NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-.
ABSTRACT
Heart failure (HF) is an underappreciated complication of diabetes. HF occurs in individuals with diabetes at higher rates, even in the absence of other HF risk factors such as coronary artery disease and hypertension. Comorbid ischemic heart disease and cardiovascular risk factors significantly contribute to the etiology of cardiomyopathy and HF in patients with diabetes. In addition, long-standing diabetes can independently cause subclinical alteration in cardiac structure and function, eventually leading to the development and progression of HF. A complex interplay between numerous mechanisms underlies the pathophysiologic links between diabetes and HF. Patients with concurrent diabetes and HF have impaired quality of life and a poor prognosis with a high risk of hospitalization and mortality. Despite the solid epidemiologic link between poor glycemic control and HF risk, the effects of intensified glycemic control in preventing HF remain controversial. Large-scale cardiovascular outcome trials published since 2015 have confirmed the efficacy and safety of sodium-glucose co-transporter-2 inhibitors (SGLT2) inhibitors in preventing HF among patients with type 2 diabetes mellitus. In addition, several dedicated major clinical trials confirmed the cardiovascular benefits of SGLT2 inhibitors in patients with established HF, regardless of left ventricular ejection fraction or diabetes status. Furthermore, high-quality data from these clinical trials transformed SGLT2 inhibitors from glucose-lowering agents to HF drugs. This chapter outlines the complex relationship between HF and diabetes, focusing on the epidemiology, pathophysiology, and prognostic implications. Additionally, we review the current knowledge on identifying subclinical cardiac remodeling, predicting HF risk, and preventing HF in diabetes. We also summarize the recent evidence and guideline recommendations for the pharmacological treatment of patients with coexisting HF and diabetes. For complete coverage of all related areas of Endocrinology, please visit our on-line FREE web-text, WWW.ENDOTEXT.ORG.
INTRODUCTION
Diabetes is a risk factor for cardiomyopathy and heart failure (HF) independent of traditional cardiovascular (CV) disease (CVD) risk factors such as hypertension and coronary artery disease (CAD) (1–4). The universal definition of HF recognizes patients with diabetes as “at risk for HF” (Stage A). Asymptomatic individuals with at least one of the following: 1) evidence of structural heart disease, 2) abnormal cardiac function, or 3) elevated cardiac natriuretic peptide or troponins are considered to have “pre-HF” (stage B). According to this classification, HF (stage C) is defined as a clinical syndrome with signs or symptoms of HF caused by an abnormality in cardiac structure and function and corroborated by elevated natriuretic peptide or objective evidence of cardiogenic congestion (pulmonary or systemic) (3,5).
The prevalence of diabetes is approximately 10.2% in the U.S. population, and HF affects 9 to 22% of patients with diabetes (6–10). In clinical trials of antidiabetic agents, HF was present in 4 to 30% of participants with diabetes (11). On the other hand, the prevalence of pre-diabetes or diabetes was 30 to 40% among individuals enrolled in HF trials (12,13).
Longstanding diabetes alters cardiac structure and function, resulting from the direct effects of abnormal myocardial metabolism and insulin resistance (IR) even without atherosclerotic CAD (14). The pathophysiologic link between diabetes and HF is complex and multifactorial, involving various abnormal biochemical pathways including but not limited to abnormal calcium signaling, deranged glucose/fatty acid metabolism, and inflammatory pathways contributing to myocardial fibrosis, stiffness, and hypertrophy (7,15,16). A complex interaction of these mechanisms can cause asymptomatic diastolic and systolic dysfunction, eventually leading to the clinical syndrome of HF. Conversely, HF is also associated with a higher prevalence of diabetes and is considered a predictor of future risk of type 2 diabetes mellitus (T2DM) (17).
Left ventricular (LV) dysfunction in patients with diabetes may present with three different HF phenotypes, such as HF with preserved LV ejection fraction (LVEF ≥ 50%; HFpEF), HF with mildly-reduced LVEF (HFmrEF; LVEF 40-49%), and HF with reduced LVEF (HFrEF; LVEF ≤40%) (3). Diagnosing HFpEF and HFmrEF is often challenging since the symptomatology of HF may overlap with other comorbidities such as obesity, lung disease, and chronic kidney disease (CKD). Therefore, the guidelines usually recommend incorporating additional objective diagnostic criteria such as elevated natriuretic peptides or imaging evidence of either structural heart disease or diastolic dysfunction (18).
The coexistence of diabetes and HF is a poor prognostic factor, posing a greater risk of HF hospitalization, all-cause mortality, and CVD mortality. For instance, epidemiologic studies indicated a 50-90% higher risk of CVD mortality in patients with HF and diabetes, regardless of HF phenotype (12,19). HF patients without DM are at increased risk of developing glycemic abnormalities. In addition, newly diagnosed pre-diabetes was associated with a significantly higher risk of all-cause and CV mortality in HF patients. These findings underscore the importance of screening for pre-diabetes or diabetes among patients with HF (17,20,21). Moreover, early assessment with echocardiography can be helpful for the detection of subclinical structural abnormalities and myocardial dysfunction in asymptomatic patients with diabetes.
The pathophysiology of diabetes-related HF is complex, and despite significant advances over the past decades, many areas are still poorly understood. Since 2015, several landmark clinical trials on sodium-glucose co-transporter-2 (SGLT2) inhibitors and glucagon-like peptide-1 receptor agonists (GLP1-RA) have revolutionized our understanding of CVD risk reduction in patients with T2DM and have led to a paradigm shift in the clinical practice recommendations for the management of T2DM (22). Incredible evidence from the CVD outcome trials (CVOTs) has confirmed the significant improvement in HF outcomes with SGLT2 inhibitors in patients with or without diabetes. These findings increased medical communities' awareness and interest in the links between diabetes and HF.
The American Diabetes Association (ADA) now recommends using SGLT2 inhibitors as first-line agents in T2DM patients with a high risk of or established HF (23). In addition, several dedicated major randomized clinical trials (RCTs) confirmed the CVD benefits of SGLT2 inhibitors in patients with established HF, regardless of LVEF or diabetes status. Moreover, these RCTs transformed SGLT2 inhibitors from glucose-lowering agents to HF drugs. The 2022 American College of Cardiology (ACC) Foundation / American Heart Association (AHA) / Heart Failure Society of America (HFSA) guideline for the management of HF recommended the use of SGLT2 inhibitors for the treatment of HF, regardless of LVEF (3).
This chapter outlines the complex relationship between HF and diabetes, focusing on the epidemiology, pathophysiology, and prognostic implications. Additionally, we review the current knowledge on identifying subclinical cardiac remodeling, predicting HF risk, and preventing HF in diabetes. We also summarize the recent evidence and guideline recommendations for the pharmacological treatment of patients with coexisting HF and diabetes.
EPIDEMIOLOGY OF DIABETES AND HF
There is a bidirectional link between diabetes and HF (24). Diabetes, either type 1 or type 2, is a strong risk factor for developing HF (25–27). In addition, HF may contribute to the pathogenesis of IR and T2DM (28). The shared underlying risk factors and the overlap of the pathophysiological mechanisms play a critical role in the frequent coexistence of T2DM and HF.
Based on the data from the National Health and Nutrition Examination Survey (NHANES) from 2015 to 2018, the prevalence of HF is 2.3% in the US general adult population (29). The prevalence of HF in individuals with diabetes ranges between 9% and 22%, depending on the characteristics of the population studied (6,8,9). Diabetes is also highly prevalent among patients with HF. In major contemporary drug trials of HF, 32% to 43% of patients with chronic HF had coexisting diabetes (12,30,31). A report from a nationwide US registry (NHANES 2005-2016) demonstrated that, among patients with HF, the prevalence rates of diagnosed and undiagnosed T2DM were 34.7% and 12.8%, respectively (32).
HF is a common but often neglected complication of diabetes (33). HF and cardiomyopathy have a heterogeneous etiology in patients with diabetes (Figure 1 and Figure 2). The strong link between diabetes and CAD, hypertension, and renal disease plays a significant role in the development of cardiomyopathy and HF in patients with diabetes (34). Moreover, HF occurs in individuals with diabetes at higher rates, even in the absence of other HF risk factors (16,35).
Diabetic Cardiomyopathy
Diabetic cardiomyopathy, which lacks a standardized clinical definition, generally refers to diabetes-related myocardial dysfunction without other potential causes (36). A report by Lundbæk in 1954 described the concept of diabetes directly causing myocardial dysfunction (37). In 1972, a landmark study by Rubler et al. described diabetic cardiomyopathy as a new clinical entity by reporting the post-mortem results of 4 patients with diabetes-related HF and dilated cardiomyopathy without other apparent causes of myocardial dysfunction (14). The initial reports of diabetic cardiomyopathy referred to a dilated LV with eccentric hypertrophy and LV systolic dysfunction (HFrEF). Nevertheless, more recent clinical studies described HFpEF with concentric LV hypertrophy and LV diastolic dysfunction as a distinct phenotype of cardiomyopathy rather than being an intermediate form between risk factors and HFrEF (38). The transition from HFpEF to HFrEF does not appear to occur as commonly as it was once presumed.
Epidemiologic Evidence
Evidence from large-scale epidemiologic studies has confirmed the strong link between diabetes and HF. For instance, reports from the prospective Framingham Heart Study in the 1970s indicated that individuals with diabetes had 2-fold (in men) to 5-fold (in women) higher risk of developing HF than individuals without diabetes after adjustment for other risk factors (2,39). Similarly, contemporary cohort studies suggested that diabetes is independently associated with a 1.7 to 2.5-fold greater risk of HF (6,40).
A recent nationwide cohort study from Sweden including >679.000 patients with T2DM and >2 million matched control subjects demonstrated that a diagnosis of T2DM was associated with HF risk even if CVD risk factors, such as glycated hemoglobin, systolic blood pressure (BP), estimated glomerular filtration rate, and lipids were within a target range (41). The study also demonstrated that CVD complications have significantly declined over the past 20 years in individuals with and without T2DM. However, the decline in the rate of HF in patients with T2DM has plateaued over recent years. One potential explanation for this finding is the obesity epidemic, as adiposity plays a major role in the development of HF in patients with diabetes. For instance, a recent analysis of 2 US cohort studies demonstrated that overall obesity, abdominal obesity, and fat mass were strongly associated with a greater risk of HF in participants with diabetes. Interestingly, a similar independent association was absent in those without T2DM (42).
Ischemic heart disease is more frequently seen in HF patients with coexistent T2DM than those without T2DM (63% vs. 47%). Moreover, ~90% of the patients with T2DM and HF of non-ischemic etiology have at least one comorbidity that can contribute to HF development, such as hypertension, atrial fibrillation, valvular disease, or pulmonary disease (43).
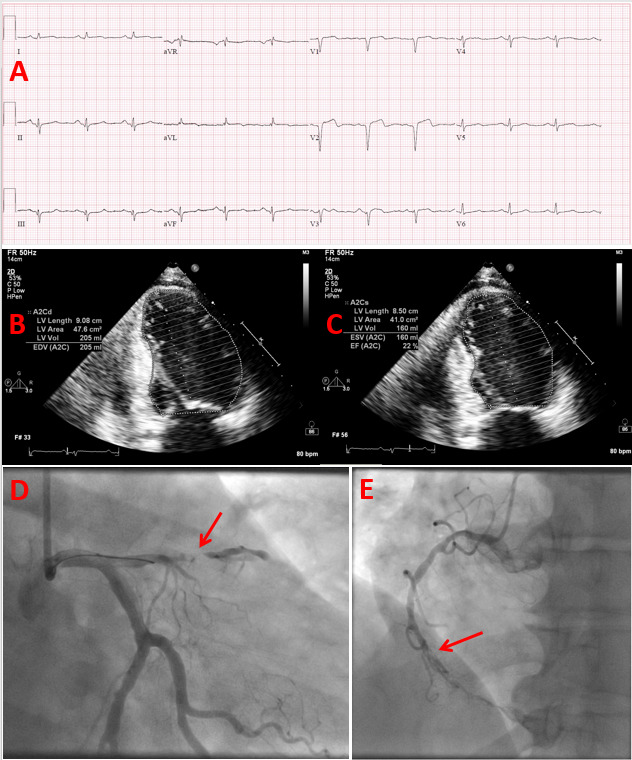
Figure 1.
Heart failure with reduced ejection fraction due to ischemic cardiomyopathy in a patient with uncontrolled type 2 diabetes. 57-year-old female patient with a history of uncontrolled type 2 diabetes (HbA1c = 12.0) and active tobacco abuse presented with a 2-day history of intermittent midsternal chest pain. (A) Her ECG on presentation demonstrated findings of acute/recent anteroseptal myocardial infarct and old/age indeterminate inferior myocardial infarct, and her serum troponin I was markedly elevated. (B and C) Her echocardiography revealed a dilated left ventricle with severely reduced systolic function, an ejection fraction of 20-25%, and akinetic anterior and inferior wall segments. Her coronary angiography, which was performed emergently, demonstrated subacute occlusion of the proximal segment of the anterior descending artery (arrow in image D) and chronic total occlusion of the middle segment of the right coronary artery (arrow in image E).
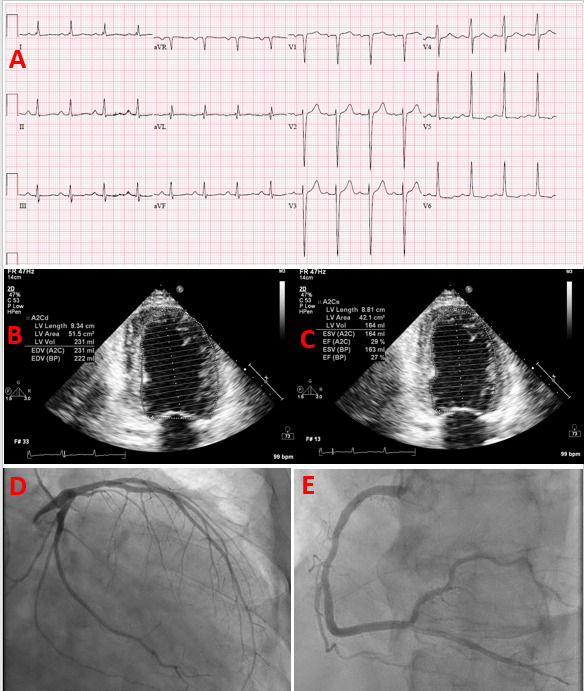
Figure 2.
Heart failure with reduced ejection fraction due to non-ischemic cardiomyopathy in a patient with uncontrolled type 2 diabetes. 59-year-old male patient with a history of hypertension (medically managed), class I obesity, hyperlipidemia, moderate alcohol consumption, and undiagnosed type 2 diabetes (HbA1c = 13.5) presented with dyspnea on exertion, orthopnea, and leg edema and he was admitted with the diagnoses of acute decompensated heart failure. (A) His ECG on presentation demonstrated left ventricular hypertrophy with repolarization abnormalities. (B and C) His echocardiography revealed a dilated left ventricle with severely reduced systolic function and diffuse hypokinesis, an ejection fraction of 25-30%, and eccentric left ventricular hypertrophy. (D and E) His coronary angiography, performed for ischemic evaluation, demonstrated no evidence of significant epicardial coronary artery disease. The patient’s non-ischemic cardiomyopathy was attributed to a mixed presentation of alcoholic and diabetic cardiomyopathy and hypertensive heart disease.
Diabetes is an independent predictor of progression from preclinical HF (stage A and stage B) to clinic HF (stage C) (44). A population-based analysis on the National Scottish Register found that HF hospitalization risk was ~2-fold higher among patients with diabetes than those without diabetes (45). A prospective cohort study, including individuals from the southeastern U.S., demonstrated that hypertension and diabetes were associated with the highest HF risk in white and black participants (46). The population-attributable risk of HF was highest for hypertension (31.8%), followed by diabetes (17%). A population-based case-control study also observed an attributable risk of HF at ~17% for diabetes, without any significant difference between HFpEF and HFrEF (47).
Epidemiologic studies have demonstrated a higher incidence of HF in men than in women with diabetes (27,40). This finding is consistent with the strong association between HF risk and male sex in the general population. However, interestingly, diabetes contributes to the future risk of HF more in women than in men, as supported by multiple epidemiologic studies (27,39). In a meta-analysis of 47 cohort studies including >12 million individuals, type 1 diabetes mellitus (T1DM) and T2DM were associated with a 47% and 9% greater excess risk of HF in women than men, respectively (48). The basis for the sex-specific disparity in HF risk attributable to diabetes remains unclear.
Prediabetes and HF Risk
Some epidemiologic studies have suggested that prediabetes may pose a risk for cardiomyopathy and HF(49). In a population-based cohort study, prediabetes was independently associated with HF with an odds ratio of 1.7 (9). A modest but significant association exists between fasting plasma glucose levels and the risk of HF independent of an individual’s diabetes status (50).
Glycemic Control and HF Risk
Glycemic exposure predicts HF risk in individuals with T1DM and T2DM (26,27,51). A population-based prospective case-control study from the Swedish National Diabetes Register evaluated the impact of glycemic control on the future risk of HF hospitalization over a mean follow-up of 7.9 years (26). Compared to a population-based control group without diabetes, patients with T1DM had a four times higher risk of HF hospitalization. Nevertheless, the risk markedly varied depending on glycemic control or comorbidities; hazard-ratio (HR) of 2.2 (1.5–3.0; p<0·0001) in patients with hemoglobin A1c (HbA1c) ≤6.9%, HR of 11.2 (8·4–14·9; p<0·0001) in patients with HbA1c ≥9.7%. Another report from the same dataset revealed that each 1% increase in HbA1c correlated with a 20% higher risk of HF in patients with myocardial infarction and T1DM (52). Among individuals with T2DM, the excess risk of HF attributable to glycemic control varies depending on the patient’s age. For instance, poor glycemic control correlates more strongly with excess risk of HF among middle-aged adults (<55 years old). In contrast, the correlation between HbA1c and the risk of HF markedly attenuates with advanced age (27).
Age at Diagnosis of Diabetes and HF Risk
Diagnosis of diabetes at a younger age correlates with a higher risk of HF (45,53,54). A report by Rawshani et al. using the data from the Swedish National Diabetes Register demonstrated that compared to a control group without diabetes, individuals with onset of T1DM before age ten years had 12 times and those with onset during young adulthood (20 to 29 years) had five times increased risk of HF. Sattar et al., using the same registry data, evaluated the association between age at diagnosis and future HF risk among participants with T2DM (54). Their analysis revealed that adults diagnosed with T2DM before 41 years of age had a five times higher risk of HF than their counterparts without diabetes. Interestingly, T2DM diagnosis after the age of 80 years did not increase the risk of HF and was associated with a lower risk of all-cause and CV mortality. Consistently, an analysis from a US cohort demonstrated that every 5-year increase in the duration of DM was independently associated with a 17% rise in the risk of incident HF (55). As expected, the association between diabetes duration and HF risk was more prominent in patients with elevated HbA1c.
The explanations behind the association of duration and age at diabetes diagnosis and future HF risk are likely multifaceted, with a variation between T1DM and T2DM. The total glycemic load, defined as the cumulative exposure to the effects of hyperglycemia, is a predictor of complications of diabetes. The main components of the total glycemic load are the glycemic variability and the duration of diabetes determined by the age of diabetes onset, more prominently in T1DM (53). Individuals who develop T2DM at a younger age are more likely to have other comorbidities such as obesity, dyslipidemia, hypertension, nephropathy, smoking, and lower socioeconomic status when compared to their counterparts without diabetes. Furthermore, this comorbidity burden likely contributes to the relative excess risk of HF observed in patients diagnosed with T2DM at a relatively young age (54). These findings highlight the significance of delaying diabetes onset as one focus of HF prevention efforts (55).
The Relationship Between Diabetes and Comorbidities
Traditional modifiable CVD risk factors, such as hypertension, obesity, dyslipidemia, and cigarette smoking, are prevalent among individuals with diabetes. Hypertension affects 66% to 76% of adults with diabetes in the US (56). According to the 2020 National Diabetes Statistics Report, 45.8% of adults with diabetes are obese (body-mass-index [BMI] of 30 to 39.9 kg/m2), and 15.5% are morbidly obese (BMI of ≥40 kg/m2) (10).
Coexisting CVD risk factors significantly contribute to the risk of HF in patients with diabetes. A large prospective cohort study, including >270,000 participants with T2DM in the Swedish National Diabetes Register, examined the relationship between five risk factors (elevated HbA1C, dyslipidemia, albuminuria, smoking, and high BP) and CVD outcomes after a median follow-up of 5.7 years (57), The analyses revealed that participants with T2DM who had no risk-factor variables outside the target ranges had a 45% higher risk of hospitalization for HF when compared to that of a control group without diabetes. However, the excess risk of hospitalization for HF was substantially higher (HR vs. control, 11.35; 95% CI, 7.16 to 18.01) when patients with T2DM had all five risk-factor variables outside the target range. These findings indicated the importance of controlling coexisting CV risk factors for preventing HF in diabetes.
Recent reports have indicated that comorbid mental disorders may increase HF risk in individuals with diabetes. A retrospective analysis of nationwide health claims data of Korean participants demonstrated an independent association between HF risk and the number of mental disorders in patients with diabetes (58).
HF as a Risk Factor for Diabetes
Patients with HF are at risk of developing incident DM over time. Data from clinical trials showed that the incidence of new-onset diabetes among patients with HF is 7 to 11% over a 3- to 5-year follow-up period (59,60). Even though the published data is sparse, some evidence has emerged over the past two decades supporting the possible independent role of HF as a risk factor for incident T2DM (61). Analyses of prospective cohort studies and clinical trial participants demonstrated that HF at baseline might predispose the future risk of new-onset diabetes (61–64). Significant predictors of incident diabetes among individuals with HF are elevated glucose and HbA1c, higher BMI and waist circumference, longer duration of HF, and higher functional class of HF (28,61–63).
IMPACT OF DIABETES ON CARDIAC STRUCTURE AND FUNCTION
The frequent coexistence of diabetes with other comorbidities, such as hypertension and obesity, makes it difficult to understand the relative contribution of each disease entity in cardiac remodeling and dysfunction in clinical practice (65). However, growing evidence has supported an independent association between diabetes and various alterations in cardiac structure and function. These asymptomatic subclinical alterations at earlier stages can be detrimental and increase the risk of developing HF and CVD morbidity and mortality in general (44). Recognizing these subclinical alterations is critical for the early identification of high-risk patients and preventing overt HF and diabetic cardiomyopathy.
