ABSTRACT
The global epidemic of type 2 diabetes remains one of the greatest health challenges of our time. The collective human and economic costs are staggering and rising. Widespread initiatives now exist to prevent diabetes wherever possible. These initiatives are singularly focused on preventing diabetes in the very highest risk group: people with prediabetes. Plasma glucose concentrations can exist over a continuum with normoglycemia on one side and diabetes mellitus on the other. Nevertheless, the concept of “prediabetes” – a state of neither normoglycemia or bonafide diabetes – has been in the clinical purview since the first formal diagnostic criteria of diabetes itself. Most can agree that prediabetes represents a high-risk state for diabetes (and for the sake of this review, high-risk for type 2 diabetes, specifically), but consensus is lacking for much else, including the diagnostic thresholds, if, when, or what to initiate as to pharmacotherapy for diabetes prevention, and whether prediabetes is actually just an earlier form of diabetes warranting similarly aggressive risk factor modification for diabetes-related complications. In this chapter, PREDIABETES, we will review the recommendations for screening, diagnosis, and intervention, largely according to the American Diabetes Association (ADA). We will also look at the pathogenesis of this highly heterogeneous dysglycemic state as well as an increasing body of evidence that treatment of prediabetes back to normoglycemia should be the goal for people with prediabetes. Lastly, the scientific evidence reviewed will be distilled into an example of a conversation intended to engage patients in this process. For complete coverage of all related areas of Endocrinology, please visit our on-line FREE web-text, WWW.ENDOTEXT.ORG.
INTRODUCTION
In 1979-1980, the National Diabetes Data Group and World Health Organization introduced the first formal diagnostic criteria for diabetes (1,2). Cross sectional observations that the presence of both microvascular disease (MVD) (3-6) and cardiovascular disease (CVD) (7,8) were higher when fasting plasma glucose (FPG) was ≥140 mg/dl and/or 2-hour post-challenge glucose (2h-PG) was ≥200 mg/dl were confirmed in longitudinal population studies, providing rationale for these cut points (9-11). Nevertheless, clear evidence that lowering plasma glucose could prevent diabetic complications was not available until the 1993 publication of the Diabetes Complications and Control Trial (DCCT) (12). The DCCT noted an inflection point between A1c 6.5-7.0% (48-53 mmol/mol) and risk for retinopathy, as well as a 76% reduction in retinopathy, in participants with type 1 diabetes randomized to intensive treatment (12). Hence, the A1c goal of <6.5-7.0% soon became – and has remained – the major benchmark of care for people with diabetes (type 1 and 2) (13).
Diagnostic criteria for diabetes have evolved over the years, lowering plasma glucose thresholds (14) and even advocating use of the A1c for diagnosis (15), while continuing to calibrate these thresholds against risk for retinopathy. Far less well known than the landmark publication of the DCCT is the re-analysis of the original data demonstrating a flaw in the models with no inflection point in A1c and risk for retinopathy noted (16). Instead, reduction in retinopathy was appreciated across the A1c range, including what is now considered the pre-diabetic A1c range. The first formal diagnostic criteria for “pre”-diabetes (i.e. impaired glucose tolerance) were introduced concurrently with those for diabetes itself (1). Diagnostic thresholds for prediabetes have been more moveable (14,15,17) and more controversial. Despite evidence demonstrating higher MVD and CVD in people with prediabetes compared to their normoglycemic peers (18-21), treatment of people with prediabetes is uncommon (22,23) as the notion of a “pre” disease presents a clinical and regulatory conundrum.
SCREENING
Much ado has been made about the cost-effectiveness of screening for prediabetes. Nevertheless, because roughly one-quarter of people with diabetes in the U.S. remain undiagnosed (24), numerous guidelines do advocate screening for dysglycemia (e.g. diabetes and prediabetes). According to the American Diabetes Association (ADA) together with the European Association for the Study of Diabetes (EASD), an informal assessment of risk factors or use of a risk assessment tool (e.g. www.diabetes.org/socrisktest) can guide who should undergo blood testing (25). Children ≥10 years old or who have gone through puberty (whichever occurs first) who are >85th% weight for height, with one or more risk factors (Table 1), should be screened. Non-pregnant adults ≥35 years without risk factors, or adults of any age who are overweight (BMI≥25 kg/m2 or BMI ≥23 kg/m2, if Asian ethnicity) and have one or more risk factors (Table 1), should be screened. The screening test should be A1c, fasting glucose, or 2-hour glucose, and repeated at least at 3-year intervals for those whose screening reveals normoglycemia and once yearly in those diagnosed with prediabetes (26).
Table 1.
Risk Factors for Prediabetes and Diabetes
DIAGNOSIS
According to the ADA and EASD, the diagnosis of prediabetes is made when the fasting plasma glucose (FPG) is 100-125 mg/dl (5.6-6.9 mmol/l; “impaired fasting glucose” (IFG)), plasma glucose concentration is 140-199 mg/dl (7.8-11.1 mmol/l; “impaired glucose tolerance” (IGT)) 2 hours after a 75 g oral glucose tolerance test (OGTT), and/or A1c 5.7-6.4% (26) (Table 2). Unlike diagnostic criteria for diabetes that are based on their predictive value for retinopathy (14), diagnostic thresholds for prediabetes are based on the likelihood of developing overt diabetes (27-30). However, discussion regarding the existing cut points is ongoing. Longitudinal data from a cohort of Israeli soldiers suggest that a fasting glucose above 87 mg/dl (4.8 mmol/l) is associated with an increased risk of future diabetes (31). Further, misclassification is common given the day-to-day variability in the fasting (15%) and 2-hour (46%) glucose concentrations (32). A1c can be confounded by a number of comorbid conditions like renal disease, anemia, and hemoglobinopathies (see www.ngsp.org/interf.asp) and must be done using a method certified by the National Glycohemoglobin Standardization Program (NGSP). Use of the 1-hour glucose value (i.e., ≥155 mg/dl post-OGTT), fructosamine, 5-androhydroglucitol among others have also been proposed, but none are standardized hence none currently recommended (33,34). Despite the fact that A1c-defined prediabetes appears to confer worse outcomes than prediabetes defined by fasting or 2-hour glucose criteria (35), the use of the A1c is not supported by the World Health Organization (WHO) for the diagnosis of prediabetes (36).
Table 2.
Current Diagnostic Criteria for Prediabetes (ADA & EASD)
PREVALENCE
The changes in diagnostic criteria over the past years make it difficult to estimate exact trends in the global burden of prediabetes. However, by combining recent data from diverse sources, the prevalence of prediabetes can roughly be approximated. In 2021, the Centers for Disease Control (CDC) estimated that 96 million Americans – 38% of the adult population – had prediabetes demonstrating an increase in the percent of the population that has prediabetes that had previously been stable (24). Discordance in the diagnostic criteria for prediabetes, regional differences in surveillance and reporting for chronic diseases, and other cultural nuances pose challenges in estimating the global burden of prediabetes. To this point, the literature is currently devoid of any estimate of global prevalence of IFG, specifically. In 2017, the International Diabetes Federation (IDF) estimated the worldwide prevalence of IGT at 318 million - a number expected to increase to 482 million by 2040 (www.diabetesatlas.org) – with no further update in 2021. Data from the National Health and Nutrition Examination Survey (NHANES) would contend that the prevalence of IFG is twice that of IGT (37) (using ADA criteria), suggesting that the worldwide prevalence of prediabetes (IFG and/or IGT) may exceed 1 billion. Most alarming is that roughly one- third of people with IGT (and possibly IFG) are between 20 and 39 years old, thus are expected to spend many years at risk for or with diabetes (www.diabetesatlas.org).
RISK FOR DIABETES
Screening for and diagnosis of prediabetes is advocated as it represents a high-risk state for the development of overt type 2 diabetes. A recent meta-analysis showed that the yearly progression rate to diabetes in individuals with prediabetes is 3.5-7.0% (vs. 2%/year in their normoglycemic counterparts) (28), with highest rates in those with combined IFG and IGT and the lowest in those with IFG by ADA (vs. WHO) definition (38). Increasing A1c is also associated with increased risk of diabetes with yearly incidence rates approximating 5% for those with an A1c of 5.7-6.0% and up to 10% for those with an A1c of 6.1-6.4% (39). Adding non-glycemic risk factors (Table 1) to the diagnosis of prediabetes markedly increases risk for diabetes, approaching 30% per year (40). Decompensation from prediabetes to diabetes appears rapid in the later stages (41) and may warrant closer monitoring for people close to the thresholds for diabetes as well as earlier risk factor modification.
