrated that empagliflozin-based treatment (n=20) resulted in a significant reduction in body weight (p=0.05), BMI (p=0.04), mean furosemide dose (p=0.05), and systolic and diastolic blood pressure (p=0.03) compared with control (non-empaglifloxin-based treatment, n=77) at 12-month follow-up. There was a statistically non-significant mean reduction in A1C of 0.6%. No serious adverse events were observed (80). Based on the study findings the investigators suggest that SGLT-2 inhibitors are suitable for use following heart transplantation (81). Reported adverse effects associated with SGLT2 use include increased risk for urinary tract infections, genital candidiasis, euglycemic diabetic ketoacidosis, and acute kidney injury. The latter presumably due to its effects on afferent arteriolar vasoconstriction and its natriuretic and diuretic effects. Distal limb amputation and Fournier gangrene associated with SGLT2i use have not consistently been demonstrated.
There have been no consensus treatment guidelines for PTDM. The choice of individual agents should be based on potential advantages and disadvantages of different classes of agents. Unless contraindicated, GLP1 receptor agonist may be considered in kidney transplant recipients with established CVD (or multiple CVD risk factors) whereas SGLT2i may be the preferred agent for those with a history of heart failure. SGLT2i use may have the added benefit of renoprotection independent of its glucose-lowering effects. Failure to achieve glycemic control despite multiple antihyperglycemic agent combination therapy generally requires initiation of insulin therapy. The 2014 international consensus guidelines on the screening, diagnosis, and management of early posttransplant hyperglycemia and PTDM is shown in Figure 3. Although evidenced-based recommendations are lacking, within the past several years newer injectable antidiabetic agents have increasingly been used. The authors’ suggested protocol for screening, diagnosis, and management of early post transplantation hyperglycemia and PTDM is shown in Figure 5 (practice varies among centers).

Figure 5.
Suggested screening and management of PTDM (opinion-based)
mo, month; AHA, American Heart Association; KDIGO, Kidney Disease Improving Global Outcomes
SUMMARY
PTDM is a common complication after solid organ transplantation and has variably been reported to be associated with increased morbidity and mortality. Risk stratification, intervention to minimize risk and early diagnosis may alleviate the incidence of PTDM and improve outcomes following solid organ transplantation. The 2014 International Consensus Guidelines suggest expanding screening tests for PTDM using postprandial glucose monitoring and HbA1C test. However, the latter should be used with caution in the early posttransplant period. A normal A1C does not exclude the diagnosis of PTDM in the presence of early posttransplant anemia and/or dynamic kidney allograft function. Whether intravenous iron therapy and/or the use of erythropoietin stimulating agent result in falsely low A1C levels remains to be studied. Currently early initiation of basal insulin therapy in patients with new onset hyperglycemia during the first post transplantation week to preserve β-cell function and progression to overt PTDM cannot be routinely recommended. Management of established late PTDM should follow the conventional approach and guidelines established for the general population. When lifestyle modification fails to achieve glycemic control, medical intervention is often necessary. The choice of one antihyperglycemic agent over the other should be based on the potential advantages and disadvantages of individual agents. Metformin appears safe in kidney transplant recipients with mild to moderate renal impairment (eGFR 30-60 mL/min). SGLT2 inhibitor has been suggested to be suitable for use following heart transplantation. Its use after kidney transplantation should be individualized. Similar to the general population, insulin therapy should be considered in individuals with suboptimal glycemic control despite multiple antihyperglycemic agent combination therapy.
REFERENCES
- 1.
- Starlz TE. Experience in renal transplantation. Philadelphia: Saunders 1964:111.
- 2.
- Gunnarsson R, Arner P, Lundgren G, et al. Steroid diabetes after renal transplantation. A preliminary report. Scan Urol Nephrol Suppl. 1977;42:191–194. [PubMed: 356208]
- 3.