Left Ventricular Hypertrophy
LV hypertrophy (LVH) is characterized by increased LV mass due to myocardial remodeling. LVH is usually caused by a complex interaction between several factors, including hypertension, diabetes, metabolic syndrome, obesity, gender, ethnicity, and genetic and neurohumoral factors (66). There are three distinct LV geometric abnormality patterns: concentric remodeling (normal LV mass with increased relative wall thickness), concentric LVH (increased LV mass and increased relative wall thickness), and eccentric LVH (increased LV mass and normal relative wall thickness) (Figure 3).
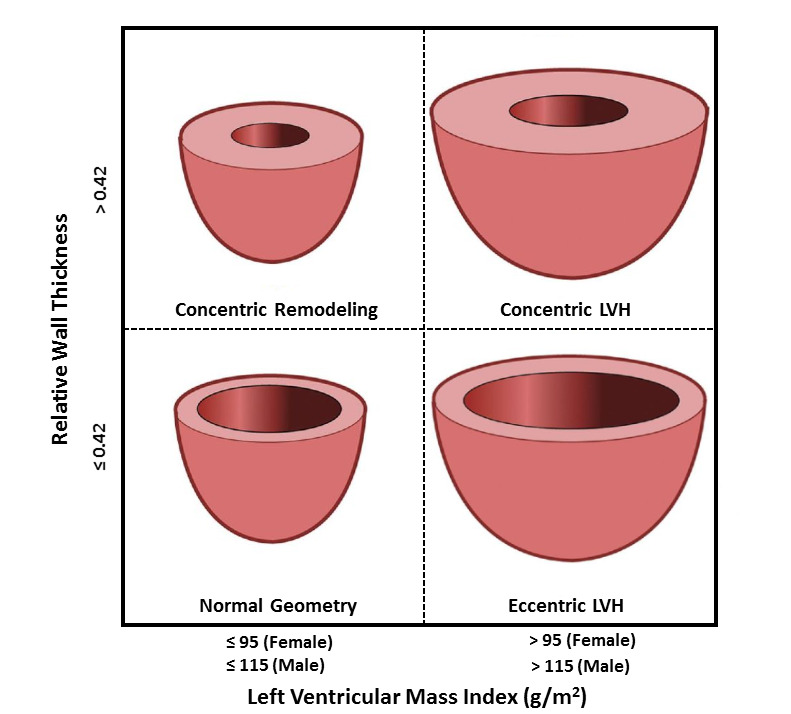
Figure 3.
Based on linear measurements, relative wall thickness and left ventricular mass index determine left ventricular geometric patterns. LVH, left ventricular hypertrophy.
LVH has long been recognized as a target organ response and a strong independent risk factor for HF, CAD, stroke, and CVD mortality (66,67). LVH leads to LV diastolic dysfunction by reduced LV compliance, impaired diastolic filling, prolonged isovolumetric relaxation, and increased LV and left atrial filling pressures (16). The universal definition of HF recognizes asymptomatic LVH, LV systolic dysfunction, and LV diastolic dysfunction as “pre-HF” to emphasize the progressive nature of HF and the importance of HF prevention (5).
LVH is common among adults with diabetes, with an estimated prevalence as high as 70% (68). A pooled analysis of 3 epidemiological cohort studies, including 2900 individuals with T2DM and no known CVD, demonstrated that 67% of the participants had at least one of the following echocardiographic abnormalities: LVH, left atrial enlargement, or diastolic dysfunction (44). Coexistent hypertension appears to be the main contributor to LVH in patients with diabetes (69). However, several studies have also demonstrated an independent association between diabetes and LVH. In the Framingham Heart Study, serum glucose, insulin levels, and IR were significantly linked to concentric LV remodeling, a finding that was more striking in women than in men (70,71). Results from a prospective cohort study with a 25-year follow-up period indicated that long-standing glycemic abnormalities have a cumulative effect on LV remodeling, and patients with early-onset diabetes tend to have a worse degree of LVH (72).
Diabetic cardiomyopathy may present with distinct LVH features (34). Thickened and stiff LV walls with normal LV volume usually characterize diabetic cardiomyopathy with HFpEF phenotype. Furthermore, at the cellular level, cardiomyocytes appear hypertrophied with normal sarcomere structure and increased collagen deposition in the interstitial space. Contrarily, diabetic cardiomyopathy with HFrEF phenotype usually manifests with eccentric LVH with dilated LV volume. At the cellular level, cardiomyocytes appear to have damage with loss of sarcomeres and replacement of some cardiomyocytes with interstitial fibrosis (38).
LV Diastolic Dysfunction
Diastolic dysfunction is common among otherwise asymptomatic individuals with diabetes, and its prevalence varies between 20% and 60% depending on the diagnostic criteria used and the population studied (73–75). Prospective cohort studies have confirmed that diabetes and poor glycemic control can independently contribute to the development of diastolic dysfunction (72). Even though diastolic dysfunction is often linked to LVH, it can occur in patients with diabetes, even in the absence of LVH (34).
Mild diastolic dysfunction (delayed myocardial relaxation) has a weak prognostic significance. However, the progression of diastolic dysfunction and increased LV filling pressure findings on echocardiography predispose to the future risk of HF and mortality in patients with diabetes (73,75). Moreover, among asymptomatic individuals with baseline diastolic dysfunction, diabetes is an independent predictor of progression to HFpEF or HFrEF (76).
LV Systolic Dysfunction
Traditionally, impaired LVEF is the primary marker of cardiomyopathy and systolic dysfunction. LVEF is a simple measure commonly used in the CV risk evaluation and management of CVD. However, LVEF does not capture the full spectrum of myocardial function (77). Global longitudinal strain (GLS) evaluated by speckle-tracking echocardiography is a robust technique that measures tissue deformation in a longitudinal direction (Figure 4). Reduced GLS is a marker of reduced contractility (75). GLS is more sensitive than conventional LVEF as a measure of systolic function and has an additional prognostic value (77,78). Therefore, it is now commonly used to detect subclinical LV systolic dysfunction.
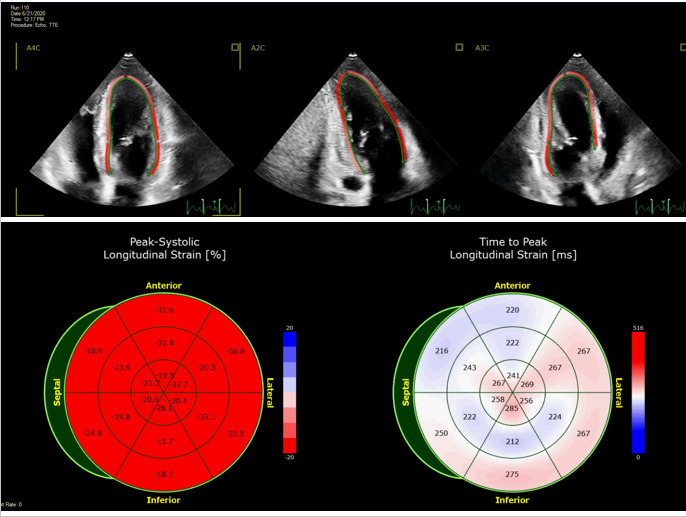
Figure 4.
Global longitudinal strain by speckle tracking echocardiography. Assessment of global longitudinal strain (GLS) in a healthy, asymptomatic individual with a GLS of -25%. The top row displays a regional strain map superimposed on the grayscale two-dimensional echocardiographic images in apical four-chamber (A4C), apical two-chamber (A2C), and apical three-chamber (A3C) views. The bottom left bullseye displays regional longitudinal strain for each segment of a 16-segment left ventricle model. Bright red denotes the most negative normal values of GLS. The bottom right bullseye shows the time (ms) between aortic valve opening and peak longitudinal strain, a measure of desynchrony, for each segment.
Impaired GLS is highly prevalent in asymptomatic, normotensive patients with diabetes and normal LVEF (67,79,80). Diabetes is associated with reduced GLS, even in adolescents and young adults with T1DM or T2DM (81,82). Moreover, an inverse correlation exists between HbA1C and GLS regardless of diabetes status, race, and sex (83). Not surprisingly, impaired GLS is a robust independent predictor of new-onset HF and mortality in patients with diabetes (67).
Diabetes can lead to clinical HF syndrome in individuals with asymptomatic LV systolic dysfunction. An RCT that included adults with asymptomatic LV systolic dysfunction demonstrated that diabetes increased the risk of HF development by 53% and doubled the risk of HF hospitalization over a median follow-up of 3 years (84).
Better glycemic control in patients with diabetes can lead to improvements in LV systolic and diastolic function indices. In a prospective cohort study of subjects with uncontrolled T2DM, lowering of average HbA1c from 10.3% to 8.3% over 12 months was associated with a 21% increase in GLS and a 24% increase in septal e’ velocity, a marker of myocardial relaxation (85).
PATHOPHYSIOLOGIC LINKS BETWEEN DIABETES AND HF
A complex interplay between numerous mechanisms underlies the pathophysiologic links between diabetes and HF. These pathophysiologic mechanisms include but are not limited to impaired cardiac insulin signaling, glucotoxicity, lipotoxicity, mitochondrial dysfunction, myocardial fibrosis, oxidative stress, impaired myocardial calcium handling, CV autonomic dysfunction, endocardial dysfunction, overactivation of the renin-angiotensin-aldosterone system (7) (Figure 5). The relative contribution of each pathophysiologic mechanism and their relationship with the phenotype of diabetic cardiomyopathy are still poorly understood. The pathophysiology of cardiomyopathy and HF vary depending on the type of diabetes (T1DM vs. T2DM) and type of HF (HFrEF vs. HFpEF) (86).
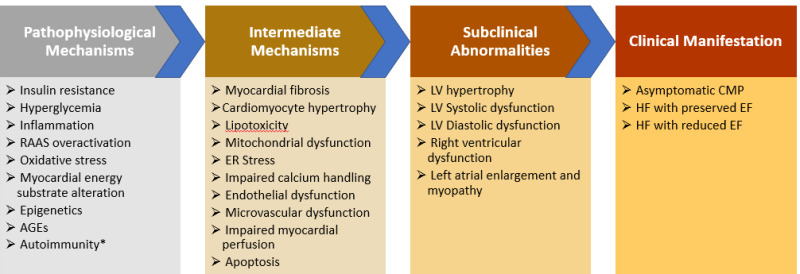
Figure 5.
Pathophysiological mechanisms, subclinical abnormalities, and clinical manifestations of diabetic cardiomyopathy. AGEs, advanced glycation end-products; CMP, cardiomyopathy; EF, ejection fraction; HF, heart failure; LV, left ventricular; RAAS, renin-angiotensin-aldosterone system. *In patients with type-1 diabetes mellitus.
Alterations in Myocardial Energy Substrate
Under normal circumstances, the heart predominantly consumes free fatty acids (FFA; ~70%) and glucose (~30%) and can adapt its choice of fuels depending on their availability (15). In T2DM, cardiomyocyte substrate utilization shifts towards FFA, and glucose utilization decreases in response to IR. As a result, the heart becomes metabolically less flexible and almost completely reliant on FFA. These alterations lead to inefficient energy metabolism since FFA oxidation requires more oxygen for energy production than glucose or ketone bodies (15,87). Moreover, the increased FFA uptake causes the accumulation of triglycerides in the cardiomyocytes and promotes lipotoxicity, mitochondrial dysfunction, oxidative stress, and apoptosis (34). A prospective study elegantly evaluated the impact of diabetes on lipid accumulation by analyzing endomyocardial biopsy samples from 158 adult heart transplant recipients (88). The investigators demonstrated that cardiomyocyte samples of transplanted healthy hearts begin to show evidence of lipid accumulation (triacylglycerol and ceramide) as early as three months after a transplant in diabetic recipients. In comparison, no lipid accumulation was present in cardiomyocyte samples of transplant recipients without diabetes. Not surprisingly, cardiomyocyte lipid accumulation was an independent predictor of early systolic and diastolic dysfunction in recipients with diabetes after 12 months of a transplant.
Hyperinsulinemia and Insulin Resistance
In broad terms, IR is defined as the inability of insulin to carry on its metabolic actions at the cellular level (34). IR is the central defect in the pathogenesis of metabolic syndrome and T2DM. Moreover, HF is a well-known insulin-resistant state, and HF risk and prognosis are markedly affected by IR (34,89).
IR increases lipolysis, hepatic lipogenesis, and hepatic gluconeogenesis. These changes lead to substrate overload and myocardial dysfunction through lipotoxicity and glucotoxicity (75). IR and related hyperinsulinemia can affect the signaling pathways involved in cardiomyocyte hypertrophy (38).
Oxidative Stress
Oxidative stress is the imbalance between the increased generation of reactive oxygen species and reduced antioxidant defense (75). Exposure to hyperglycemia induces oxidative stress by activating NADPH oxidase, promoting mitochondrial production of superoxides, and increasing the formation of advanced glycation end products (AGEs) due to nonenzymatic glycation and oxidation of proteins and lipids (34,87).
Oxidative stress contributes to increased cardiac remodeling, reduced cardiac contractility and relaxation, and impaired cardiomyocyte calcium handling (75). Moreover, oxidative stress contributes to myocardial dysfunction by causing protein and DNA damage, increasing myocardial inflammation, and impairing intracellular signaling pathways (34).
Endoplasmic Reticulum Stress and Impaired Calcium Handling
Myocardial intracellular calcium levels regulate myocardial contractility during a cardiac cycle. Alterations in the complex mechanism of calcium handling can impact myocardial contraction and relaxation (90). The endoplasmic reticulum has a major role in Ca2+ handling, lipid synthesis, and protein folding and modification (91). Moreover, cytosolic Ca2+ levels regulate cellular metabolism and cell signaling. Hyperglycemia and IR trigger endoplasmic reticulum stress, leading to unfolded proteins accumulating and impairing Ca2+ handling. In diabetic cardiomyopathy, impaired Ca2+ reuptake by the endoplasmic reticulum prolongs diastolic relaxation time (91). Studies on animal models have indicated that impaired cardiomyocyte Ca2+ handling plays a crucial role in the pathophysiology of diabetic cardiomyopathy (7,90).
Endothelial and Microvascular Dysfunction
Endothelial dysfunction, which disturbs endothelial-cardiomyocyte communication and vascular function, is common in patients with diabetes and CVD (15). Diabetes induces the deposition of AGEs in the endothelial and smooth muscle cells of the myocardial microvasculature (92). Furthermore, the deposition of AGEs triggers vascular inflammation, which reduces endothelial nitric oxide production. Low myocardial nitric oxide bioavailability levels predispose to concentric LV remodeling and diastolic dysfunction (38). Diabetes has also been linked to capillary rarefaction and pericyte loss. Microcirculatory rarefaction can impair myocardial perfusion, reduce coronary flow reserve, lead to tissue hypoxia, increase myocardial stiffness, and decrease contractility (38,93).
Inflammation
Systemic inflammation is a central component of the association between obesity, diabetes, CAD, and HF (34). In individuals with obesity, the expanding adipose tissue recruits immune cells and causes overproduction of proinflammatory cytokines, leading to obesity-mediated chronic inflammation (94). Chronic low-grade inflammation predisposes to IR and T2DM and contributes to diabetes-related complications (94). Similarly, systemic inflammation is highly prevalent in patients with HF, contributing to the development, progression, and poor prognosis of HF regardless of LVEF (95). Animal model studies have shown a complex interaction between various inflammatory pathways implicated in cardiac inflammation and the development of diabetic cardiomyopathy (96,97). One potential link between HF and diabetes is S100A12, an inflammatory protein that increases with hyperglycemic stress. A prospective cohort study including 1345 patients with T2DM demonstrated an independent association between increased A100A12 and risk of HF hospitalization (98).
Epicardial Adipose Tissue Expansion
Diabetes and obesity can independently contribute to the expansion and transformation of epicardial adipose tissue (86,99). Epicardial adipose tissue expansion has been associated with LV systolic and diastolic dysfunction in patients with T2DM (100). Epicardial fat volume correlates with myocardial fibrosis (101), vascular stiffness (102), and reduced coronary microcirculation (103). Epicardial adipose tissue expansion and transformation contribute to the alterations in the cardiac structure and function through several pathophysiological mechanisms such as proinflammatory effects of adipokines (i.e., leptin, tumor necrosis factor-a, interleukin-1b and interleukin-6) secreted from epicardial fat and oxidative stress induced by reactive oxygen species released from adipocytes (86,104).
Autoimmunity
Autoimmunity is implicated in the pathogenesis of CVD among patients with T1DM (38,105). A recent report from a prospective cohort study showed that participants with T1DM and uncontrolled glycemia (HbA1c 9.0%) have a high prevalence of cardiac autoantibodies with an antibody profile similar to that seen in patients with chronic Chagas cardiomyopathy (106). Moreover, cardiac autoantibody positivity predicted elevated high-sensitivity C-reactive protein and the future risk of CVD events. Interestingly, cardiac autoimmunity was lower in participants with controlled T1DM (HbA1c <7.0%) compared to those with uncontrolled (HbA1c 9.0%). The specific role of cardiac autoimmunity in the development and progression of diabetic cardiomyopathy remains to be further explored.
Overactivation of the Renin-Angiotensin-Aldosterone System
Overactivation of the renin-angiotensin-aldosterone system (RAAS) constitutes a robust pathophysiologic link between diabetes, obesity, hypertension, and HF (34). The typical features of RAAS overactivation are elevated serum angiotensin and aldosterone levels and the upregulation of angiotensin and mineralocorticoid receptors (91,107). There is a bidirectional relationship between RAAS activation and dysglycemia. Hyperglycemia and IR can activate the RAAS, and in return, RAAS induces systemic and cardiac IR and contributes to oxidative stress in cardiomyocytes by increasing the activity of NADPH oxidase (7).
Autonomic Dysfunction
Autonomic nervous system dysfunction is highly prevalent in patients with diabetes. CV autonomic neuropathy (CAN) differentially impacts the cardiac innervation's sympathetic and parasympathetic components, leading to sympathetic overactivation in the earlier stages (16). This imbalance is believed to contribute to the pathogenesis of CVD. CAN induces LV remodeling, LV systolic and diastolic dysfunction, and myocardial ischemia (108–110). CAN is usually asymptomatic at earlier stages, and at advanced stages, it may present with resting tachycardia, orthostatic hypotension, abnormal BP regulation, blunted heart rate response to exercise, and impaired heart rate variability (34,111).
Myocardial Fibrosis
Myocardial fibrosis, detected by histopathology or cardiac MRI, is a hallmark of diabetes-induced cardiac remodeling and related myocardial dysfunction. In patients with diabetes, the degree of myocardial fibrosis directly correlates with HbA1c levels (112). Diabetes-induced myocardial fibrosis is characterized by the remodeling of extracellular matrix with the deposition and crosslinking of stiff collagen, progressive elimination of muscular fibrils, perivascular fibrosis, basement membrane thickening, coronary microvascular sclerosis, and formation of microaneurysms (16). Myocardial fibrosis is often considered an end product of various pathophysiologic abnormalities such as hyperglycemia, hyperinsulinemia, oxidative stress, CAN, inflammation, and the overactivation of RAAS.
Apoptosis
Apoptosis, the process of programmed cell death, has been implicated in the development of diabetic cardiomyopathy and HF (113). Studies from animal models and human subjects have demonstrated a strong association between hyperglycemia and cardiomyocyte apoptosis (114,115). The primary drivers of cardiomyocyte apoptosis in diabetes are oxidative stress, endoplasmic reticulum stress, and dysregulation of autophagy, the lysosomal process that degrades and recycles cellular proteins and organelles (34,116).
PROGNOSTIC IMPLICATIONS OF DIABETES IN HF
Data from epidemiologic studies and clinical trials have consistently demonstrated that individuals with concurrent diabetes and HF have impaired quality of life, are at high risk of hospitalization and mortality, and have an overall poor prognosis (33,117). In addition, coexistent prediabetes also appears to increase the risk of morbidity and mortality in patients with HF as well (118).
A large meta-analysis including >380,000 subjects with acute and chronic HF from 43 registries and clinical trials revealed that diabetes was associated with a 28% increased risk of all-cause mortality and ~35% increased risk of both CV death and hospitalization (mostly from HF) over a three year follow up (119). Interestingly, the adverse impact of diabetes on the risk of hospitalization and mortality did not differ according to the LVEF group (≤35% vs. >35%) but was higher in patients with chronic HF than those with acute HF. Observational studies have suggested a U-shaped relationship between HbA1c and mortality in patients with coexisting HF and diabetes. Aguliar et al. reported that HbA1c of 7.1% to ≤7.8% was associated with the lowest risk of mortality when compared with the other quantiles of HbA1c in a cohort of ambulatory patients with HF who were receiving medical therapy for diabetes in the early 2000s (120).
Several potential mechanisms have been proposed to explain the prognostic impacts of diabetes in HF. Diabetes is linked to multimorbidity, which significantly alters HF outcomes. Moreover, diabetes induces myocardial fibrosis, inflammation, and endothelial dysfunction, leading to higher LV pressures, poor functional status, and impaired exercise capacity in patients with HF (121–125). Diabetes predisposes neurohumoral overactivation and alterations of renal sodium handling, which may lead to congestion, cardiorenal syndrome, and impaired diuretic responsiveness (121). Also, hyperglycemia in patients with diabetes upregulates SGLT2, which leads to increased renal sodium absorption and volume expansion (121). Moreover, the increased burden of ischemic heart disease and other diabetes-related comorbidities, such as CKD, contribute to the detrimental effects of HF (126).
Data from the Swedish Heart Failure Registry demonstrated that the diabetes-associated mortality risk is more pronounced in individuals with HF of ischemic etiology than those with nonischemic etiology (43). The 2-year survival rate was less than 50% among those with HF, T2DM, and ischemic heart disease.
The presence of preexisting microvascular disease is an independent risk factor for the risk of future HF events in patients with T2DM (44). In addition, microvascular disease portends an increased risk of mortality and morbidity in patients with HF. A post hoc analysis of a large RCT demonstrated a significant association between a history of microvascular complications and future risk of adverse events among study subjects with diabetes and HFpEF (127).
