NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-.
ABSTRACT
Hyperprolactinemia is the most common hypothalamic-pituitary dysfunction, being an important cause of irregular menses and infertility amongst young women. Clinical and laboratory investigation is crucial to determine hyperprolactinemia etiology and to indicate the proper treatment. Prolactinoma is the most common cause of pathological hyperprolactinemia. Physiological and pharmacological causes must be ruled out. Macroprolactinemia is a laboratorial pitfall and must be ruled out in asymptomatic hyperprolactinemic individuals. Usually, treatment is not necessary. Hook effect is another laboratory pitfall that may underestimate prolactin levels confounding the differential diagnosis between macroprolactinomas and pseudoprolactinomas. Clinical treatment with dopamine agonists is effective in 80 to 90% of hyperprolactinemic patients leading to normal serum prolactin levels and tumor reduction. Normoprolactinemia after dopamine agonist (DA) withdrawal is possible in around 30% of cases. Hypogonadism and infertility are usually reversed upon prolactin level normalization due to DA treatment, allowing pregnancy in most patients. In micro and intrasellar macroprolactinomas, DA can be withdrawal after pregnancy confirmation. Dopamine agonists are usually well tolerated. Nevertheless, valvopathy and psychiatric side effects should be actively evaluated. Surgical treatment may be indicated in resistant/intolerant patients and symptomatic apoplectic tumors. Radiotherapy is rarely performed and must be reserved to control tumors growing in an aggressive fashion. Temozolomide is an alternative treatment for resistant/aggressive prolactinomas not responding to high doses of dopamine agonists, multiple surgeries, and radiotherapy. For complete coverage of all related areas of Endocrinology, please visit our on-line FREE web-text, WWW.ENDOTEXT.ORG.
INTRODUCTION
Prolactin secreting pituitary tumors are the most common type of hormone secreting pituitary tumors (1). They are also the only pituitary tumors which can be effectively managed primarily by medical means (2). Therefore, their identification and diagnosis are imperative to avoid unnecessary pituitary surgery.
PROLACTIN SECRETION
Prolactin is secreted by the lactotrophs in the anterior pituitary gland. Prolactin secretion is regulated by the hypothalamus. Unlike the other anterior pituitary hormones, however, the hypothalamic influence is predominantly of tonic inhibition (3).
The hypothalamus secretes prolactin-release-inhibiting factors (PIF) and prolactin-releasing factors (PRF). PIF is predominantly exert by dopamine, with GABA having a minor role (4). Prolactin controls its own secretion through a short loop negative feedback, stimulating tuberoinfundibular dopamine (TIDA) cells. Nevertheless, the nature of the physiological PRF is unclear. Thyrotropin-releasing hormone (TRH), vasoactive intestinal peptide (VIP), serotonin, histamine, oxytocin, and estrogens can act as a PRF. Other neurotransmitters and neuropeptides can also modulate prolactin secretion including endothelin, TGF beta1, angiotensin, somatostatin, substance P, neurotensin, calcitonin, EGF, natriuretic atrial peptide, bombesin, cholecystokinin, acetylcholine and vasopressin (5).
CAUSES OF HYPERPROLACTINEMIA
Disorders causing elevated prolactin levels is shown in Table 1. The causes of hyperprolactinemia may be considered, in a simplified fashion, as resulting from four basic abnormalities. In some patients, however, it is not possible to elucidate the cause of hyperprolactinemia, discussed below (6). Also, stress from venipuncture can cause slight increase in serum prolactin levels in asymptomatic individuals and a normal serum prolactin measured after resting for 30 minutes can rule out this condition (7). Nevertheless, routinely resting before venipuncture is usually not recommended (8). In addition, prolactin should not be measured after a seizure, which could increase hormonal level (6).
Table 1.
Etiology of Hyperprolactinemia
| Pituitary Disease Prolactinomas Acromegaly Clinically nonfunctioning pituitary adenomas Empty Sella syndrome Hypophysitis Hypothalamic Disease Craniopharyngiomas Meningiomas Germinomas Other tumors Sarcoidosis Langerhans cell histiocytosis Neuroaxis irradiation Vascular Pituitary Stalk Section Medications Phenothiazines Butyrophenones Atypical Antipsychotics Tricyclic Antidepressants Serotonin Reuptake Inhibitors Reserpine Methyldopa Verapamil Metoclopramide Neurogenic Chest wall/Breast lesions Spinal Cord lesions Other Pregnancy Breast-feeding Hypothyroidism Renal Insufficiency Adrenal Insufficiency Ectopic prolactin production Familial hyperprolactinemia (mutated prolactin receptor) Untreated phenylketonuria Macroprolactinoma Idiopathic |
Hypothalamic Dopamine Deficiency
Diseases of the hypothalamus, such as tumors, arterio-venous malformations, and inflammatory processes such as sarcoidosis result in either diminished synthesis or release of dopamine. Furthermore, certain drugs (e.g., alpha-methyldopa and reserpine) are capable of depleting the central dopamine stores.
Defective Transport Mechanisms
Transection of the pituitary stalk results in impaired transport of dopamine from the hypothalamus to the lactotrophs. Pituitary or stalk tumors with abnormal blood supplies, or their pressure effects, may interfere with the circulatory pathway from the hypothalamus down the pituitary stalk to the normal lactotrophs, or a tumor, producing effective dopamine deficiency due to a functional stalk section. Besides tumors, infections, and inflammatory diseases such as hypophysitis, sarcoidosis, and tuberculosis can also cause pituitary stalk disconnection.
Lactotroph Insensitivity to Dopamine
Dopamine receptors have been found on human pituitary lactotroph adenoma cells. Receptor sensitivity to dopamine could be diminished, which would explain the lack of response to increased endogenous dopamine stimulation. However, an obvious response of the receptors to pharmacologic dopamine agonists (DA) makes this possibility less likely. Certain drugs act as dopamine-receptor-blocking agents, including phenothiazines (e.g., chlorpromazine), butyrophenones (haloperidol), and benzamides (metoclopramide, sulpiride, and domperidone). These drugs block the effects of endogenous dopamine and thus release lactotrophs from their hypothalamic inhibition. This sequence of events results in hyperprolactinemia. Table 2 summarizes the most common drugs causing hyperprolactinemia (5,9).