- Araki M, Flechner SM, Ismail HR, et al. Posttransplant diabetes mellitus in kidney transplant recipients receiving calcineurin or mTOR inhibitor drugs. Transplantation. 2006;81(3):335–341. [PubMed: 16477217]
- 4.
- Crutchlow MF, Bloom RD. Transplant-associated hyperglycemia: a new look at an old problem. Clin J Am Soc Nephrol. 2007;2(2):343–355. [PubMed: 17699434]
- 5.
- Bloom RD, Crutchlow MF. New-onset diabetes mellitus in the kidney recipients: diagnosis and management strategies. Clinical J Am Soc Nephrol. 2008;3:S38–48. [PMC free article: PMC3152270] [PubMed: 18309002]
- 6.
- Pham PT, Pham PVC, Lipshutz G. New onset diabetes mellitus after solid organ transplantation. Endocrinol Metab Clin North Am. 2007;36(4):873–890. [PubMed: 17983926]
- 7.
- Sharif A, Hecking M, de Vries AP, et al. Proceedings from an international consensus meeting on posttransplantation diabetes mellitus: recommendations and future directions. Am J Transplant. 2014; 14(9):1992-2000.
- 8.
- Pham PT, Pham PM, Pham SV, Pham PA, Pham PC. New onset diabetes after transplantation (NODAT): an overview. Diabetes Metab Syndr Obes. 2011;4:175–86. [PMC free article: PMC3131798] [PubMed: 21760734]
- 9.
- Davidson J, Wilkinson AH, Dantal J, et al. New-onset diabetes after transplantation: 2003 International Consensus Guidelines. Transplantation. 2003;7:SS3–SS24. [PubMed: 12775942]
- 10.
- American Diabetes Association. Standards of Medical Care in Diabetes 2011. Diabetes Care. 2011;34:S11. [PMC free article: PMC3006050] [PubMed: 21193625]
- 11.
- Ng JM, Cooke M, Bhandari S, et al. The effect of iron and erythropoietin treatment on the A1C of patients with diabetes and chronic kidney disease. Diabetes Care. 2010;33(11):2310–2313. [PMC free article: PMC2963485] [PubMed: 20798337]
- 12.
- Baid S, Cosimi AB, Farrell ML, et al. Posttransplant diabetes mellitus in liver transplant recipients: risk factors, temporal relationship with hepatitis C virus allograft hepatitis, and impact on mortality. Transplantation. 2001;72:1066–1072. [PubMed: 11579302]
- 13.
- Knobler H, Stagnaro-Green A, Wallenstein S, et al. Higher incidence of diabetes in liver transplant recipients with hepatitis C. J Clin Gastroenterol. 1998;26:30–33. [PubMed: 9492860]
- 14.
- Ye X, Kuo H-T, Sampaio MS, Jiang Y, Bunnapradist S. Risk factors for the development of new-onset diabetes mellitus after transplant in adult lung transplant recipients. Clin Transplant. 2010 [PubMed: 21175848]
- 15.
- Lieber SR, Lee RA, Jiang Y, et al. The Impact of PostTransplant Diabetes Mellitus on Liver Transplant Outcomes. Clin Transplant. 2019 Jun;33(6):e13554. [PMC free article: PMC6995642] [PubMed: 30927288] [CrossRef]
- 16.
- Yusen RD, Edwards LB, Anne I, Dipchand AI, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-third Adult Lung and Heart-Lung Transplant Report-2016; Focus Theme: Primary Diagnostic Indications for Transplant. J Heart Lung Transplant. 2016 Oct;35(10):1170–1184. Epub 2016 Sep 13. [PubMed: 27772669] [CrossRef]
- 17.
- Cosio FG, Pesavento TE, Osei K, et al. Posttransplant diabetes mellitus: increasing incidence in renal allograft recipients transplanted in recent years. Kid Int. 2001;59(2):732–737. [PubMed: 11168956]
- 18.