PREVENTION OF HF IN PATIENTS WITH DIABETES
The critical importance of HF prevention is underscored by HF staging, where risk factors such as hypertension and diabetes are classified as stage A (at risk for HF) (3). Because of the detrimental prognostic impact of clinical HF, the prevention of asymptomatic cardiac remodeling and dysfunction (pre-HF, stage B) and symptomatic HF (stage C) are among the primary goals of the management of patients with diabetes (128).
Prevention of HF by Preventing Diabetes
Preventing the onset of diabetes during young adulthood or middle age is an effective strategy for reducing HF risk later in life. An analysis of a large pooled US cohort evaluated the cumulative and relative impact of the absence of five modifiable HF risk factors (diabetes, hypertension, obesity, dyslipidemia, smoking) in middle age (45 to 55 years of age) (129). The data showed that the absence of diabetes in middle age strongly predicted HF-free survival, with a more than 60% lower risk of incident HF than those with diabetes. In addition, subjects without diabetes, hypertension, and obesity at ages 45 to 55 years, compared to those with all 3 of these risk factors, had an average >10 years longer HF-free survival and 13 years longer overall survival (129).
Prediction of HF Risk in Patients with DM
HF risk stratification is essential for prevention in patients with DM or prediabetes who do not have ASCVD. Even though echocardiography can detect cardiac remodeling in patients with diabetes, its use is not routinely recommended for asymptomatic individuals because of concerns about cost-effectiveness (4). However, measuring natriuretic peptides or high-sensitivity cardiac troponin can help identify patients with pre-HF or those at risk of progression to HF. Therefore, patients with diabetes should have a measurement of natriuretic peptides or high-sensitivity cardiac troponin yearly to identify high-risk individuals and assist with HF prevention (4).
Several risk prediction tools and algorithms have been developed to predict incident HF in patients with dysglycemia. Pandey et al. described a simple biomarker-based risk score including high-sensitivity cardiac troponin T ≥6 ng/l, high-sensitivity C-reactive protein ≥3 mg/l, N-terminal pro-B-type natriuretic peptide (NT-proBNP) ≥125 pg/ml, and LVH by ECG, with each abnormal parameter counting as 1 point. This risk score was tested in participants from 3 major US cohort studies. This risk score had good risk stratification for predicting 5-year incident HF risk in patients with both diabetes and prediabetes (130). More complex risk prediction tools (WATCH-DM and TRS-HFDM) incorporating a more extensive list of clinical variables have also been developed. Validation studies on participants from different clinical trials demonstrated that these risk scores could stratify HF risk among patients with T2DM (131–133). However, prospective studies have not evaluated these risk scores, and their clinical utility remains uncertain.
Glycemic control, obesity management, and BP control are well-established therapeutic options to lower the risk of microvascular or macrovascular complications in patients with diabetes. The clinical implications of these therapeutic options in HF prevention will be reviewed here.
Impact of Blood Pressure Control on HF Prevention
Diabetes and hypertension commonly coexist because of the overlap of underlying risk factors and pathophysiological mechanisms (134,135). The coexistence of diabetes and hypertension synergistically contributes to the risk of microvascular and macrovascular complications and CVD. Therefore, BP control with lifestyle modifications and antihypertensive medications is a primary target for reducing the risk of complications due to coexisting diabetes and hypertension (134). The ACC/AHA guidelines recommend initiating an antihypertensive agent when patients with diabetes have an office BP of ≥140/90 mmHg. The recommended BP target is below 140/90 mmHg for low-risk patients and below 130/80 for individuals with established or high risk for atherosclerotic CVD (136).
BP lowering has strong benefits in preventing HF among individuals with diabetes (35). However, the magnitude of this benefit appears to be smaller in patients with diabetes than in those without diabetes. Large meta-analyses of RCTs of BP-lowering therapy demonstrated that every 10 mmHg reduction in systolic BP (SBP) was associated with a 16% to 25% lower risk of HF among individuals with diabetes and 25% to 43% risk reduction among those without diabetes (137,138). The landmark ACCORD BP (Action to Control Cardiovascular Risk in Diabetes Blood Pressure) trial compared the impact of intensive (SBP goal <120 mmHg) versus standard BP control (SBP <140 mmHg) on major adverse CV events (MACE) in hypertensive patients with diabetes (139). In this trial, intensive BP control did not improve the risk of MACE or HF but increased the risk of adverse events. Therefore, the 2018 European Society of Cardiology (ESC)/European Society of Hypertension guidelines for the management of arterial hypertension recommended avoiding a target SBP <120 mmHg in patients with diabetes (140).
The antihypertensive drug classes with strong CV risk reduction in individuals with diabetes are thiazide diuretics, angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and dihydropyridine calcium channel blockers. Therefore, any of these agents can be considered a first-line therapy for lowering BP in individuals with diabetes. Because of their renal protection benefits, an ACE inhibitor or an ARB should be a part of the initial therapy for those with albuminuria (136,141). It should be noted that beta-blockers are not among the first-line antihypertensive agents for patients with or without diabetes. Because there is insufficient evidence on the mortality benefits of beta-blockers when used for the sole purpose of BP reduction in the absence of HF or CAD (136,141). The landmark Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack (ALLHAT) has been the largest trial designed to assess the relative efficacy of different antihypertensive agents in protection against CVD. The results of this trial demonstrated that chlorthalidone (a thiazide diuretic) was superior to lisinopril (an ACE inhibitor) and amlodipine (a calcium-channel blocker) in the prevention of HF among patients with or without diabetes (142,143). A subsequent large meta-analysis of RCTs revealed that diuretics and renin-angiotensin system blockers were independently superior to other antihypertensive agents in the prevention of HF among patients with diabetes (138).
Loop diuretics are considered to have a neutral effect on glycemic control in patients with diabetes. Evidence from posthoc analysis and meta-analysis of antihypertensive drug trials has shown a small but significant association between thiazide diuretic use and higher fasting plasma glucose (144). A meta-analysis of 26 RCTs demonstrated that thiazide diuretic use was associated with a 4.6 mg/dL higher fasting plasma glucose than non-thiazide agents or placebo in patients with hypertension (145). A possible link between thiazide diuretic use and new-onset diabetes in patients with hypertension was supported by some studies (146) but not all (147). Overall, the CV benefits of thiazide diuretics outweigh the risk of new-onset diabetes in non-diabetic individuals and the risk of uncontrolled glycemia in patients with diabetes (144). Therefore, neither hypertension nor HF management guidelines recommend avoidance of thiazide diuretics in patients with or at risk for diabetes.
The ACC/AHA guidelines recommend the use of steroidal mineralocorticoid receptor antagonists (MRA, i.e., spironolactone and eplerenone) as an add-on therapy to the first-line antihypertensive agents in the treatment of resistant hypertension (136). Besides modest BP reduction, these agents can provide anti-fibrotic, anti-inflammatory, and anti-proteinuric benefits (148,149). The clinical CV benefits of these agents have been proven in patients with HFrEF. However, we lack large-scale RCTs demonstrating their CV benefits in primary prevention settings among patients with hypertension or diabetes. In a small RCT including 140 patients with T2DM and high CVD risk, adding high-dose eplerenone to standard treatment reduced LV mass and decreased NT-ProBNP and a circulating serum marker of myocardial fibrosis (150). Our knowledge of MRAs in patients with diabetes has expanded with two Phase III landmark RCTs evaluating the cardiorenal benefits of Fineranone, a recently discovered non-steroidal MRA with high affinity and specificity for the mineralocorticoid receptor (151). FIDELIO-DKD and FIGARO-DKD trials demonstrated that fineranone therapy on top of maximally tolerated RAAS inhibitor treatment was renally protective and reduced the risk of the primary endpoint of CVD outcomes in patients with T2DM and CKD (152,153). More specifically, fineranone therapy significantly reduced the risk of hospitalization for HF (HR; 0.71 [95% CI, 0.56-0.90]) in the FIGARO-DKD trial (153). Based on these results, the U.S. Food and Drug Administration (FDA) recently approved fineranone to reduce the risk of estimated glomerular filtration rate (eGFR) decline, end-stage renal disease, CVD death, non-fatal MI, and HF hospitalization in patients with CKD and T2DM (151).
Obesity Management and Lifestyle Modifications
Studies have consistently demonstrated that purposeful weight reduction, achieved via diet, exercise, or bariatric surgery, has favorable impacts on glycemic control, IR, BP, and lipid profiles and reduces the need for antidiabetics in obese individuals with T2DM (34,154,155). Moreover, weight reduction can delay the progression from prediabetes to T2DM (156). The ADA recommends lifestyle modification to achieve at least a 5% weight loss for all overweight or obese individuals with prediabetes or diabetes (157). Also, the guidelines emphasize the need for an individualized medical nutrition therapy program for individuals with diabetes to achieve treatment goals. The recommended exercise regimen for most individuals with T1DM and T2DM is at least 150 minutes of moderate to vigorous aerobic activity per week, spread over at least three days/week, and an additional 2-3 sessions/week of resistance exercise (157).
Despite the well-established favorable effects of weight loss in patients with diabetes, the role of lifestyle changes and weight loss in preventing HF among diabetic patients remains uncertain. A meta-analysis of 36 prospective cohort studies published before 2014 demonstrated that achieving recommended physical activity levels (150 minutes of moderate‐intensity aerobic activity per week) was associated with reduced risk of incident HF (relative risk; 0.81 [0.76, 0.86]) in patients with diabetes (112).
The Look AHEAD (Action for Health in Diabetes) has been the largest RCT evaluating the CV effects of an intensive lifestyle intervention that promoted weight loss through decreased caloric intake and increased physical activity in overweight or obese participants with T2DM (154). The study subjects in the intensive lifestyle intervention group lost 8.6% of body weight at one year and, by the end of the 10-year follow-up, maintained a modest (6%) weight loss compared to 3.5% in the control group. Despite the achieved relative weight loss and improved physical fitness and HbA1c, the intensive lifestyle intervention did not reduce the risk of CVD mortality and morbidity, including HF risk, which was a secondary outcome. A post hoc analysis by Pandey et al. evaluated the impact of cardiorespiratory fitness and the degree of weight loss on the HF risk among the Look AHEAD trial participants with an extended follow-up period (158). The investigators observed a 20% reduction in incident HF risk as a response to a 10% reduction in BMI over a 4-year follow-up. Moreover, a higher baseline and improvement of cardiorespiratory fitness over time predicted a lower risk of incident HF. Interestingly, subgroup analysis revealed a more significant correlation between baseline fitness and incident HFpEF than incident HFrEF. Future dedicated studies are needed to explore the HF risk reduction effects of more intense weight loss and exercise training interventions to promote sustained improvements in body weight and cardiorespiratory fitness in patients with T2DM (158).
Metabolic surgeries, when performed as a part of a comprehensive weight management strategy, are effective treatment options for achieving more significant and durable weight loss in individuals with severe obesity (159). Individuals with T2DM and severe obesity who undergo metabolic surgeries experience improvement in glycemic control and insulin sensitivity and may have remission of diabetes (159). Evidence from RCTs and observational studies has demonstrated that metabolic surgery, as compared to conventional lifestyle modifications and medical therapy, can reduce overall CV risk and improve the quality of life in individuals with T2DM and severe obesity (160–162). The impact of metabolic surgeries on incident HF risk has not yet been evaluated in large-scale RCTs. In a large nationwide prospective observational study of obese individuals without a known history of HF, metabolic surgery was associated with a >50% reduction in the risk of incident HF (163). Another retrospective cohort study from the Cleveland Clinic Health System in the U.S. demonstrated that metabolic surgery among patients with T2DM and obesity was associated with approximately 40% relative reduction of major adverse CV event risk and more than 60% relative reduction of incident HF risk over a median follow-up of 3.9 years (164).
Impact of Glycemic Control
In patients with T1DM and T2DM, intensive glycemic control significantly reduces the risk and severity of microvascular complications (34). However, despite the solid epidemiologic link between poor glycemic control and HF risk, the effects of intensified glycemic control in preventing HF remain controversial. Early clinical trials that established the CV benefits and risks of intensive glycemic control did not include HF as a primary endpoint. However, post hoc or secondary outcome analyses of prospective trials have shed light on the relationship between glycemic control and HF prevention.
In the UK Prospective Study of Diabetes (UKPDS) published more than 20 years ago, intensive glycemic control with metformin, sulfonylureas, or insulin was compared to conventional therapy in adults with recently diagnosed T2DM. A post hoc analysis of the main trial demonstrated that each 1% reduction in mean HbA1c was associated with a 16% decrease in incident HF (165). However, similar results were not replicated by subsequent large-scale RCTs such as the ACCORD (166,167), the ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (168), and the VADT (Veterans Affair Diabetes Trial) (169) which failed to show any reduction in HF risk with intensive glucose control in patients with T2DM. Consistently, a meta-analysis of all these trials showed no overall effect of intensive glucose control on HF risk despite a modest (9%) reduction in the risk of major CV outcomes (170). These observations confirmed that blood-glucose-lowering and improvement of HbA1c are insufficient targets for preventing HF in patients with diabetes.
The treatment section below discusses the impact of specific antidiabetic agents on the prevention of HF in high-risk patients.
TREATMENT OF HF IN PATIENTS WITH DIABETES
The primary objectives in managing HF are to reduce mortality, prevent HF hospitalization, and improve patients’ clinical status, quality of life, and functional capacity (18). The major components of managing HF are lifestyle changes, education and support for HF self-management, monitoring, control of the underlying causes and associated comorbidities, pharmacologic therapy, cardiac rehabilitation, device therapies, mechanical circulatory support, and cardiac transplantation (171). The major society guidelines for the management of patients with HF include the ACC/AHA/HFSA guidelines published in 2022 (3) and the European Society of Cardiology (ESC) guidelines published in 2021 (18).
The main components of lifestyle changes recommended for patients with HF are physical exercise, smoking cessation, restriction of or abstinence from alcohol consumption, dietary modifications, and avoidance of obesity (18). ACC/AHA/HFSA and ESC guidelines strongly recommend regular aerobic exercise and exercise training to improve functional capacity and symptoms in patients with HF who can participate. The ACC/AHA/HFSA guideline indicated that avoiding excessive dietary sodium intake is reasonable (class IIa) for patients with symptomatic HF to reduce congestive symptoms (3). However, because of a lack of affirmative evidence from clinical trials, the guidelines did not provide precise recommendations about the limit of daily sodium intake and whether it should vary depending on the type, stage, or severity of HF or comorbidities.
Diuretic Therapy in HF
Diuretics increase urinary sodium excretion and reduce physical signs and symptoms of congestion in HF patients by inhibiting sodium or chloride reabsorption in the renal tubules. Loop diuretics such as bumetanide, furosemide, and torsemide act in the loop of Henle, whereas thiazide diuretics such as hydrochlorothiazide, metolazone, chlorthalidone, and potassium-sparing diuretics such as spironolactone, eplerenone, and triamterene act in the distal position of the tubule (172). Loop diuretics, which produce a shorter and more intense diuresis than thiazides, are usually the preferred agents for achieving and maintaining euvolemia and reducing the risk of HF hospitalization in patients with HF. Large-scale RCTs have not evaluated the effects of the loop and thiazide diuretics on mortality and morbidity in patients with HF.
First-Line Pharmacological Treatment of HFrEF
Neurohumoral antagonists (RAAS inhibitors and beta-blockers) and SGLT2 inhibitors with proven morbidity and mortality benefits are the cornerstone of guideline-directed medical therapy for patients with chronic HFrEF (173). The guidelines generally do not recommend specific therapeutic approaches in patients with diabetes compared to those without diabetes.
RAAS AND NEPRILYSIN INHIBITORS
The guidelines from AHA/ACC/HFSA (3) and ESC (18) recommend (Class I) the use of angiotensin receptor-neprilysin inhibitor (ARNI; sacubitril/valsartan) as a first-line therapy for patients with HFrEF (New York Heart Association [NYHA] Class II or III). ACE inhibitors (Class I recommendation) are recommended to reduce the risk of morbidity and mortality in patients with HFrEF when using ARNI is not feasible. ARBs are considered acceptable vasodilator treatment options (class I recommendation) as a first-line alternative to ACE inhibitors for patients intolerant of ACE inhibitors because of cough or angioedema and when using ARNI is not feasible.
Subgroup analysis or meta-analysis of major HF trials demonstrated that the effectiveness of RAAS and neprilysin inhibitors in HF does not vary based on patients’ diabetes status. Therapy with ACE inhibitors, ARBs, or an ARNI reduces the risk of morbidity and mortality among HF patients without significant effect variation based on diabetes status (33,61,122,174). In addition, ARNI therapy provides a similar degree of natriuretic peptide improvement and cardiac reverse remodeling in patients with or without diabetes (175).
Post hoc analysis of RCTs revealed that ACE inhibitors and ARBs might reduce the risk of incident diabetes in patients with HFrEF (59,61,176). However, the data on the impact of these agents on glycemic control in patients with HF and preexisting diabetes remains limited. Neprilysin inhibition with sacubitril appears to have more favorable effects on glycemic control than ACE inhibitors (61). In the PARADIGM-HF trial, HFrEF patients enrolled in the enalapril and sacubitril-valsartan arms experienced an average of 0.16% and 0.26% HbA1c reduction after one year of treatment (177). In addition, sacubitril-valsartan use was associated with a 29% reduction in new insulin use compared to enalapril.
Diabetes confers a higher risk of diabetic nephropathy and CKD. Diabetic nephropathy can lead to increased renal sodium retention and a higher risk of hyperkalemia. Therefore, it is critical to monitor serum electrolytes and creatinine when starting or escalating the dose of RAAS inhibitors in patients with HF and diabetes (178). Of note, ARNI therapy may cause a higher rate of hypotension than ACE inhibitors or ARB (4).
BETA-BLOCKERS
Beta-blocker therapy is recommended (class I) for all patients with stable, symptomatic HFrEF (3,18). Beta-blocker therapy reduces the risk of death and hospitalization and improves LVEF and clinical status in patients with HFrEF. The ACC/AHA/HFSA guidelines recommend using one of the three beta-blockers with proven mortality benefits (e.g., metoprolol succinate, carvedilol, and bisoprolol).
Based on RCTs, the morbidity and mortality benefits of beta-blockers are similar in HFrEF patients with or without diabetes (174,179,180). A prospective cohort study from the UK suggested that increasing beta-blocker dose was associated with a more significant prognostic advantage in HF patients with diabetes than those without diabetes (181).
Data from some old observational studies and clinical trials raised concerns for a slight increase in the risk of new-onset diabetes associated with using propranolol, a first-generation non-selective beta-blocker, to treat hypertension (182,183). However, such an adverse effect concern is not present with newer-generation beta-blockers in HF populations (184). Compared to other beta-blockers, carvedilol use may even reduce HbA1c, fasting insulin levels, and risk of new-onset diabetes in patients with HFrEF (60,184).
Beta-blockers can potentially mask hypoglycemia symptoms by preventing palpitations and tremors and could prolong recovery from hypoglycemia by reducing glucose production in the liver (178). A post hoc analysis of the ACCORD trial demonstrated a significant association between beta-blocker use and severe hypoglycemia risk in patients with diabetes (185). However, a post hoc analysis of the MERIT-HF trial (Metoprolol CR/XL Randomized Intervention Trial in Chronic Heart Failure) did not show a similar association between beta-blocker use and hypoglycemia events in patients with HF and coexisting T2DM (186).
MINERALCORTICOID RECEPTOR INHIBITORS
MRAs (i.e., spironolactone or eplerenone) are recommended for all patients with HFrEF (LVEF ≤35%) and NYHA class II to IV symptoms if eGFR is >30 ml/min/1.73 m2 and serum potassium is <5 mEq/L (3,18). Similar to other RAAS inhibitors, the clinical benefits of MRAs have been consistent in HF patients with or without diabetes (187). Based on limited data, MRAs appear to have a neutral effect on glycemic parameters and diabetes risk in individuals with HFrEF (64,188). MRAs can cause hyperkalemia; therefore, monitoring electrolytes while initiating or maintaining MRA therapy is crucial.
SGLT2 INHIBITORS
SGLT2 inhibitors are recommended (class I) to reduce CVD mortality and HF hospitalization in patients with symptomatic HFrEF, irrespective of the presence of T2DM (3,18). Empagliflozin, Dapagliflozin, and Sotagliflozin are the SGLT2 inhibitors approved by the U.S. FDA to reduce CVD death and HF hospitalization in patients with HF across the range of LVEF (HFrEF, HFpEF, HFmrEF).
Dedicated landmark trials proved the CV benefits of SGLT2 inhibitors in patients with HFrEF, regardless of T2DM status (Table 1). The DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure) (189) and EMPEROR-Reduced (EMPagliflozin outcomE tRial in Patients With chrOnic heaRt Failure With Reduced Ejection Fraction) (190) trials enrolled symptomatic chronic HFrEF (LVEF ≤40%, NYHA class II to IV, and elevated natriuretic peptides) and were already on guideline-directed medical therapy. Patients with T1DM and advanced CKD were excluded. In these trials, compared to placebo, SGLT2 inhibitors reduced the composite of CVD death and HF hospitalization by ~25%. In addition, SGLT2 inhibitors slowed the progression of renal disease. The SGLT2 inhibitors' CV benefits are independent of their glucose-lowering effects (191). In addition, SGLT2 inhibitor therapy appears to improve the clinical stability and functional status of patients with HF. An analysis from the EMPEROR-Reduced trial demonstrated that empagliflozin therapy was associated with improvement in the NYHA class and requirement of diuretic intensification when compared to placebo (192).
Sotagliflozin is a dual SGLT1/SGLT2 inhibitor that increases urinary glucose excretion by SGLT2 inhibition and delays intestinal glucose absorption by SGLT1 inhibition. The SOLOIST-WHF (Effect of Sotagliflozin on Cardiovascular Events in Patients With Type 2 Diabetes And Worsening Heart Failure) trial evaluated the efficacy and safety of sotagliflozin in patients with T2DM who were hospitalized with HF (HFpEF and HFrEF) (190). In this trial, Sotagliflozin therapy initiated before or shortly after hospital discharge reduced the combined endpoint of CVD death, HF hospitalization, or urgent HF visits by 33%.