Stimulation of Lactotrophs
Hypothyroidism may be associated with hyperprolactinemia. If hypothyroidism results in increased TRH production, then TRH (which can act as a PRF) could lead to hyperprolactinemia. Estrogens act directly at the pituitary level, causing stimulation of lactotrophs, and thus enhance prolactin secretion. Furthermore, estrogens increase the mitotic activity of lactotrophs, increasing cell numbers. Pregnancy and lactation are physiological causes of hyperprolactinemia. Injury to the chest wall can also lead to hyperprolactinemia. This results from abnormal stimulation of the reflex associated with the rise in prolactin that is seen normally in lactating women during suckling (6).
CLINICAL MANIFESTATIONS OF HYPERPROLACTINEMIA
The symptoms associated with hyperprolactinemia may be due to several factors: the direct effects of excess prolactin, such as the induction of galactorrhea (10) or hypogonadism (11), the effects of the structural lesion causing the disorder (i.e. the pituitary tumor), leading to, for example, headaches, visual field defects, or external ophthalmoplegia (12); or associated dysfunction of the secretion of other anterior pituitary hormones.
The incidence of galactorrhea in hyperprolactinemic patients is between 30% and 80%, depending on the skill of the examiner and the degree of estrogen deficiency. Approximately 50% of women with galactorrhea, however, have normal prolactin levels. As mentioned below, it is particularly those patients with very high prolactin levels, i.e., greater than 100ng/mL (2000mU/L), who often have no galactorrhea. Thus, galactorrhea is an inconsistent marker of hyperprolactinemia (10).
Women with hyperprolactinemia usually present with menstrual abnormalities – amenorrhea or oligomenorrhea – or regular cycles with infertility. Occasionally, patients may present with menorrhagia. Menstrual disorders are often not seen with mild hyperprolactinemia but it is unusual not having menstrual problems if serum prolactin is greater than 180 ng/mL (3,600 mU/L) (13).
In contrast, men often present late in the course of the disease with symptoms of expansion of their pituitary tumor (i.e., headaches, visual defects, and external ophthalmoplegia) or symptoms from secondary adrenal or thyroid failure. Nevertheless, these men can present with sexual impairment for many years before their diagnosis. It is unknown if macroprolactinomas are more commonly seen in men due to these delayed diagnosis and/or if prolactinoma´s pathogenesis is different in men (14). In contrast to women in whom microprolactinomas are most commonly seen, macroprolactinomas are usually found in men and the serum prolactin levels are usually much higher than those in women (15).
Occasionally, the syndrome may occur in prepubertal or peripubertal children, when it may present with delayed or arrested puberty or with headache and/or visual field defects or with growth arrest. Children and adolescents often present with aggressive prolactinomas, especially in boys (16). Genetic conditions such as MEN 1 and familial isolated pituitary adenomas (FIPA) should be considered (17).
DIFFERENTIAL DIAGNOSIS
It is important to exclude other causes of hyperprolactinemia: pregnancy, lactation, hypothyroidism, use of drugs that either deplete central dopamine or block dopamine receptors, and renal or hepatic failure. Ruling out these important causes, and any hypothalamic lesion, three common diagnostic possibilities remain: the presence of a microadenoma, macroadenoma, or no visible tumor at all. If patients do not harbor an identifiable tumor, they are described as having idiopathic hyperprolactinemia. It is likely, however, that patients with this condition may harbor small microprolactinomas, which were undetected with less sensitive imaging tools used in the past, and even with magnetic resonance imaging (MRI) (3).
A microadenoma is described as having a maximum diameter of up to 10mm (the maximal diameter of the normal pituitary gland) while a macroadenoma has a diameter larger than 10 mm. Giant adenomas are defined as the presence of the largest diameter of the tumor being larger than 4 cm (18). A microadenoma is often visualized using MRI. Usually, the serum prolactin level is below 200ng/mL (4000mU/L) in patients with microadenomas. A macroadenoma that secretes prolactin is usually associated with a serum prolactin level of more than 200ng/mL (4000mU/L). If the patient has a macroadenoma and a serum prolactin level of less than 200ng/mL (4000mU/L), consideration should be given to the possibility that a nonfunctioning pituitary adenoma (pseudo-prolactinoma) is present, the hyperprolactinemia resulting from deprivation of some lactotrophs of dopaminergic inhibition (3). However, a laboratory artifact may lead to a wrong differential diagnosis between macroprolactinomas and pseudoprolactinomas. When serum prolactin is evaluated by two-site immunometric assays, large amounts of prolactin saturate both the capture and the signal antibodies, impairing their binding, causing serum prolactin to be underestimated (the so-called “high-dose hook effect”). Therefore, patients bearing macroprolactinomas with extremely high serum prolactin levels (generally >1,000 ng/ml [>180,000 mU/L]) may present falsely low levels, e.g., 30-120 ng/ml (600-2,400 mU/L) range, causing the patient to be misdiagnosed as harboring a nonfunctioning pituitary adenoma. In order to avoid unnecessary surgery (treatment of choice for nonfunctioning tumors), prolactin assays with serum dilution are recommended in patients with macroadenomas who may harbor a prolactinomas (19). Recent assays do not present hook effect at PRL concentrations as high as 295,000 mIU/L (around 14,750 ng/mL) (20).
Another condition that interferes in the parallelism of serum PRL concentrations and prolactinoma dimensions is cystic prolactinomas, defined if 45% of lesion is the predominantly cystic sellar lesions (>50% of the volume being cystic) (21). In these lesions, serum prolactin levels are lower, but usually greater than 94 ng/mL. Differential diagnosis is important to determine the correct therapeutic intervention and includes Rathke’s cleft cysts, non-prolactin secreting cystic adenomas, craniopharyngiomas, and arachnoid cysts (22).
Another laboratory pitfall concerns the presence of high serum prolactin levels in subjects with few or no symptoms related to prolactin excess. Human prolactin circulates as monomeric prolactin and as larger forms, which are indistinguishable by routine assays. Monomeric prolactin is the most common form, but serum prolactin can be elevated due to the presence of aggregates with low biological activity, such as big-big prolactin, leading to so-called macroprolactinemia. The presence of molecular aggregates with low biological activity, macroprolactin, should be suspected when high serum prolactin levels are detected in patients without or with few signs and symptoms related to hyperprolactinemia. Precipitation with polyethylene glycol (PEG) is an excellent screening method. Chromatography confirms the presence of macroprolactin but is an expensive and time-consuming method; it is performed only when PEG precipitation results are inconclusive. Macroprolactinemia is a common finding, some reporting it as frequently as 8 to 42% of all cases; other centers find that it is extremely rare. Big-big prolactin biological activity is still controversial in the literature (23). Studies in vitro with rat Nb2 cell bioassays show either the presence or the absence of biological activity. A recent study using a human prolactin receptor-mediated assay compared with rat Nb2 cell assay showed that the activity displayed by macroprolactin toward the rat receptor may be inappropriate because it is not observed in the human prolactin receptor-mediated assay, consistent with the apparent absence of bioactivity in vivo (24). Most patients with macroprolactinemia do not manifest clinical features related to hyperprolactinemia, and do not need any treatment. Therefore, in order to avoid unnecessary medical or even surgical procedures, macroprolactin screening is important to consider when clinical features and serum prolactin assay results are not consonant with one another. Moreover, standardization of monomeric prolactin levels after PEG precipitation is crucial to evaluate conditions that could be associated with macroprolactinemia, as prolactinomas. Therefore, monomeric prolactin levels point to real hyperprolactinemia, even in the presence of macroprolactinemia (25).