- Kasiske BL, Snyder JJ, Gilbertson D, et al. Diabetes mellitus after kidney transplantation in the United States. Am J transplant. 2003;3(2):178–185. [PubMed: 12603213]
- 19.
- Martinez-Castelao A, Hernandez MD, Pascual J, et al. Detection and treatment of post kidney transplant hyperglycemia: a Spanish multicenter cross-sectional study. Transplant Proc. 2005;37:3813–3816. [PubMed: 16386547]
- 20.
- Quaglia M1. The Role of TCF7L2 rs7903146 in Diabetes After Kidney Transplant: Results From a Single-Center Cohort and Meta-Analysis of the Literature. Transplantation. 2016;100(8):1750–1758. Terrazzino S, Musetti C, et al. [PubMed: 26555947]
- 21.
- Jenssen T, Hartmann A. Post-transplant diabetes mellitus in patients with solid organ transplants. Nat Rev Endocrinol. 2019;15(3):172–188. [PubMed: 30622369]
- 22.
- Xue M, Lv C, Chen X, et al. Donor liver steatosis: A risk factor for early new-onset diabetes after liver transplantation. J Diabetes Investig. 2017 Mar;8(2):181–187. [PMC free article: PMC5334314] [PubMed: 27511316] [CrossRef]
- 23.
- Elens L, Sombogaard F, Hesselink D, et al. Single-nucleotide polymorphism in P450 oxidoreductase and peroxisome proliferator-activated receptor are associated with the development of new onset diabetes after transplantation in kidney transplant recipients treated with tacrolimus. Pharmacogenet Genomics. 2013; 23:649-657 (21)
- 24.
- El Essawy B, Kandeel F. Pre, peri and posttransplant diabetes mellitus. Current opinion Nephrol Hypertens. 2018; 28: 47-57 (22)
- 25.
- Joss N, Staatz CE, Thomson AH, et al. Predictors of new onset diabetes after renal transplantation 2007;21(1): 136- 143.
- 26.
- Malik RF, Jia Y, Mansour SG, et al. Post-transplant Diabetes Mellitus in Kidney Transplant Recipients: A Multicenter Study. Kidney360. 2021 Jun 2;2(8):1296-1307.
- 27.
- Kuo H-T, Sampaio MS, Vincenti F, et al. Associations of pretransplant diabetes mellitus, New-Onset Diabetes Mellitus after Transplant, and acute rejection with transplant outcomes: an analysis of the Organ Procurement and Transplant Network/United Network for Organ Sharing (OPTN/UNOS) database. 2010;56(6): 1026-1028.
- 28.
- Burroughs TE, Swindle J, Takemoto S, et al. Diabetic complications associated with new-onset diabetes mellitus in renal transplant recipients. Transplantation. 2007;83(8):1027. [PubMed: 17452891]
- 29.
- Kuo HT, Lum E, Martin P, et al. Effect of diabetes and acute rejection on liver transplant outcomes, an analysis of the OPTN/UNOS database. Liver Transpl. 2016;22(16):796–804. [PubMed: 26850091]
- 30.
- Roccaro GA, Goldberg DS, Hwang WT, et al. Sustained post transplantation diabetes is associated with long-term major cardiovascular events following liver transplantation. Am J Transplant. 2018;18(1):207–215. [PMC free article: PMC5740009] [PubMed: 28640504]
- 31.
- Peláez-Jaramillo MJ, Cárdenas-Mojica AA, Gaete PV, Mendivil CO. Post-Liver Transplantation Diabetes Mellitus: A Review of Relevance and Approach to Treatment. Diabetes Ther. 2018 Apr;9(2):521–543. [PMC free article: PMC6104273] [PubMed: 29411291] [CrossRef]
- 32.
- Foroutan F, Alba AC, Guyatt G, et al. Predictors of 1-year mortality in heart transplant recipients: a systematic review and meta-analysis. Heart. 2018 Jan;104(2):151–160. [PubMed: 28855271]
- 33.