Despite the guideline recommendations and strong clinical trial evidence supporting their benefits, SGLT2 inhibitor therapy utilization among patients with HF remains low. A recent nationwide retrospective cohort study analyzed the STLT2 inhibitor prescription patterns among patients hospitalized with HFrEF between July 2021 and July 2022 (193). Only 20% of eligible patients in this cohort were prescribed SGLT2 inhibitors at discharge. Moreover, the utilization was low even among patients with multiple indications, such as comorbid CKD and T2DM.
Table 1.
Trials of SGLT2 Inhibitors in Patients with HF
| Medication | Trial | Publication Year | Patient Characteristics | History of T2DM | Follow-up Period | Primary Outcome* (HR, 95% CI) |
|---|---|---|---|---|---|---|
| Empagliflozin | EMPEROR-REDUCED (190) | 2020 | Symptomatic stable HF (LVEF ≤40%) | 50% | 16 months | 0.75 (0.65 – 0.86) |
| Empagliflozin | EMPEROR-PRESERVED (194) | 2021 | Symptomatic stable HF (LVEF > 40%) | 49% | 26 months | 0.79 (0.69 – 0.90) |
| Dapagliflozin | DAPA-HF(189) | 2019 | Symptomatic stable HF (LVEF ≤40%) | 42% | 18 months | 0.74 (0.0.65 - 0.85) |
| Dapagliflozin | DELIVER(118) | 2022 | Symptomatic stable HF (LVEF > 40%) | 45% | 2.3 years | 0.82 (0.73 – 0.92) |
| Sotagliflozin | SOLOIST-WHF (195) | 2021 | Recently hospitalized HF (All LVEF groups) | 100% | 9 months | 0.67 (0.52 – 0.85) |
- *
Primary outcome was a composite of cardiovascular death or hospitalization for HF.
Device Therapies for HFrEF
Implantable cardioverter-defibrillator (ICD) is strongly recommended (Class I) for primary prevention of sudden cardiac death in patients with symptomatic HFrEF who have an LVEF ≤35% despite guideline-directed medical therapy for >3 months (3,18). HFrEF patients with diabetes carry a significantly higher risk of sudden cardiac death than those without diabetes (122). This observation highlights the importance of considering ICD in appropriately selected cases with HFrEF and diabetes. Strong evidence from major ICD trials has confirmed the sudden cardiac death risk reduction benefits of ICDs in individuals with coexisting HFrEF and diabetes (196,197).
Cardiac resynchronization therapy (CRT) is a well-established therapeutic modality in patients with HFrEF and prolonged QRS duration. In appropriately selected cases, CRT with biventricular pacing can improve LV systolic function and reduce the risk of morbidity and mortality through its ability to reverse the remodeling of LV (198). In major CRT trials, HFrEF patients with and without diabetes experienced similar overall effectiveness of CRT for reducing mortality and HF hospitalization (199–201). However, observational studies suggested that the magnitude of LV reverse remodeling and improvement of systolic and diastolic function may be less pronounced in individuals with diabetes than in those without diabetes (198,202,203).
Treatment of HFpEF
Until recently, the management of patients with HFpEF and HFmrEF lacked specific therapies shown to improve morbidity and mortality definitively. Clinical trials of pharmacologic agents with proven benefits in HFrEF have predominantly revealed neutral results in populations with HFpEF (30,31,204).
The guidelines focus on aggressive management of risk factors and comorbidities, exercise training, and symptom management with diuretics when volume overload findings are present in patients with HFpEF (3). The landscape of medical management of HFpEF has dramatically changed with the data from clinical trials evaluating the safety and efficacy of SGLT2 inhibitor therapy in patients with HFpEF (Table 1). The EMPEROR-Preserved (Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction) enrolled 5988 symptomatic patients with HF with LVEF >40% and elevated natriuretic peptides (194). In this trial, compared to placebo, empagliflozin therapy reduced the primary composite outcome of CVD death or HF hospitalization by 21%, primarily driven by a 29% reduction in HF hospitalization. SGLT2 inhibitor therapy was beneficial regardless of the presence or absence of T2DM. Based on the results of the EMPEROR-Preserved trial, the 2022 ACC/AHA/HFSA guidelines recommended (class IIa) SGLT2 inhibitor therapy to reduce CV death and HF hospitalization in patients with HFpEF and HFmrEF (3). Since the publication of these guidelines, the DELIVER (Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure) trial was completed (118). This trial demonstrated similar benefits of dapagliflozin therapy in patients with HF and LVEF >40%. Data from the PRESERVED-HF trial, a relatively small-size multicentric RCT, evaluated the impact of dapagliflozin on the quality of life and symptoms in patients with HFpEF. In this trial, 12 weeks of dapagliflozin treatment significantly improved patient-reported physical limitations and symptoms and objectively measured exercise tolerance (205).
The 2022 ACC/AHA/HFSA guidelines indicate that based on a subgroup analysis of RCTs, ARBs, MRAs, and ARNI (in appropriately selected patients) might be considered (Class IIb) in patients with HFpEF or HFmrEF to decrease hospitalizations (3).
PHARMACOLOGIC THERAPY OF T2DM IN PATIENTS WITH HF
Lifestyle therapy is essential to managing patients with diabetes and established or high risk for HF. We point the readers to documents from ADA and ACC/AHA for detailed review and recommendations on lifestyle therapy in this patient population (3,4,157).
In this chapter, we provide a focused review of the effects of glucose-lowering agents from a HF perspective. Glycemic control is essential in patients with diabetes who have additional CV risk factors or established CVD. The ADA Standards of Medical Care in Diabetes recommend a holistic, multifactorial, and patient-centered approach when choosing antidiabetic medications. As per the guidelines, antidiabetic therapy should be selected according to patient-specific goals such as cardiorenal protection or achieving and maintaining glycemic and weight management goals. Moreover, considering comorbidities, such as HF and CKD, is essential when determining management goals (23,141).
SGLT2 Inhibitors
In light of the evidence from CVOTs showing the benefits of CVD risk reduction and renal protection, the ADA recommends using GLP1-RAs or SGLT2 inhibitors as first-line agents in T2DM patients with high-risk or established ASCVD (23). In addition, SGLT2 inhibitors are the preferred first-line antidiabetic in patients with known HF or CKD (eGFR <60 ml/min/1.73 m2 or albuminuria). This approach is a change from before, as the guidelines no longer require first-line metformin therapy before initiating SGLT2 inhibitors or GLP1-RAs when the therapy is started with the goal of cardiorenal risk reduction in high-risk patients with T2DM. (Table 2).
Table 2.
Pharmacologic Therapy with a Goal of Cardiorenal Risk Reduction in High-Risk Patients with T2DM
| Risk Profile | First-line Therapy | Second-line Therapy if A1C is Above the Target |
|---|---|---|
| ASCVD* Indicators of High Risk** | GLP1-RA with proven CVD benefit Or SGLT2 inhibitor with proven CVD benefit | GLP1-RA with proven CVD benefit Or SGLT2 inhibitor with proven CVD benefit |
| Heart Failure | SGLT2 inhibitor with proven HF benefit | Follow the algorithm for the achievement of glycemic and weight management goals. |
| CKD*** | SGLT2 Inhibitor with proven CKD benefits | GLP1-RA with proven CVD benefit |
- *
ASCVD, atherosclerotic cardiovascular disease: Individuals with established cardiovascular disease such as myocardial infarction, stroke, revascularization procedure, amputation, or symptomatic/asymptomatic coronary artery disease. **Indicators of high risk: ≥55 years of age with two or more additional risk factors such as obesity, hypertension, dyslipidemia, smoking, and albuminuria. ***CKD, chronic kidney disease: GFR <60 ml/min/1.73 m2 or albuminuria (albumin-creatinine ratio ≥30 mg/g). GLP1-RA, glucagon-like peptide-1 receptor agonists; A1C, hemoglobin A1c, SGLT2, sodium-glucose co-transporter -2.
A wealth of high-quality data proves the HF benefits of SGLT2 inhibitors in patients with T2DM. Since the publication of EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) trial results in 2015, (206) several large-scale CVOTs have revolutionized our understanding of the prevention of CV events and HF in patients with T2DM (Table 1 and 3).
In the EMPA-REG OUTCOME, empagliflozin, in the CANVAS (Canagliflozin Cardiovascular Assessment Study), canagliflozin and in the DECLARE-TIMI 58 (Dapagliflozin Effect on Cardiovascular Events–Thrombolysis in Myocardial Infarction 58), dapagliflozin significantly reduced incident HF events as a secondary end-point in patients with established or high risk for CVD (206–208). The CREDENCE (Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation) trial, designed to assess renal outcomes of canagliflozin, also showed a reduction in hospitalization for HF (HR:0.69, p<0.001) (209). In the VERTIS-CV (Cardiovascular Outcomes Following Ertugliflozin Treatment in Patients with Type 2 Diabetes Mellitus and Atherosclerotic Cardiovascular Disease) trial, ertugliflozin was non-inferior to placebo in regards to major CVD events in 8264 patients with T2DM and established CVD. In this trial, Ertugliflozin reduced the risk of HF hospitalization (an exploratory secondary outcome) by 30% (hazard ratio 0.70 [95% CI, 0.54 to 0.90]) (210).
A meta-analysis of 6 RCTs (EMPAREG-OUTCOME, CANVAS, DECLARE-TIMI 58, and CREDENCE, VERTIS-CV) explored the CV benefits of 4 SGLT2 inhibitors in a combined sample size of 46969 patients with T2DM. In this meta-analysis, SGLT2 inhibitor therapy was associated with a reduced risk of MACE (HR, 0.90; 95% CI, 0.85-0.95), kidney outcomes (HR, 0.62; 95% CI, 0.56-0.70), and a combined outcome of CVD death and HF hospitalization (HR, 0.78; 95% CI, 0.73-0.84) (118).
The SCORE trial (Effect of Sotagliflozin on Cardiovascular and Renal Events in Patients with Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk) evaluated the CV benefits of sotagliflozin, a dual SGLT1/SGLT2 inhibitor, against placebo in 10584 patients with T2DM, CKD, and risk of CVD. In this trial, Sotagliflozin reduced the coprimary end-point of MACE (HR; 0.84 [95% CI, 0.72 to 0.99]) and secondary end-point of HF hospitalization (HR; 0.67 [0.55–0.82]) (210).
In Europe and Japan, SGLT-2 inhibitors are approved as adjunctive therapy for T1DM in patients with a BMI of at least 27 kg/m2. This recommendation is primarily based on the assumption that CV and renal protective effects of SGLT2 inhibitors can be generalized to T1DM populations. However, we still lack large-scale RCTs assessing the cardiorenal benefits of SGLT2 inhibitors in T1DM (211). Considering the high risk of diabetic ketoacidosis and the lack of proven diabetic ketoacidosis risk mitigation strategies, the U.S. FDA denied the approval of SGLT2 inhibitors for T1DM in the U.S.
Table 3.
Heart Failure Hospitalization Risk with SGLT-2 Inhibitors in Patients with T2DM
| Medication | Trial | Publication Year | Patient Characteristics | History of HF | Follow-up Period | HF hospitalization (HR, 95% CI) |
|---|---|---|---|---|---|---|
| Empagliflozin | EMPA-REG OUTCOME (206) | 2015 | Established CVD | 10% | 3.1 years | 0.65 (0.50 - 0.85) |
| Empagliflozin | EMPA-KIDNEY (212) | 2023 | CKD with or without albuminuria* | Not reported | 2 years | 0.84 (0.67 – 1.07) ** [p=0.15] |
| Canagliflozin | CANVAS Program (207) | 2017 | CV risk factors (34%) Established CVD (66%) | 14% | 3.2 years | 0.67 (0.52 - 0.87) |
| Canagliflozin | CREDENCE (209) | 2019 | CKD with albuminuria | 15% | 2.6 years | 0.61 (0.47 - 0.80) |
| Dapagliflozin | DECLARE-TIMI 58(208) | 2019 | CV risk factors (59%) Established CVD (41%) | 10% | 4.2 years | 0.73 (0.61 - 0.88) |
| Dapagliflozin | DAPA-CKD (213) | 2020 | CKD with albuminuria | 11% | 2.4 years | 0.71 (0.55 – 0.92) ** |
| Ertugliflozin | VERTIS-CV (210) | 2020 | Established CVD | 24% | 3.5 years | 0.70 (0.54 - 0.90) |
| Sotagliflozin | SCORED (214) | 2020 | CKD with a high risk for CVD | 31% | 16 months | 0.67 (0.55 – 0.82) |
- *
Estimated eGFR of 20 to 45 ml/min/1.73 m2, or eGFR of 45 to 90 ml/min/1.73 m2 with a urinary albumin-to-creatinine ratio of ≥ 200 mg/g. **Composite secondary outcome of cardiovascular death and heart failure hospitalization. CKD, chronic kidney disease; CVD, cardiovascular disease.
Glucagon-like Peptide-1 Receptor Agonists
As mentioned above, GLP1-RAs are among the first-line agents for glucose-lowering and cardiorenal risk reduction in T2DM patients with indicators of high-risk or established ASCVD (23). In addition, GLP1-RAs are recommended as second-line therapy in patients with CKD if SGLT2 inhibitor therapy is contraindicated or not tolerated or if the HbA1c remains above target despite using an SGLT2 inhibitor.
Landmark CVOTs have confirmed the favorable effects of GLP1-RAs on the risk of major CV events such as CV death, nonfatal myocardial infarction, or nonfatal stroke in patients with T2DM. However, individual trials revealed mostly neutral results regarding the effect of GLP1-RAs on HF outcomes in patients with T2DM and at risk for HF (Table 4) (215,216). Efpeglenatide was the only GLP-RA that significantly reduced HF hospitalization as a secondary end-point in a major CVOT, including T2DM patients with a history of CVD or CKD (217). U.S. FDA has not yet approved this agent.
Some early small-size trials raised concerns for increased risk of hospitalization or arrhythmia in response to GLP1-RA treatment in patients HFrEF (218). However, A recent meta-analysis of 7 RCTs, including 54,092 ambulatory patients with T2DM, revealed that GLP1-RAs reduced the composite of HF hospitalization and CVD death (HR 0.84, 95% CI: 0.76-0.92.) in patients without a prior history of HF. However, compared to placebo, GLP1-RAs did not reduce the same outcome in patients with a previous history of HF (219). Based on available data, GLP1-RAs may potentially prevent HF in patients with no history of HF. However, GLP1-RAs do not appear to reduce HF-related events in patients with a history of HF and coexisting diabetes (218).
GLP1-RAs (i.e., liraglutide and semaglutide) have also been approved as a medical therapy for weight loss in non-diabetic individuals with overweight or obesity. And in a landmark trial, treatment with tirzepatide, a novel combined glucose-dependent insulinotropic polypeptide and GLP1-RA, led to substantial and sustained weight loss and improvement of cardiometabolic parameters in individuals with obesity (220). Several ongoing CVOTs are expected to shed light on the CV-related efficacy and safety of semaglutide or tirzepatide therapy in people with obesity or overweight but without diabetes.
STEP-HFpEF (Effect of Semaglutide 2.4 mg Once Weekly on Function and Symptoms in Subjects with Obesity-related Heart Failure with Preserved Ejection Fraction) trial has been the first RCT demonstrating the benefits of weight loss with a GLP1-RA in non-diabetic patients with HFpEF and obesity (BMI kg/m2) (221). In this trial, semaglutide once-weekly therapy for one year led to 13.3% weight loss and was associated with significant reductions in HF-related symptoms and physical limitations. In addition, patients treated with semaglutide experienced significant improvement in 6-minute wall distance and reduction levels of natriuretic peptide and c-reactive protein. Interestingly, fewer serious adverse events were observed with semaglutide than placebo. This trial was not powered to evaluate mortality and HF hospitalization outcomes properly. Another ongoing trial is investigating the impact of semaglutide in patients with HFpEF, obesity, and T2DM.
Metformin
As per the recent guidelines, metformin remains the preferred initial pharmacologic agent for treating T2DM if the treatment goal is achieving and maintaining glycemic control and weight management (23). The efficacy and safety of metformin in patients with HF have not been evaluated in dedicated prospective CVOTs. Therefore, metformin is no longer considered the first-line agent for cardiorenal renal risk reduction in T2DM patients with established or increased risk of HF. However, metformin can be used for glucose lowering in patients with T2DM and stable HF if eGFR remains >30 ml/min/1.73 m2. However, it should be avoided in hospitalized patients with HF (141).
Data from retrospective studies and post hoc analysis of prospective RCTs predominantly supported the benefits and safety of metformin in HF populations (222). A meta-analysis of 9 observational studies conducted in the 2000s and early 2010s showed reduced mortality and no change in safety outcomes with the use of metformin compared to sulfonylurea therapy (predominantly) in HF populations (223). It should be noted that the U.S. FDA removed HF from the contraindication list of metformin in 2006 since the lactic acidosis risk is rare, and the benefits of metformin use in patients with HF were supported by observational studies (61).
Table 4.
Effect of Glucose-Lowering Drugs on Heart Failure Hospitalization Risk in Patients with T2DM
| Drug Class | Medication | HF Hospitalization |
|---|---|---|
| SGLT-2 Inhibitors | Empagliflozin | 35% reduced risk |
| Canagliflozin | 33% reduced risk | |
| Dapagliflozin | 27% reduced risk | |
| Ertugliflozin | 30% reduced risk* | |
| Dual SGLT1/SGLT2 Inhibitor | Sotagliflozin | 33% reduced risk |
| GLP-1 Receptor Agonists | Liraglutide | Neutral effect |
| Lixisenatide | Neutral effect | |
| Semaglutide | Neutral effect | |
| Albiglutide | Neutral effect | |
| Exenatide | Neutral effect | |
| Efpeglenatide | 39% reduced risk | |
| DPP-4 Inhibitors | Saxagliptin | 27% increased risk |
| Alogliptin | Neutral effect** | |
| Sitagliptin | Neutral effect | |
| Vildagliptin | Neutral effect | |
| Linagliptin | Neutral effect | |
| Thiazolidinediones | Rosiglitazone | Increased risk*** |
| Pioglitazone | 41% Increased risk |
- *
Exploratory secondary outcome (primary outcome of MACE was similar to placebo). **Possible increase in the risk of HF hospitalization in patients without a history of HF at baseline (HR 1.76, 1·07–2·90). *** Increased risk of HF hospitalization and HF-related death (HR 2.10, 1.35-3.27).
DPP4 Inhibitors
CV safety and HF outcomes of DPP4 inhibitors have been examined in several large-scale CVOTs (Table 4). In the Savor-TIMI-53 trial, there was no significant difference between Saxagliptin and placebo regarding the primary outcome of CV events. However, saxagliptin use was associated with a 27% relative increase in the risk of HF hospitalizations (224). In the EXAMINE (Cardiovascular Outcomes Study of Alogliptin in Patients With Type 2 Diabetes and Acute Coronary Syndrome) trial, alogliptin use showed a non-significant trend towards (3.9% vs. 3.3%, p = 0.22) increased risk of HF hospitalizations in the entire study population with a history of T2DM and recent acute coronary syndrome (225). However, the subgroup analysis showed an unexpected increase (2.2% vs. 1.3%, p = 0.026) in the risk of HF hospitalizations among subjects without a previous history of HF at baseline. Contrarily, a similar increase in the risk of HF hospitalization was not observed with other DPP4 inhibitors in dedicated CVOTs (226,227). The mechanism of increased HF hospitalization risk with saxagliptin and alogliptin remains unknown.
A recent retrospective comparative-effectiveness study using a US health insurance data set compared the CVD outcomes among patients with T2DM who were prescribed a DPP4 inhibitor vs. an SGLT2 inhibitor (193). In this cohort, the DPP4 inhibitor therapy group experienced a higher risk of MACE and hospitalization for HF than those initiated on an SGLT2 inhibitor.
DPP4 inhibitors have become less popular due to their cost and limited effectiveness in CV risk reduction compared to SGLT2 inhibitors and GLP1-RAs. Therefore, they are not among the preferred first- or second-line agents for managing T2DM in patients with HF. Based on available data, saxagliptin should be avoided in patients with known or at risk for HF. Moreover, HF risk is listed in the prescribing precautions of alogliptin.
Insulin
No solid evidence exists to suggest that, in T2DM, improving glycemic control with insulin lowers HF risk in at-risk patients or improves outcomes in those with established HF. Even though some observational studies suggested an increased risk of CV mortality and HF in T2DM patients treated with insulin (228), limited prospective trial data did not support such an association. The ORIGIN Trial (Outcome Reduction With Initial Glargine Intervention) was a CVOT that examined the early use of basal insulin (glargine) versus standard care in patients with T2DM or prediabetes and a high risk of CVD. The study found that insulin glargine was neutral with regards to CV outcomes and HF events (229).
Sulfonylureas
Sulfonylureas (i.e., glipizide, glyburide, and glimepiride) are widely used glucose-lowering agents that promote weight gain and fluid retention (4). No dedicated RCTs evaluated the efficacy and safety of sulfonylureas in patients with HF. Data from post hoc analysis of prospective trials and observational studies have suggested either no change or worsening of HF outcomes with the use of sulfonylureas in patients with or without known HF. A recent population-based cohort study including older diabetic patients hospitalized for HF between 2006 and 2014 demonstrated that sulfonylurea initiation was associated with increased risk of future HF hospitalization (HR: 1.22; 95% CI: 1.00-1.48; P = 0.050) and mortality (HR: 1.24; 95% CI: 1.00-1.52; P = 0.045) (193). Based on current evidence, SGLT2 inhibitors, GLP1-RAs, and metformin are strongly preferable; sulfonylurea use should be avoided in patients with established or high risk for HF (4,141).
Thiazolidinediones
Thiazolidinediones (rosiglitazone and pioglitazone) have been effective oral antidiabetics in treating T2DM, significantly reducing HbA1c. However, they carry a risk of HF exacerbation and thus should be avoided in patients with established or at risk for HF. (Table 4). Therefore, thiazolidinediones should not be used in patients with pre-HF or clinical HF (4).
