NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-.
ABSTRACT
The coexistence of diabetes and hypertension is known to have a multiplicative effect on adverse clinical outcomes with respect to both microvascular and macrovascular disease. Effective management of diabetes should therefore include a multifaceted approach combining optimal control of blood pressure and lipids with appropriate glycemic control. The pathophysiology of hypertension in diabetes involves maladaptive changes in the autonomic nervous system, vascular endothelial dysfunction, enhanced activation of the renin-angiotensin-aldosterone system, immune function alterations, and harmful environmental factors. Multiple high-quality randomized controlled trials have shown improvement in morbidity with lowering of elevated blood pressure in people with diabetes. Attention must be paid to individual risk factors and co-morbidities with a goal of less than 130/80 mm Hg in most patients with diabetes who are at higher risk of cardiovascular disease (CVD) than those without diabetes. Good glycemic control, optimizing weight, and promotion of exercise as well as lessening harmful environment factors such as air pollution exposure are integral components of the approach to blood pressure control in these patients. Judicious selection of therapy and consideration of relevant side-effect profiles is paramount. The potential for both beneficial and detrimental drug interactions must be kept in mind and drug combinations should be chosen after due deliberation. Angiotensin converting enzyme inhibitors and angiotensin receptor blockers remain preferred agents for initiation of antihypertensive therapy, while combined use of these agents is not recommended due to poor renal outcomes. With the advent of newer antidiabetic agents such as SGLT inhibitors and GLP1 receptor agonists, consideration should be given to their antihypertensive, renal, and cardiovascular disease lowering properties when initiating therapy for glycemic control. For complete coverage of all related areas of Endocrinology, please visit our on-line FREE web-text, WWW.ENDOTEXT.ORG.
INTRODUCTION
Statistics from the Centers for Disease Control and Prevention (CDC) and National Health and Nutritional Examination Survey (NHANES) database show that the incidence of Type 2 diabetes mellitus (T2DM) has risen steeply in the last few decades. It is estimated that diabetes affects 34.2 million people in US 10.5% of US population. 73.6% of individuals with diabetes aged 18 years or more have hypertension. The coexistence of hypertension and diabetes in a large population of patients is not coincidental; individuals with T2DM often display a constellation of metabolic derangements termed the cardiometabolic or cardiorenal metabolic syndrome (1). This syndrome comprises a cluster of CVD risk factors including T2DM, hypertension, dyslipidemia, central obesity, and chronic kidney disease. The coexistence of hypertension and diabetes in these individuals substantially increases the risk for cardiovascular disease (CVD), cerebrovascular accident (CVA), retinopathy, and nephropathy (2). The rising prevalence of obesity and sedentary lifestyles in the US are the major driver of both diabetes and hypertension and the resulting health care costs are a serious public health concern. Increasingly, the role of environmental factors such as food deserts and environmental pollution in the promotion of diabetes, hypertension, and CVD is being appreciated. These harmful environmental factors especially affect minorities and other disadvantaged populations.
The increasing prevalence of T2DM in the general population has expectedly been paralleled by a rise in microvascular and macrovascular complications. Despite major advances in healthcare delivery, diabetes mellitus continues to be the leading cause of blindness, end stage renal disease (ESRD), and non-traumatic lower limb amputations in the US as well as the seventh leading cause of death as of 2017 (1). While optimal glycemic control remains paramount in the prevention of microvascular complications (retinopathy, nephropathy, and neuropathy), concurrent cardiometabolic derangements such as hypertension and dyslipidemia play a pivotal role in the initiation and progression of macrovascular disease (ischemic heart disease, stroke, and peripheral vascular disease) (3). Effective management of diabetes should therefore include a multifaceted approach combining optimal control of blood pressure and lipids with appropriate glycemic control (4). This chapter will focus on the management of hypertension in patients with diabetes.
PATHOPHYSIOLOGY OF HYPERTENSION IN DIABETES
The pathophysiology of hypertension in diabetes can be traced to maladaptive changes and complex interactions between the autonomic nervous system, a maladaptive immune system, enhanced activation of the renin-angiotensin-aldosterone system (RAAS) as well as adverse environmental factors. The factors listed below play a major role in the pathogenesis of hypertension and have been targeted for therapeutic interventions (2,5).