- Kim HJ, Jung SH, Kim JJ, et al. New-onset diabetes mellitus after heart transplantation: incidence, risk factors, and impact on clinical outcome. Circ J. 2017:806–814. [PubMed: 28344200]
- 34.
- Christie JD, Edwards LB, Kucheryavaya AY, et al. The registry of the international society of heart and lung transplantation: twenty-seventh official adult transplant report 2010. J Heart Lung Transplant. 2010:1105–1118. [PubMed: 20870165]
- 35.
- Hackman KL, Snell GI, Bach LA. Poor glycemic control is associated with decreased survival in lung transplant recipients. Transplantation. 2017:2200–2205. [PubMed: 27798516]
- 36.
- Hackman KL, Bailey MJ, Snell GI. Diabetes is a major risk factor for mortality after lung transplant. Am J Transplant. 2014;14:438–445. [PubMed: 24401019]
- 37.
- Wilkinson AH, Davidson J, Dotta F, et al. Guidelines for the treatment and management of new-onset diabetes after transplantation. Clin Transplant. 2005;19:291–298. [PubMed: 15877787]
- 38.
- Nathan DM, Davidson MB, DeFronzo RA, et al. Impaired fasting glucose and impaired glucose tolerance. Diabetes Care. 2007;30:753. [PubMed: 17327355]
- 39.
- Pham PT, Pham SV, Lee M, et al. Evaluation of the potential kidney transplant candidates. In: Pham PT, Pham PC. Quick Guide to Kidney Transplantation: From Initial Evaluation to Long-Term Post-Transplant Care. First Edition. Lippincott, Williams and Wilkins 2019; pp 40-58.
- 40.
- Chakkera HA, Weil EJ, Pham PT, et al. Can new-onset diabetes after kidney transplant be prevented? Diabetes Care. 2013;36:1406–1412. [PMC free article: PMC3631828] [PubMed: 23613600]
- 41.
- Pham PT, Edling KL, Chakkera HA, et al. Screening strategies and predictive diagnostic tools for the development of newonset diabetes mellitus after transplantation: an overview. Diabetes Metab Syndr Obes. 2012;5:379–87. [PMC free article: PMC3496371] [PubMed: 23152690]
- 42.
- Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. [PMC free article: PMC1370926] [PubMed: 11832527]
- 43.
- Kim Y, Kim JR, Choi H, et al. Patients with persistent newonset diabetes after transplantation have greater weight gain after kidney transplantation. J Korean Med Sci. 2013 Oct;28(10):1431–4. [PMC free article: PMC3792595] [PubMed: 24133345]
- 44.
- Sharif A, Moore R, Baboolal K. Influence of lifestyle modification in renal transplant recipients with postprandial hyperglycemia. Transplantation. 2008;85:353–358. [PubMed: 18301331]
- 45.
- Kuningas K, Driscoll J, Reena Mair R, et al. Comparing Glycemic Benefits of Active Versus Passive Lifestyle Intervention in Kidney Allograft Recipients: A Randomized Controlled Trial. Transplantation. 2020 Jul;104(7):1491–1499. [PubMed: 31568390] [CrossRef]
- 46.
- Hecking M, Werzowa J, Haidinger M, et al. Novel views on new-onset diabetes after transplantation: development, prevention and treatment. Nephrol Dial Transplant. 2013 Mar;28(3):550–66. [PMC free article: PMC4375396] [PubMed: 23328712]
- 47.
- Hecking M, Haidinger M, Doller D, et al. Early basal insulin therapy decreases new-onset diabetes after transplantation. J Am Soc Nephrol. 2012;23:739–749. [PMC free article: PMC3312499] [PubMed: 22343119]
- 48.
- Schwaiger E, Krenn S, Kurnikowski A et al. Early Postoperative Basal Insulin Therapy versus Standard of Care for the Prevention of Diabetes Mellitus after Kidney Transplantation: A Multicenter Randomized Trial. J Am Soc Nephrol. 2021 Aug;32(8):2083-2098. doi: 10.1681/ASN.2021010127. 552. [CrossRef]
- 49.