A recent study looked at the prevalence of prediabetes and risk of developing diabetes in 3412 individuals between 71 and 90 years of age (42). The prevalence of diabetes in this population was very high with 44% meeting the criteria based on A1C, 59% based on fasting glucose, 73% based on either A1c or fasting glucose, and 29% based on both A1c and fasting glucose. After a median 5-year follow-up only 9% of individuals with prediabetes based on A1c developed diabetes and only 8% of individuals with prediabetes based on fasting glucose developed diabetes. In individuals with prediabetes based on both A1c and fasting glucose levels 12% developed diabetes during the 5-year follow-up period. Many of the individuals with prediabetes regressed to normal glycemia. Thus, in the elderly the risk of progressing from prediabetes to diabetes appears to be lower than in middle aged individuals.
SUBTYPES & PATHOGENESIS
Not long ago, the universal teaching was that post-prandial hyperglycemia always preceded fasting hyperglycemia in the evolution of diabetes (Figure 1). The past decade has ushered in compelling evidence that this is not always the case. IFG can be isolated or precede IGT, IGT can be isolated or precede IFG, or they can be concurrent in the prediabetic state (27,29,43) (Figure 1). This realization has sparked rigorous investigations into the pathogenesis of the subtypes - IFG, IGT and IFG/IGT - as discreet prediabetic states. Early studies used the homeostasis model assessment (HOMA) to delineate IFG from IGT, concluding that IFG was more insulin resistant than IGT (43). Most noteworthy is the fact that this conclusion is inherently flawed since HOMA relies on FPG (i.e., HOMA-IR = FPG x FPI / 22.5) and IFG is defined by FPG. Fortunately, more rigorous investigations have followed.

Figure 1.
A) Former concept as to the pathophysiology of prediabetes and diabetes >10 years ago; B) Current knowledge as to the pathophysiology of prediabetes and diabetes <10 years
In some individuals, type 2 diabetes seems to develop as a consequence of inherent beta cell dysfunction (44). In others, development of insulin resistance precedes defects in the pancreatic beta cells (44,45). These findings underscore that prediabetes (like type 2 diabetes) is not a single disease entity, but rather multiple diseases with different pathologies (Table 3) and trajectories for disease development. This notion is supported by longitudinal data from the Whitehall II Study illustrating that the underlying disease mechanisms for individuals developing type 2 diabetes differ depending on whether diabetes is diagnosed by increased fasting or 2-hour plasma glucose levels (44). Further, this heterogeneity in the disease process is present decades before the clinical onset of diabetes. Defects unique to IFG and IGT may be collective or unique when IFG and IGT exist in combination (46).
Table 3.
Overview of the Distinguishing Features of IFG vs. IGT
Impaired Fasting Glucose (IFG)
THE ROLE OF THE LIVER
In healthy humans, circulating plasma glucose concentration is maintained in a narrow range by the liver’s ability to regulate its direction of glucose flux (47). By virtue of hepatic insulin resistance (48), decreased hepatic glucose clearance (49), or lower glucose effectiveness (50), endogenous glucose production (EGP) becomes abnormal in the development of isolated IFG (48,51-54). EGP, as measured by glucose rate of appearance (Ra), has been reported as 8-25% higher in people with IFG vs. normal glucose tolerant (NGT) controls in some studies (46,54), or “inappropriately” comparable to NGT (given the higher circulating glucose and insulin levels in IFG) in others (48,55). It is clear that the liver, rather than muscle, plays a distinctive role in the pathogenesis of IFG.
THE ROLE OF THE BETA CELL
Unique defects in beta cell function are seen in concert with the defects in the liver in people with isolated IFG. Collective data suggest that beta cell function may be intrinsically impaired, vs. acquired, in IFG. This notion is supported by epidemiologic studies showing diminished insulin response to glucose in normoglycemic individuals who later develop isolated IFG (56) and that this defect may be seen as long as 18 years before they are diagnosed with diabetes (44). Furthermore, beta cell dysfunction has been demonstrated in individuals with isolated IFG and normal peripheral insulin sensitivity (48,51).
The exact manner of beta cell dysfunction in IFG appears specific to 1st vs. 2nd phase insulin secretion (55,57). It should be pointed out, however, that 1st phase insulin secretion is only appreciated in response to an intravenous glucose challenge bringing its physiologic relevance into question. Studies carefully examining insulin secretion in IFG (vs. NGT or IGT) have uniformly noted decrements in response to intravenous, but not oral, glucose challenges (46,48,51,54,55). Collectively, these data imply a dependence on the incretin hormones to maintain normal insulin secretion in IFG that may diverge from the role of the incretin hormones to facilitate insulin secretion in IGT.
OTHER DISTINGUISHING AND NON-DISTINGUISHING FEATURES OF IFG
Despite the implication of different roles for the incretin hormones in conferring IFG vs. IGT, existing data are conflicting (51,58). Likewise, plasma glucagon concentrations (51), adipose tissue mass and function (59) do not appear different, and other pathogenic features such as intramuscular lipids have not been compared between the subtypes of prediabetes. Of note, people with IFG tend to be male and younger – whereas those with IGT female and older - and have slight differences in their risk factors for CVD (43,60,61).
Impaired Glucose Tolerance (IGT)
THE ROLE OF SKELETAL MUSCLE
Despite reports of greater hepatic fat in people with IGT vs. IFG (62), skeletal muscle, rather than liver, has been implicated as the site of insulin resistance in isolated IGT. Glucose rate of disappearance (Rd; a measure of muscle insulin sensitivity) has been shown to be 42-48% lower in IGT vs. NGT (48,55) with only minimal impairments seen in IFG (54). Because of the larger contribution of muscle (vs. liver) to whole-body insulin sensitivity, people with isolated IGT demonstrate on average 15-30% lower whole body insulin sensitivity compared to those with isolated IFG (51,52,57).
THE ROLE OF THE BETA CELL
In contrast to IFG, beta cell dysfunction appears to be acquired rather than intrinsic in IGT. For example, long-term population studies have not noted early defects in people destined to develop isolated IGT (56). Nevertheless, beta cell dysfunction has been repeatedly observed in people with established IGT, particularly when significant whole body and skeletal muscle insulin resistance co-exists (51,56,63,64). The exact manner of beta cell dysfunction in IGT appears specific to 2nd vs. 1st phase insulin secretion (55,57) and is observed whether or not the incretin-axis is invoked during the assessment.
A1c-Defined Prediabetes
Recent trends in medical practice have seen the 2-hour OGTT fall from grace and be replaced by the A1c, even for the diagnosis and surveillance of prediabetes. Being that A1c is a composite of fasting and post-prandial glucose concentrations, it cannot delineate IFG from IGT nor any of the pathology unique to either. Alpha-hydroxybuytric acid, linoleoyl-glycerophosphocholine, and oleic acid have been shown predictive of 2-hour glucose values in three European cohort studies (65), hence may hold value if the pathophysiologic differences between IFG and IGT are to guide clinical decision-making in the future. Currently, the strategies for diabetes prevention do not discriminate between the subtypes of prediabetes.
CLINICAL TRIALS AIMED AT PREVENTING OR DELAYING DIABETES
With the global surge in the prevalence of type 2 diabetes, focus on its prevention has intensified. Clinical trials for diabetes prevention around the globe have universally enrolled participants with untreated prediabetes (mostly IGT) due to their high risk for acquiring overt diabetes (28). Approaches for the prevention of diabetes have included intensive lifestyle modification (66-68) (Figure 2) or drug therapy using glucose-lowering medications (69-76) (Figure 3) or anti-obesity medications (77-81) (Figure 4). Lifestyle interventions have utilized a low fat (<30% calories from fat; <10% from saturated fat) hypocaloric diet and moderate intensity exercise ~150 minutes per week for the purpose of 5-7% weight reduction. With the exception of the NAVIGATOR Trial (75), collective results demonstrate that diabetes incidence can be reduced by 20-89% over 2.4-6 years in a wide range of ethnic groups.