Sedentary Lifestyle, Excessive Caloric Intake and Insulin Resistance
Sedentary lifestyle and excessive caloric intake can lead to increased adiposity which has been associated with increased risk of worsening insulin resistance. Insulin resistance has been linked in turn to an increased vascular oxidative stress, inflammation, and endothelial dysfunction characterized by diminished vascular nitric oxide bioactivity, all of which promote vascular stiffness resulting in a persistent elevation of blood pressure and the promotion of CVD (6,7).
Elevated Intravascular Volume
Intravascular volume is strongly influenced by total body sodium content. Sodium is the major extracellular cation in human beings, and possesses osmotic activity which helps determine effective arterial blood volume. A mismatch between sodium intake and sodium loss can result in a positive sodium balance. The ensuing increase in intravascular sodium concentration stimulates an influx of water along the osmotic gradient, thus raising intravascular volume. Elevated intravascular volume consequently increases venous return to the heart boosting cardiac output in accordance with the Frank Starling Law, and this process eventually leads to elevated arterial pressure (8). There is also increasing evidence that increased activation of sodium inward transport in endothelial cells contribute to increased vascular stiffness and elevated blood pressure in states of obesity and insulin resistance as exists in most patients with T2DM (7).
Increased blood pressure (BP) from intravascular volume expansion is typically corrected by a rise in glomerular filtration and compensatory urinary salt excretion. This phenomenon of increased salt excretion in a state of elevated blood pressure has been termed pressure natriuresis. Unfortunately, this mechanism alone cannot correct persistently elevated blood pressure, principally because of secondary changes within the kidney microvasculature and maladaptive changes within the glomerular apparatus itself that lower glomerular filtration and stimulate sodium reabsorption. These changes are most apparent in overt chronic kidney disease (CKD) and end stage renal disease (ESRD), both of which are characterized by concurrent volume overload and sustained hypertension. Hypertension in CKD/ESRD is often difficult to control and requires restoration of normal vascular volume, which can be achieved by means of diuretics or dialysis (8,9).
Premature Vascular Aging
Changes in vessel lumen elasticity affect the ease with which blood can flow through arteries. Minimal reductions in lumen diameter can lead to exponentially increased resistance to blood flow. Patients with hypertension often demonstrate structural and functional changes that adversely alter the lumen of small arteries and arterioles. The vascular remodeling, low grade inflammation, vascular fibrosis and stiffening seen with hypertension in individuals with diabetes can arise as a response to elevated BP. Patients with diabetes thus manifest accelerated premature vascular aging characterized by impaired endothelial mediated relaxation, enhanced vascular smooth muscle contraction and resistance as well as vascular stiffness (7). These maladaptive vascular changes both contribute to the development of hypertension and accelerate the harmful effects of hypertension on vessel integrity (8,10).
Autonomic Nervous System Dysregulation
The autonomic nervous system is an important determinant of BP. Both the sympathetic and the parasympathetic systems are involved in the regulation of BP. Increased sympathetic activity leads to an increase in heart rate, force of contraction of ventricles, peripheral vascular resistance, and fluid retention. These physiological actions combine to promote BP elevation. Decreased parasympathetic outflow also results in increased heart rate and relative sympathetic hyperactivity thus contributing to an elevation in BP. Dysregulation of these pathways is seen with central obesity, insulin resistance, and sleep apnea. Hypertension associated with these disorders is often accompanied by increased sympathetic activity, an activated RAAS, and resistant hypertension. Furthermore, activation of the sympathetic nervous system also promotes insulin resistance and risk of T2DM. The autonomic dysfunction seen with T2DM can also contribute to these changes and thus worsen hypertension. The relevance of these pathways in the pathogenesis of hypertension and diabetes is demonstrated by the observation that interruption of the central sympathetic outflow by renal denervation is associated with improved insulin sensitivity, better glycemic control, and reductions in BP (2,8).