Enlargement of the pituitary fossa on a skull X-ray may represent the expansion of the fossa by the macroadenoma, but care should be exercised to exclude the possibility of cisternal herniation (a partially empty fossa) as a cause for the enlargement. CT and MRI scans are useful and will also demonstrate any hypothalamic-pituitary pathologies, including solid and cystic lesions (26).
CHANGES IN THE BREAST DUE TO PROLACTIN
A woman with amenorrhea due to hyperprolactinemia does not develop the breast atrophy seen in postmenopausal women or women with amenorrhea who are gonadotropin-deficient or have primary ovarian failure. On examination, the breast and areola are well developed and the Montgomery tubercles are hyperplastic. If the breast is correctly examined, first by expressing it from the periphery towards the areola to empty milk ducts, followed by squeezing and lifting the areola (rather than the nipple itself) to empty the milk sinuses, galactorrhea can usually be found.
In patients with extremely high prolactin levels and hypogonadism, galactorrhea may not be found, as minimum estrogen levels are necessary for this physical sign to occur. In male patients with hyperprolactinemia, there is usually no gynecomastia, but milk may be expressed from an entirely normal-sized male breast. The incidence of galactorrhea in men with hyperprolactinemia is low, less than 30% (i.e., it is much less common than in women). Nevertheless, the presence of galactorrhea in a man with a pituitary mass is an important clinical clue to the presence of hyperprolactinemia and possible prolactinomas (3).
HYPERPROLACTINEMIC HYPOGONADISM
The pathogenesis of the hypogonadal state in hyperprolactinemia is poorly understood. Results from an animal model study suggest that hyperprolactinemia inhibits gonadotropin-releasing hormone (GnRH) pulsatility by reducing kisspeptin input, considered the major controlling point of reproduction (27). Moreover, kisspeptin administration restored hypothalamic-pituitary-ovarian in two hyperprolactinemic women (28).
In men, testosterone levels are usually low but can occasionally be normal, while in women, a hypoestrogenic state may occur, with loss of ovulation. The clinical features in hyperprolactinemic women, however, differ from those in the postmenopausal state since breast atrophy is absent and gonadotropin levels are not elevated.
Suppression of Gonadal Function in Hyperprolactinemia
In addition to GnRH inhibition, via kisspeptin, suppression of gonadotropin secretion through inhibition of positive estrogen feedback on luteinizing hormone (LH) secretion in women (29), an increase in adrenal androgen secretion (30), and blockade of the effects of gonadotropins at the gonadal level contribute to suppression of gonadal function in hyperprolactinemia (31,32) . Reduction in the normal LH pulsatility, essential for normal gonadal function, also occurs. Prolactin may interfere with LH and FSH action at the gonad, blocking progesterone synthesis, and may stimulate adrenal androgen secretion (29-32).
IMAGING OF THE PITUITARY
The anatomy of the pituitary is optimally assessed by contrast-enhanced MRI. MRI allows imaging of the optic chiasm, the cavernous sinuses, the pituitary (both the normal gland and tumors), and its stalk. In addition, aneurysms of the carotid are immediately obvious. Thus, MRI allows accurate measurement of the size of the pituitary and of any tumor and its relationship to the optic chiasm and cavernous sinuses. Cisternal herniation is also readily seen. If MRI is not available, CT scanning is also helpful but the resolution is less good and it is less satisfactory for delineating the relationship of the diaphragm sellae with the optic chiasm. There is little place for routine skull X-ray other than for delineating bony structures (26). For additional information see the chapter on the radiology of the pituitary.
TREATMENT OF HYPERPROLACTINEMIA
Therapeutic strategy must consider several aspects, such as the patient’s clinical presentation, the differences between microadenomas and macroadenomas concerning their natural history, the desire for pregnancy, and the patient’s treatment preference, if applicable. Medical treatment with dopamine agonist (DA) drugs is currently the gold standard approach both for microprolactinomas and macroprolactinomas. Pituitary surgery, usually by the transsphenoidal approach is generally reserved for prolactinomas resistant to DA drugs. For microadenomas, the results in the hands of most experienced surgeons are similar, with about 80% having serum prolactin normalization. However, approximately 25% develop recurrence of hyperprolactinemia by five years after surgery even with the most experienced transsphenoidal surgeons. Surgical results in macroprolactinomas are much poorer, mainly in big and/or invasive tumors. Radiotherapy for prolactinomas generally brings poor results, especially regarding normoprolactinemia restoration, and is currently reserved only for macroadenomas refractory both to medical and surgical treatment (3).
Dopamine Agonist Drug Therapy
The first DA ergot compound to be used in clinical practice was bromocriptine, a peptide ergot. It was introduced in the early 1970s in Europe and thus there is more than 40 years of experience of the use of such compounds in the treatment of hyperprolactinemia. Bromocriptine has the advantage of having a long duration of action compared to dopamine itself or oral compounds such a levo-dopa (33).
Bromocriptine has a similar mode of action to dopamine in stimulating dopamine receptors on the prolactin-secreting pituitary cells – D2 receptors. Stimulation of these receptors leads to inhibition of both prolactin secretion and synthesis. Figure 1 illustrates a successful case of prolactinoma treatment with bromocriptine. Subsequently a variety of other compounds have been developed which are useful additions. These include quinagolide and cabergoline (3).

Figure 1.
A 34-year-old patient with amenorrhea and hyperprolactinemia (PRL 1630 ng/ml) had a sella CT imaging depicting a pituitary mass on the left (A). Bromocriptine was introduced and after 30 days on 7.5 mg a day, PRL dropped to 32 ng/mL with menses restoration. A repeat CT demonstrated a decrease in tumor size (B).