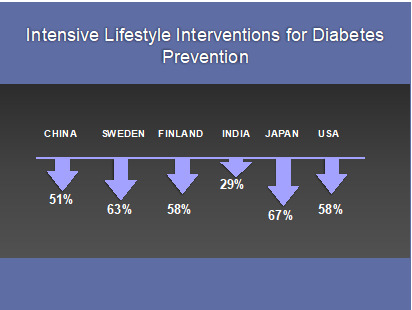
Figure 2.
Major trials using intensive lifestyle interventions for diabetes prevention
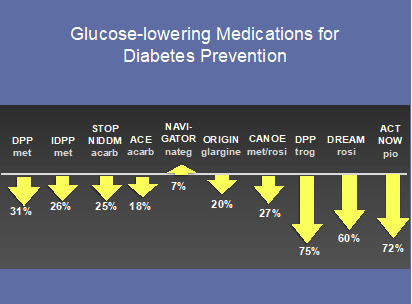
Figure 3.
Major trials using glucose-lowering medications for diabetes prevention
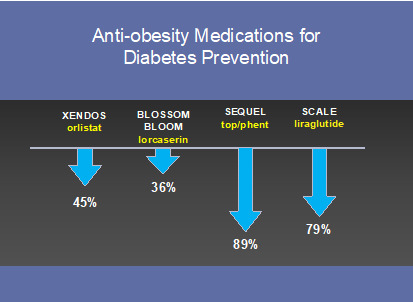
Figure 4.
Major trials using anti-obesity medications for diabetes prevention
Despite success amongst the various strategies employed, only intensive lifestyle modification has been universally advocated. The lifestyle curriculum designed for the U.S. Diabetes Prevention Program (DPP) serves as the foundation for the National DPP (NDPP) – the translational effort of bringing clinical trial results to the real world (www.cdc.gov/diabetes/prevention). A recent meta-analysis of 63 publications stemming from international real-world translations of clinical trial lifestyle curriculum demonstrated a 3% reduction in absolute risk and 29% reduction in relative risk for active participants, even when weight loss was modest (82). Likewise, the National Health Service Diabetes Prevention Programme (NHS DPP) began implementation across the United Kingdom in 2016 (83). Evaluation of the program showed a consistent ~40% reduction in onset of diabetes over 13.4 months, including when the curriculum was delivered by lay volunteers (84). Initiation of metformin in people with pre-diabetes is recommended for those younger than 65 years old with a body mass index (BMI) ≥25 kg/m2 (85). To date, only ~0.7% of people with prediabetes in the U.S. are treated with metformin (23). It should be noted that no medication is approved by the U.S. Food and Drug Administration (FDA) for the treatment of prediabetes – not even metformin – as the FDA does not recognize prediabetes as a disease. In fact, the mere notion of a “pre” disease creates a clinical and regulatory conundrum. In 2008, the FDA issued guidance for industry developing drugs for the treatment or prevention of diabetes stating that it would consider approving pharmacotherapy for prediabetes if the drug could show “clinical benefit” (e.g. a delay or lessening in micro- or macrovascular complications) (https://www.fda.gov/downloads/ Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071624.pdf). Increasing evidence shows this may be possible.
COMPLICATIONS OF PREDIABETES
It is alluring to imagine an A1c threshold below which patients are fully protected from diabetic complications (86). This quest has proven less straightforward than is widely acknowledged. People with prediabetes can suffer the same micro-, macrovascular, and non-vascular complications as people with diabetes, just at a lower incidence rate. Further, data exist and studies ongoing to show clear benefit from early intervention for people with prediabetes (www.clinicaltrials.gov/prediabetes).
Microvascular
Diabetes remains a leading cause of blindness, kidney failure, and amputations around the world. Benchmarks for diabetes care are explicitly based on the prevention of such microvascular complications (13). Nonetheless, complications of diabetes increase with increasing glycemia, even in the prediabetic glucose range. For example, nearly 10% of DPP participants had diabetic retinopathy, without diabetes, in a cross sectional analysis (19). Moreover, data from NHANES suggests the steepest increase in risk for retinopathy occurs at an A1c of 5.5% (18), which would be considered normoglycemia by current ADA and WHO criteria. Polyneuropathy has also been reported as more prevalent in prediabetes, affecting 13% of people with IGT and 11.3% with IFG compared to 7.4% with NGT (21). Lastly, microalbuminuria doubles in prevalence with the onset of IFG or IGT, whereas its progression appears slower at the diagnostic threshold for overt diabetes (87). Recent trends reveal chronic kidney disease (defined as a glomerular filtration rate (GFR) < 60 ml/min/1.73 m2) is now as prevalent in people with prediabetes as diabetes itself (88).
Perhaps more surprising than the incidence and prevalence of microvascular disease in people with prediabetes are data showing benefit from early interventions. For example, the DPP Outcomes Study (DPPOS) demonstrated a 21% lower prevalence of the composite microvascular endpoint (retinopathy, nephropathy and/or neuropathy) in women who had been randomized to the intensive lifestyle intervention and followed 15 years post-randomization and a 28% lower prevalence across the treatment groups when diabetes was prevented (89). In the roughly 600 participants with prediabetes that entered the Swedish Obesity Study (SOS), the composite microvascular endpoint was 82% lower in those who underwent bariatric surgery a median of 19 years after their procedure – an effect size that was much greater than for those who entered the study with either diabetes or normoglycemia (90). Lastly, retinopathy was shown reduced by 40% in the 30-year follow-up of the Da Qing Study – a study that rendered a meager average of 1.8 kg weight loss during the intervention period (91). Altogether, there is increasing evidence that people with prediabetes are at risk for classic complications of diabetes and these can be prevented with early intervention (Figure 5).
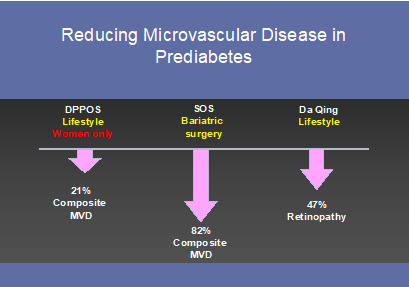
Figure 5.
Trials demonstrating a reduction in microvascular disease in people with prediabetes
Macrovascular
In 2010, a meta-analysis by Ford et al. illustrated an approximate 20% increased risk of cardiovascular disease (CVD) in people with prediabetes, irrespective of type (IFG or IGT), criteria used to define it, or the development of diabetes (20). As a continuous variable, however, CVD risk appears more closely related to 2-hour than fasting glucose (92). In 2018, serial cross sectional data from NHANES showed surprising similarity in the prevalence of myocardial infarction and stroke in people with prediabetes vs. diabetes (88) likely due to the dramatic fall in incident myocardial infarction and stroke in people with diabetes (93). This finding implies that CVD may now be as common in people with prediabetes as with diabetes (recently reviewed by (94)). It should be recognized that whether the elevated glucose is causing the increased risk of CVD in individuals with prediabetes is uncertain as prediabetes is associated with other factors such as obesity, insulin resistance, dyslipidemia, hypertension, hypercoagulation, and inflammation that could be playing important roles in increasing the risk of CVD.
As with microvascular disease, data do exist that early intervention also prevents macrovascular disease in people with prediabetes (Figure 6). The first study to contend that this may be the case came from a post-hoc analysis of STOP-NIDDM – a trial that used acarbose to prevent or delay diabetes in people with prediabetes. This analysis showed a highly unexpected 49% lower probability of any CV event in the group randomized to acarbose (95). Interestingly, the trial was repeated, powered with benefit as the a priori hypothesis and did not succeed at recapitulating the prior findings (73). Differences in medication dosage and ethnic admixture may or may not explain the discrepancy. Nevertheless, pioglitazone has been shown to reduce CV events over 4.8 years in insulin-resistant people 6 months post-stroke with an average A1c of 5.8% (96). Likewise, the Da Qing Study revealed a 33% lower CV mortality and 26% lower all-cause mortality, whilst still preventing diabetes, 30 years into the post-randomization follow-up (91). CV data from the DPPOS is expected shortly with great anticipation that prediabetes may finally be recognized as an earlier form of diabetes warranting intervention. While the effect of lowering glucose levels in individuals with prediabetes is uncertain given the high risk of CVD in this population aggressive treatment of dyslipidemia and hypertension is indicated given the large number of studies showing benefits.