- Boudreaux JP, McHugh L, Canafax DM, et al. The impact of cyclosporine and combination immunosuppression on the incidence of post transplant diabetes in renal allograft recipients. Transplantation. 1987;44(3):376–381. [PubMed: 3307061]
- 50.
- Heisel O, Heisel R, Balshaw R, et al. New onset diabetes in patients receiving calcineurin inhibitors: a systematic review and meta-analysis. Am J Transplant. 2004;4(4):583–595. [PubMed: 15023151]
- 51.
- Vincenti F, Friman S, Scheuermann E, et al. Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus. Am J Transplant. 2007 Jun;7(6):1506–14. [PubMed: 17359512]
- 52.
- Rathi M, Rajkumar V, Rao N, et al. Conversion from tacrolimus to cyclosporine in patients with new-onset diabetes after renal transplant: an open-label randomized prospective pilot study. Transplant Proc. 2015 May;47(4):1158–61. [PubMed: 26036543]
- 53.
- Wissing KM, Abramowicz D, Weekers L, et al. Prospective randomized study of conversion from tacrolimus to cyclosporine A to improve glucose metabolism in patients with posttransplant diabetes mellitus after renal transplantation. Am J Transplant. 2018;18(7):1726–1734. [PubMed: 29337426]
- 54.
- Pascual J, Royuela A, Galeano C, et al. Very early steroid withdrawal or avoidance for kidney transplant recipients: A systematic review. Nephroll Dial Transplant. 2012;27:825–832. [PubMed: 21785040]
- 55.
- Pascual J, Galeano C, Royuela A, et al. A systematic review on steroid withdrawal between 3 and 6 months after kidney transplantation. Transplantation. 2010;90:343–349. [PubMed: 20574419]
- 56.
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32(1):193. [PMC free article: PMC2606813] [PubMed: 18945920]
- 57.
- The 2022 American Diabetes Association. Standards of Medical Care in Diabetes-2022 Abridged for Primary Care Providers. 10.2337/cd22-as01. [CrossRef]
- 58.
- Alnasrallah B, Goh TL, Chan LW, et al. Transplantation and diabetes (Transdiab): a pilot randomised controlled trial of metformin in impaired glucose tolerance after kidney transplantation. BMC Nephrol. 2019 Apr 29;20(1):147. [PMC free article: PMC6489311] [PubMed: 31035960] [CrossRef]
- 59.
- Maedler K, Carr RD, Bosco D, et al. Sulfonylurea induced beta-cell apoptosis in cultured human islets. The J of Clin End and Metab. 2005;90:501–506. [PubMed: 15483097]
- 60.
- Mu J, Petrov A, Eiermann GJ, Woods J, et al. Inhibition of DPP-4 with sitagliptin improves glycemic control and restores islet cell mass and function in a rodent model of type 2 diabetes. Eur J Pharmacol. 2009;623(1-3):148–154. [PubMed: 19765579]
- 61.
- Cho JM, Jang HW, Cheon H, et al. A novel dipeptidyl peptidase IV inhibitor DA-1229 ameliorates streptozocin induced diabetes by increasing β-cell replication and neogenesis. Diabetes Res Clin Pract. 2011;91(1):72–79. [PubMed: 21093089]
- 62.
- Halden TAS, Asberg A, Vik K, et al. Short-term efficacy and safety of sitagliptin treatment in long-term stable renal recipients with new-onset diabetes after transplantation. Nephrol Dial Transplant. 2014;14:115–123.
- 63.
- Haidinger M, Werzowa J, Hecking M, et al. Efficacy and safety of vildagliptin in new-onset diabetes after kidney transplantation. A randomized, double-blind, placebo controlled trial. Am J Transplant. 2014;14:115–123. [PubMed: 24279801]
- 64.