Cabergoline has an extremely long biological half-life and thus, generally only needs to be administered either once or twice per week, with a weekly dose of 0.5 to 2.0 mg. Doses higher than 2 mg per week may be used in refractory cases. In addition to its long biological half-life, cabergoline is generally better tolerated than bromocriptine, increasing the patient’s adherence. Therefore, cabergoline is currently considered the first-choice drug for the treatment of prolactinomas, except for patients wishing pregnancy in the short-term (see below). In a study comparing bromocriptine (2.5 to 5.0 mg twice daily) to cabergoline (0.5 to 1.0 mg twice weekly) in 459 hyperprolactinemic women with amenorrhea, stable normoprolactinemia was achieved in 83% of patients on cabergoline and in 59% patients on bromocriptine. Ovulatory cycles or pregnancy occurred in 72% of cases on cabergoline and in 52% of cases on bromocriptine. Drug withdrawal due to adverse effects was reported in 3% of patients on cabergoline and in 12% of patients on bromocriptine. Regarding tumor size, a decrease in at least 50% was obtained in 64% of patients on bromocriptine and in 93% of patients on cabergoline (as demonstrated in Figure 2). This important beneficial effect of DA treatment can rapidly relieve mass effects symptoms such as visual impairment, without the need of surgical decompression (34).
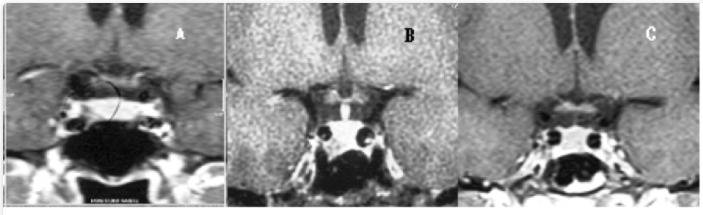
Figure 2.
A 32 yrs-old female patient with hyperprolactinemia (PRL 80 ng/mL), irregular menses and galactorrhea had a sellar MR showing a pituitary lesion of 0.8 cm on maximal diameter (A). After cabergoline introduction (0.5 mg/week), PRL levels normalized, paralleled with menses restoration and remission of galactorrhea. Tumoral dimensions reduction are shown: tumoral maximal diameter of 0.6 cm after two years of treatment (B) and no lesion visualization after seven years on cabergoline (C).
Surgical therapy of large prolactin-secreting pituitary tumors is unsatisfactory since it is capable of normalizing serum prolactin levels or gonadal function in fewer than 20% of patients, particularly those with high prolactin levels, so the problem should be solved by another therapeutic approach.
SIDE EFFECTS
Side effects of DA therapy usually occur at the start of treatment and frequently disappear with continued therapy. If treatment is started with full doses or increased too quickly, dizziness, nausea, and postural hypotension may occur. To avoid such effects, DA must always be taken during a meal. Administration should be started at night, with a snack, when the patient retires to bed. Doses can be gradually increased afterwards.
Cabergoline and pergolide were associated with a higher risk of cardiac valvopathy in patients with Parkinson´s disease. These DA also have an agonist effect on serotonin receptor 5HT2B, present in fibroblast of cardiac valves and chordae tendineae. Fibroblast’s proliferation occurs after this receptor activation, leading to valve insufficiency, especially of tricuspid and pulmonary valves. This proposed mechanism was already described in carcinoid syndrome. Nevertheless, mean cabergoline dose for Parkinson patients is 3 mg a day, much higher than the usual dose for hyperprolactinemia. Stiles et al in 2018 published a meta-analysis including 836 cabergoline-treated hyperprolactinemic patients and 1388 healthy controls from 13 published studies and there was an increase in tricuspid regurgitation of any degree (35). Although moderate and severe tricuspid regurgitation was heavily influenced by data from one center (36), data from this meta-analysis could not rule out an effect on cardiac valvular dysfunction of cabergoline used at “endocrine” dosages to treat hyperprolactinemia. British Society of Echocardiography, the British Heart Valve Society and the Society for Endocrinology recommend that a standard transthoracic echocardiogram should be performed before a patient starts DA therapy for hyperprolactinemia, repeating this exam at 5 years after starting cabergoline in patients taking a total weekly dose less than or equal to 2 mg, or annually if the dose is greater than 2 mg a week(37).
Quinagolide is the only non-ergot derived DA and is only available in Europe, while pergolide has now been withdrawn.
Recently, impulse control disorders (ICD) have been associated with DA treatment, due to the dopamine receptor type 3. There are some case reports of patients harboring prolactinomas who presented ICD during DA treatment, with different doses and length of time and an active approach to screen psychiatry disorders before and during DA treatment is recommended (38). In five series of cases, summing up 543 patients with prolactinoma, ICD frequency varied from 8 to 61%, with hypersexuality being the most common ICD and associated with male gender (39). DA withdrawal led to an improvement of psychiatric symptoms. Patients must be evaluated by psychiatry and DA treatment should be reevaluated on an individualized basis (40).
CAN A DOPAMINE AGONIST DRUG BE WITHDRAWN WITHOUT RECURRENCE?
One of the drawbacks of medical treatment of prolactinomas is the need for long-term therapy in the majority of the cases. As a matter of fact, treatment with bromocriptine and other DA drugs generally is considered as “symptomatic”, since DA discontinuation often leads to recurrence of hyperprolactinemia and to tumor regrowth in most patients at least after short-term use.
Nevertheless, remission and normoprolactinemia after DA withdrawal can occur, especially after long-term treatment. Concerning long-term therapy with bromocriptine, a retrospective study showed that 25.8% of 62 patients with microprolactinomas and 15.9% of 69 patients with macroprolactinomas treated with bromocriptine for a median time of 47 months had persistent normoprolactinemic after a median time of 44 months after drug withdrawal (41). Another study encompassed a large cohort of hyperprolactinemic patients on cabergoline. The drug was discontinued in patients who attained normoprolactinemia, with at least 50% of tumor reduction or disappearance on image, with at least 2 years of follow-up after cabergoline withdrawal. Serum prolactin remained normal in 76%, 70% and 64% of patients with “idiopathic” hyperprolactinemia, microprolactinomas, and macroprolactinomas, respectively (42). This great discrepancy between results with bromocriptine and cabergoline was not confirmed by a meta-analysis, including 743 patients from nineteen studies: the pooled proportion of patients with remission was 21%. Stratifying those results, remission was obtained in 32% for idiopathic hyperprolactinemia, 21% for micro, and 16% for macroprolactinomas. The probability of success was higher when DA treatment lasted at least two years. A trend of cabergoline superiority over bromocriptine was observed albeit with no statistical significance (43). Hu et al performed a meta-analysis about prolactinoma remission, including 637 patients (492 micro and 123 macroprolactinomas treated with CAB, and found the pooled proportion of patients with recurrence of 65% in a random effects model. Patients who received the lowest CAB dose and presented a significant reduction in tumor size before withdrawal were more likely to achieve the best success (44). More recently, a third meta-analysis included 1106 patients, 727 harboring microprolactinoma and 306 macroprolactinoma, treated with BRC or CAB. The overall success rate after DA withdrawal reached 36.6% (95% CI 29.4–44.2%). Better remission rate was related to CAB treatment, especially in patients with a duration of treatment longer than two years, to low-dose CAB maintenance, and to a significant reduction in tumor size before withdrawal. (45). A second attempt to DA withdrawal was also described, with lower rates of success (46).