European Medicines Agencies and the U.S. FDA restricted the use of rosiglitazone, citing concerns about increased HF exacerbation after the publication of the results of the RECORD trial (Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycaemia in Diabetes) (230). Similarly, the PROactive study (PROspective pioglitAzone Clinical Trial In macroVascular Events) showed an improvement in MACE but an increased risk of HF events with pioglitazone therapy in high-risk patients with T2DM (231). The worsening HF outcomes with the treatment of thiazolidinediones have mainly been attributed to their effect on fluid retention. Some studies have raised concerns about the adverse impact of thiazolidinediones on myocardial metabolism and remodeling (61,222). Because of the availability of better options and their potential unfavorable side effect profile, the popularity of thiazolidinediones has significantly declined.
CONCLUSIONS
HF and cardiomyopathy have a heterogeneous etiology in patients with diabetes. Diabetes-related comorbidities, such as CAD and hypertension, contribute to the pathogenesis of HF in patients with diabetes. The pathophysiologic link between diabetes and HF is multifactorial, involving various abnormal biochemical pathways. A complex interaction of these mechanisms contributes to the development of asymptomatic diastolic and systolic dysfunction, eventually leading to the clinical syndrome of HF.
The coexistence of diabetes and HF is a poor prognostic factor, and it poses a higher risk of HF hospitalization, all-cause mortality, and CVD mortality. Therefore, the prevention of asymptomatic cardiac remodeling and progression into symptomatic HF are among the primary goals of the clinical management of patients with diabetes. BP lowering has substantial benefits in preventing HF among individuals with diabetes. Despite the well-established favorable effects of weight loss, the role of lifestyle changes and weight loss in preventing HF among diabetic patients remains uncertain. Observational data demonstrated a significant reduction in HF outcomes in response to metabolic surgeries among patients with diabetes and morbid obesity.
Guideline-directed medical therapy for HF has robust morbidity and mortality benefits in individuals with or without diabetes. Subgroup analysis or meta-analysis of major HF RCTs demonstrated that the effectiveness of HF therapies such as beta-blockers, RAAS inhibitors, ARNI, and MRAs do not vary based on patients’ diabetes status, and these therapies lead to similar reductions in morbidity and mortality among HF patients with or without diabetes.
Since 2015, several landmark clinical trials of SGLT2 inhibitors and GLP1-RA have revolutionized our understanding of CVD risk reduction in patients with T2DM and have led to a paradigm shift in the clinical practice recommendations for managing T2DM. SGLT-2 inhibitors are the preferred agents in the glucose-lowering regimen independent of baseline HbA1c in T2DM patients with known or at risk for HF.
Several dedicated major clinical trials confirmed the CVD benefits of SGLT2 inhibitors in patients with established HF, regardless of LVEF or diabetes status. High-quality data from these clinical trials transformed SGLT2 inhibitors from a glucose-lowering agent to a HF drug. Moreover, SGLT2 inhibitors are the only medication class with U.S. FDA approval and strong guideline indication (class I or IIa) for all HF patients across different LVEF groups.
ACKNOWLEDGMENT
We thank Halis Kaan Akturk for his contribution to the previous version of this chapter.
REFERENCES
- 1.
- Chen YT, Vaccarino V, Williams CS, Butler J, Berkman LF, Krumholz HM. Risk factors for heart failure in the elderly: A prospective community- based study. Am. J. Med. 1999;106(6):605–612. [PubMed: 10378616]
- 2.
- Kannel WBB, Hjortland M, Castelli WPP. Role of diabetes in congestive heart failure: The Framingham study. Am. J. Cardiol. 1974;34(1):29–34. [PubMed: 4835750]
- 3.
- Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, Fang JC, Fedson SE, Fonarow GC, Hayek SS, Hernandez AF, Khazanie P, Kittleson MM, Lee CS, Link MS, Milano CA, Nnacheta LC, Sandhu AT, Stevenson LW, Vardeny O, Vest AR, Yancy CW. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022;145(18):E895–E1032. [PubMed: 35363499]
- 4.
- Pop-Busui R, Januzzi JL, Bruemmer D, Butalia S, Green JB, Horton WB, Knight C, Levi M, Rasouli N, Richardson CR. Heart Failure: An Underappreciated Complication of Diabetes. A Consensus Report of the American Diabetes Association. Diabetes Care 2022;45(7):1670–1690. [PMC free article: PMC9726978] [PubMed: 35796765]
- 5.
- Bozkurt B, Coats AJ, Tsutsui H, Abdelhamid M, Adamopoulos S, Albert N, Anker SD, Atherton J, Böhm M, Butler J, Drazner MH, Felker GM, Filippatos G, Fonarow GC, Fiuzat M, Gomez-Mesa JE, Heidenreich P, Imamura T, Januzzi J, Jankowska EA, Khazanie P, Kinugawa K, Lam CSP, Matsue Y, Metra M, Ohtani T, Francesco Piepoli M, Ponikowski P, Rosano GMC, Sakata Y, SeferoviĆ P, Starling RC, Teerlink JR, Vardeny O, Yamamoto K, Yancy C, Zhang J, Zieroth S. Universal Definition and Classification of Heart Failure: A Report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition o. J. Card. Fail. 2021;27(4):387–413. [PubMed: 33663906]
- 6.
- Nichols GA, Gullion CM, Koro CE, Ephross SA, Brown JB. The incidence of congestive heart failure in type 2 diabetes: An update. Diabetes Care 2004;27(8):1879–1884. [PubMed: 15277411]
- 7.
- Jia G, Hill MAA, Sowers JRR. Diabetic cardiomyopathy: An update of mechanisms contributing to this clinical entity. Circ. Res. 2018;122(4):624–638. [PMC free article: PMC5819359] [PubMed: 29449364]
- 8.
- Bertoni AG, Hundley WG, Massing MW, Bonds DE, Burke GL, Goff DC. Heart Failure Prevalence, Incidence, and Mortality in the Elderly with Diabetes. Diabetes Care 2004;27(3):699–703. [PubMed: 14988288]
- 9.
- Thrainsdottir IS, Aspelund T, Thorgeirsson G, Gudnason V, Hardarson T, Malmberg K, Sigurdsson G, Rydén L. The association between glucose abnormalities and heart failure in the population-based Reykjavík Study. Diabetes Care 2005;28(3):612–616. [PubMed: 15735197]
- 10.
- Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2020.
- 11.
- Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsbøll T. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016;375(19):1834–1844. [PubMed: 27633186]
- 12.
- McMurray JJV, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR. Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure. N. Engl. J. Med. 2014;371(11):993–1004. [PubMed: 25176015]
- 13.
- Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJV, Michelson EL, Olofsson B, Östergren J, Yusuf S. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: The CHARM-overall programme. Lancet 2003;362(9386):759–766. [PubMed: 13678868]
- 14.
- Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am. J. Cardiol. 1972;30(6):595–602. [PubMed: 4263660]
- 15.
- Maack C, Lehrke M, Backs J, Heinzel FRR, Hulot JSS, Marx N, Paulus WJJ, Rossignol P, Taegtmeyer H, Bauersachs J, Bayes-Genis A, Brutsaert D, Bugger H, Clarke K, Cosentino F, De Keulenaer G, Cas ADD, González A, Huelsmann M, Iaccarino G, Lunde IGG, Lyon ARR, Pollesello P, Rena G, Riksen NPP, Rosano G, Staels B, Van Laake LWW, Wanner C, Farmakis D, Filippatos G, Ruschitzka F, Seferovic P, De Boer RAA, Heymans S. Heart failure and diabetes: Metabolic alterations and therapeutic interventions: A state-of-The-Art review from the Translational Research Committee of the Heart Failure Association-European Society of Cardiology. Eur. Heart J. 2018;39(48):4243–4254. [PMC free article: PMC6302261] [PubMed: 30295797]
- 16.
- Murtaza G, Virk HUHUH, Khalid M, Lavie CJJ, Ventura H, Mukherjee D, Ramu V, Bhogal S, Kumar G, Shanmugasundaram M, Paul TKK. Diabetic cardiomyopathy - A comprehensive updated review. Prog. Cardiovasc. Dis. 2019;62(4):315–326. [PubMed: 30922976]
- 17.
- Amato L, Paolisso G, Cacciatore F, Ferrara N, Ferrara P, Canonico S, Varricchio M, Rengo F. Congestive heart failure predicts the development of non-insulin-dependent diabetes mellitus in the elderly. The Osservatorio Geriatrico Regione Campania Group. Diabetes Metab. 1997;23(3):213–8. [PubMed: 9233998]
- 18.
- McDonagh TA, Metra M, Adamo M, Baumbach A, Böhm M, Burri H, Čelutkiene J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gardner RS, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Piepoli MF, Price S, Rosano GMC, Ruschitzka F, Skibelund AK, de Boer RA, Schulze PC, Abdelhamid M, Aboyans V, Adamopoulos S, Anker SD, Arbelo E, Asteggiano R, Bauersachs J, Bayes-Genis A, Borger MA, Budts W, Cikes M, Damman K, Delgado V, Dendale P, Dilaveris P, Drexel H, Ezekowitz J, Falk V, Fauchier L, Filippatos G, Fraser A, Frey N, Gale CP, Gustafsson F, Harris J, Iung B, Janssens S, Jessup M, Konradi A, Kotecha D, Lambrinou E, Lancellotti P, Landmesser U, Leclercq C, Lewis BS, Leyva F, Linhart A, Løchen ML, Lund LH, Mancini D, Masip J, Milicic D, Mueller C, Nef H, Nielsen JC, Neubeck L, Noutsias M, Petersen SE, Petronio AS, Ponikowski P, Prescott E, Rakisheva A, Richter D, Schlyakhto E, Seferovic P, Senni M, Sitges M, Sousa-Uva M, Tocchetti CG, Touyz R, Tschoepe C, Waltenberger J, Krim M, Hayrapetyan H, Moertl D, Mustafayev I, Kurlianskaya A, Depauw M, Kušljugić Z, Gatzov P, Agathangelou P, Melenovský V, Løgstrup BB, Mostafa AM, Uuetoa T, Lassus J, Logeart D, Kipiani Z, Chrysohoou C, Sepp R, Ingimarsdóttir IJ, O’Neill J, Gotsman I, Iacoviello M, Bajraktari G, Lunegova O, Kamzola G, Massih TA, Benlamin H, Žaliaduonyte D, Noppe S, Moore A, Vataman E, Boskovic A, Bennis A, Manintveld OC, Kostovska ES, Gulati G, Straburzyńska-Migaj E, Silva-Cardoso J, Rimbaş RC, Lopatin Y, Foscoli M, Stojkovic S, Goncalvesova E, Fras Z, Segovia J, Lindmark K, Maeder MT, Bsata W, Abid L, Altay H, Voronkov L, Davies C, Abdullaev T, Baigent CN, Antoniou S, Collet JP, Halvorsen S, Koskinas KC. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021;42(36):3599–3726. [PubMed: 34447992]
- 19.
- Targher G, Dauriz M, Laroche C, Temporelli PL, Hassanein M, Seferovic PM, Drozdz J, Ferrari R, Anker S, Coats A, Filippatos G, Crespo-Leiro MG, Mebazaa A, Piepoli MF, Maggioni A Pietro, Tavazzi L. In-hospital and 1-year mortality associated with diabetes in patients with acute heart failure: results from the ESC-HFA Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2017;19(1):54–65. [PubMed: 27790816]
- 20.
- Demant MN, Gislason GH, Køber L, Vaag A, Torp-Pedersen C, Andersson C. Association of heart failure severity with risk of diabetes: A Danish nationwide cohort study. Diabetologia 2014;57(8):1595–1600. [PubMed: 24849568]
- 21.
- Pavlović A, Polovina M, Ristić A, Seferović JP, Veljić I, Simeunović D, Milinković I, Krljanac G, Ašanin M, Oštrić-Pavlović I, Seferović PM. Long-term mortality is increased in patients with undetected prediabetes and type-2 diabetes hospitalized for worsening heart failure and reduced ejection fraction. Eur. J. Prev. Cardiol. 2019;26(1):72–82. [PubMed: 30335505]
- 22.
- Wilcox T, De Block C, Schwartzbard AZ, Newman JD. Diabetic Agents, From Metformin to SGLT2 Inhibitors and GLP1 Receptor Agonists: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020;75(16):1956–1974. [PMC free article: PMC7219531] [PubMed: 32327107]
- 23.
- Elsayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, Kahan S, Khunti K, Leon J, Lyons SK, Perry M Lou, Prahalad P, Pratley RE, Seley JJ, Stanton RC, Gabbay RA. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes—2023. Diabetes Care 2023;46(supp):S140–S157. [PMC free article: PMC9810476] [PubMed: 36507650]
- 24.
- Marsico F, Gargiulo P, Marra AM, Parente A, Paolillo S. Glucose Metabolism Abnormalities in Heart Failure Patients: Insights and Prognostic Relevance. Heart Fail. Clin. 2019;15(3):333–340. [PubMed: 31079691]
- 25.
- Braunwald E. Diabetes, heart failure, and renal dysfunction: The vicious circles. Prog. Cardiovasc. Dis. 2019;62(4):298–302. [PubMed: 31377223]
- 26.
- Rosengren A, Vestberg D, Svensson AM, Kosiborod M, Clements M, Rawshani A, Pivodic A, Gudbjörnsdottir S, Lind M. Long-term excess risk of heart failure in people with type 1 diabetes: A prospective case-control study. Lancet Diabetes Endocrinol. 2015;3(11):876–885. [PubMed: 26388415]
- 27.
- Rosengren A, Edqvist J, Rawshani A, Sattar N, Franzén S, Adiels M, Svensson AM, Lind M, Gudbjörnsdottir S. Excess risk of hospitalisation for heart failure among people with type 2 diabetes. Diabetologia 2018;61(11):2300–2309. [PMC free article: PMC6182656] [PubMed: 30094466]
- 28.
- Tenenbaum A, Motro M, Fisman EZ, Leor J, Freimark D, Boyko V, Mandelzweig L, Adler Y, Sherer Y, Behar S. Functional class in patients with heart failure is associated with the development of diabetes. Am. J. Med. 2003;114(4):271–275. [PubMed: 12681453]
- 29.
- Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, Baker-Smith CM, Beaton AZ, Boehme AK, Buxton AE, Commodore-Mensah Y, Elkind MSV, Evenson KR, Eze-Nliam C, Fugar S, Generoso G, Heard DG, Hiremath S, Ho JE, Kalani R, Kazi DS, Ko D, Levine DA, Liu J, Ma J, Magnani JW, Michos ED, Mussolino ME, Navaneethan SD, Parikh NI, Poudel R, Rezk-Hanna M, Roth GA, Shah NS, St-Onge MP, Thacker EL, Virani SS, Voeks JH, Wang NY, Wong ND, Wong SS, Yaffe K, Martin SS. Heart Disease and Stroke Statistics - 2023 Update: A Report from the American Heart Association. Circulation 2023;147(8):E93–E621. [PubMed: 36695182]
- 30.
- Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, van Veldhuisen DJ, Zannad F, Zile MR, Desai AS, Claggett B, Jhund PS, Boytsov SA, Comin-Colet J, Cleland J, Düngen H-D, Goncalvesova E, Katova T, Kerr Saraiva JF, Lelonek M, Merkely B, Senni M, Shah SJ, Zhou J, Rizkala AR, Gong J, Shi VC, Lefkowitz MP. Angiotensin–Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2019;381(17):1609–1620. [PubMed: 31475794]
- 31.
- Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O’Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM. Spironolactone for Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2014;370(15):1383–1392. [PubMed: 24716680]
- 32.
- Ishikawa Y, Lewis RD, Laing EM, Anderson AK, Zhang D, Quyyumi AA, Dunbar SB, Trivedi-Kapoor R, Sattler ELP. Prevalence and trends of type 2 diabetes mellitus and prediabetes among community-dwelling heart failure patients in the United States. Diabetes Res. Clin. Pract. 2022;184. doi:.10.1016/J.DIABRES.2022.109191 [PubMed: 35041861] [CrossRef]
- 33.
- Kristensen SL, Preiss D, Jhund PS, Squire I, Cardoso JS, Merkely B, Martinez F, Starling RC, Desai AS, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, McMurray JJV, Packer M. Risk Related to Pre-Diabetes Mellitus and Diabetes Mellitus in Heart Failure with Reduced Ejection Fraction: Insights from Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure Trial. Circ. Hear. Fail. 2016;9(1):1–12. [PMC free article: PMC4718182] [PubMed: 26754626]
- 34.
- Oktay AA, Akturk HK, Esenboğa K, Javed F, Polin NM, Jahangir E. Pathophysiology and Prevention of Heart Disease in Diabetes Mellitus. Curr. Probl. Cardiol. 2018;43(3):68–110. [PubMed: 29471918]
- 35.
- Bensimhon HF, Cavender MA. Hypertension Treatment in Diabetes: Focus on Heart Failure Prevention. Heart Fail. Clin. 2019;15(4):551–563. [PubMed: 31472890]
- 36.
- Albakri A. Diabetic cardiomyopathy: a review of literature on clinical status and meta-analysis of tissue doppler diagnostic method and the clinical value of intensive glycemic control. Med. Clin. Arch. 2018;2(4):1–12.
- 37.
- Lundbæk K. DIABETIC ANGIOPATHY. A SPECIFIC VASCULAR DISEASE. Lancet 1954;263(6808):377–379. [PubMed: 13131862]
- 38.
- Seferović PM, Paulus WJ. Clinical diabetic cardiomyopathy: A two-faced disease with restrictive and dilated phenotypes. Eur. Heart J. 2015;36(27):1718–1727. [PubMed: 25888006]
- 39.
- Kannel WB, McGee DL. Diabetes and Cardiovascular Disease: The Framingham Study. JAMA 1979;241(19):2035–2038. [PubMed: 430798]
- 40.
- Uijl A, Koudstaal S, Direk K, Denaxas S, Groenwold RHH, Banerjee A, Hoes AW, Hemingway H, Asselbergs FW. Risk factors for incident heart failure in age- and sex-specific strata: a population-based cohort using linked electronic health records. Eur. J. Heart Fail. 2019;21(10):1197–1206. [PMC free article: PMC7074015] [PubMed: 30618162]
- 41.
- Sattar N, McMurray J, Borén J, Rawshani A, Omerovic E, Berg N, Halminen J, Skoglund K, Eliasson B, Gerstein HC, McGuire DK, Bhatt D, Rawshani A. Twenty Years of Cardiovascular Complications and Risk Factors in Patients With Type 2 Diabetes: A Nationwide Swedish Cohort Study. Circulation 2023;147(25):1872–1886. [PubMed: 37154040]
- 42.
- Patel K V., Segar MW, Lavie CJ, Kondamudi N, Neeland IJ, Almandoz JP, Martin CK, Carbone S, Butler J, Powell-Wiley TM, Pandey A. Diabetes Status Modifies the Association Between Different Measures of Obesity and Heart Failure Risk Among Older Adults: A Pooled Analysis of Community-Based NHLBI Cohorts. Circulation 2022;145(4):268. [PMC free article: PMC8792339] [PubMed: 34860539]
- 43.
- Johansson I, Dahlström U, Edner M, Näsman P, Rydén L, Norhammar A. Prognostic Implications of Type 2 Diabetes Mellitus in Ischemic and Nonischemic Heart Failure. J. Am. Coll. Cardiol. 2016;68(13):1404–1416. [PubMed: 27659462]
- 44.
- Echouffo-Tcheugui JB, Ndumele CE, Zhang S, Florido R, Matsushita K, Coresh J, Skali H, Shah AM, Selvin E. Diabetes and Progression of Heart Failure: The Atherosclerosis Risk In Communities (ARIC) Study. J. Am. Coll. Cardiol. 2022;79(23):2285–2293. [PMC free article: PMC10125541] [PubMed: 35680178]
- 45.
- McAllister DA, Read SH, Kerssens J, Livingstone S, McGurnaghan S, Jhund P, Petrie J, Sattar N, Fischbacher C, Kristensen SL, McMurray J, Colhoun HM, Wild SH. Incidence of hospitalization for heart failure and case-fatality among 3.25 million people with and without diabetes mellitus. Circulation 2018;138(24):2774–2786. [PMC free article: PMC6287897] [PubMed: 29950404]
- 46.
- Kubicki DM, Xu M, Akwo EA, Dixon D, Muñoz D, Blot WJ, Wang TJ, Lipworth L, Gupta DK. Race and Sex Differences in Modifiable Risk Factors and Incident Heart Failure. JACC Hear. Fail. 2020;8(2):122–130. [PMC free article: PMC6995453] [PubMed: 32000962]
- 47.
- Chamberlain AM, Boyd CM, Manemann SM, Dunlay SM, Gerber Y, Killian JM, Weston SA, Roger VL. Risk Factors for Heart Failure in the Community: Differences by Age and Ejection Fraction. Am. J. Med. 2020;133(6):e237–e248. [PMC free article: PMC7558500] [PubMed: 31747542]
- 48.
- Ohkuma T, Komorita Y, Peters SAE, Woodward M. Diabetes as a risk factor for heart failure in women and men: a systematic review and meta-analysis of 47 cohorts including 12 million individuals. Diabetologia 2019;62(9):1550–1560. [PMC free article: PMC6677875] [PubMed: 31317230]
- 49.
- Sinha A, Ning H, Cameron N, Bancks M, Carnethon MR, Allen NB, Wilkins JT, Lloyd-jones DM, Khan SS. Atherosclerotic Cardiovascular Disease or Heart Failure: First Cardiovascular Event in Adults With Prediabetes and Diabetes. J Card Fail. 2022. doi:.10.1016/j.cardfail.2022.10.426 [PubMed: 36343785] [CrossRef]
- 50.
- Held C, Gerstein HC, Yusuf S, Zhao F, Hilbrich L, Anderson C, Sleight P, Teo K, ONTARGET/TRANSCEND Investigators. Glucose levels predict hospitalization for congestive heart failure in patients at high cardiovascular risk. Circulation 2007;115(11):1371–5. [PubMed: 17339550]
- 51.
- Nelson AJ, Peterson ED, Pagidipati NJ. Atherosclerotic cardiovascular disease and heart failure: Determinants of risk and outcomes in patients with diabetes. Prog. Cardiovasc. Dis. 2019;62(4):306–314. [PubMed: 31301314]
- 52.