Renin Angiotensin Aldosterone System (RAAS)
The RAAS pathway plays a central role in maintaining normal BP. RAAS activation is closely linked to the pathogenesis of hypertension via the cardiovascular and renal effects of elevations, particularly of plasma aldosterone level. Angiotensin II is a potent vasoconstrictor and acts directly to increase vascular smooth muscle tone. Angiotensin II also stimulates secretion of aldosterone, which promotes sodium and water retention, leading to elevated blood pressure through volume expansion. Obesity is associated with elevated plasma aldosterone levels, even in the absence of elevation of angiotensin levels. This elevation is thought to be related, in part, to secretion of aldosterone releasing factors from the expanded adipose tissue (7). Understanding the physiology of RAAS is essential as it is the principal target for angiotensin converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), and increasingly mineralocorticoid receptor antagonists, which are cornerstones of BP management in individuals with diabetes (8).
Renin is a proteolytic enzyme secreted by the juxtaglomerular cells in the kidney. Renin cleaves circulating angiotensinogen to angiotensin I. ACE within the lung capillaries then converts angiotensin I into angiotensin II. Production and release of renin is tightly regulated by many interdependent factors such as renal perfusion pressure, sodium chloride concentration in distal tubule of nephron, and stimulation of renin secreting cells by the sympathetic nervous system.
Physiologic activation of RAAS is seen with renal hypoperfusion due to hypovolemia. Release of renin from the juxtaglomerular apparatus results in a cascade of events, culminating in increased production of angiotensin II. Angiotensin II then raises blood pressure through direct vasoconstriction and by stimulation of aldosterone secretion leading to sodium and water retention and restoration of intravascular volume (8).
Obesity and insulin resistance are associated with inappropriate activation of RAAS and the sympathetic nervous system. Increased adiposity has been linked with high levels of plasma aldosterone suggesting that RAAS may be chronically overactive in obesity (7). Angiotensin II and aldosterone have been shown to inhibit insulin metabolic signaling in classical insulin sensitive tissues and this likely plays a role in impaired endothelial-mediated vascular relaxation and the development of hypertension. Angiotensin II and aldosterone may also promote insulin resistance through non-genomic mechanisms such as activation of serine kinases and increased serine phosphorylation of insulin receptor substrate 1, reduced phosphatidylinositol 3-kinase engagement and protein kinase B stimulation, diminished insulin metabolic signaling, and impaired nitric oxide mediated vascular relaxation. (11,12). Increasingly it is recognized that elevated aldosterone in conjunction with hyperinsulinemia, as often exists in obesity and insulin resistance, promote vascular stiffness and associated increases in hypertension and CVD (7).
Renal Dysfunction
Renal dysfunction appears to share a reciprocal relationship with hypertension in diabetic individuals. While hypertension itself is recognized as a risk factor for chronic kidney disease in the setting of diabetes, it is important to note that diabetic nephropathy also contributes to development of hypertension. This reciprocal relationship is most obvious in type 1 diabetics without pre-existing hypertension. Longitudinal studies have shown that microalbuminuria precedes hypertension in this population, and the prevalence of hypertension rises progressively with worsening kidney disease, approaching 90% in type 1 diabetics with end stage renal disease. Proposed mechanisms include volume expansion secondary to increased renal sodium reabsorption, peripheral vasoconstriction arising from endothelial dysfunction, dysregulated activation of the RAAS, upregulation of endothelin1, and downregulation of nitric oxide (13).
Role of Innate and Adaptive Immunity
There is emerging evidence that innate immunity and acquired immunity are involved in angiotensin II and aldosterone-induced hypertension and vascular disease (6). Animal studies suggest that intact T cell function is required for full expression of these adverse effects and that T cells and macrophages mediate the oxidative injury associated with these effects. On the other hand, the protective properties of T regulatory cells in animal models suggests a potential therapeutic role for these cells, although at this time such interventions are limited to the research setting.
Environmental and Socioeconomic Factors
There are marked disparities in hypertension between White and Black Americans. This disparity is increasing despite higher levels of awareness and treatment of their hypertension amongst Black Americans as compared to their White counterparts (14). This disparity has been magnified with the recent Covid-19 pandemic with disproportionate levels of morbidity and mortality amongst communities of color. Some have suggested that this disparity in Covid outcomes are related to similar environmental, economic, and social inequities as those that promote hypertension, obesity, and diabetes (15).