Although the exact mechanism of prolactinomas remission is not completely understood, it could also be linked to the natural history of the disease. Periodic withdrawal of DA is recommended, especially in cases with normal serum PRL levels and tumor reduction. Prolactinoma remission is also described after pregnancy. It is suggested that estrogen-induced necrosis could lead to tumor size and PRL decrease after delivery (3).
PROLACTINOMAS RESISTANT TO DOPAMINE AGONISTS
About 15% of patients with prolactinomas are resistant to DA therapy. The main mechanism is reduction of D2R tumor expression (47,48), although D2R polymorphism (49,50) and filamin A (51) low expression could also be implied. If a patient has been responsive and then becomes unresponsive, a pituitary carcinoma should be ruled out. The approach to the resistant prolactinoma includes pituitary surgery, radiotherapy and drugs such as temozolomide (52) and pasireotide, which has been used in only a few reported cases (53). Pituitary surgery, usually by transsphenoidal approach, aims for complete tumor removal or at least a vast debulking, which may lead to serum prolactin normalization with DA reintroduction in partially resistant cases. Surgery is more effective in microadenomas and non-invasive macroadenomas. In a meta-analysis, a surgical remission rate of 74.7% in micro and 34% in macroadenomas was shown. Nevertheless, the recurrence rate was 18% and 23% in micro and macroadenomas, respectively, leading to an even lower long-term remission rate (54,55). Radiotherapy is indicated in aggressive cases not controlled by surgery or drugs (54,56). The alkylating agent temozolomide has efficacy in aggressive pituitary adenomas and carcinomas, mainly the prolactin secreting ones. Complete and partial response was obtained in 56% of 32 cases reviewed in the literature (52). Figure 3 illustrates sellar MR of a young man with an invasive/aggressive macroprolactinoma, resistant do dopamine agonist, multiple surgeries and radiotherapy.

Figure 3.
26 yrs-old man complaining of visual disturbance was submitted to a sellar MRI (A): sellar mass with 5 cm in the maximal diameter with supra, infra and left parasellar invasion. Serum PRL levels were above 1000 ng/mL. After one year on cabergoline, 0.5 mg/day, there was no tumor reduction and PRL levels were around 800 ng/mL. He was then submitted to transsphenoidal surgery and radiotherapy in another medical service. Sellar MRI (B) two months after the surgery showed a pituitary mass with 3.1 cm in the maximal diameter. Despite chiasmal decompression, visual dysfunction was not reversed and, during his follow-up, anterior pituitary function was lost. PRL levels on cabergoline, 1.5 mg/week, were 250 ng/ml. After two more years, sellar MRI depicted a lesion of 2.9 cm. in the maximal diameter (C). There was a progressive rise of PRL levels despite increased cabergoline doses (0.5 mg/d) and another sellar MRI (D), after two years, depicted a lesion of 3.1 cm in the maximal diameter. Another surgery was indicated.
Fertility in Women
The efficacy of hyperprolactinemia/prolactinoma´s treatment allow fertility and pregnancy in many women. There are, however, several important considerations that must be recognized by both the physician and patient. It includes tumor growth risk and possible teratogenic sequelae of fetal exposure to bromocriptine and other drugs (57).
There is little doubt that patients with pituitary tumors run a small, but significant, risk of expansion of the tumor during pregnancy. It is very difficult, however, to assess the absolute risk. With microadenomas, which did not undergo previous surgery or radiotherapy, the incidence seems to be 2.5%. In patients with macroadenomas not operated on or irradiated, the incidence is higher, 18.1% (58,59). However, the risk of complications may be lower in women previously operated or irradiated. This risk is unrelated to bromocriptine therapy prior to pregnancy but may occur when fertility is induced with other drugs, including exogenous gonadotropins and clomiphene, and even when no drug therapy has been employed in patients with pre-existing pituitary adenomas.
In practice, the problem of pregnancy is not great, since the vast majority of women who present with hyperprolactinemia only have microadenomas. To avoid major problems, it is extremely important that patients undergo careful endocrine, neuroradiologic, and neuro-ophthalmologic evaluation prior to treatment. If there is no suprasellar extension, and if the patient harbors only a microadenoma, then the risk of clinically significant swelling of the pituitary is extremely small; it is therefore suggested that the patient be evaluated clinically in every trimester throughout pregnancy, with no routine serum prolactin assessment. If the patient has a macroadenoma and suprasellar extension, transsphenoidal decompression can be considered, mainly for resistant cases. However, in those patients with a good response to DA in terms of prolactin normalization and tumor shrinkage within sellar boundaries, at least one year before pregnancy, the drug can be withdrawn and reintroduced if tumor re-growth is observed. If such an approach fails, pituitary surgery or premature delivery, if feasible, would be indicated. Additionally, in the case of tumor apoplexy, high-dose dexamethasone may improve clinical symptoms and also may reduce the chances of fetal respiratory distress if premature delivery is needed (2,59).
In recent years, pregnancies have been described in patients using other DA such as quinagolide and cabergoline. Although there is no evidence of increased frequency of abortions or malformations in cabergoline-induced pregnancies, the drug’s long action, which persists up to three weeks after its withdrawal, associated with fewer (albeit increasing) data when compared to bromocriptine (around 1000 versus over 6,000 pregnancies), limit the confidence that it can be used for patients who wish to conceive or its use during pregnancy. In the United States, bromocriptine is the only FDA approved drug for inducing pregnancy in hyperprolactinemic women. Cabergoline’s label does not recommend use during pregnancy. Nevertheless, experience of pregnancy induced by cabergoline is accumulating. Incidence of premature delivery, multiple births, and malformations is not different from women who used bromocriptine or from the general population (60). However, the issue of spontaneous abortion is still under debate (60,61). Quinagolide use was related to abortions and malformations (58).