- Nyström T, Sartipy U, Contardi A, Lind M, Bellocco R, Eliasson B, Svensson AM, Holzmann MJ. Glycated Hemoglobin A1c Levels in Type 1 Diabetes Mellitus and Outcomes after Myocardial Infarction. Circulation 2019;139(20):2380–2382. [PubMed: 31082296]
- 53.
- Rawshani A, Sattar N, Franzén S, Rawshani A, Hattersley AT, Svensson AM, Eliasson B, Gudbjörnsdottir S. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, register-based cohort study. Lancet 2018;392(10146):477–486. [PMC free article: PMC6828554] [PubMed: 30129464]
- 54.
- Sattar N, Rawshani A, Franzén S, Rawshani A, Svensson AM, Rosengren A, Mcguire DK, Eliasson B, Gudbjörnsdottir S. Age at Diagnosis of Type 2 Diabetes Mellitus and Associations With Cardiovascular and Mortality Risks: Findings From the Swedish National Diabetes Registry. Circulation 2019;139(19):2228–2237. [PubMed: 30955347]
- 55.
- Echouffo-Tcheugui JB, Zhang S, Florido R, Hamo C, Pankow JS, Michos ED, Goldberg RB, Nambi V, Gerstenblith G, Post WS, Blumenthal RS, Ballantyne CM, Coresh J, Selvin E, Ndumele CE. Duration of Diabetes and Incident Heart Failure The ARIC (Atherosclerosis Risk In Communities) Study. JACC Heart Fail 2021. doi:.10.1016/j.jchf.2021.06.005 [PMC free article: PMC8629143] [PubMed: 34325890] [CrossRef]
- 56.
- Shin D, Bohra C, Kongpakpaisarn K. Impact of the Discordance Between the American College of Cardiology/American Heart Association and American Diabetes Association Recommendations on Hypertension in Patients With Diabetes Mellitus in the United States. Hypertension 2018;72(2):256–259. [PubMed: 29941518]
- 57.
- Rawshani A, Rawshani A, Franzén S, Sattar N, Eliasson B, Svensson AM, Zethelius B, Miftaraj M, McGuire DK, Rosengren A, Gudbjörnsdottir S. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2018;379(7):633–644. [PubMed: 30110583]
- 58.
- Yoo TK, Han K Do, Rhee EJ, Lee WY. Impact of mental disorders on the risk of heart failure among Korean patients with diabetes: a cohort study. Cardiovasc. Diabetol. 2023;22(1):1–13. [PMC free article: PMC10197825] [PubMed: 37208672]
- 59.
- Yusuf S, Ostergren JB, Gerstein HC, Pfeffer MA, Swedberg K, Granger CB, Olofsson B, Probstfield J, McMurray J V, Mortality C in HF-A of R in, Morbidity Program I. Effects of candesartan on the development of a new diagnosis of diabetes mellitus in patients with heart failure.[Erratum appears in Circulation. 2005 Oct 25;112(7):e292]. Circulation 2005;112(1):48–53. [PubMed: 15983242]
- 60.
- Torp-Pedersen C, Metra M, Charlesworth A, Spark P, Lukas MA, Poole-Wilson PA, Swedberg K, Cleland JGF, Di Lenarda A, Remme WJ, Scherhag A. Effects of metoprolol and carvedilol on pre-existing and new onset diabetes in patients with chronic heart failure: Data from the Carvedilol or Metoprolol European Trial (COMET). Heart 2007;93(8):968–973. [PMC free article: PMC1994413] [PubMed: 17237130]
- 61.
- Dunlay SM, Givertz MM, Aguilar D, Allen LA, Chan M, Desai AS, Deswal A, Dickson VV, Kosiborod MN, Lekavich CL, McCoy RG, Mentz RJ, Piña IL. Type 2 Diabetes Mellitus and Heart Failure: A Scientific Statement From the American Heart Association and the Heart Failure Society of America: This statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update. Circulation 2019;140(7):e294–e324. [PubMed: 31167558]
- 62.
- Sud M, Wang X, Austin PC, Lipscombe LL, Newton GE, Tu J V., Vasan RS, Lee DS. Presentation blood glucose and death, hospitalization, and future diabetes risk in patients with acute heart failure syndromes. Eur. Heart J. 2015;36(15):924–931. [PMC free article: PMC6371700] [PubMed: 25572328]
- 63.
- Preiss D, Zetterstrand S, McMurray JJV, Östergren J, Michelson EL, Granger CB, Yusuf S, Swedberg K, Pfeffer MA, Gerstein HC, Sattar N. Predictors of development of diabetes in patients with chronic heart failure in the Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity (CHARM) program. Diabetes Care 2009;32(5):915–920. [PMC free article: PMC2671104] [PubMed: 19196892]
- 64.
- Preiss D, Van Veldhuisen DJ, Sattar N, Krum H, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B, Zannad F, McMurray JJV. Eplerenone and new-onset diabetes in patients with mild heart failure: Results from the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF). Eur. J. Heart Fail. 2012;14(8):909–915. [PubMed: 22611047]
- 65.
- Ernande L, Audureau E, Jellis CL, Bergerot C, Henegar C, Sawaki D, Czibik G, Volpi C, Canoui-Poitrine F, Thibault H, Ternacle J, Moulin P, Marwick TH, Derumeaux G. Clinical Implications of Echocardiographic Phenotypes of Patients With Diabetes Mellitus. J. Am. Coll. Cardiol. 2017;70(14):1704–1716. [PubMed: 28958326]
- 66.
- Yildiz M, Oktay AA, Stewart MH, Milani R V., Ventura HO, Lavie CJ. Left ventricular hypertrophy and hypertension. Prog. Cardiovasc. Dis. 2020;63:10–21. [PubMed: 31759953]
- 67.
- Wang Y, Yang H, Huynh Q, Nolan M, Negishi K, Marwick TH. Diagnosis of Nonischemic Stage B Heart Failure in Type 2 Diabetes Mellitus: Optimal Parameters for Prediction of Heart Failure. JACC Cardiovasc. Imaging 2018;11(10):1390–1400. [PubMed: 29778859]
- 68.
- Dawson A, Morris AD, Struthers AD. The epidemiology of left ventricular hypertrophy in type 2 diabetes mellitus. Diabetologia 2005;48(10):1971–1979. [PubMed: 16094529]
- 69.
- Hamid A, Yimer WK, Oshunbade AA, Kamimura D, Clark D, Fox ER, Min YI, Muntner P, Shimbo D, Pandey A, Shah AM, Mentz RJ, Jones DW, Bertoni AG, Hall JE, Correa A, Butler J, Hall ME. Impact of Diabetes and Hypertension on Left Ventricular Structure and Function: The Jackson Heart Study. J. Am. Hear. Assoc. Cardiovasc. Cerebrovasc. Dis. 2023;12(6):26463. [PMC free article: PMC10111514] [PubMed: 36880997]
- 70.
- Velagaleti RS, Gona P, Chuang ML, Salton CJ, Fox CS, Blease SJ, Yeon SB, Manning WJ, O’Donnell CJ. Relations of insulin resistance and glycemic abnormalities to cardiovascular magnetic resonance measures of cardiac structure and function: the Framingham Heart Study. Circ. Cardiovasc. Imaging 2010;3(3):257–63. [PMC free article: PMC3057083] [PubMed: 20208015]
- 71.
- Rutter MK, Parise H, Benjamin EJ, Levy D, Larson MG, Meigs JB, Nesto RW, Wilson PWF, Vasan RS. Impact of glucose intolerance and insulin resistance on cardiac structure and function: Sex-related differences in the Framingham Heart Study. Circulation 2003;107(3):448–454. [PubMed: 12551870]
- 72.
- Kishi S, Gidding SS, Reis JP, Colangelo LA, Venkatesh BA, Armstrong AC, Isogawa A, Lewis CE, Wu C, Jacobs DR, Liu K, Lima JAC. Association of Insulin Resistance and Glycemic Metabolic Abnormalities With LV Structure and Function in Middle Age: The CARDIA Study. JACC Cardiovasc. Imaging 2017;10(2):105–114. [PubMed: 27544896]
- 73.
- From AM, Scott CG, Chen HH. The development of heart failure in patients with diabetes mellitus and pre-clinical diastolic dysfunction a population-based study. J. Am. Coll. Cardiol. 2010;55(4):300–5. [PMC free article: PMC3878075] [PubMed: 20117433]
- 74.
- Galderisi M. Diastolic Dysfunction and Diabetic Cardiomyopathy. Evaluation by Doppler Echocardiography. J. Am. Coll. Cardiol. 2006;48(8):1548–1551. [PubMed: 17045886]
- 75.
- Marwick TH, Ritchie R, Shaw JE, Kaye D. Implications of Underlying Mechanisms for the Recognition and Management of Diabetic Cardiomyopathy. J. Am. Coll. Cardiol. 2018;71(3):339–351. [PubMed: 29348027]
- 76.
- Zhang L, Liebelt JJ, Madan N, Shan J, Taub CC. Comparison of Predictors of Heart Failure With Preserved Versus Reduced Ejection Fraction in a Multiracial Cohort of Preclinical Left Ventricular Diastolic Dysfunction. Am. J. Cardiol. 2017;119(11):1815–1820. [PubMed: 28450040]
- 77.
- Potter E, Marwick TH. Assessment of Left Ventricular Function by Echocardiography: The Case for Routinely Adding Global Longitudinal Strain to Ejection Fraction. JACC Cardiovasc. Imaging 2018;11(2P1):260–274. [PubMed: 29413646]
- 78.
- Smiseth OA, Torp H, Opdahl A, Haugaa KH, Urheim S. Myocardial strain imaging: how useful is it in clinical decision making? Eur. Heart J. 2016;37(15):1196–1207. [PMC free article: PMC4830908] [PubMed: 26508168]
- 79.
- Holland DJ, Marwick TH, Haluska BA, Leano R, Hordern MD, Hare JL, Fang ZY, Prins JB, Stanton T. Subclinical LV dysfunction and 10-year outcomes in type 2 diabetes mellitus. Heart 2015;101(13):1061–1066. [PubMed: 25935767]
- 80.
- Enomoto M, Ishizu T, Seo Y, Yamamoto M, Suzuki H, Shimano H, Kawakami Y, Aonuma K. Subendocardial Systolic Dysfunction in Asymptomatic Normotensive Diabetic Patients. Circ. J. 2015;79(8):1749–1755. [PubMed: 26016923]
- 81.
- Haley JE, Zhiqian G, Philip KR, Nicolas ML, Thomas KR, Lawrence DM, Elaine UM. Reduction in myocardial strain is evident in adolescents and young adults with obesity and type 2 diabetes. Pediatr. Diabetes 2020;21(2):243–250. [PubMed: 31825129]
- 82.
- Rakha S, Aboelenin HM. Left ventricular functions in pediatric patients with ten years or more type 1 diabetes mellitus: Conventional echocardiography, tissue Doppler, and two-dimensional speckle tracking study. Pediatr. Diabetes 2019;20(7):946–954. [PubMed: 31355962]
- 83.
- Skali H, Shah A, Gupta DK, Cheng S, Claggett B, Liu J, Bello N, Aguilar D, Vardeny O, Matsushita K, Selvin E, Solomon S. Cardiac Structure and Function Across the Glycemic Spectrum in Elderly Men and Women Free of Prevalent Heart Disease: The Atherosclerosis Risk in the Community Study. Circ. Hear. Fail. 2015;8(3):448–454. [PMC free article: PMC4439326] [PubMed: 25759458]
- 84.
- Rørth R, Jhund PS, Mogensen UM, Kristensen SL, Petrie MC, Køber L, McMurray JJV. Risk of incident heart failure in patients with diabetes and asymptomatic left ventricular systolic dysfunction. Diabetes Care 2018;41(6):1285–1291. [PubMed: 29626073]
- 85.
- Leung M, Wong VW, Hudson M, Leung DY. Impact of Improved Glycemic Control on Cardiac Function in Type 2 Diabetes Mellitus. Circ. Cardiovasc. Imaging 2016;9(3):e003643. [PubMed: 26962125]
- 86.
- Packer M. Differential Pathophysiological Mechanisms in Heart Failure With a Reduced or Preserved Ejection Fraction in Diabetes. JACC Hear. Fail. 2021;9(8):535–549. [PubMed: 34325884]
- 87.
- Bertero E, Maack C. Metabolic remodelling in heart failure. Nat. Rev. Cardiol. 2018;15(8):457–470. [PubMed: 29915254]
- 88.
- Marfella R, Amarelli C, Cacciatore F, Balestrieri ML, Mansueto G, D’Onofrio N, Esposito S, Mattucci I, Salerno G, De Feo M, D’Amico M, Golino P, Maiello C, Paolisso G, Napoli C. Lipid Accumulation in Hearts Transplanted From Nondiabetic Donors to Diabetic Recipients. J. Am. Coll. Cardiol. 2020;75(11):1249–1262. [PubMed: 32192650]
- 89.
- Doehner W, Rauchhaus M, Ponikowski P, Godsland IF, von Haehling S, Okonko DO, Leyva F, Proudler AJ, Coats AJS, Anker SD. Impaired insulin sensitivity as an independent risk factor for mortality in patients with stable chronic heart failure. J. Am. Coll. Cardiol. 2005;46(6):1019–26. [PubMed: 16168285]
- 90.
- Sanganalmath SK, Dubey S, Veeranki S, Narisetty K, Krishnamurthy P. The interplay of inflammation, exosomes and Ca2+ dynamics in diabetic cardiomyopathy. Cardiovasc. Diabetol. 2023;22(1):1–22. [PMC free article: PMC9942322] [PubMed: 36804872]
- 91.
- Jia G, Whaley-Connell A, Sowers JR. Diabetic cardiomyopathy: a hyperglycaemia- and insulin-resistance-induced heart disease. Diabetologia 2018;61(1):21–28. [PMC free article: PMC5720913] [PubMed: 28776083]
- 92.
- Falcão-Pires I, Hamdani N, Borbély A, Gavina C, Schalkwijk CG, van der Velden J, van Heerebeek L, Stienen GJM, Niessen HWM, Leite-Moreira AF, Paulus WJ. Diabetes mellitus worsens diastolic left ventricular dysfunction in aortic stenosis through altered myocardial structure and cardiomyocyte stiffness. Circulation 2011;124(10):1151–9. [PubMed: 21844073]
- 93.
- Hinkel R, Hoewe A, Renner S, Ng J, Lee S, Klett K, Kaczmarek V, Moretti A, Laugwitz KL, Skroblin P, Mayr M, Milting H, Dendorfer A, Reichart B, Wolf E, Kupatt C. Diabetes Mellitus–Induced Microvascular Destabilization in the Myocardium. J. Am. Coll. Cardiol. 2017;69(2):131–143. [PubMed: 28081822]
- 94.
- DeFuria J, Belkina AC, Jagannathan-Bogdan M, Snyder-Cappione J, Carr JD, Nersesova YR, Markham D, Strissel KJ, Watkins A a, Zhu M, Allen J, Bouchard J, Toraldo G, Jasuja R, Obin MS, McDonnell ME, Apovian C, Denis G V, Nikolajczyk BS. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc. Natl. Acad. Sci. U. S. A. 2013;110(13):5133–8. [PMC free article: PMC3612635] [PubMed: 23479618]
- 95.
- Murphy SP, Kakkar R, McCarthy CP, Januzzi JL. Inflammation in Heart Failure: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020;75(11):1324–1340. [PubMed: 32192660]
- 96.
- Vulesevic B, McNeill B, Giacco F, Maeda K, Blackburn NJR, Brownlee M, Milne RW, Suuronen EJ. Methylglyoxal-induced endothelial cell loss and inflammation contribute to the development of diabetic cardiomyopathy. Diabetes 2016;65(6):1699–1713. [PMC free article: PMC4878427] [PubMed: 26956489]
- 97.
- Tan X, Hu L, Shu Z, Chen L, Li X, Du M, Sun D, Mao X, Deng S, Huang K, Zhang F. Role of CCR2 in the development of streptozotocin-treated diabetic cardiomyopathy. Diabetes 2019;68(11):2063–2073. [PMC free article: PMC6804626] [PubMed: 31439648]
- 98.
- Gellen B, Thorin-Trescases N, Thorin E, Gand E, Ragot S, Montaigne D, Pucheu Y, Mohammedi K, Gatault P, Potier L, Liuu E, Hadjadj S, Saulnier PJ, Hadjadj S, Marechaud R, Ragot S, Piguel X, Saulnier PJ, Javaugue V, Gand E, Hulin-Delmotte C, Llatty P, Ducrocq G, Roussel R, Rigalleau V, Pucheu Y, Zaoui P, Montaigne D, Halimi JM, Gatault P, Sosner P, Gellen B. Increased serum S100A12 levels are associated with higher risk of acute heart failure in patients with type 2 diabetes. ESC Hear. Fail. 2022;9(6):3909–3919. [PMC free article: PMC9773733] [PubMed: 36637406]
- 99.
- Groves EM, Erande AS, Le C, Salcedo J, Hoang KC, Kumar S, Mohar DS, Saremi F, Im J, Agrawal Y, Nadeswaran P, Naderi N, Malik S. Comparison of epicardial adipose tissue volume and coronary artery disease severity in asymptomatic adults with versus without diabetes mellitus. Am. J. Cardiol. 2014;114(5):686–691. [PMC free article: PMC4216806] [PubMed: 25037677]
- 100.
- Christensen RH, Hansen CS, von Scholten BJ, Jensen MT, Pedersen BK, Schnohr P, Vilsbøll T, Rossing P, Jørgensen PG. Epicardial and pericardial adipose tissues are associated with reduced diastolic and systolic function in type 2 diabetes. Diabetes, Obes. Metab. 2019;21(8):2006–2011. [PubMed: 31050126]
- 101.
- Ng ACT, Strudwick M, van der Geest RJ, Ng ACC, Gillinder L, Goo SY, Cowin G, Delgado V, Wang WYS, Bax JJ. Impact of Epicardial Adipose Tissue, Left Ventricular Myocardial Fat Content, and Interstitial Fibrosis on Myocardial Contractile Function. Circ. Cardiovasc. Imaging 2018;11(8):e007372. [PubMed: 30354491]
- 102.
- Al-Talabany S, Mordi I, Graeme Houston J, Colhoun HM, Weir-McCall JR, Matthew SZ, Looker HC, Levin D, Belch JJF, Dove F, Khan F, Lang CC. Epicardial adipose tissue is related to arterial stiffness and inflammation in patients with cardiovascular disease and type 2 diabetes. BMC Cardiovasc. Disord. 2018;18(1):1–8. [PMC free article: PMC5809843] [PubMed: 29433433]
- 103.
- Nakanishi K, Fukuda S, Tanaka A, Otsuka K, Taguchi H, Shimada K. Relationships Between Periventricular Epicardial Adipose Tissue Accumulation, Coronary Microcirculation, and Left Ventricular Diastolic Dysfunction. Can. J. Cardiol. 2017;33(11):1489–1497. [PubMed: 28974326]
- 104.
- Lavie CJ, Oktay AA, Pandey A. Pericardial Fat and CVD: Is All Fat Created Equally? JACC Cardiovasc. Imaging 2017;10(9):1028–1030. [PubMed: 28330656]
- 105.
- Hoffman WH, Sharma M, Cihakova D, Talor M V., Rose NR, Mohanakumar T, Passmore GG. Cardiac antibody production to self-antigens in children and adolescents during and following the correction of severe diabetic ketoacidosis. Autoimmunity 2016;49(3):188–196. [PubMed: 26911924]
- 106.
- Sousa GR, Pober D, Galderisi A, Lv H, Yu L, Pereira AC, Doria A, Kosiborod M, Lipes MA. Glycemic Control, Cardiac Autoimmunity, and Long-Term Risk of Cardiovascular Disease in Type 1 Diabetes Mellitus. Circulation 2019;139(6):730–743. [PMC free article: PMC6361693] [PubMed: 30586738]
- 107.
- Miller JA, Floras JS, Zinman B, Skorecki KL, Logan AG. Effect of hyperglycaemia on arterial pressure, plasma renin activity and renal function in early diabetes. Clin. Sci. 1996;90(3):189–195. [PubMed: 8777824]
- 108.
- Spallone V, Ziegler D, Freeman R, Bernardi L, Frontoni S, Pop-Busui R, Stevens M, Kempler P, Hilsted J, Tesfaye S, Low P, Valensi P. Cardiovascular autonomic neuropathy in diabetes: Clinical impact, assessment, diagnosis, and management. Diabetes. Metab. Res. Rev. 2011;27(7):639–653. [PubMed: 21695768]
- 109.
- Pop-Busui R, Cleary PA, Braffett BH, Martin CL, Herman WH, Low PA, Lima JAC, Bluemke DA. Association between cardiovascular autonomic neuropathy and left ventricular dysfunction: DCCT/EDIC study (diabetes control and complications trial/epidemiology of diabetes interventions and complications). J. Am. Coll. Cardiol. 2013;61(4):447–454. [PMC free article: PMC3616477] [PubMed: 23265339]
- 110.
- Sacre JW, Franjic B, Jellis CL, Jenkins C, Coombes JS, Marwick TH. Association of cardiac autonomic neuropathy with subclinical myocardial dysfunction in type 2 diabetes. JACC Cardiovasc. Imaging 2010;3(12):1207–1215. [PubMed: 21163448]
- 111.
- Vinik AI, Casellini C, Parson HK, Colberg SR, Nevoret ML. Cardiac autonomic neuropathy in diabetes: A predictor of cardiometabolic events. Front. Neurosci. 2018;12(AUG):591. [PMC free article: PMC6119724] [PubMed: 30210276]
- 112.
- Salvador DB, Gamba MR, Gonzalez-Jaramillo N, Gonzalez-Jaramillo V, Raguindin PFN, Minder B, Gräni C, Wilhelm M, Stettler C, Doria A, Franco OH, Muka T, Bano A. Diabetes and Myocardial Fibrosis: A Systematic Review and Meta-Analysis. JACC Cardiovasc. Imaging 2022;15(5):796–808. [PubMed: 35512952]
- 113.
- Ouyang C, You J, Xie Z. The interplay between autophagy and apoptosis in the diabetic heart. J. Mol. Cell. Cardiol. 2014;71(2014):71–80. [PubMed: 24513079]
- 114.