Foods that are traditionally considered healthy and promoted as components of the DASH diet (16) are often unavailable to people living in these communities due to either lack of access or reasons of affordability. Instead, they become consumers of cheap high salt and high caloric foods, a process that naturally leads to obesity and hypertension (15). Furthermore, lack of safe outdoor spaces discourages exercise and targeted advertising increases poor health decisions such as smoking. These effects are further reinforced by a study of Black and White Americans living in the same environmental setting (long term integrated neighborhoods). In the Exploring Health Disparities in Integrated Communities-South Western Baltimore (EHDIC-SWB) study it was found that although the odds ratio for hypertension was higher in Blacks in the sample population, it was decreased by roughly 30% as compared to NHANES data. The authors concluded that social and environmental exposure explained a substantial proportion of race differences in persons with hypertension and diabetes (17).
BLOOD PRESSURE MEASUREMENT AND MONITORING
Accurate measurement of BP is key for both diagnosis and effective management of hypertension. BP measurement is most often conducted in the medical office, where it can be performed either through the auscultatory technique of listening to Korotkoff sounds or the oscillometric technique employed in automated devices. Use of oscillometric devices has largely replaced the auscultatory method primarily for reasons of convenience and concerns over inter-observer variability with manual measurements. However, it is important to remember that even automated measurements can be erroneous if certain precautions are not taken. Measurements should be made in the seated position after the patient has rested for 3-5 minutes, and preferably with an empty bladder. No exertion, physical exercise, eating, smoking or exposure to stress for at least 30 minutes before BP reading. Three readings within a period of 2 weeks will be ideal. The device used should be calibrated regularly to ensure reliable readings. Improper cuff size is a common source of erroneous readings. It is recommended that cuff bladder length be equal to the patient’s arm circumference measured at the midpoint of acromion and olecranon process and the width be equal to about one-half of the arm circumference. Use of a cuff that is too small is more common because of the rising incidence of obesity and results in overestimation of blood pressure. Despite using all these precautions, there can be significant variability between individual readings and American Heart Association (AHA) recommends obtaining at least two readings during each clinic visit (18).
Ambulatory blood pressure monitoring (ABPM) is a fully-automated non-invasive modality that involves placement of a blood pressure cuff on the non-dominant arm with measurements every 15 to 30 minutes over the course of a 24-hour period. Compared to in-office blood pressure measurement, ABPM has higher prognostic value for target organ damage and cardiovascular outcomes (19). The primary advantage of ABPM lies in its comprehensive nature unlike office monitoring that relies on single measurements. This format permits detection of distinct blood pressure patterns such as sustained, white-coat, masked, and nocturnal hypertension, as well as non-dipping or reverse-dipping patterns that cannot be detected with office measurements alone. These patterns are associated with varying cardiovascular outcomes and must therefore be managed quite differently. White-coat hypertension denotes a situation wherein office measurements are in the hypertensive range but ABPM readings are consistently normal. This phenomenon is attributed to the effect of an observer at the time of measurement; it is associated with minimal cardiovascular risk and is not an indication for antihypertensive therapy. It should be noted however that individuals with white-coat are at elevated risk for developing sustained hypertension and should therefore be monitored periodically. On the other hand, masked hypertension refers to a situation where office measurements are normal but ABPM shows readings in the hypertensive range. This phenomenon is associated with increased cardiovascular risk comparable to that seen with sustained hypertension. Importantly, masked hypertension is more common in diabetic individuals and obese patients. It is assumed that these patients benefit from aggressive antihypertensive therapy although no randomized controlled trials have been performed to confirm such expectations (20).
Blood pressure normally displays a physiologic circadian rhythm, dipping by more than 10% during the night relative to daytime readings. Patients in whom blood pressure drops by less than 10% are said to have a non-dipping pattern. This non-dipping pattern is more prevalent in diabetic individuals and has been associated with cardiovascular autonomic neuropathy. Its contribution to progression of diabetic complications is more controversial. Hyperglycemia itself can influence the normal nocturnal dip through its effect on circulating plasma volume, blood flow distribution and renal hemodynamics (21).
BLOOD PRESSURE TARGETS IN PATIENTS WITH DIABETES
The importance of rigorous blood pressure control in prevention of diabetes-related morbidity cannot be overemphasized. This holds true for macrovascular as well as microvascular complications and is supported by a mounting body of evidence. The United Kingdom Prospective Diabetes Study (UKPDS), showed 44, 32, and 34 percent reductions in risks for stroke, diabetes related deaths, and retinopathy respectively with blood pressure reduction (target blood pressure <150/85 mm Hg). A linear relationship between systolic blood pressure reduction and adverse outcomes was seen in readings as low as 120 mm Hg (22,23).