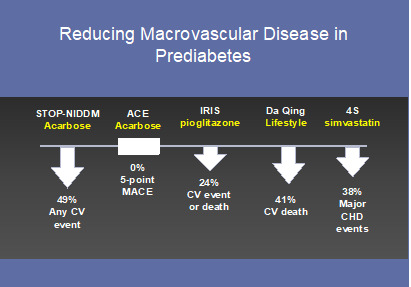
Figure 6.
Trials demonstrating a reduction in macrovascular disease in people with prediabetes
Not Necessarily Vascular
Although risk factor modification largely focuses on preventing the classic complications of diabetes, greater attention is being paid to a much larger scope of possible comorbidities. A recent study elaborated on structural brain abnormalities in people with prediabetes that are linked to dementia, stroke, and depression and hypothesized that glucose-lowering may reverse the abnormalities (97). Functionally, these brain changes lead to slower processing speeds and cognitive deficits (98). Mild cognitive impairments are accelerated by the presence of prediabetes leading to frank dementia (99). Unequivocally, cognitive impairments and dementia dramatically reduce quality of life for both patients and their care-takers. Fortunately, patient-reported outcomes are becoming increasing revered as a scientific endpoint and may provide additional rationale for treating prediabetes. The much-anticipated long term outcomes from the DPPOS (expected 2020-2025) also include examining treatment effect on cognition, aspects of aging, quality of life, health care utilization and cancer.
RESTORATION OF NORMOGLYCEMIA
In clinical trials to date, interventions were deemed successful if diabetes was prevented or delayed, yet many participants remained with prediabetes. Arguably, prevention of diabetes and its complications lies in the restoration of normoglycemia rather than in the maintenance of prediabetes. This was confirmed by a post-hoc analysis from the Diabetes Prevention Program Outcomes Study (DPPOS) (100). This analysis demonstrated a 56% lower risk of diabetes 10 years from randomization among those who were able to achieve normoglycemia during DPP vs. those who remained with prediabetes. Additionally, restoration of normoglycemia reduced prevalence of microvascular disease (101) and CV risk factors despite less use of medication to lower lipids and blood pressure (102). The concept that diabetes and CV risk can be significantly reduced over the long-term through the pursuit of normoglycemia represents a major shift in our current thinking and has quickly gained consensus as the goal for people with prediabetes (103,104). Clinical predictors (105) and calculators as to the likelihood of regression (106) can be used to select and activate patients. Importantly, restoration of normoglycemia – as opposed to “diabetes prevention” – is clinically actionable.
Exactly how normoglycemia should be achieved is far less clear. Data from the DPP would contend that only lifestyle modification, not metformin, is useful in achieving normoglycemia in people with prediabetes (105) (Figure 7). Of note, lifestyle modification has been shown particularly effective in women (107) and the elderly (108). The thiazolidinediones (TZD’s) have also demonstrated their ability to restore normoglycemia in people with prediabetes (71,72,109) and may gain greater acceptance in this population now that their CV safety has been established. An increasing number of trials are focused on the ability of medication or lifestyle to not only prevent or delay onset of diabetes, but restore normoglycemia (79,110,111).
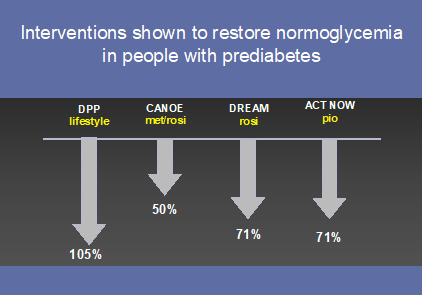
Figure 7.
Interventions that have restored normoglycemia in people with prediabetes
TRANSLATING INFORMATION INTO CONVERSATION
As we follow the recommended steps for screening and diagnosis of prediabetes outlined above, the next step in beginning the conversation with a patient with prediabetes is educating them about what the diagnosis means. An A1c of 5.7-6.0% carries up to a 25%/5-year risk, whereas an A1c 6.0-6.4% carries up to a 50%/5-year risk, and prediabetes period carries up to a 70% lifetime risk of diabetes. Further, people with prediabetes can suffer complications of diabetes even if they never convert. Early intervention can prevent diabetes by more than 50% if normoglycemia can be attained – even if transiently. Intensive lifestyle modification and a number of glucose-lowering and anti-obesity medications have been shown as capable to achieve this. Metformin is recommended for younger, overweight people with prediabetes even though it may not achieve normoglycemia as readily. Micro- and macrovascular risk factor modification is critical. Plasma glucose concentrations should be followed and re-screening for diabetes done annually.
CONCLUSION
In the light of the global burden of prediabetes affecting close to one billion people, the high progression rates to type 2 diabetes, and the increased risk of both micro- and macrovascular complications and death (112), efforts focused on preventing progression to diabetes and its complications are crucial. Although both intensive lifestyle intervention and various medications have proven to be effective for prevention or delay of diabetes in people with prediabetes, their uptake has been slow. This is true even in light of emerging data showing the vast benefits of early interventions. Our best bet to recognize prediabetes as a disease is probably by calling it what it is: early diabetes (94) and treat it as such, eradicating the term “prediabetes” for good.
REFERENCES
- 1.
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28(12):1039–1057. [PubMed: 510803]
- 2.
- World Health Organization. Report of the Expert Committee on Diabetes. WHO Technical Report Seies. Vol 646. Geneva, Switzerland 1980.
- 3.
- Fabre J, Balant LP, Dayer PG, Fox HM, Vernet AT. The kidney in maturity onset diabetes mellitus: a clinical study of 510 patients. Kidney Int. 1982;21(5):730–738. [PubMed: 7109459]
- 4.
- Haffner SM, Mitchell BD, Pugh JA, Stern MP, Kozlowski MK, Hazuda HP, Patterson JK, Klein R. Proteinuria in Mexican Americans and non-Hispanic whites with NIDDM. Diabetes Care. 1989;12(8):530–536. [PubMed: 2776587]
- 5.
- Hamman RF, Mayer EJ, Moo-Young GA, Hildebrandt W, Marshall JA, Baxter J. Prevalence and risk factors of diabetic retinopathy in non-Hispanic whites and Hispanics with NIDDM. San Luis Valley Diabetes Study. Diabetes. 1989;38(10):1231–1237. [PubMed: 2792575]
- 6.
- Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol. 1984;102(4):527–532. [PubMed: 6367725]
- 7.
- Siitonen O, Uusitupa M, Pyorala K, Lansimies E, Voutilainen E. Aortic calcifications and their relationship to coronary heart disease and cardiovascular risk factors in patients with newly diagnosed non-insulin-dependent diabetes and in nondiabetic subjects. Cardiology. 1987;74(5):335–343. [PubMed: 3652078]
- 8.
- Uusitupa M, Siitonen O, Pyorala K, Aro A, Hersio K, Penttila I, Voutilainen E. The relationship of cardiovascular risk factors to the prevalence of coronary heart disease in newly diagnosed type 2 (non-insulin-dependent) diabetes. Diabetologia. 1985;28(9):653–659. [PubMed: 3905476]
- 9.
- Keen H, Jarrett RJ, McCartney P. The ten-year follow-up of the Bedford survey (1962-1972): glucose tolerance and diabetes. Diabetologia. 1982;22(2):73–78. [PubMed: 7060852]
- 10.
- O'Sullivan JB, Mahan CM. Prospective study of 352 young patients with chemical diabetes. N Engl J Med. 1968;278(19):1038–1041. [PubMed: 5644965]
- 11.
- Sayegh HA, Jarrett RJ. Oral glucose-tolerance tests and the diagnosis of diabetes: results of a prospective study based on the Whitehall survey. Lancet. 1979;2(8140):431–433. [PubMed: 89497]
- 12.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. [PubMed: 8366922]
- 13.
- American Diabetes Association. 6. Glycemic Targets: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42 Suppl 1:S61–S70. [PubMed: 30559232]
- 14.
- American Diabetes Association. clinical practice recommendations 1997. Diabetes Care. 1997;20 Suppl 1:S1–70. [PubMed: 9028710]
- 15.
- American Diabetes Association. Standards of medical care in diabetes--2010. Diabetes Care. 2010;33 Suppl 1:S11–61. [PMC free article: PMC2797382] [PubMed: 20042772]
- 16.