Fertility in Men
Hyperprolactinemic men exhibit reduced quality of seminal fluid, including the total sperm count, the sperm kinetic index, and sperm nuclear DNA integrity. Cabergoline significantly increased those indexes, mainly after 12 months of treatment (62).
Interestingly enough, it was shown that clomiphene citrate administration can increase testosterone levels in men, even when serum prolactin levels were elevated. Fertility restoration is an additional advantage of this approach, compared to testosterone replacement (63).
Conclusion
Dopamine-agonist therapy for hyperprolactinemia leads to a reversal of the hyperprolactinemic hypogonadal state without risk of the development of pituitary insufficiency, thus allowing pregnancy in former infertile patients. Dopamine-agonist therapy is effective not only in patients with microadenomas but also in the majority of patients with large prolactin-secreting tumors in reducing tumor size. Moreover, emerging evidence points to the possibility of drug withdrawal after long-term treatment. Surgery, radiotherapy and antiblastic drugs, as temozolomide, should be reserved for resistant/aggressive cases.
REFERENCES
- 1.
- Daly AF, Beckers A. The Epidemiology of Pituitary Adenomas. Endocrinol Metab Clin North Am. 2020;49:347–355. [PubMed: 32741475]
- 2.
- Melmed S, Casanueva FF, Hoffman AR, Kleinberg DL, Montori VM, Schlechte JA, Wass JA, Society E. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:273–288. [PubMed: 21296991]
- 3.
- BRONSTEIN, M.D. Disorders of Prolactin Secretion and Prolactinomas. In: De, LJ G, JL J, eds. Endocrinology. Vol 1. Philadelphia: Elsevier Saunders; 2010:485-510.
- 4.
- Grattan DR. 60 YEARS OF NEUROENDOCRINOLOGY: The hypothalamo-prolactin axis. J Endocrinol. 2015;226:T101–122. [PMC free article: PMC4515538] [PubMed: 26101377]
- 5.
- Torre DL, Falorni A. Pharmacological causes of hyperprolactinemia. Ther Clin Risk Manag. 2007;3:929–951. [PMC free article: PMC2376090] [PubMed: 18473017]
- 6.
- ME M. Disorders of prolactin secretion. Endocrinol Metab Clin North Am. 2001;30:585–610. [PubMed: 11571932]
- 7.
- Jacobs S, Brown SA, Mason J, Wahby V, Kasl S, Ostfeld A. Psychological distress, depression and prolactin response in stressed persons. J Human Stress. 1986;12:113–118. [PubMed: 3559194]
- 8.
- Ferriani RA, Silva de Sá MF. Effect of venipuncture stress on plasma prolactin levels. Int J Gynaecol Obstet. 1985;23:459–462. [PubMed: 2868939]
- 9.
- Molitch ME. Drugs and prolactin. Pituitary. 2008;11:209–218. [PubMed: 18404390]
- 10.
- Kleinberg DL, Noel GL, Frantz AG. Galactorrhea: a study of 235 cases, including 48 with pituitary tumors. N Engl J Med. 1977;296:589–600. [PubMed: 840242]
- 11.
- Franks S, Murray MA, Jequier AM, Steele SJ, Nabarro JD, Jacobs HS. Incidence and significance of hyperprolactinaemia in women with amenorrhea. Clin Endocrinol (Oxf). 1975;4:597–607. [PubMed: 1104218]
- 12.
- Abe T, Matsumoto K, Kuwazawa J, Toyoda I, Sasaki K. Headache associated with pituitary adenomas. Headache. 1998;38:782–786. [PubMed: 11279904]
- 13.
- Mah PM, Webster J. Hyperprolactinemia: etiology, diagnosis, and management. Semin Reprod Med. 2002;20:365–374. [PubMed: 12536359]
- 14.
- Duskin-Bitan H, Shimon I. Prolactinomas in males: any differences? Pituitary. 2020;23:52–57. [PubMed: 31802331]
- 15.
- Ciccarelli A, Guerra E, De Rosa M, Milone F, Zarrilli S, Lombardi G, Colao A. PRL secreting adenomas in male patients. Pituitary. 2005;8:39–42. [PubMed: 16411067]
- 16.
- Arya VB, Aylwin SJB, Hulse T, Ajzensztejn M, Kalitsi J, Kalogirou N, Bodi I, Thomas N, Hampton T, Kapoor RR, Buchanan CR. Prolactinoma in childhood and adolescence-Tumour size at presentation predicts management strategy: Single centre series and a systematic review and meta-analysis. Clin Endocrinol (Oxf). 2021;94:413–423. [PubMed: 33340135]
- 17.
- Barry S, Korbonits M. Update on the Genetics of Pituitary Tumors. Endocrinol Metab Clin North Am. 2020;49:433–452. [PubMed: 32741481]
- 18.
- Maiter D, Delgrange E. Therapy of endocrine disease: the challenges in managing giant prolactinomas. Eur J Endocrinol. 2014;170:R213–227. [PubMed: 24536090]
- 19.
- Frieze TW, Mong DP, Koops MK. "Hook effect" in prolactinomas: case report and review of literature. Endocr Pract. 2002;8:296–303. [PubMed: 12173917]
- 20.
- Fahie-Wilson M, Bieglmayer C, Kratzsch J, Nusbaumer C, Roth HJ, Zaninotto M, Plebani M, Hubbuch A, Schneider E. Roche Elecsys Prolactin II assay: reactivity with macroprolactin compared with eight commercial assays for prolactin and determination of monomeric prolactin by precipitation with polyethylene glycol. Clin Lab. 2007;53:485–492. [PubMed: 17821956]
- 21.
- Faje A, Chunharojrith P, Nency J, Biller BM, Swearingen B, Klibanski A. Dopamine Agonists Can Reduce Cystic Prolactinomas. J Clin Endocrinol Metab. 2016;101:3709–3715. [PubMed: 27459530]
- 22.
- Nakhleh A, Shehadeh N, Hochberg I, Zloczower M, Zolotov S, Taher R, Daoud Naccache D. Management of cystic prolactinomas: a review. Pituitary. 2018;21:425–430. [PubMed: 29654440]
- 23.
- Hattori N. Macroprolactinemia: a new cause of hyperprolactinemia. J Pharmacol Sci. 2003;92:171–177. [PubMed: 12890882]
- 24.