The Hypertension On Target (HOT) trial showed a reduction in CVD with lowering of diastolic blood pressure. Interestingly however, this benefit was only seen in patients with diabetes, suggesting the need for establishing a different and perhaps more aggressive blood pressure target in this population subgroup (24).
The Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) trial was the first study designed specifically to address blood pressure control in subjects with diabetes. The results were impressive, showing significant reduction in microvascular events, cardiovascular deaths, and all-cause mortality with aggressive reduction in both systolic and diastolic blood pressure (mean achieved blood pressure of 134/74 mm Hg versus 140/76 mm Hg) (25).
Major medical societies including the American Diabetes Association (ADA) recommend a target blood pressure of less than 130/80 mm Hg for patients with diabetes. The first trial to seek justification for this recommendation was the Normotensive Appropriate Blood Pressure Control in Diabetes (ABCD) trial. Although no specific blood pressure target was pursued, the mean attained blood pressure of 128/75 mm Hg in the intensive treatment group, was under the systolic target of 130 mm Hg. Over a follow up of five years, no significant difference was seen in creatinine clearance (primary outcome) or cardiovascular events when compared to the placebo group (mean blood pressure 137/81). The intensive treatment group did manifest significant reductions in progression of retinopathy, albuminuria, and absolute risk of stroke (26,27).
The notion of a systolic blood pressure goal of less than 130 mm Hg was challenged by the ACCORD blood pressure trial. This large randomized control trial compared a systolic target of <120 mm Hg (intensive therapy) to a systolic target of <140 mm Hg (standard therapy). With more than 4500 patients and a mean follow up of 4.7 years, no significant difference was seen between the two groups in terms of combined CVD outcomes (heart attack, stroke, and cardiovascular death). Importantly, similar to the results of the ABCD trial, a 40 percent reduction was seen in stroke risk (28). This study was confounded by factors that do not allow for recommendations based on the outcomes of this study.
The most recent large-scale randomized control trial that examined a lower systolic blood pressure goal was the Systolic Blood Pressure Intervention Trial (SPRINT). This trial compared the benefit of treatment to a systolic blood pressure target of less than 120 mm Hg (intensive-treatment group) with the treatment to a target of less than 140 mm Hg (standard-treatment group). At 1 year, the intensive-treatment group had a mean systolic blood pressure of 121.4 mm Hg versus the standard-treatment group with a mean systolic blood pressure of 136.2 mm Hg. The results showed significantly lower rates of fatal and nonfatal cardiovascular events and death from any cause in the intensive-treatment group. Serious adverse events possibly or definitely related to the intervention were statistically more frequent in the intensive-treatment group with a hazard ratio of 1.88 (P<0.001). This study included 9361 participants with a median follow up of 3.26 years; however, patients with diabetes were excluded. The SPRINT trial therefore supports a lower goal but cannot be applied directly to the diabetic population because of its study design (29).
Some experts have suggested that the ACCORD trial was underpowered to show a significant difference for the primary endpoint. A recently pooled analysis merged the data from the SPRINT and ACCORD trials and looked at the same primary endpoint that was used in SPRINT. The primary endpoint differed from the ACCORD trial in that it included unstable angina and acute decompensated heart failure in addition to myocardial infarction, stroke and CVD death. The final analysis showed a significant favorable effect for the intensive treatment group in both patients with and without diabetes. This suggests that there may not be a differential beneficial effect of intensive blood pressure lowering (i.e., to less than 130/80 mm Hg) in patients with T2DM (30). It must also be noted that both the SPRINT and ACCORD trials involved BP measurements under strictly controlled conditions that would be expected to yield lower readings compared to conventional clinic settings. This observation raises questions about whether more liberal targets might be used in real world settings to achieve comparable cardiovascular benefits.