- Boerner BP, Miles CD, Shivaswamy V. Efficacy and safety of sitagliptin for the treatment of new-onset diabetes after renal transplantation. Int J Endocrinol. 2014;2014:617638. Epub 2014 Apr 10. [PMC free article: PMC4003765] [PubMed: 24817885] [CrossRef]
- 65.
- Werzowa J, Hecking M, Haidinger M, et al. Vildagliptin and pioglitazone in patients with impaired glucose tolerance after kidney transplantation: a randomized, placebo-controlled clinical trial. Transplantation. 2013;95(3):456–462. [PubMed: 23380864]
- 66.
- Abdelaziz TS. Ali AY1, Fatthy M. Efficacy and safety of Dipeptidyl Peptidase-4 Inhibitors in kidney transplant recipients with Post-transplant diabetes mellitus (PTDM)-a systematic review and Meta-Analysis. Curr Diabetes Rev. 2019 Mar 21; doi[Epub ahead of print] [PubMed: 30907326] [CrossRef]
- 67.
- Gueler I, Mueller S, Helmschrott M, et al. Effects of vildagliptin (Galvus®) therapy in patients with type 2 diabetes mellitus after heart transplantation. Drug Des Devel Ther. 2013 Apr 8;7:297–303. [PMC free article: PMC3623547] [PubMed: 23630415]
- 68.
- Scirica BM, Braunwald E, Raz I, et al. Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation. 2014 Oct 28;130(18):1579–1588. Epub 2014 Sep 4. [PubMed: 25189213] [CrossRef]
- 69.
- Zannad F, Cannon CP. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015 May 23;385(9982):2067–76. Epub 2015 Mar 10. [PubMed: 25765696] [CrossRef]
- 70.
- McGuire DK, Van de Werf F, Armstrong PW, et al. Association Between Sitagliptin Use and Heart Failure Hospitalization and Related Outcomes in Type 2 Diabetes Mellitus: Secondary Analysis of a Randomized Clinical Trial. JAMA Cardiol. 2016 May 1;1(2):126–135. [PubMed: 27437883] [CrossRef]
- 71.
- Pinelli NR, Patel A, Salinitri FD. Coadministration of liraglutide with tacrolimus in kidney transplant recipients: a case series. Diabetes Care. 2013 Oct;36(10):e171–172. [PMC free article: PMC3781489] [PubMed: 24065848]
- 72.
- Sadhu AR, Schwartz SS, Herman ME. The rationale for use of incretins in the management of New Onset Diabetes after Transplantation (NODAT). Endocr Pract. 2015 Jul;21(7):814–822. [PubMed: 25786557]
- 73.
- Jin J, Jin L, Luo K, et al. Effect of Empagliflozin on Tacrolimus-Induced Pancreas Islet Dysfunction and Renal Injury. Am J Transplant. 2017;17(10):2601–2616. [PubMed: 28422431]
- 74.
- Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019 Jan 5;393(10166):31–39. Epub 2018 Nov 10. [PubMed: 30424892] [CrossRef]
- 75.
- Zelniker TA, Braunwald E. Mechanisms of Cardiorenal Effects of Sodium-Glucose Cotransporter 2 Inhibitors: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020 Feb 4;75(4):422–434. doiErratum in: J Am Coll Cardiol. 2020 Sep 22;76(12):1505. PMID: 32000955. [PubMed: 32000955] [CrossRef]
- 76.
- Yang S, He W, Zhao L, Mi Y. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with kidney outcomes in patients with type 2 diabetes: A systematic review and network meta-analysis. PLoS One. 2022 Apr 14;17(4):e0267025. doiPMCID: PMC9009659. [PMC free article: PMC9009659] [PubMed: 35421174] [CrossRef]
- 77.