- Lachin JM, Genuth S, Nathan DM, Zinman B, Rutledge BN, Group DER. Effect of glycemic exposure on the risk of microvascular complications in the diabetes control and complications trial--revisited. Diabetes. 2008;57(4):995–1001. [PubMed: 18223010]
- 17.
- American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2004;27 Suppl 1:S15–35. [PubMed: 14693923]
- 18.
- Cheng YJ, Gregg EW, Geiss LS, Imperatore G, Williams DE, Zhang X, Albright AL, Cowie CC, Klein R, Saaddine JB. Association of A1C and fasting plasma glucose levels with diabetic retinopathy prevalence in the U.S. population: Implications for diabetes diagnostic thresholds. Diabetes Care. 2009;32(11):2027–2032. [PMC free article: PMC2768189] [PubMed: 19875604]
- 19.
- Diabetes Prevention Program Research Group. The prevalence of retinopathy in impaired glucose tolerance and recent-onset diabetes in the Diabetes Prevention Program. Diabet Med. 2007;24(2):137–144. [PMC free article: PMC2267935] [PubMed: 17257275]
- 20.
- Ford ES, Zhao G, Li C. Pre-diabetes and the risk for cardiovascular disease: a systematic review of the evidence. J Am Coll Cardiol. 2010;55(13):1310–1317. [PubMed: 20338491]
- 21.
- Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A, Group KS. Prevalence of polyneuropathy in pre-diabetes and diabetes is associated with abdominal obesity and macroangiopathy: the MONICA/KORA Augsburg Surveys S2 and S3. Diabetes Care. 2008;31(3):464–469. [PubMed: 18039804]
- 22.
- Li Y GL, Burrows NR, Rolka DB, Albright A. Awareness of pre-diabetes – United States, 2005-2010. . MMWR. 2013;62(11):209–212.
- 23.
- Moin T, Li J, Duru OK, Ettner S, Turk N, Keckhafer A, Ho S, Mangione CM. Metformin prescription for insured adults with prediabetes from 2010 to 2012: a retrospective cohort study. Ann Intern Med. 2015;162(8):542–548. [PMC free article: PMC4682357] [PubMed: 25894024]
- 24.
- Centers for Disease Control and Prevention. https://www
.cdc.gov/diabetes /data/statistics-report/index .html. Accessed March 1, 2022. - 25.
- American Diabetes Association Professional Practice Committee. Draznin B, Aroda VR, Bakris G, Benson G, Brown FM, Freeman R, Green J, Huang E, Isaacs D, Kahan S, Leon J, Lyons SK, Peters AL, Prahalad P, Reusch JEB, Young-Hyman D, Das S, Kosiborod M. 3. Prevention or Delay of Type 2 Diabetes and Associated Comorbidities: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45 Supplement_1:S39–S45. [PubMed: 34964876]
- 26.
- American Diabetes Association Professional Practice Committee. Draznin B, Aroda VR, Bakris G, Benson G, Brown FM, Freeman R, Green J, Huang E, Isaacs D, Kahan S, Leon J, Lyons SK, Peters AL, Prahalad P, Reusch JEB, Young-Hyman D, Das S, Kosiborod M. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45 Supplement_1:S17–S38. [PubMed: 34964875]
- 27.
- de Vegt F, Dekker JM, Jager A, Hienkens E, Kostense PJ, Stehouwer CD, Nijpels G, Bouter LM, Heine RJ. Relation of impaired fasting and postload glucose with incident type 2 diabetes in a Dutch population: The Hoorn Study. JAMA. 2001;285(16):2109–2113. [PubMed: 11311100]
- 28.
- Engberg S, Vistisen D, Lau C, Glumer C, Jorgensen T, Pedersen O, Borch-Johnsen K. Progression to impaired glucose regulation and diabetes in the population-based Inter99 study. Diabetes Care. 2009;32(4):606–611. [PMC free article: PMC2660484] [PubMed: 19114617]
- 29.
- Meigs JB, Muller DC, Nathan DM, Blake DR, Andres R. Baltimore Longitudinal Study of A. The natural history of progression from normal glucose tolerance to type 2 diabetes in the Baltimore Longitudinal Study of Aging. Diabetes. 2003;52(6):1475–1484. [PubMed: 12765960]
- 30.
- Soderberg S, Zimmet P, Tuomilehto J, de Courten M, Dowse GK, Chitson P, Stenlund H, Gareeboo H, Alberti KG, Shaw J. High incidence of type 2 diabetes and increasing conversion rates from impaired fasting glucose and impaired glucose tolerance to diabetes in Mauritius. J Intern Med. 2004;256(1):37–47. [PubMed: 15189364]
- 31.
- Tirosh A, Shai I, Tekes-Manova D, Israeli E, Pereg D, Shochat T, Kochba I, Rudich A. Israeli Diabetes Research G. Normal fasting plasma glucose levels and type 2 diabetes in young men. N Engl J Med. 2005;353(14):1454–1462. [PubMed: 16207847]
- 32.
- Mooy JM, Grootenhuis PA, de Vries H, Kostense PJ, Popp-Snijders C, Bouter LM, Heine RJ. Intra-individual variation of glucose, specific insulin and proinsulin concentrations measured by two oral glucose tolerance tests in a general Caucasian population: the Hoorn Study. Diabetologia. 1996;39(3):298–305. [PubMed: 8721775]
- 33.
- Bergman M, Manco M, Sesti G, Dankner R, Pareek M, Jagannathan R, Chetrit A, Abdul-Ghani M, Buysschaert M, Olsen MH, Nilsson PM, Medina JL, Roth J, Groop L, Del Prato S, Raz I, Ceriello A. Petition to replace current OGTT criteria for diagnosing prediabetes with the 1-hour post-load plasma glucose>/=155mg/dl (8.6mmol/L). Diabetes Res Clin Pract. 2018;146:18–33. [PubMed: 30273707]
- 34.
- Juraschek SP, Steffes MW, Selvin E. Associations of alternative markers of glycemia with hemoglobin A(1c) and fasting glucose. Clin Chem. 2012;58(12):1648–1655. [PMC free article: PMC3652236] [PubMed: 23019309]
- 35.
- Vistisen D, Witte DR, Brunner EJ, Kivimaki M, Tabak A, Jorgensen ME, Faerch K. Risk of Cardiovascular Disease and Death in Individuals With Prediabetes Defined by Different Criteria: The Whitehall II Study. Diabetes Care. 2018;41(4):899–906. [PMC free article: PMC6463620] [PubMed: 29453200]
- 36.
- World Health Organization. Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus. Geneva, Switzerland2011:1-25.
- 37.
- Cowie CC, Rust KF, Ford ES, Eberhardt MS, Byrd-Holt DD, Li C, Williams DE, Gregg EW, Bainbridge KE, Saydah SH, Geiss LS. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988-1994 and 2005-2006. Diabetes Care. 2009;32(2):287–294. [PMC free article: PMC2628695] [PubMed: 19017771]
- 38.
- Morris DH, Khunti K, Achana F, Srinivasan B, Gray LJ, Davies MJ, Webb D. Progression rates from HbA1c 6.0-6.4% and other prediabetes definitions to type 2 diabetes: a meta-analysis. Diabetologia. 2013;56(7):1489–1493. [PubMed: 23584433]
- 39.
- Zhang X, Gregg EW, Williamson DF, Barker LE, Thomas W, Bullard KM, Imperatore G, Williams DE, Albright AL. A1C level and future risk of diabetes: a systematic review. Diabetes Care. 2010;33(7):1665–1673. [PMC free article: PMC2890379] [PubMed: 20587727]
- 40.
- Rasmussen SS, Glumer C, Sandbaek A, Lauritzen T, Borch-Johnsen K. Progression from impaired fasting glucose and impaired glucose tolerance to diabetes in a high-risk screening programme in general practice: the ADDITION Study, Denmark. Diabetologia. 2007;50(2):293–297. [PubMed: 17143605]
- 41.
- Ferrannini E, Nannipieri M, Williams K, Gonzales C, Haffner SM, Stern MP. Mode of onset of type 2 diabetes from normal or impaired glucose tolerance. Diabetes. 2004;53(1):160–165. [PubMed: 14693710]
- 42.