In conclusion, multiple high-quality randomized controlled trials have shown improvement in morbidity with correction of elevated BP in people with diabetes. Patients with T2DM appear to be particularly susceptible to the deleterious effects of hypertension in initiation and progression of CVD. In the treatment of hypertension in patients with diabetes attention must be paid to individual risk factors, co-morbidities, and patient preferences when considering lower treatment targets. A lower blood pressure target, for instance, might be more appropriate for a young person who would likely benefit from a reduction in stroke risk and reduced progression of retinopathy without experiencing unwanted side effects of hypotension, syncope, and hyperkalemia that are encountered more commonly in the older population and those with multiple co-morbidities.
Key outcome studies and results are summarized in table 1.
Table 1.
Key Outcome Studies and Results
| Outcome Study | Intervention | Results |
|---|---|---|
| United Kingdom Prospective Diabetes Study (UKPDS) | Blood pressure reduction (< 150/85 mmHg) | 44% risk reduction in stroke 32% risk reduction in diabetes related deaths 34% risk of retinopathy |
| Hypertension On Target Trial (HOT) | Lower diastolic blood pressure | Reduction in CVD |
| Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation trial (ADVANCE) | Reduced systolic and diastolic blood pressure (134/74mmHg vs 140/76mmHg) | Reduction in microvascular events, cardiovascular deaths, and all-cause mortality |
| Normotensive Appropriate Blood Pressure Control in Diabetes Trial (ABCD) | Intensive blood pressure control (128/75mmHg vs 137/81mmHg) | Reduction in progression of retinopathy, albuminuria, and absolute risk of stroke No difference in creatinine clearance or cardiovascular events |
| ACCORD Study Group Trial | Intensive blood pressure control (Systolic target <120mmHg vs <140mmHg) | 40% risk reduction for stroke No difference for combined CVD outcomes (heart attack, stroke, and cardiovascular death) |
| Systolic Blood Pressure Intervention Trial (SPRINT) | Intensive blood pressure control (Systolic target <120mmHg vs <140mmHg) Achieved mean blood pressure 121.4mmHg vs 136.2mmHg | Reduced rates of fatal and nonfatal cardiovascular evens and death Increased adverse events related to intensive group |
TREATMENT OF HYPERTENSION
Treatment of hypertension in patients with diabetes is challenging as these patients can develop resistant hypertension. Moreover, individuals with diabetes have a higher incidence of cardiac and renal comorbidities that can lower tolerance to aggressive antihypertensive therapy. An effective treatment regimen must therefore address all aspects of the complex metabolic derangements seen in this population group (4).
This section will focus chiefly on the treatment of hypertension in association with T2DM. We will examine treatment strategies by drug class, critically reviewing the advantages and disadvantages of each. The importance of accurately measuring BP and using proper techniques needs to be emphasized, especially considering the lifelong implications for the patient. Once a diagnosis of hypertension has been established in a patient with diabetes, it is imperative that aggressive treatment be initiated in a timely manner. It is also worth noting that with some exceptions, the degree of blood pressure reduction achieved is of greater importance than the class of antihypertensive employed.
The various classes of antihypertensive drug that are commonly employed in diabetic individuals are summarized in table 2. The overall approach to hypertension in a diabetic patient is outlined in figure 1.