- Glezer A, Soares CR, Vieira JG, Giannella-Neto D, Ribela MT, Goffin V, Bronstein MD. Human macroprolactin displays low biological activity via its homologous receptor in a new sensitive bioassay. J Clin Endocrinol Metab. 2006;91:1048–1055. [PubMed: 16384849]
- 25.
- Bronstein MD. Editorial: is macroprolactinemia just a diagnostic pitfall? Endocrine. 2012;41:169–170. [PubMed: 22286912]
- 26.
- Rennert J, Doerfler A. Imaging of sellar and parasellar lesions. Clin Neurol Neurosurg. 2007;109:111–124. [PubMed: 17126479]
- 27.
- Sonigo C, Bouilly J, Carré N, Tolle V, Caraty A, Tello J, Simony-Conesa FJ, Millar R, Young J, Binart N. Hyperprolactinemia-induced ovarian acyclicity is reversed by kisspeptin administration. J Clin Invest. 2012;122:3791–3795. [PMC free article: PMC3461919] [PubMed: 23006326]
- 28.
- Millar RP, Sonigo C, Anderson RA, George J, Maione L, Brailly-Tabard S, Chanson P, Binart N, Young J. Hypothalamic-Pituitary-Ovarian Axis Reactivation by Kisspeptin-10 in Hyperprolactinemic Women With Chronic Amenorrhea. J Endocr Soc. 2017;1:1362–1371. [PMC free article: PMC5686678] [PubMed: 29264460]
- 29.
- Glass MR SR, Butt WR, Edwards RL, London DR. An abnormality of osterogen feedback in amenorrhoea-galactorrhoea. BMJ. 1975;3:274–275. [PMC free article: PMC1674237] [PubMed: 1170923]
- 30.
- Feitus FA. GBaMM. Stat5-mediated regulation of the human type II 3beta-hydrosteroid dehydrogenase/delat-5-delta4isomerase gene activation by prolactin. Molecular Endocrinology. 1999;13:1084–1093. [PubMed: 10406460]
- 31.
- Demura R, Ono M, Demura H, Shizume K, Oouchi H. Prolactin directly inhibits basal as well as gonadotropin-stimulated secretion of progesterone and 17 beta-estradiol in the human ovary. J Clin Endocrinol Metab. 1982;54:1246–1250. [PubMed: 7076799]
- 32.
- Dorrington JH, Gore-Langton RE. Antigonadal action of prolactin: further studies on the mechanism of inhibition of follicle-stimulating hormone-induced aromatase activity in rat granulosa cell cultures. Endocrinology. 1982;110:1701–1707. [PubMed: 6804209]
- 33.
- Thorner MO, McNeilly AS, Hagan C, Besser GM. Long-term treatment of galactorrhoea and hypogonadism with bromocriptine. Br Med J. 1974;2:419–422. [PMC free article: PMC1610452] [PubMed: 4600593]
- 34.
- Webster J, Piscitelli G, Polli A, Ferrari CI, Ismail I, Scanlon MF. A comparison of cabergoline and bromocriptine in the treatment of hyperprolactinemic amenorrhea. Cabergoline Comparative Study Group. N Engl J Med. 1994;331:904–909. [PubMed: 7915824]
- 35.
- Stiles CE, Tetteh-Wayoe ET, Bestwick J, Steeds RP, Drake WM. A meta-analysis of the prevalence of cardiac valvulopathy in hyperprolactinemic patients treated with Cabergoline. J Clin Endocrinol Metab. 2018 [PubMed: 30215804]
- 36.
- Colao A, Galderisi M, Di Sarno A, Pardo M, Gaccione M, D'Andrea M, Guerra E, Pivonello R, Lerro G, Lombardi G. Increased prevalence of tricuspid regurgitation in patients with prolactinomas chronically treated with cabergoline. J Clin Endocrinol Metab. 2008;93:3777–3784. [PubMed: 18682513]
- 37.
- Steeds R, Stiles C, Sharma V, Chambers J, Lloyd G, Drake W. Echocardiography and monitoring patients receiving dopamine agonist therapy for hyperprolactinaemia: A joint position statement of the British Society of Echocardiography, the British Heart Valve Society and the Society for Endocrinology. Clin Endocrinol (Oxf). 2019;90:662–669. [PubMed: 30818417]
- 38.
- Noronha S, Stokes V, Karavitaki N, Grossman A. Treating prolactinomas with dopamine agonists: always worth the gamble? Endocrine. 2016;51:205–210. [PubMed: 26336835]
- 39.
- De Sousa SMC, Baranoff J, Rushworth RL, Butler J, Sorbello J, Vorster J, Thompson T, McCormack AI, Inder WJ, Torpy DJ. Impulse Control Disorders in Dopamine Agonist-Treated Hyperprolactinemia: Prevalence and Risk Factors. J Clin Endocrinol Metab. 2020:105. [PubMed: 31580439]
- 40.
- Ioachimescu AG, Fleseriu M, Hoffman AR, Vaughan Iii TB, Katznelson L. Psychological effects of dopamine agonist treatment in patients with hyperprolactinemia and prolactin-secreting adenomas. Eur J Endocrinol. 2019;180:31–40. [PubMed: 30400048]
- 41.
- Passos VQ, Souza JJ, Musolino NR, Bronstein MD. Long-term follow-up of prolactinomas: normoprolactinemia after bromocriptine withdrawal. J Clin Endocrinol Metab. 2002;87:3578–3582. [PubMed: 12161478]
- 42.
- Colao A, Di Sarno A, Cappabianca P, Di Somma C, Pivonello R, Lombardi G. Withdrawal of long-term cabergoline therapy for tumoral and nontumoral hyperprolactinemia. N Engl J Med. 2003;349:2023–2033. [PubMed: 14627787]
- 43.
- Dekkers OM, Lagro J, Burman P, Jørgensen JO, Romijn JA, Pereira AM. Recurrence of hyperprolactinemia after withdrawal of dopamine agonists: systematic review and meta-analysis. J Clin Endocrinol Metab. 2010;95:43–51. [PubMed: 19880787]
- 44.
- Hu J, Zheng X, Zhang W, Yang H. Current drug withdrawal strategy in prolactinoma patients treated with cabergoline: a systematic review and meta-analysis. Pituitary. 2015;18:745–751. [PubMed: 25500765]
- 45.
- Xia MY, Lou XH, Lin SJ, Wu ZB. Optimal timing of dopamine agonist withdrawal in patients with hyperprolactinemia: a systematic review and meta-analysis. Endocrine. 2018;59:50–61. [PubMed: 29043560]
- 46.