- Rooney MR, Rawlings AM, Pankow JS, Echouffo Tcheugui JB, Coresh J, Sharrett AR, Selvin E. Risk of Progression to Diabetes Among Older Adults With Prediabetes. JAMA Intern Med. 2021;181(4):511–519. [PMC free article: PMC7871207] [PubMed: 33555311]
- 43.
- Tripathy D, Carlsson M, Almgren P, Isomaa B, Taskinen MR, Tuomi T, Groop LC. Insulin secretion and insulin sensitivity in relation to glucose tolerance: lessons from the Botnia Study. Diabetes. 2000;49(6):975–980. [PubMed: 10866050]
- 44.
- Faerch K, Witte DR, Tabak AG, Perreault L, Herder C, Brunner EJ, Kivimaki M, Vistisen D. Trajectories of cardiometabolic risk factors before diagnosis of three subtypes of type 2 diabetes: a post-hoc analysis of the longitudinal Whitehall II cohort study. Lancet Diabetes Endocrinol. 2013;1(1):43–51. [PubMed: 24622266]
- 45.
- Tabak AG, Jokela M, Akbaraly TN, Brunner EJ, Kivimaki M, Witte DR. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Lancet. 2009;373(9682):2215–2221. [PMC free article: PMC2726723] [PubMed: 19515410]
- 46.
- Perreault L, Bergman BC, Playdon MC, Dalla Man C, Cobelli C, Eckel RH. Impaired fasting glucose with or without impaired glucose tolerance: progressive or parallel states of prediabetes? Am J Physiol Endocrinol Metab. 2008;295(2):E428–435. [PMC free article: PMC2519761] [PubMed: 18523123]
- 47.
- Clore JN, Glickman PS, Helm ST, Nestler JE, Blackard WG. Evidence for dual control mechanism regulating hepatic glucose output in nondiabetic men. Diabetes. 1991;40(8):1033–1040. [PubMed: 1860555]
- 48.
- Abdul-Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA. Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes. 2006;55(5):1430–1435. [PubMed: 16644701]
- 49.
- Jani R, Molina M, Matsuda M, Balas B, Chavez A, DeFronzo RA, Abdul-Ghani M. Decreased non-insulin-dependent glucose clearance contributes to the rise in fasting plasma glucose in the nondiabetic range. Diabetes Care. 2008;31(2):311–315. [PubMed: 18000182]
- 50.
- Perreault L, Faerch K, Kerege AA, Bacon SD, Bergman BC. Hepatic glucose sensing is impaired, but can be normalized, in people with impaired fasting glucose. J Clin Endocrinol Metab. 2014;99(7):E1154–1162. [PMC free article: PMC4079303] [PubMed: 24731008]
- 51.
- Faerch K, Vaag A, Holst JJ, Glumer C, Pedersen O, Borch-Johnsen K. Impaired fasting glycaemia vs impaired glucose tolerance: similar impairment of pancreatic alpha and beta cell function but differential roles of incretin hormones and insulin action. Diabetologia. 2008;51(5):853–861. [PubMed: 18317726]
- 52.
- Meyer C, Pimenta W, Woerle HJ, Van Haeften T, Szoke E, Mitrakou A, Gerich J. Different mechanisms for impaired fasting glucose and impaired postprandial glucose tolerance in humans. Diabetes Care. 2006;29(8):1909–1914. [PubMed: 16873801]
- 53.
- Tripathy D, Almgren P, Tuomi T, Groop L. Contribution of insulin-stimulated glucose uptake and basal hepatic insulin sensitivity to surrogate measures of insulin sensitivity. Diabetes Care. 2004;27(9):2204–2210. [PubMed: 15333485]
- 54.
- Weyer C, Bogardus C, Pratley RE. Metabolic characteristics of individuals with impaired fasting glucose and/or impaired glucose tolerance. Diabetes. 1999;48(11):2197–2203. [PubMed: 10535454]
- 55.
- Bock G, Dalla Man C, Campioni M, Chittilapilly E, Basu R, Toffolo G, Cobelli C, Rizza R. Pathogenesis of pre-diabetes: mechanisms of fasting and postprandial hyperglycemia in people with impaired fasting glucose and/or impaired glucose tolerance. Diabetes. 2006;55(12):3536–3549. [PubMed: 17130502]
- 56.
- Faerch K, Vaag A, Holst JJ, Hansen T, Jorgensen T, Borch-Johnsen K. Natural history of insulin sensitivity and insulin secretion in the progression from normal glucose tolerance to impaired fasting glycemia and impaired glucose tolerance: the Inter99 study. Diabetes Care. 2009;32(3):439–444. [PMC free article: PMC2646025] [PubMed: 19056613]
- 57.
- Festa A, D'Agostino R Jr, Hanley AJ, Karter AJ, Saad MF, Haffner SM. Differences in insulin resistance in nondiabetic subjects with isolated impaired glucose tolerance or isolated impaired fasting glucose. Diabetes. 2004;53(6):1549–1555. [PubMed: 15161760]
- 58.
- Laakso M, Zilinskaite J, Hansen T, Boesgaard TW, Vanttinen M, Stancakova A, Jansson PA, Pellme F, Holst JJ, Kuulasmaa T, Hribal ML, Sesti G, Stefan N, Fritsche A, Haring H, Pedersen O, Smith U, Consortium E. Insulin sensitivity, insulin release and glucagon-like peptide-1 levels in persons with impaired fasting glucose and/or impaired glucose tolerance in the EUGENE2 study. Diabetologia. 2008;51(3):502–511. [PubMed: 18080106]
- 59.
- Abdul-Ghani MA, Molina-Carrion M, Jani R, Jenkinson C, Defronzo RA. Adipocytes in subjects with impaired fasting glucose and impaired glucose tolerance are resistant to the anti-lipolytic effect of insulin. Acta Diabetol. 2008;45(3):147–150. [PubMed: 18357404]
- 60.
- Hanefeld M, Koehler C, Henkel E, Fuecker K, Schaper F, Temelkova-Kurktschiev T. Post-challenge hyperglycaemia relates more strongly than fasting hyperglycaemia with carotid intima-media thickness: the RIAD Study. Risk Factors in Impaired Glucose Tolerance for Atherosclerosis and Diabetes. Diabet Med. 2000;17(12):835–840. [PubMed: 11168325]
- 61.
- Williams JW, Zimmet PZ, Shaw JE, de Courten MP, Cameron AJ, Chitson P, Tuomilehto J, Alberti KG. Gender differences in the prevalence of impaired fasting glycaemia and impaired glucose tolerance in Mauritius. Does sex matter? Diabet Med. 2003;20(11):915–920. [PubMed: 14632717]
- 62.
- Kantartzis K, Machann J, Schick F, Fritsche A, Haring HU, Stefan N. The impact of liver fat vs visceral fat in determining categories of prediabetes. Diabetologia. 2010;53(5):882–889. [PubMed: 20099057]
- 63.
- Ahren B, Pacini G. Impaired adaptation of first-phase insulin secretion in postmenopausal women with glucose intolerance. Am J Physiol. 1997;273(4 Pt 1):E701–707. [PubMed: 9357798]
- 64.
- Festa A, Williams K, D'Agostino R Jr, Wagenknecht LE, Haffner SM. The natural course of beta-cell function in nondiabetic and diabetic individuals: the Insulin Resistance Atherosclerosis Study. Diabetes. 2006;55(4):1114–1120. [PubMed: 16567536]
- 65.
- Cobb J, Eckhart A, Motsinger-Reif A, Carr B, Groop L, Ferrannini E. alpha-Hydroxybutyric Acid Is a Selective Metabolite Biomarker of Impaired Glucose Tolerance. Diabetes Care. 2016;39(6):988–995. [PubMed: 27208342]
- 66.
- Kosaka K, Noda M, Kuzuya T. Prevention of type 2 diabetes by lifestyle intervention: a Japanese trial in IGT males. Diabetes Res Clin Pract. 2005;67(2):152–162. [PubMed: 15649575]
- 67.
- Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V. Indian Diabetes Prevention P. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia. 2006;49(2):289–297. [PubMed: 16391903]
- 68.
- Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M. Finnish Diabetes Prevention Study G. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–1350. [PubMed: 11333990]
- 69.