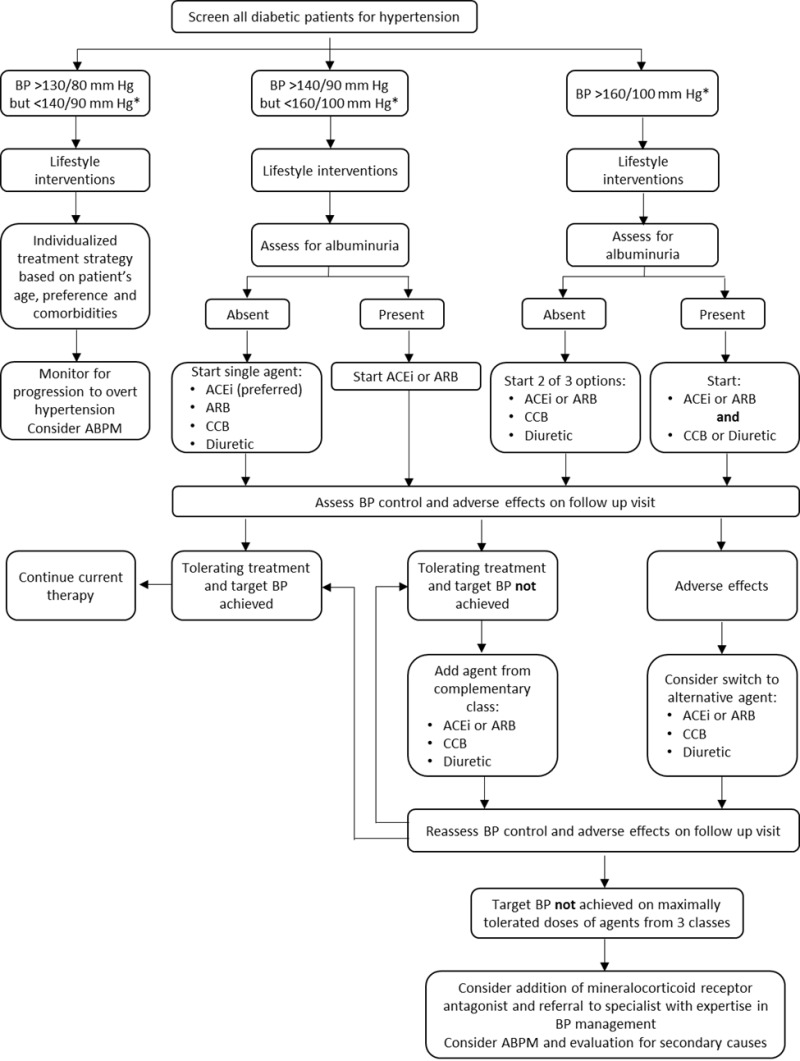
Figure 1.
Approach to hypertension in the diabetic patient
Table 2.
Summary of Antihypertensive Agents with Emphasis on Patients with Diabetes
| Class with representative examples | Preferred use | Notable side effects | Contraindications | Effect on insulin resistance and/or glycemic control |
|---|---|---|---|---|
| ACE inhibitor* Lisinopril Ramipril Benazepril | Diabetics Also preferred in: Proteinuric CKD HFrEF Established CAD | Hyperkalemia Acute kidney injury (up to 25% rise in creatinine is expected) Angioedema Cough Teratogenicity | Pregnancy Avoid concomitant use with aliskiren or ARB | Improved |
| ARB Telmisartan Valsartan Losartan Irbesartan Candesartan | Diabetics who are intolerant of ACE inhibitors Also preferred in: Proteinuric CKD HFrEF Established CAD | Hyperkalemia Acute kidney injury (up to 25% rise in creatinine is expected) Teratogenicity | Pregnancy Avoid concomitant use with aliskiren or ACE inhibitor | Improved |
| Direct renin inhibitor** Aliskiren | Diabetics with proteinuric CKD who are intolerant of both ACE inhibitors and ARBs | Hyperkalemia Acute kidney injury Teratogenicity | Pregnancy Avoid concomitant use with ACE inhibitor or ARB | Unknown |
| Thiazide-like diuretic Chlorthalidone Indapamide HCTZ | Hypervolemic or edematous patients Must be used before diagnosing “resistant hypertension” | Photosensitivity Hyponatremia Hypokalemia Hypomagnesemia Hyperuricemia Orthostatic hypotension | Pregnancy Use with caution in cirrhotic patients (risk of hyponatremia) Ineffective in advanced CKD-GFR<30 | Worsened with HCTZ Indapamide has positive effect |
| Dihydropyridine calcium channel blocker* Nicardipine Amlodipine | Patients who are already on preferred agents but not at target blood pressure | Peripheral edema | None but should not be initiated until other preferred agents have been started | Neutral |
| Beta adrenergic blocker Carvedilol Nebivolol Metoprolol | Preferred in: History of myocardial infarction HFrEF | Orthostatic hypotension Acute decompensation of heart failure Bronchospasm Hypoglycemia unawareness Depression Impotence | Avoid in active bronchospasm, vasospastic disorders Avoid if pheochromocytoma suspected (until adequate alpha blockade) Use with caution in PVD | Worsened with non-vasodilating agents like metoprolol and not with Carvedilol and nebivolol |
| Mineralocorticoid receptor blocker Spironolactone Eplerenone Finerenone | Preferred in: HFpEF and HFrEF Resistant hypertension Primary aldosteronism | Hyperkalemia Gynecomastia (with spironolactone) | Avoid in pregnancy Caution if using with ACE, ARB or renin inhibitors | Improved with spironolactone, unknown with other agents |
Preferred agents within each class are bolded. Preference is based on available evidence from randomized control trials.