- Zannad F, Ferreira JP, Pocock SJ, et al. Cardiac and Kidney Benefits of Empagliflozin in Heart Failure Across the Spectrum of Kidney Function: Insights From EMPEROR-Reduced. Circulation. 2021 Jan 26;143(4):310–321. [PMC free article: PMC7834910] [PubMed: 33095032] [CrossRef]
- 78.
- Halden TAS, Kvitne KE, Karsten Midtvedt K, et al. Efficacy and Safety of Empagliflozin in Renal Transplant Recipients With Posttransplant Diabetes Mellitus. Diabetes Care. 2019 Jun;42(6):1067–1074. [PubMed: 30862658]
- 79.
- Mahling M, Schork A, Nadalin S, et al. Sodium-Glucose Cotransporter 2 (SGLT2) Inhibition in kidney transplant recipients with diabetes mellitus. Kidney Blood Press Res. 2019;44(5):984–992. [PubMed: 31437852]
- 80.
- Cehic MG, Christopher A, Muir CA, et al. Efficacy and Safety of Empagliflozin in the Management of Diabetes Mellitus in Heart Transplant Recipients. Transplant Direct. 2019 May;5(5):e450. [PMC free article: PMC6511439] [PubMed: 31165085] [CrossRef]
- 81.
- Cehic MG, Nundall N, Greenfield JR, et al. Management Strategies for Posttransplant Diabetes Mellitus after Heart Transplantation: A Review. J Transplant. 2018 Jan 29;2018:1025893. doi: 10.1155/2018/1025893. eCollection 2018. [CrossRef]
- Sustained Virological Response Is Associated with a Decreased Risk of Posttransplant Diabetes Mellitus in Liver Transplant Recipients with Hepatitis C-Related Liver Disease.[Liver Transpl. 2018]Sustained Virological Response Is Associated with a Decreased Risk of Posttransplant Diabetes Mellitus in Liver Transplant Recipients with Hepatitis C-Related Liver Disease.Roccaro GA, Mitrani R, Hwang WT, Forde KA, Reddy KR. Liver Transpl. 2018 Dec; 24(12):1665-1672.
- Risk factors and outcomes associated with posttransplant diabetes mellitus in kidney transplant recipients.[Transplant Proc. 2010]Risk factors and outcomes associated with posttransplant diabetes mellitus in kidney transplant recipients.Siraj ES, Abacan C, Chinnappa P, Wojtowicz J, Braun W. Transplant Proc. 2010 Jun; 42(5):1685-9.
- Posttransplantation Diabetes Mellitus Among Solid Organ Recipients in a Danish Cohort.[Transpl Int. 2022]Posttransplantation Diabetes Mellitus Among Solid Organ Recipients in a Danish Cohort.Dos Santos Q, Hornum M, Terrones-Campos C, Crone CG, Wareham NE, Soeborg A, Rasmussen A, Gustafsson F, Perch M, Soerensen SS, et al. Transpl Int. 2022; 35:10352. Epub 2022 Apr 5.
- Review An update review of post-transplant diabetes mellitus: Concept, risk factors, clinical implications and management.[Diabetes Obes Metab. 2024]Review An update review of post-transplant diabetes mellitus: Concept, risk factors, clinical implications and management.Kanbay M, Copur S, Topçu AU, Guldan M, Ozbek L, Gaipov A, Ferro C, Cozzolino M, Cherney DZI, Tuttle KR. Diabetes Obes Metab. 2024 Jul; 26(7):2531-2545. Epub 2024 Apr 1.
- Review Post-Transplantation Diabetes Mellitus.[Diabetes Ther. 2020]Review Post-Transplantation Diabetes Mellitus.Ahmed SH, Biddle K, Augustine T, Azmi S. Diabetes Ther. 2020 Apr; 11(4):779-801. Epub 2020 Feb 24.
- Diabetes Mellitus After Solid Organ Transplantation - EndotextDiabetes Mellitus After Solid Organ Transplantation - Endotext
Your browsing activity is empty.
Activity recording is turned off.
See more...