- Kuethe F, Sigusch HH, Bornstein SR, Hilbig K, Kamvissi V, Figulla HR. Apoptosis in patients with dilated cardiomyopathy and diabetes: A feature of diabetic cardiomyopathy? Horm. Metab. Res. 2007;39(9):672–676. [PubMed: 17846975]
- 115.
- He C, Zhu H, Li H, Zou MH, Xie Z. Dissociation of Bcl-2-Beclin1 complex by activated AMPK enhances cardiac autophagy and protects against cardiomyocyte apoptosis in diabetes. Diabetes 2013;62(4):1270–1281. [PMC free article: PMC3609561] [PubMed: 23223177]
- 116.
- Ljubkovic M, Gressette M, Bulat C, Cavar M, Bakovic D, Fabijanic D, Grkovic I, Lemaire C, Marinovic J. Disturbed fatty acid oxidation, endoplasmic reticulum stress, and apoptosis in left ventricle of patients with type 2 diabetes. Diabetes 2019;68(10):1924–1933. [PubMed: 31391173]
- 117.
- Van Den Berge JC, Constantinescu AA, Boiten HJ, Van Domburg RT, Deckers JW, Akkerhuis KM. Short- and long-term prognosis of patients with acute heart failure with and without diabetes: Changes over the last three decades. Diabetes Care 2018;41(1):143–149. [PubMed: 28982652]
- 118.
- Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, Shah SJ, Desai AS, Jhund PS, Belohlavek J, Chiang C-E, Borleffs CJW, Comin-Colet J, Dobreanu D, Drozdz J, Fang JC, Alcocer-Gamba MA, Al Habeeb W, Han Y, Cabrera Honorio JW, Janssens SP, Katova T, Kitakaze M, Merkely B, O’Meara E, Saraiva JFK, Tereshchenko SN, Thierer J, Vaduganathan M, Vardeny O, Verma S, Pham VN, Wilderäng U, Zaozerska N, Bachus E, Lindholm D, Petersson M, Langkilde AM. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022;387(12):1089–1098. [PubMed: 36027570]
- 119.
- Dauriz M, Mantovani A, Bonapace S, Verlato G, Zoppini G, Bonora E, Targher G. Prognostic impact of diabetes on long-term survival outcomes in patients with heart failure: A meta-analysis. Diabetes Care 2017;40(11):1597–1605. [PubMed: 29061587]
- 120.
- Aguilar D, Bozkurt B, Ramasubbu K, Deswal A. Relationship of Hemoglobin A1C and Mortality in Heart Failure Patients With Diabetes. J. Am. Coll. Cardiol. 2009;54(5):422–428. [PMC free article: PMC2753214] [PubMed: 19628117]
- 121.
- McHugh K, DeVore AD, Wu J, Matsouaka RA, Fonarow GC, Heidenreich PA, Yancy CW, Green JB, Altman N, Hernandez AF. Heart Failure With Preserved Ejection Fraction and Diabetes: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019;73(5):602–611. [PubMed: 30732715]
- 122.
- MacDonald MR, Petrie MC, Varyani F, Ostergren J, Michelson EL, Young JB, Solomon SD, Granger CB, Swedberg K, Yusuf S, Pfeffer MA, McMurray JJ V, CHARM Investigators. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur. Heart J. 2008;29(11):1377–85. [PubMed: 18413309]
- 123.
- Aguilar D, Deswal A, Ramasubbu K, Mann DL, Bozkurt B. Comparison of Patients With Heart Failure and Preserved Left Ventricular Ejection Fraction Among Those With Versus Without Diabetes Mellitus. Am. J. Cardiol. 2010;105(3):373–377. [PMC free article: PMC2813214] [PubMed: 20102951]
- 124.
- Kristensen SL, Mogensen UM, Jhund PS, Petrie MC, Preiss D, Win S, Køber L, McKelvie RS, Zile MR, Anand IS, Komajda M, Gottdiener JS, Carson PE, McMurray JJ V. Clinical and Echocardiographic Characteristics and Cardiovascular Outcomes According to Diabetes Status in Patients With Heart Failure and Preserved Ejection FractionClinical Perspective. Circulation 2017;135(8):724–735. [PubMed: 28052977]
- 125.
- Lindman BR, Dávila-Román VG, Mann DL, McNulty S, Semigran MJ, Lewis GD, De Las Fuentes L, Joseph SM, Vader J, Hernandez AF, Redfield MM. Cardiovascular phenotype in HFpEF patients with or without diabetes: A RELAX trial ancillary study. J. Am. Coll. Cardiol. 2014;64(6):541–549. [PMC free article: PMC4133145] [PubMed: 25104521]
- 126.
- Cavender MA, Steg PG, Smith SC, Eagle K, Ohman EM, Goto S, Kuder J, Im K, Wilson PWF, Bhatt DL. Impact of Diabetes Mellitus on Hospitalization for Heart Failure, Cardiovascular Events, and Death: Outcomes at 4 Years from the Reduction of Atherothrombosis for Continued Health (REACH) Registry. Circulation 2015;132(10):923–931. [PubMed: 26152709]
- 127.
- Sandesara PB, O’Neal WT, Kelli HM, Samman-Tahhan A, Hammadah M, Quyyumi AA, Sperling LS. The prognostic significance of diabetes and microvascular complications in patients with heart failure with preserved ejection fraction. Diabetes Care 2018;41(1):150–155. [PMC free article: PMC5741155] [PubMed: 29051160]
- 128.
- Bassi N, Fonarow GC. Prevention of Heart Failure in Patients with Diabetes: Role of Diabetes Medications. Curr. Cardiol. Rep. 2018;20(11):1–7. [PubMed: 30259198]
- 129.
- Ahmad FS, Ning H, Rich JD, Yancy CW, Lloyd-Jones DM, Wilkins JT. Hypertension, Obesity, Diabetes, and Heart Failure–Free Survival: The Cardiovascular Disease Lifetime Risk Pooling Project. JACC Hear. Fail. 2016;4(12):911–919. [PMC free article: PMC5582802] [PubMed: 27908389]
- 130.
- Pandey A, Vaduganathan M, Patel K V, Ayers C, Ballantyne CM, Kosiborod MN, Carnethon M, Defilippi C, Mcguire DK, Khan SS, Caughey MC, De Lemos JA, Everett BM. Biomarker-Based Risk Prediction of Incident Heart Failure in Pre-Diabetes and Diabetes. JACC Heart Fail 2021. doi:.10.1016/j.jchf.2020.10.013 [PubMed: 33422434] [CrossRef]
- 131.
- Berg DD, Wiviott SD, Scirica BM, Gurmu Y, Mosenzon O, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Johanson P, Johansson PA, Langkilde AM, Raz I, Braunwald E, Sabatine MS. Heart Failure Risk Stratification and Efficacy of Sodium-Glucose Cotransporter-2 Inhibitors in Patients With Type 2 Diabetes Mellitus. Circulation 2019;140(19):1569–1577. [PMC free article: PMC6829059] [PubMed: 31474116]
- 132.
- Segar MW, Patel K V, Hellkamp AS, Vaduganathan M, Lokhnygina Y, Green JB, Wan S-H, Kolkailah AA, Holman RR, Peterson ED, Kannan V, Willett DL, McGuire DK, Pandey A. Validation of the WATCH-DM and TRS-HF DM Risk Scores to Predict the Risk of Incident Hospitalization for Heart Failure Among Adults With Type 2 Diabetes: A Multicohort Analysis. J. Am. Hear. Assoc. J Am Hear. Assoc 2022;11:24094. [PMC free article: PMC9238735] [PubMed: 35656988]
- 133.
- Segar MW, Vaduganathan M, Patel K V., McGuire DK, Butler J, Fonarow GC, Basit M, Kannan V, Grodin JL, Everett B, Willett D, Berry J, Pandey A. Machine learning to predict the risk of incident heart failure hospitalization among patients with diabetes: The WATCH-DM risk score. Diabetes Care 2019;42(12):2298–2306. [PMC free article: PMC7364669] [PubMed: 31519694]
- 134.
- Yildiz M, Esenboğa K, Oktay AA. Hypertension and diabetes mellitus: highlights of a complex relationship. Curr. Opin. Cardiol. 2020;35(4):397–404. [PubMed: 32371623]
- 135.
- Khangura DS, Waqar Salam M, Brietzke SA, Sowers JR. Hypertension in Diabetes. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A, Hershman JM, Kaltsas G, Koch C, Kopp P, Korbonits M, McLachlan R, Morley JE, New M, Perreault L, Purnell J, Rebar R, Singer F, Trence DL, Vinik A, Wilson DP, eds. Endotext. South Dartmouth (MA): MDText.com, Inc.; 2000; 2018.
- 136.
- Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA, Williamson JD, Wright JT. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary. JACC 2018;71(19):e127–e248. [PubMed: 29146535]
- 137.
- Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: A systematic review and meta-analysis. Lancet 2016;387(10022):957–967. [PubMed: 26724178]
- 138.
- Thomopoulos C, Parati G, Zanchetti A. Effects of blood-pressure-lowering treatment on outcome incidence in hypertension:10-Should blood pressure management differ in hypertensive patients with and without diabetes mellitus? Overview and meta-analyses of randomized trials. J. Hypertens. 2017;35(5):922–944. [PubMed: 28141660]
- 139.
- Accord-BP-Study-Group. Effects of Intensive Blood-Pressure Control in Type 2 Diabetes Mellitus. N. Engl. J. Med. 2010;362(17):1575–1585. [PMC free article: PMC4123215] [PubMed: 20228401]
- 140.
- Williams B, Mancia G, Spiering W, Rosei EA, Azizi M, Burnier M, Clement DL, Coca A, De Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I, De Backer G, Heagerty AM, Agewall S, Bochud M, Borghi C, Boutouyrie P, Brguljan J, Bueno H, Caiani EG, Carlberg B, Chapman N, Cífková R, Cleland JGF, Collet JP, Coman IM, De Leeuw PW, Delgado V, Dendale P, Diener HC, Dorobantu M, Fagard R, Farsang C, Ferrini M, Graham IM, Grassi G, Haller H, Hobbs FDR, Jelakovic B, Jennings C, Katus HA, Kroon AA, Leclercq C, Lovic D, Lurbe E, Manolis AJ, McDonagh TA, Messerli F, Muiesan ML, Nixdorff U, Olsen MH, Parati G, Perk J, Piepoli MF, Polonia J, Ponikowski P, Richter DJ, Rimoldi SF, Roffi M, Sattar N, Seferovic PM, Simpson IA, Sousa-Uva M, Stanton A V., Van De Borne P, Vardas P, Volpe M, Wassmann S, Windecker S, Zamorano JL. 2018 ESC/ESH Guidelines for themanagement of arterial hypertension. Eur. Heart J. 2018;39(33):3021–3104. [PubMed: 30165516]
- 141.
- Elsayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Das SR, Hilliard ME, Isaacs D, Johnson EL, Kahan S, Khunti K, Kosiborod M, Leon J, Lyons SK, Perry M Lou, Prahalad P, Pratley RE, Seley JJ, Stanton RC, Gabbay RA. 10. Cardiovascular Disease and Risk Management: Standards of Care in Diabetes—2023. Diabetes Care 2023;46(January):S158–S190. [PMC free article: PMC9810475] [PubMed: 36507632]
- 142.
- Whelton PK, Barzilay J, Cushman WC, Davis BR, Ilamathi E, Kostis JB, Leenen FHH, Louis GT, Margolis KL, Mathis DE, Moloo J, Nwachuku C, Panebianco D, Parish DC, Pressel S, Simmons DL, Thadani U. Clinical outcomes in antihypertensive treatment of type 2 diabetes, impaired fasting glucose concentration, and normoglycemia: Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Arch. Intern. Med. 2005;165(12):1401–1409. [PubMed: 15983290]
- 143.
- Wright JT, Probstfield JL, Cushman WC, Pressel SL, Cutler JA, Davis BR, Einhorn PT, Rahman M, Whelton PK, Ford CE, Haywood LJ, Margolis KL, Oparil S, Black HR, Alderman MH. ALLHAT findings revisited in the context of subsequent analyses, other trials, and meta-analyses. Arch. Intern. Med. 2009;169(9):832–842. [PMC free article: PMC2803011] [PubMed: 19433694]
- 144.
- Scheen AJ. Type 2 Diabetes and Thiazide Diuretics. Curr. Diab. Rep. 2018;18(2):1–13. [PubMed: 29399724]
- 145.
- Zhang X, Zhao Q. Association of Thiazide-Type Diuretics With Glycemic Changes in Hypertensive Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Clinical Trials. J. Clin. Hypertens. 2016;18(4):342–351. [PMC free article: PMC8031670] [PubMed: 26395424]
- 146.
- Elliott WJ, Meyer PM. Incident diabetes in clinical trials of antihypertensive drugs: a network meta-analysis. Lancet 2007;369(9557):201–207. [PubMed: 17240286]
- 147.
- Yang Y, Xu H. Comparing six antihypertensive medication classes for preventing new-onset diabetes mellitus among hypertensive patients: a network meta-analysis. J. Cell. Mol. Med. 2017;21(9):1742–1750. [PMC free article: PMC5571556] [PubMed: 28230330]
- 148.
- Williams B, Macdonald TM, Morant S, Webb DJ, Sever P, McInnes G, Ford I, Cruickshank JK, Caulfield MJ, Salsbury J, Mackenzie I, Padmanabhan S, Brown MJ. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): A randomised, double-blind, crossover trial. Lancet 2015;386(10008):2059–2068. [PMC free article: PMC4655321] [PubMed: 26414968]
- 149.
- Agarwal R, Kolkhof P, Bakris G, Bauersachs J, Haller H, Wada T, Zannad F. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur. Heart J. 2021;42(2):152–161. [PMC free article: PMC7813624] [PubMed: 33099609]
- 150.
- Brandt-Jacobsen NH, Lav Madsen P, Johansen ML, Rasmussen JJ, Forman JL, Holm MR, Rye Jørgensen N, Faber J, Rossignol P, Schou M, Kistorp C. Mineralocorticoid Receptor Antagonist Improves Cardiac Structure in Type 2 Diabetes: Data From the MIRAD Trial. JACC Hear. Fail. 2021;9(8):550–558. [PubMed: 34325885]
- 151.
- Pandey AK, Bhatt DL, Cosentino F, Marx N, Rotstein O, Pitt B, Pandey A, Butler J, Verma S. Non-steroidal mineralocorticoid receptor antagonists in cardiorenal disease. Eur. Heart J. 2022;43(31):2931–2945. [PubMed: 35713973]
- 152.
- Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, Kolkhof P, Nowack C, Schloemer P, Joseph A, Filippatos G. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2020;383(23):2219–2229. [PubMed: 33264825]
- 153.
- Pitt B, Filippatos G, Agarwal R, Anker SD, Bakris GL, Rossing P, Joseph A, Kolkhof P, Nowack C, Schloemer P, Ruilope LM. Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. N. Engl. J. Med. 2021;385(24):2252–2263. [PubMed: 34449181]
- 154.
- The (Look AHEAD Researh Group). Cardiovascular Effects of Intensive Lifestyle Intervention in Type 2 Diabetes. N. Engl. J. Med. 2013;369(2):145–154. [PMC free article: PMC3791615] [PubMed: 23796131]
- 155.
- Wing RR. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch. Intern. Med. 2010;170(17):1566–75. [PMC free article: PMC3084497] [PubMed: 20876408]
- 156.
- Diabetes Prevention Program Research Group. Reduction in the Incidence of Type 2 Diabetes with Lifestyle Intervention or Metformin. N. Engl. J. Med. 2002;346(6):393–403. [PMC free article: PMC1370926] [PubMed: 11832527]
- 157.
- Elsayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, Kahan S, Khunti K, Leon J, Lyons SK, Perry M Lou, Prahalad P, Pratley RE, Seley JJ, Stanton RC, Young-Hyman D, Gabbay RA. 5. Facilitating Positive Health Behaviors and Well-being to Improve Health Outcomes: Standards of Care in Diabetes—2023. Diabetes Care 2023;46(January):S68–S96. [PMC free article: PMC9810478] [PubMed: 36507648]
- 158.
- Pandey A, Patel K V, Bahnson JL, Gaussoin SA, Martin CK, Balasubramanyam A, Johnson KC, Mcguire DK, Bertoni AG, Kitzman D, Berry JD. Association of Intensive Lifestyle Intervention, Fitness, and Body Mass Index with Risk of Heart Failure in Overweight or Obese Adults with Type 2 Diabetes Mellitus: An Analysis from the Look AHEAD Trial. Circulation 2020;141(16):1295–1306. [PMC free article: PMC9976290] [PubMed: 32134326]
- 159.
- Pareek M, Schauer PR, Kaplan LM, Leiter LA, Rubino F, Bhatt DL. Metabolic Surgery: Weight Loss, Diabetes, and Beyond. J. Am. Coll. Cardiol. 2018;71(6):670–687. [PubMed: 29420964]
- 160.
- Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Nanni G, Castagneto M, Bornstein S, Rubino F. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 Year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 2015;386(9997):964–973. [PubMed: 26369473]
- 161.
- Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, Navaneethan SD, Singh RP, Pothier CE, Nissen SE, Kashyap SR. Bariatric Surgery versus Intensive Medical Therapy for Diabetes — 5-Year Outcomes. N. Engl. J. Med. 2017;376(7):641–651. [PMC free article: PMC5451258] [PubMed: 28199805]
- 162.
- Fisher DP, Johnson E, Haneuse S, Arterburn D, Coleman KJ, O’Connor PJ, O’Brien R, Bogart A, Theis MK, Anau J, Schroeder EB, Sidney S. Association between Bariatric Surgery and Macrovascular Disease Outcomes in Patients with Type 2 Diabetes and Severe Obesity. JAMA - J. Am. Med. Assoc. 2018;320(15):1570–1582. [PMC free article: PMC6233803] [PubMed: 30326126]
- 163.
- Sundström J, Bruze G, Ottosson J, Marcus C, Näslund I, Neovius M. Weight Loss and Heart FailureClinical Perspective. Circulation 2017;135(17):1577–1585. [PMC free article: PMC5404408] [PubMed: 28258170]
- 164.
- Aminian A, Zajichek A, Arterburn DE, Wolski KE, Brethauer SA, Schauer PR, Kattan MW, Nissen SE. Association of Metabolic Surgery with Major Adverse Cardiovascular Outcomes in Patients with Type 2 Diabetes and Obesity. JAMA - J. Am. Med. Assoc. 2019;322(13):1271–1282. [PMC free article: PMC6724187] [PubMed: 31475297]
- 165.
- Stratton IM, Adler AI, Neil HAW, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. Br. Med. J. 2000;321(7258):405–412. [PMC free article: PMC27454] [PubMed: 10938048]
- 166.
- Gerstein HC, Miller ME, Byington RP, Goff DC, Bigger JT. Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 2008;358(24):2545–2559. [PMC free article: PMC4551392] [PubMed: 18539917]
- 167.
- Gerstein HC, Miller ME, Genuth S, Ismail-Beigi F, Buse JB, Goff DC, Probstfield JL, Cushman WC, Ginsberg HN, Thomas Bigger J, Grimm RH, Byington RP, Rosenberg YD, Friedewald WT. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N. Engl. J. Med. 2011;364(9):818–828. [PMC free article: PMC4083508] [PubMed: 21366473]
- 168.
- Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, De Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2008;358(24):2560–2572. [PubMed: 18539916]
- 169.
- Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD. Glucose Control and Vascular Complications in Veterans with Type 2 Diabetes. N. Engl. J. Med. 2009;360(2):129–139. [PubMed: 19092145]
- 170.
- Turnbull FM, Abraira C, Anderson RJ, Byington RP, Chalmers JP, Duckworth WC, Evans GW, Gerstein HC, Holman RR, Moritz TE, Neal BC, Ninomiya T, Patel AA, Paul SK, Travert F, Woodward M. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia 2009;52(11):2288–2298. [PubMed: 19655124]
- 171.
- Colucci WS. Overview of the management of heart failure with reduced ejection fraction in adults. In: UpToDate.; 2020.
- 172.
- Ponikowski P, Voors AAA, Anker SDD, Bueno H, Cleland JGFGF, Coats AJSJS, Falk V, González-Juanatey JRR, Harjola VPP, Jankowska EAA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JTT, Pieske B, Riley JPP, Rosano GMCMC, Ruilope LMM, Ruschitzka F, Rutten FHH, Van Der Meer P, Sisakian HSS, Isayev E, Kurlianskaya A, Mullens W, Tokmakova M, Agathangelou P, Melenovsky V, Wiggers H, Hassanein M, Uuetoa T, Lommi J, Kostovska ESS, Juilliere Y, Aladashvili A, Luchner A, Chrysohoou C, Nyolczas N, Thorgeirsson G, Weinstein JMM, Lenarda AD Di, Aidargaliyeva N, Bajraktari G, Beishenkulov M, Kamzola G, Abdel-Massih T, Celutkiene J, Noppe S, Cassar A, Vataman E, AbirKhalil S, van Pol P, Mo R, Straburzynska-Migaj E, Fonseca C, Chioncel O, Shlyakhto E, Zavatta M, Otasevic P, Goncalvesova E, Lainscak M, Molina BDD, Schaufelberger M, Suter T, Yılmaz MBB, Voronkov L, Davies C. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2016;37(27):2129-2200m.
- 173.
- van der Meer P, Gaggin HK, Dec GW. ACC/AHA Versus ESC Guidelines on Heart Failure: JACC Guideline Comparison. J. Am. Coll. Cardiol. 2019;73(21):2756–2768. [PubMed: 31146820]
- 174.
- Shekelle PG, Rich MW, Morton SC, Atkinson SW, Tu W, Maglione M, Rhodes S, Barrett M, Fonarow GC, Greenberg B, Heidenreich PA, Knabel T, Konstam MA, Steimle A, Stevenson LW. Efficacy of angiotensin-converting enzyme inhibitors and beta-blockers in the management of left ventricular systolic dysfunction according to race, gender, and diabetic status: A meta-analysis of major clinical trials. J. Am. Coll. Cardiol. 2003;41(9):1529–1538. [PubMed: 12742294]
- 175.
- Khan MS, Felker GM, Piña IL, Camacho A, Bapat D, Ibrahim NE, Maisel AS, Prescott MF, Ward JH, Solomon SD, Januzzi JL, Butler J. Reverse Cardiac Remodeling Following Initiation of Sacubitril/Valsartan in Patients With Heart Failure With and Without Diabetes. JACC Hear. Fail. 2021;9(2):137–145. [PubMed: 33309581]
- 176.