- Vilar L, Albuquerque JL, Gadelha PS, Rangel Filho F, Siqueira AM, da Fonseca MM, Viana KF, Gomes BS, Lyra R. Second Attempt of Cabergoline Withdrawal in Patients with Prolactinomas after a Failed First Attempt: Is it Worthwhile? Front Endocrinol (Lausanne). 2015;6:11. [PMC free article: PMC4316769] [PubMed: 25699020]
- 47.
- Passos VQ, Fortes MA, Giannella-Neto D, Bronstein MD. Genes differentially expressed in prolactinomas responsive and resistant to dopamine agonists. Neuroendocrinology. 2009;89:163–170. [PubMed: 18791324]
- 48.
- Molitch ME. Pharmacologic resistance in prolactinoma patients. Pituitary. 2005;8:43–52. [PubMed: 16411068]
- 49.
- Filopanti M, Lania AG, Spada A. Pharmacogenetics of D2 dopamine receptor gene in prolactin-secreting pituitary adenomas. Expert Opin Drug Metab Toxicol. 2010;6:43–53. [PubMed: 19929252]
- 50.
- Bueno C, Trarbach EB, Bronstein MD, Glezer A. Cabergoline and prolactinomas: lack of association between DRD2 polymorphisms and response to treatment. Pituitary. 2017;20:295–300. [PubMed: 27848079]
- 51.
- Peverelli E, Mantovani G, Vitali E, Elli FM, Olgiati L, Ferrero S, Laws ER, Della Mina P, Villa A, Beck-Peccoz P, Spada A, Lania AG. Filamin-A is essential for dopamine d2 receptor expression and signaling in tumorous lactotrophs. J Clin Endocrinol Metab. 2012;97:967–977. [PubMed: 22259062]
- 52.
- Chen C, Yin S, Zhang S, Wang M, Hu Y, Zhou P, Jiang S. Treatment of aggressive prolactinoma with temozolomide: A case report and review of literature up to date. Medicine (Baltimore). 2017;96:e8733. [PMC free article: PMC5708963] [PubMed: 29381964]
- 53.
- Sari R, Altinoz MA, Ozlu EBK, Sav A, Danyeli AE, Baskan O, Er O, Elmaci I. Treatment Strategies for Dopamine Agonist-Resistant and Aggressive Prolactinomas: A Comprehensive Analysis of the Literature. Horm Metab Res. 2021;53:413–424. [PubMed: 34282593]
- 54.
- Gillam MP, Molitch ME, Lombardi G, Colao A. Advances in the treatment of prolactinomas. Endocr Rev. 2006;27:485–534. [PubMed: 16705142]
- 55.
- Primeau V, Raftopoulos C, Maiter D. Outcomes of transsphenoidal surgery in prolactinomas: improvement of hormonal control in dopamine agonist-resistant patients. Eur J Endocrinol. 2012;166:779–786. [PubMed: 22301915]
- 56.
- Ježková J, Hána V, Kosák M, Kršek M, Liščák R, Vymazal J, Pecen L, Marek J. Role of gamma knife radiosurgery in the treatment of prolactinomas. Pituitary. 2019;22:411–421. [PubMed: 31222579]
- 57.
- Glezer A, Bronstein MD. Prolactinomas: how to handle prior to and during pregnancy? Minerva Endocrinol. 2017 [PubMed: 29265784]
- 58.
- Huang W, Molitch ME. Pituitary Tumors in Pregnancy. Endocrinol Metab Clin North Am. 2019;48:569–581. [PubMed: 31345524]
- 59.
- Glezer A, Bronstein MD. Prolactinomas in pregnancy: considerations before conception and during pregnancy. Pituitary. 2019 [PubMed: 31792668]
- 60.
- Sant' Anna BG. Musolino NRC, Gadelha MR, Marques C, Castro M, Elias PCL, Vilar L, Lyra R, Martins MRA, Quidute ARP, Abucham J, Nazato D, Garmes HM, Fontana MLC, Boguszewski CL, Bueno CB, Czepielewski MA, Portes ES, Nunes-Nogueira VS, Ribeiro-Oliveira A, Francisco RPV, Bronstein MD, Glezer A. A Brazilian multicentre study evaluating pregnancies induced by cabergoline in patients harboring prolactinomas. Pituitary. 2020;23:120–128. [PubMed: 31728906]
- 61.
- Hurault-Delarue C, Montastruc JL, Beau AB, Lacroix I, Damase-Michel C. Pregnancy outcome in women exposed to dopamine agonists during pregnancy: a pharmacoepidemiology study in EFEMERIS database. Arch Gynecol Obstet. 2014;290:263–270. [PubMed: 24664257]
- 62.
- De Rosa M, Zarrilli S, Di Sarno A, Milano N, Gaccione M, Boggia B, Lombardi G, Colao A. Hyperprolactinemia in men: clinical and biochemical features and response to treatment. Endocrine. 2003;20:75–82. [PubMed: 12668871]
- 63.
- Ribeiro RS, Abucham J. Recovery of persistent hypogonadism by clomiphene in males with prolactinomas under dopamine agonist treatment. Eur J Endocrinol. 2009;161:163–169. [PubMed: 19359408]
- Review Prolactinoma Management.[Endotext. 2000]Review Prolactinoma Management.Molitch ME, Drummond J, Korbonits M. Endotext. 2000
- Review Hyperprolactinemia and prolactinoma.[Handb Clin Neurol. 2014]Review Hyperprolactinemia and prolactinoma.Romijn JA. Handb Clin Neurol. 2014; 124:185-95.
- Review Prolactinomas.[Endocrinol Metab Clin North Am...]Review Prolactinomas.Glezer A, Bronstein MD. Endocrinol Metab Clin North Am. 2015 Mar; 44(1):71-8. Epub 2014 Nov 6.
- Review Hyperprolactinemia/Prolactinomas in the Postmenopausal Period: Challenges in Diagnosis and Management.[Neuroendocrinology. 2019]Review Hyperprolactinemia/Prolactinomas in the Postmenopausal Period: Challenges in Diagnosis and Management.Pekić S, Medic Stojanoska M, Popovic V. Neuroendocrinology. 2019; 109(1):28-33. Epub 2018 Oct 22.
- Review Hyperprolactinemia: pathophysiology and management.[Treat Endocrinol. 2003]Review Hyperprolactinemia: pathophysiology and management.Verhelst J, Abs R. Treat Endocrinol. 2003; 2(1):23-32.
- Hyperprolactinemia - EndotextHyperprolactinemia - Endotext
Your browsing activity is empty.
Activity recording is turned off.
See more...