- Buchanan TA, Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, Ochoa C, Tan S, Berkowitz K, Hodis HN, Azen SP. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk hispanic women. Diabetes. 2002;51(9):2796–2803. [PubMed: 12196473]
- 70.
- Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. Group S-NTR. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002;359(9323):2072–2077. [PubMed: 12086760]
- 71.
- DeFronzo RA, Tripathy D, Schwenke DC, Banerji M, Bray GA, Buchanan TA, Clement SC, Henry RR, Hodis HN, Kitabchi AE, Mack WJ, Mudaliar S, Ratner RE, Williams K, Stentz FB, Musi N, Reaven PD, Study AN. Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med. 2011;364(12):1104–1115. [PubMed: 21428766]
- 72.
- Dream Trial Investigators. Gerstein HC, Yusuf S, Bosch J, Pogue J, Sheridan P, Dinccag N, Hanefeld M, Hoogwerf B, Laakso M, Mohan V, Shaw J, Zinman B, Holman RR. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet. 2006;368(9541):1096–1105. [PubMed: 16997664]
- 73.
- Holman RR, Coleman RL, Chan JCN, Chiasson JL, Feng H, Ge J, Gerstein HC, Gray R, Huo Y, Lang Z, McMurray JJ, Ryden L, Schroder S, Sun Y, Theodorakis MJ, Tendera M, Tucker L, Tuomilehto J, Wei Y, Yang W, Wang D, Hu D, Pan C, Group ACES. Effects of acarbose on cardiovascular and diabetes outcomes in patients with coronary heart disease and impaired glucose tolerance (ACE): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017;5(11):877–886. [PubMed: 28917545]
- 74.
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Diabetes Prevention Program Research G. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. [PMC free article: PMC1370926] [PubMed: 11832527]
- 75.
- Navigator Study Group. Holman RR, Haffner SM, McMurray JJ, Bethel MA, Holzhauer B, Hua TA, Belenkov Y, Boolell M, Buse JB, Buckley BM, Chacra AR, Chiang FT, Charbonnel B, Chow CC, Davies MJ, Deedwania P, Diem P, Einhorn D, Fonseca V, Fulcher GR, Gaciong Z, Gaztambide S, Giles T, Horton E, Ilkova H, Jenssen T, Kahn SE, Krum H, Laakso M, Leiter LA, Levitt NS, Mareev V, Martinez F, Masson C, Mazzone T, Meaney E, Nesto R, Pan C, Prager R, Raptis SA, Rutten GE, Sandstroem H, Schaper F, Scheen A, Schmitz O, Sinay I, Soska V, Stender S, Tamas G, Tognoni G, Tuomilehto J, Villamil AS, Vozar J, Califf RM. Effect of nateglinide on the incidence of diabetes and cardiovascular events. N Engl J Med. 2010;362(16):1463–1476. [PubMed: 20228402]
- 76.
- Origin Trial Investigators. Gerstein HC, Bosch J, Dagenais GR, Diaz R, Jung H, Maggioni AP, Pogue J, Probstfield J, Ramachandran A, Riddle MC, Ryden LE, Yusuf S. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367(4):319–328. [PubMed: 22686416]
- 77.
- Bohula EA, Scirica BM, Inzucchi SE, McGuire DK, Keech AC, Smith SR, Kanevsky E, Murphy SA, Leiter LA, Dwyer JP, Corbalan R, Hamm C, Kaplan L, Nicolau JC, Ophuis TO, Ray KK, Ruda M, Spinar J, Patel T, Miao W, Perdomo C, Francis B, Dhadda S, Bonaca MP, Ruff CT, Sabatine MS, Wiviott SD. Investigators C-TSC. Effect of lorcaserin on prevention and remission of type 2 diabetes in overweight and obese patients (CAMELLIA-TIMI 61): a randomised, placebo-controlled trial. Lancet. 2018;392(10161):2269–2279. [PubMed: 30293771]
- 78.
- Garvey WT, Ryan DH, Henry R, Bohannon NJ, Toplak H, Schwiers M, Troupin B, Day WW. Prevention of type 2 diabetes in subjects with prediabetes and metabolic syndrome treated with phentermine and topiramate extended release. Diabetes Care. 2014;37(4):912–921. [PMC free article: PMC4392900] [PubMed: 24103901]
- 79.
- le Roux CW, Astrup A, Fujioka K, Greenway F, Lau DC, Van Gaal L, Ortiz RV, Wilding JP, Skjoth TV, Manning LS, Pi-Sunyer X, Obesity S, Prediabetes NNSG. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet. 2017 [PubMed: 28237263]
- 80.
- Nesto R, Fain R, Li Y, Shanahan W. Evaluation of lorcaserin on progression of prediabetes to type 2 diabetes and reversion to euglycemia. Postgrad Med. 2016;128(4):364–370. [PubMed: 27116910]
- 81.
- Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27(1):155–161. [PubMed: 14693982]
- 82.
- Galaviz KI, Weber MB, Straus A, Haw JS, Narayan KMV, Ali MK. Global Diabetes Prevention Interventions: A Systematic Review and Network Meta-analysis of the Real-World Impact on Incidence, Weight, and Glucose. Diabetes Care. 2018;41(7):1526–1534. [PMC free article: PMC6463613] [PubMed: 29934481]
- 83.
- National Health Service. https://www
.england.nhs .uk/wp-content/uploads /2016/08/dpp-faq.pdf. Accessed March 1, 2022. - 84.
- Sampson M, Clark A, Bachmann M, Garner N, Irvine L, Howe A, Greaves C, Auckland S, Smith J, Turner J, Rea D, Rayman G, Dhatariya K, John WG, Barton G, Usher R, Ferns C, Pascale M. Norfolk Diabetes Prevention Study G. Lifestyle Intervention With or Without Lay Volunteers to Prevent Type 2 Diabetes in People With Impaired Fasting Glucose and/or Nondiabetic Hyperglycemia: A Randomized Clinical Trial. JAMA Intern Med. 2021;181(2):168–178. [PMC free article: PMC7607494] [PubMed: 33136119]
- 85.
- American Diabetes Association. 3. Prevention or Delay of Type 2 Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42 Suppl 1:S29–S33. [PubMed: 30559229]
- 86.
- Sattar N, Preiss D. HbA1c in type 2 diabetes diagnostic criteria: addressing the right questions to move the field forwards. Diabetologia. 2012;55(6):1564–1567. [PubMed: 22398646]
- 87.
- Tapp RJ, Shaw JE, Zimmet PZ, Balkau B, Chadban SJ, Tonkin AM, Welborn TA, Atkins RC. Albuminuria is evident in the early stages of diabetes onset: results from the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Am J Kidney Dis. 2004;44(5):792–798. [PubMed: 15492944]
- 88.
- Ali MK, Bullard KM, Saydah S, Imperatore G, Gregg EW. Cardiovascular and renal burdens of prediabetes in the USA: analysis of data from serial cross-sectional surveys, 1988-2014. Lancet Diabetes Endocrinol. 2018;6(5):392–403. [PMC free article: PMC6615033] [PubMed: 29500121]
- 89.
- Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015;3(11):866–875. [PMC free article: PMC4623946] [PubMed: 26377054]
- 90.
- Carlsson LM, Sjoholm K, Karlsson C, Jacobson P, Andersson-Assarsson JC, Svensson PA, Larsson I, Hjorth S, Neovius M, Taube M, Carlsson B, Peltonen M. Long-term incidence of microvascular disease after bariatric surgery or usual care in patients with obesity, stratified by baseline glycaemic status: a post-hoc analysis of participants from the Swedish Obese Subjects study. Lancet Diabetes Endocrinol. 2017;5(4):271–279. [PMC free article: PMC5394228] [PubMed: 28237791]
- 91.
- Gong Q, Zhang P, Wang J, Ma J, An Y, Chen Y, Zhang B, Feng X, Li H, Chen X, Cheng YJ, Gregg EW, Hu Y, Bennett PH, Li G. Da Qing Diabetes Prevention Study G. Morbidity and mortality after lifestyle intervention for people with impaired glucose tolerance: 30-year results of the Da Qing Diabetes Prevention Outcome Study. Lancet Diabetes Endocrinol. 2019;7(6):452–461. [PMC free article: PMC8172050] [PubMed: 31036503]
- 92.