- Vermes E, Ducharme A, Bourassa MG, Lessard M, White M, Tardif JC. Enalapril reduces the incidence of diabetes in patients with chronic heart failure: Insight from the studies of left ventricular dysfunction (SOLVD). Circulation 2003;107(9):1291–1296. [PubMed: 12628950]
- 177.
- Seferovic JP, Claggett B, Seidelmann SB, Seely EW, Packer M, Zile MR, Rouleau JL, Swedberg K, Lefkowitz M, Shi VC, Desai AS, McMurray JJV, Solomon SD. Effect of sacubitril/valsartan versus enalapril on glycaemic control in patients with heart failure and diabetes: a post-hoc analysis from the PARADIGM-HF trial. Lancet Diabetes Endocrinol. 2017;5(5):333–340. [PMC free article: PMC5534167] [PubMed: 28330649]
- 178.
- Seferović PM, Petrie MC, Filippatos GS, Anker SD, Rosano G, Bauersachs J, Paulus WJ, Komajda M, Cosentino F, de Boer RA, Farmakis D, Doehner W, Lambrinou E, Lopatin Y, Piepoli MF, Theodorakis MJ, Wiggers H, Lekakis J, Mebazaa A, Mamas MA, Tschöpe C, Hoes AW, Seferović JP, Logue J, McDonagh T, Riley JP, Milinković I, Polovina M, van Veldhuisen DJ, Lainscak M, Maggioni AP, Ruschitzka F, McMurray JJV. Type 2 diabetes mellitus and heart failure: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018;20(5):853–872. [PubMed: 29520964]
- 179.
- Bell DSH, Lukas MA, Holdbrook FK, Fowler MB. The effect of carvedilol on mortality risk in heart failure patients with diabetes: Results of a meta-analysis. Curr. Med. Res. Opin. 2006;22(2):287–296. [PubMed: 16466600]
- 180.
- Haas SJ, Vos T, Gilbert RE, Krum H. Are β-blockers as efficacious in patients with diabetes mellitus as in patients without diabetes mellitus who have chronic heart failure? A meta-analysis of large-scale clinical trials. Am. Heart J. 2003;146(5):848–853. [PubMed: 14597934]
- 181.
- Witte KK, Drozd M, Walker AMN, Patel PA, Kearney JC, Chapman S, Sapsford RJ, Gierula J, Paton MF, Lowry J, Kearney MT, Cubbon RM. Mortality reduction associated with β-adrenoceptor inhibition in chronic heart failure is greater in patients with diabetes. Diabetes Care 2018;41(1):136–142. [PubMed: 28982651]
- 182.
- Barzilay JI, Davis BR, Whelton PK. The glycemic effects of antihypertensive medications. Curr. Hypertens. Rep. 2014;16(1):1–6. [PubMed: 24338675]
- 183.
- Helgeland A, Leren P, Foss OP, Hjermann I, Holme I, Lund-Larsen PG. Serum glucose levels during long-term observation of treated and untreated men with mild hypertension. The Oslo study. Am. J. Med. 1984;76(5):802–805. [PubMed: 6720727]
- 184.
- Wai B, Kearney LG, Hare DL, Ord M, Burrell LM, Srivastava PM. Beta blocker use in subjects with type 2 diabetes mellitus and systolic heart failure does not worsen glycaemic control. Cardiovasc. Diabetol. 2012;11(14):1–5. [PMC free article: PMC3298480] [PubMed: 22330091]
- 185.
- Tsujimoto T, Sugiyama T, Shapiro MF, Noda M, Kajio H. Risk of Cardiovascular Events in Patients with Diabetes Mellitus on β-Blockers. Hypertension 2017;70(1):103–110. [PMC free article: PMC5739105] [PubMed: 28559400]
- 186.
- Deedwania PC, Giles TD, Klibaner M, Ghali JK, Herlitz J, Hildebrandt P, Kjekshus J, Spinar J, Vitovec J, Stanbrook H, Wikstrand J. Efficacy, safety and tolerability of metoprolol CR/XL in patients with diabetes and chronic heart failure: Experiences from MERIT-HF. Am. Heart J. 2005;149(1):159–167. [PubMed: 15660048]
- 187.
- Eschalier R, McMurray JJV, Swedberg K, Van Veldhuisen DJ, Krum H, Pocock SJ, Shi H, Vincent J, Rossignol P, Zannad F, Pitt B. Safety and efficacy of eplerenone in patients at high risk for hyperkalemia and/or worsening renal function: Analyses of the EMPHASIS-HF study subgroups (eplerenone in mild patients hospitalization and survival study in heart failure). J. Am. Coll. Cardiol. 2013;62(17):1585–1593. [PubMed: 23810881]
- 188.
- Korol S, White M, O’Meara E, Rouleau JL, White-Guay B, Dorais M, Ahmed A, de Denus S, Perreault S. Is there a potential association between spironolactone and the risk of new-onset diabetes in a cohort of older patients with heart failure? Eur. J. Clin. Pharmacol. 2019;75(6):837–847. [PubMed: 30758517]
- 189.
- McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang C-E, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O’Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde A-M. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019;381(21):1995–2008. [PubMed: 31535829]
- 190.
- Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi D-J, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner-La Rocca H-P, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde M-F, Spinar J, Squire I, Taddei S, Wanner C, Zannad F. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020;383(15):1413–1424. [PubMed: 32865377]
- 191.
- Braunwald E. Gliflozins in the Management of Cardiovascular Disease. N. Engl. J. Med. 2022;386(21):2024–2034. [PubMed: 35613023]
- 192.
- Packer M, Anker SD, Butler J, Filippatos G, Ferreira JP, Pocock SJ, Carson P, Anand I, Doehner W, Haass M, Komajda M, Miller A, Pehrson S, Teerlink JR, Brueckmann M, Jamal W, Zeller C, Schnaidt S, Zannad F. Effect of Empagliflozin on the Clinical Stability of Patients with Heart Failure and a Reduced Ejection Fraction: The EMPEROR-Reduced Trial. Circulation 2021;143(4):326–336. [PMC free article: PMC7834905] [PubMed: 33081531]
- 193.
- Khan MS, Solomon N, DeVore AD, Sharma A, Felker GM, Hernandez AF, Heidenreich PA, Matsouaka RA, Green JB, Butler J, Yancy CW, Peterson PN, Fonarow GC, Greene SJ. Clinical Outcomes With Metformin and Sulfonylurea Therapies Among Patients With Heart Failure and Diabetes. JACC Hear. Fail. 2022;10(3):198–210. [PubMed: 34895861]
- 194.
- Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner–La Rocca H-P, Choi D-J, Chopra V, Chuquiure-Valenzuela E, Giannetti N, Gomez-Mesa JE, Janssens S, Januzzi JL, Gonzalez-Juanatey JR, Merkely B, Nicholls SJ, Perrone S V., Piña IL, Ponikowski P, Senni M, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Carson P, Lam CSP, Marx N, Zeller C, Sattar N, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021;385(16):1451–1461. [PubMed: 34449189]
- 195.
- Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Voors AA, Metra M, Lund LH, Komajda M, Testani JM, Wilcox CS, Ponikowski P, Lopes RD, Verma S, Lapuerta P, Pitt B. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N. Engl. J. Med. 2021;384(2):117–128. [PubMed: 33200892]
- 196.
- Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N. Engl. J. Med. 2005;352(3):225–237. [PubMed: 15659722]
- 197.
- Wittenberg SM, Cook JR, Hall WJ, McNitt S, Zareba W, Moss AJ. Comparison of efficacy of implanted cardioverter-defibrillator in patients with versus without diabetes mellitus. Am. J. Cardiol. 2005;96(3):417–419. [PubMed: 16054472]
- 198.
- Tan ESJ, Lim J, Chan SP, Seow JTK, Singh D, Yeo WT, Lim TW, Kojodjojo P, Seow SC. Effect of Diabetes Mellitus on Cardiac Resynchronization Therapy and to Prognosis in Heart Failure (from the Prospective Evaluation of Asian With Cardiac Resynchronization Therapy for Heart Failure Study). Am. J. Cardiol. 2019;124(6):899–906. [PubMed: 31326077]
- 199.
- Ghali J k., Boehmer J, Feldman AM, Saxon LA, Demarco T, Carson P, Yong P, Galle EG, Leigh J, Ecklund FL, Bristow MR. Influence of Diabetes on Cardiac Resynchronization Therapy With or Without Defibrillator in Patients With Advanced Heart Failure. J. Card. Fail. 2007;13(9):769–773. [PubMed: 17996827]
- 200.
- Hoppe UC, Freemantle N, Cleland JGF, Marijianowski M, Erdmann E. Effect of cardiac resynchronization on morbidity and mortality of diabetic patients with severe heart failure. Diabetes Care 2007;30(3):722–724. [PubMed: 17327349]
- 201.
- Sun H, Guan Y, Wang L, Zhao Y, Lv H, Bi X, Wang H, Zhang X, Liu L, Wei M, Song H, Su G. Influence of diabetes on cardiac resynchronization therapy in heart failure patients: A meta-analysis. BMC Cardiovasc. Disord. 2015;15(1). doi:.10.1186/s12872-015-0018-0 [PMC free article: PMC4461910] [PubMed: 25880202] [CrossRef]
- 202.
- Höke U, Thijssen J, Van Bommel RJ, Van Erven L, Van Der Velde ET, Holman ER, Schalij MJ, Bax JJ, Delgado V, Marsan NA. Influence of diabetes on left ventricular systolic and diastolic function and on long-term outcome after cardiac resynchronization therapy. Diabetes Care 2013;36(4):985–991. [PMC free article: PMC3609501] [PubMed: 23223348]
- 203.
- Kahr PC, Trenson S, Schindler M, Kuster J, Kaufmann P, Tonko J, Hofer D, Inderbitzin DT, Breitenstein A, Saguner AM, Flammer AJ, Ruschitzka F, Steffel J, Winnik S. Differential effect of cardiac resynchronization therapy in patients with diabetes mellitus: a long-term retrospective cohort study. ESC Hear. Fail. 2020;7(5):2773–2783. [PMC free article: PMC7524059] [PubMed: 32652900]
- 204.
- Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A. Irbesartan in patients with heart failure and preserved ejection fraction. N. Engl. J. Med. 2008;359(23):2456–2467. [PubMed: 19001508]
- 205.
- Nassif ME, Windsor SL, Borlaug BA, Kitzman DW, Shah SJ, Tang F, Khariton Y, Malik AO, Khumri T, Umpierrez G, Lamba S, Sharma K, Khan SS, Chandra L, Gordon RA, Ryan JJ, Chaudhry SP, Joseph SM, Chow CH, Kanwar MK, Pursley M, Siraj ES, Lewis GD, Clemson BS, Fong M, Kosiborod MN. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: a multicenter randomized trial. Nat. Med. 2021;27(11):1954–1960. [PMC free article: PMC8604725] [PubMed: 34711976]
- 206.
- Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 2015;373(22):2117–2128. [PubMed: 26378978]
- 207.
- Neal B, Perkovic V, Mahaffey KW, De Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 2017;377(7):644–657. [PubMed: 28605608]
- 208.
- Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2019;380(4):347–357. [PubMed: 30415602]
- 209.
- Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, De Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N. Engl. J. Med. 2019;380(24):2295–2306. [PubMed: 30990260]
- 210.
- Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, Charbonnel B, Frederich R, Gallo S, Cosentino F, Shih WJ, Gantz I, Terra SG, Cherney DZI, McGuire DK. Cardiovascular Outcomes with Ertugliflozin in Type 2 Diabetes. N. Engl. J. Med. 2020;383(15):1425–1435. [PubMed: 32966714]
- 211.
- Akturk HK, Rewers A, Garg SK. SGLT inhibition: A possible adjunctive treatment for type 1 diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 2018;25(4):246–250. [PubMed: 29794497]
- 212.
- Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023;388(2):117–127. [PMC free article: PMC7614055] [PubMed: 36331190]
- 213.
- Heerspink HJL, Stefánsson B V., Correa-Rotter R, Chertow GM, Greene T, Hou F-F, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjöström CD, Toto RD, Langkilde A-M, Wheeler DC. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020;383(15):1436–1446. [PubMed: 32970396]
- 214.
- Bhatt DL, Szarek M, Pitt B, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Inzucchi SE, Kosiborod MN, Cherney DZI, Dwyer JP, Scirica BM, Bailey CJ, Díaz R, Ray KK, Udell JA, Lopes RD, Lapuerta P, Steg PG. Sotagliflozin in Patients with Diabetes and Chronic Kidney Disease. N. Engl. J. Med. 2021;384(2):129–139. [PubMed: 33200891]
- 215.
- Marso SP, Daniels GH, Frandsen KB, Kristensen P, Mann JFE, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2016;375(4):311–322. [PMC free article: PMC4985288] [PubMed: 27295427]
- 216.
- Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 2019;394(10193):121–130. [PubMed: 31189511]
- 217.
- Gerstein HC, Sattar N, Rosenstock J, Ramasundarahettige C, Pratley R, Lopes RD, Lam CSP, Khurmi NS, Heenan L, Del Prato S, Dyal L, Branch K. Cardiovascular and Renal Outcomes with Efpeglenatide in Type 2 Diabetes. N. Engl. J. Med. 2021;385(10):896–907. [PubMed: 34215025]
- 218.
- Ferreira JP, Sharma A, Butler J, Packer M, Zannad F, Vasques-nóvoa F, Leite-moreira A, Neves JS. Glucagon-Like Peptide-1 Receptor Agonists Across the Spectrum of Heart Failure. 2023;(July):1–6. [PubMed: 37409733]
- 219.
- Ferreira JP, Saraiva F, Sharma A, Vasques-Nóvoa F, Angélico-Gonçalves A, Leite AR, Borges-Canha M, Carvalho D, Packer M, Zannad F, Leite-Moreira A, Neves JS. Glucagon-like peptide 1 receptor agonists in patients with type 2 diabetes with and without chronic heart failure: A meta-analysis of randomized placebo-controlled outcome trials. Diabetes, Obes. Metab. 2023;25(6):1495–1502. [PubMed: 36722252]
- 220.
- Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, Alves B, Kiyosue A, Zhang S, Liu B, Bunck MC, Stefanski A. Tirzepatide Once Weekly for the Treatment of Obesity. N. Engl. J. Med. 2022;387(3):205–216. [PubMed: 35658024]
- 221.
- Kosiborod MN, Abildstrøm SZ, Borlaug BA, Butler J, Rasmussen S, Davies M, Hovingh GK, Kitzman DW, Lindegaard ML, Møller D V, Shah SJ, Treppendahl MB, Verma S, Abhayaratna W, Ahmed FZ, Chopra V, Ezekowitz J, Fu M, Ito H, Lelonek M, Melenovsky V, Merkely B, Núñez J, Perna E, Schou M, Senni M, Sharma K, Van der Meer P, von Lewinski D, Wolf D, Petrie MC, STEP-HFpEF Trial Committees and Investigators. Semaglutide in Patients with Heart Failure with Preserved Ejection Fraction and Obesity. N. Engl. J. Med. 2023:1–16. [PubMed: 37622681]
- 222.
- Kenny HC, Abel ED. Heart Failure in Type 2 Diabetes Mellitus: Impact of Glucose-Lowering Agents, Heart Failure Therapies, and Novel Therapeutic Strategies. Circ. Res. 2019;124(1):121–141. [PMC free article: PMC6447311] [PubMed: 30605420]
- 223.
- Eurich DT, Weir DL, Majumdar SR, Tsuyuki RT, Johnson JA, Tjosvold L, Vanderloo SE, McAlister FA. Comparative safety and effectiveness of metformin in patients with diabetes mellitus and heart failure: systematic review of observational studies involving 34,000 patients. Circ. Heart Fail. 2013;6(3):395–402. [PubMed: 23508758]
- 224.
- Scirica BM, Braunwald E, Raz I, Cavender MA, Morrow DA, Jarolim P, Udell JA, Mosenzon O, Im K, Umez-Eronini AA, Pollack PS, Hirshberg B, Frederich R, Lewis BS, McGuire DK, Davidson J, Steg PG, Bhatt DL. Heart failure, saxagliptin, and diabetes mellitus: Observations from the SAVOR-TIMI 53 randomized trial. Circulation 2014;130(18):1579–1588. [PubMed: 25189213]
- 225.
- Zannad F, Cannon CP, Cushman WC, Bakris GL, Menon V, Perez AT, Fleck PR, Mehta CR, Kupfer S, Wilson C, Lam H, White WB. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: A multicentre, randomised, double-blind trial. Lancet 2015;385(9982):2067–2076. [PubMed: 25765696]
- 226.
- White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N. Engl. J. Med. 2013;369(14):1327–1335. [PubMed: 23992602]
- 227.
- McGuire DK, Van De Werf F, Armstrong PW, Standl E, Koglin J, Green JB, Bethel MA, Cornel JH, Lopes RD, Halvorsen S, Ambrosio G, Buse JB, Josse RG, Lachin JM, Pencina MJ, Garg J, Lokhnygina Y, Holman RR, Peterson ED. Association between sitagliptin use and heart failure hospitalization and related outcomes in type 2 diabetes mellitus: Secondary analysis of a randomized clinical trial. JAMA Cardiol. 2016;1(2):126–135. [PubMed: 27437883]
- 228.
- Hippisley-Cox J, Coupland C. Diabetes treatments and risk of heart failure, cardiovascular disease, and all cause mortality: Cohort study in primary care. BMJ 2016;354. doi:.10.1136/bmj.i3477 [PMC free article: PMC4948032] [PubMed: 27413012] [CrossRef]
- 229.
- Gerstein HC, Bosch J, Dagenais GR, Díaz R, Jung H, Maggioni AP. Basal insulin and cardiovascular and other outcomes in dysglycemia. N. Engl. J. Med. 2012;367(4):319–328. [PubMed: 22686416]
- 230.
- Home PD, Pocock SJ, Beck-Nielsen H, Curtis PS, Gomis R, Hanefeld M, Jones NP, Komajda M, McMurray JJ. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet 2009;373(9681):2125–2135. [PubMed: 19501900]
- 231.
- Dormandy JA, Charbonnel B, Eckland DJA, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefèbvre PJ, Murray GD, Standl E, Wilcox RG, Wilhelmsen L, Betteridge J, Birkeland K, Golay A, Heine RJ, Korányi L, Laakso M, Mokáň M, Norkus A, Pirags V, Podar T, Scheen A, Scherbaum W, Schernthaner G, Schmitz O, Škrha J, Smith U, Tatoň J. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial in macroVascular Events): A randomised controlled trial. Lancet 2005;366(9493):1279–1289. [PubMed: 16214598]
- ABSTRACT
- INTRODUCTION
- EPIDEMIOLOGY OF DIABETES AND HF
- IMPACT OF DIABETES ON CARDIAC STRUCTURE AND FUNCTION
- PATHOPHYSIOLOGIC LINKS BETWEEN DIABETES AND HF
- PROGNOSTIC IMPLICATIONS OF DIABETES IN HF
- PREVENTION OF HF IN PATIENTS WITH DIABETES
- TREATMENT OF HF IN PATIENTS WITH DIABETES
- PHARMACOLOGIC THERAPY OF T2DM IN PATIENTS WITH HF
- CONCLUSIONS
- ACKNOWLEDGMENT
- REFERENCES
- Review Sodium Glucose Cotransporter-2 Inhibition in Heart Failure: Potential Mechanisms, Clinical Applications, and Summary of Clinical Trials.[Circulation. 2017]Review Sodium Glucose Cotransporter-2 Inhibition in Heart Failure: Potential Mechanisms, Clinical Applications, and Summary of Clinical Trials.Lytvyn Y, Bjornstad P, Udell JA, Lovshin JA, Cherney DZI. Circulation. 2017 Oct 24; 136(17):1643-1658.
- Review Potential Therapeutic Benefits of Sodium-Glucose Cotransporter 2 Inhibitors in the Context of Ischemic Heart Failure: A State-of-the-Art Review.[Cardiovasc Hematol Agents Med ...]Review Potential Therapeutic Benefits of Sodium-Glucose Cotransporter 2 Inhibitors in the Context of Ischemic Heart Failure: A State-of-the-Art Review.Gitto M, Vrachatis DA, Condorelli G, Papathanasiou K, Reimers B, Deftereos S, Stefanini GG. Cardiovasc Hematol Agents Med Chem. 2022; 20(2):90-102.
- Lessons from a pre-specified meta-analysis of sodium-glucose cotransporter-2 (SGLT2) inhibitors in heart failure: Time for new clinical recommendations.[Glob Cardiol Sci Pract. 2023]Lessons from a pre-specified meta-analysis of sodium-glucose cotransporter-2 (SGLT2) inhibitors in heart failure: Time for new clinical recommendations.Kotit S. Glob Cardiol Sci Pract. 2023 May 11; 2023(2):e202314. Epub 2023 May 11.
- Sympatholytic Mechanisms for the Beneficial Cardiovascular Effects of SGLT2 Inhibitors: A Research Hypothesis for Dapagliflozin's Effects in the Adrenal Gland.[Int J Mol Sci. 2021]Sympatholytic Mechanisms for the Beneficial Cardiovascular Effects of SGLT2 Inhibitors: A Research Hypothesis for Dapagliflozin's Effects in the Adrenal Gland.Lymperopoulos A, Borges JI, Cora N, Sizova A. Int J Mol Sci. 2021 Jul 19; 22(14). Epub 2021 Jul 19.
- Review Mechanisms and Perspectives of Sodium-Glucose Co-transporter 2 Inhibitors in Heart Failure.[Front Cardiovasc Med. 2021]Review Mechanisms and Perspectives of Sodium-Glucose Co-transporter 2 Inhibitors in Heart Failure.Zeng Q, Zhou Q, Liu W, Wang Y, Xu X, Xu D. Front Cardiovasc Med. 2021; 8:636152. Epub 2021 Feb 10.
- Diabetes, Cardiomyopathy, and Heart Failure - EndotextDiabetes, Cardiomyopathy, and Heart Failure - Endotext
Your browsing activity is empty.
Activity recording is turned off.
See more...