- Qiao Q, Pyorala K, Pyorala M, Nissinen A, Lindstrom J, Tilvis R, Tuomilehto J. Two-hour glucose is a better risk predictor for incident coronary heart disease and cardiovascular mortality than fasting glucose. Eur Heart J. 2002;23(16):1267–1275. [PubMed: 12175663]
- 93.
- Gregg EW, Li Y, Wang J, Burrows NR, Ali MK, Rolka D, Williams DE, Geiss L. Changes in diabetes-related complications in the United States, 1990-2010. N Engl J Med. 2014;370(16):1514–1523. [PubMed: 24738668]
- 94.
- Perreault L, Faerch K, Gregg EW. Can Cardiovascular Epidemiology and Clinical Trials Close the Risk Management Gap Between Diabetes and Prediabetes? Curr Diab Rep. 2017;17(9):77. [PubMed: 28766246]
- 95.
- Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. Group S-NTR. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003;290(4):486–494. [PubMed: 12876091]
- 96.
- Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M, Guarino PD, Lovejoy AM, Peduzzi PN, Conwit R, Brass LM, Schwartz GG, Adams HP Jr, Berger L, Carolei A, Clark W, Coull B, Ford GA, Kleindorfer D, O'Leary JR, Parsons MW, Ringleb P, Sen S, Spence JD, Tanne D, Wang D, Winder TR, Investigators IT. Pioglitazone after Ischemic Stroke or Transient Ischemic Attack. N Engl J Med. 2016;374(14):1321–1331. [PMC free article: PMC4887756] [PubMed: 26886418]
- 97.
- van Agtmaal MJM, Houben A, de Wit V, Henry RMA, Schaper NC, Dagnelie PC, van der Kallen CJ, Koster A, Sep SJ, Kroon AA, Jansen JFA, Hofman PA, Backes WH, Schram MT, Stehouwer CDA. Prediabetes Is Associated With Structural Brain Abnormalities: The Maastricht Study. Diabetes Care. 2018;41(12):2535–2543. [PubMed: 30327356]
- 98.
- van Bussel FC, Backes WH, van Veenendaal TM, Hofman PA, van Boxtel MP, Schram MT, Sep SJ, Dagnelie PC, Schaper N, Stehouwer CD, Wildberger JE, Jansen JF. Functional Brain Networks Are Altered in Type 2 Diabetes and Prediabetes: Signs for Compensation of Cognitive Decrements? The Maastricht Study. Diabetes. 2016;65(8):2404–2413. [PubMed: 27217484]
- 99.
- Xu W, Caracciolo B, Wang HX, Winblad B, Backman L, Qiu C, Fratiglioni L. Accelerated progression from mild cognitive impairment to dementia in people with diabetes. Diabetes. 2010;59(11):2928–2935. [PMC free article: PMC2963552] [PubMed: 20713684]
- 100.
- Perreault L, Pan Q, Mather KJ, Watson KE, Hamman RF, Kahn SE. Diabetes Prevention Program Research G. Effect of regression from prediabetes to normal glucose regulation on long-term reduction in diabetes risk: results from the Diabetes Prevention Program Outcomes Study. Lancet. 2012;379(9833):2243–2251. [PMC free article: PMC3555407] [PubMed: 22683134]
- 101.
- Perreault L, Pan Q, Schroeder EB, Kalyani RR, Bray GA, Dagogo-Jack S, White NH, Goldberg RB, Kahn SE, Knowler WC, Mathioudakis N, Dabelea D. Diabetes Prevention Program Research G. Regression From Prediabetes to Normal Glucose Regulation and Prevalence of Microvascular Disease in the Diabetes Prevention Program Outcomes Study (DPPOS). Diabetes Care. 2019;42(9):1809–1815. [PMC free article: PMC6702603] [PubMed: 31320445]
- 102.
- Perreault L, Temprosa M, Mather KJ, Horton E, Kitabchi A, Larkin M, Montez MG, Thayer D, Orchard TJ, Hamman RF, Goldberg RB. Diabetes Prevention Program Research G. Regression from prediabetes to normal glucose regulation is associated with reduction in cardiovascular risk: results from the Diabetes Prevention Program outcomes study. Diabetes Care. 2014;37(9):2622–2631. [PMC free article: PMC4140157] [PubMed: 24969574]
- 103.
- Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, Dagogo-Jack S, DeFronzo RA, Einhorn D, Fonseca VA, Garber JR, Garvey WT, Grunberger G, Handelsman Y, Hirsch IB, Jellinger PS, McGill JB, Mechanick JI, Rosenblit PD, Umpierrez GE. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm - 2019 Executive Summary. Endocr Pract. 2019;25(1):69–100. [PubMed: 30742570]
- 104.
- Phillips LS, Olson DE. Diabetes: normal glucose levels should be the goal. Nat Rev Endocrinol. 2012;8(9):510–512. [PubMed: 22847240]
- 105.
- Perreault L, Kahn SE, Christophi CA, Knowler WC, Hamman RF. Diabetes Prevention Program Research G. Regression from pre-diabetes to normal glucose regulation in the diabetes prevention program. Diabetes Care. 2009;32(9):1583–1588. [PMC free article: PMC2732165] [PubMed: 19587364]
- 106.
- Herman WH, Pan Q, Edelstein SL, Mather KJ, Perreault L, Barrett-Connor E, Dabelea DM, Horton E, Kahn SE, Knowler WC, Lorenzo C, Pi-Sunyer X, Venditti E, Ye W. Diabetes Prevention Program Research G. Impact of Lifestyle and Metformin Interventions on the Risk of Progression to Diabetes and Regression to Normal Glucose Regulation in Overweight or Obese People With Impaired Glucose Regulation. Diabetes Care. 2017;40(12):1668–1677. [PMC free article: PMC5711336] [PubMed: 29021207]
- 107.
- Perreault L, Ma Y, Dagogo-Jack S, Horton E, Marrero D, Crandall J, Barrett-Connor E. Diabetes Prevention P. Sex differences in diabetes risk and the effect of intensive lifestyle modification in the Diabetes Prevention Program. Diabetes Care. 2008;31(7):1416–1421. [PMC free article: PMC2453677] [PubMed: 18356403]
- 108.
- Diabetes Prevention Program Research Group. Crandall J, Schade D, Ma Y, Fujimoto WY, Barrett-Connor E, Fowler S, Dagogo-Jack S, Andres R. The influence of age on the effects of lifestyle modification and metformin in prevention of diabetes. J Gerontol A Biol Sci Med Sci. 2006;61(10):1075–1081. [PMC free article: PMC1783677] [PubMed: 17077202]
- 109.
- Zinman B, Harris SB, Neuman J, Gerstein HC, Retnakaran RR, Raboud J, Qi Y, Hanley AJ. Low-dose combination therapy with rosiglitazone and metformin to prevent type 2 diabetes mellitus (CANOE trial): a double-blind randomised controlled study. Lancet. 2010;376(9735):103–111. [PubMed: 20605202]
- 110.
- Coppell K, Freer T, Abel S, Whitehead L, Tipene-Leach D, Gray AR, Merriman T, Sullivan T, Krebs J, Perreault L. What predicts regression from pre-diabetes to normal glucose regulation following a primary care nurse-delivered dietary intervention? A study protocol for a prospective cohort study. BMJ Open. 2019;9(12):e033358. [PMC free article: PMC6924756] [PubMed: 31822546]
- 111.
- Ritchie ND, Sauder KA, Kaufmann PG, Perreault L. Patient-Centered Goal-Setting in the National Diabetes Prevention Program: A Pilot Study. Diabetes Care. 2021;44(11):2464–2469. [PMC free article: PMC8546276] [PubMed: 34404739]
- 112.
- Saydah SH, Loria CM, Eberhardt MS, Brancati FL. Subclinical states of glucose intolerance and risk of death in the U.S. Diabetes Care. 2001;24(3):447–453. [PubMed: 11289466]
Publication Details
Author Information and Affiliations
Publication History
Last Update: March 3, 2022.
Copyright
This electronic version has been made freely available under a Creative Commons (CC-BY-NC-ND) license. A copy of the license can be viewed at http://creativecommons.org/licenses/by-nc-nd/2.0/.
Publisher
MDText.com, Inc., South Dartmouth (MA)
NLM Citation
Perreault L. Prediabetes. [Updated 2022 Mar 3]. In: Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-.