- *
All agents in this class are considered equivalent
- **
Only agent currently approved in this class
Abbreviations: ACE: Angiotensin converting enzyme; ARB: Angiotensin receptor blocker; CAD: Coronary artery disease; CKD: Chronic kidney disease; HCTZ: Hydrochlorothiazide; HFpEF: Heart failure with preserved ejection fraction; HFrEF: Heart failure with reduced ejection fraction; PVD: Peripheral vascular disease. GFR: Glomerular Filtration rate
Lifestyle Modification
Lifestyle modification is a very important and often overlooked aspect of treatment of diabetes and hypertension. Changes to lifestyle that appear to have health benefits include:
- Reducing salt intake to less than 1.5 g/day
- Increasing consumption of fruits and vegetables (8-10 servings per day)
- Increasing consumption of low- fat dairy products (2-3 servings per day)
- Increasing activity levels/ engaging in regular aerobic physical activity (e.g., brisk walking 30 min/day)
- Losing excess weight
- Avoiding excessive alcohol consumption (less than 2 drinks (30 ml ethanol)/day for men and less than 1 drink (15ml of ehanol)/day for women)
Lifestyle modification may be used as a sole treatment modality in patients with BP <140/90, but ideally should be combined with pharmacotherapy in patients with systolic blood pressure (SBP) ≥ 140 and/or diastolic blood pressure (DBP) ≥ 90 mm Hg. It is generally agreed that lifestyle modification has modest antihypertensive effects, yielding an effective blood pressure reduction of 5-10 mm Hg. Nevertheless, ancillary benefits of improved cardiovascular fitness, reduced adiposity, and the possibility of future reduction in medication doses make such interventions an indispensable part of the management of these patients.
Angiotensin Converting Enzyme Inhibitors
ACE inhibitors inhibit the angiotensin converting enzyme and thus prevent conversion of angiotensin 1 to angiotensin II. This along with other mechanisms leads to decreased peripheral resistance and lowering of BP. ACE inhibitors selectively dilate the efferent renal arterioles and therefore lower intraglomerular pressure. This hemodynamic effect is reno-protective in patients with diabetic kidney disease. An acute rise in serum creatinine may occur at the onset of ACE inhibitor therapy. Elevation of serum creatinine by up to 30% above baseline is acceptable and does not mandate stopping therapy but does underscore the need for careful monitoring. The beneficial effects of ACE inhibitors on renal and cardiac function are widely recognized (31,32,33) and these agents are prescribed almost reflexively as initial antihypertensive treatment in patients with concomitant diabetes and hypertension (34). However, it must be noted that the primary advantage of ACE inhibitors over other classes of antihypertensive agents, lies in their proven ability to slow the progression of proteinuria.
ACE inhibitors possess a favorable side effect profile and are well-tolerated in general. Use of these agents is not associated with adverse alterations in lipid profile, glucose levels, and uric acid levels, such as those seen with other antihypertensive agents. As noted above creatinine elevation is frequently observed and should not require cessation of therapy unless excessive. On the other hand, dry persistent cough, another common side effect, is a reasonable cause for discontinuation of therapy. Patients with long standing diabetes, diabetic nephropathy, and hyporeninemic hypoaldosteronism/ type 4 renal tubular acidosis can develop hyperkalemia with these drugs. Angioedema – a severe hypersensitivity reaction more commonly observed in the African American population, is also associated with ACE inhibitor use and the drug should be permanently discontinued in such patients. Further, ACE inhibitors have teratogenic potential by interfering with fetal kidney development and caution must be exercised while using ACE inhibitors in females of child bearing age (33).
Due to their potential benefits and favorable risk benefit profile, ACE inhibitors have been established as the benchmark by which newer classes of antihypertensive agents are judged, especially in patients with diabetes and diabetic kidney disease.
Angiotensin Receptor Blockers (ARBs)
ARBs exert similar salutary effects as ACE inhibitors, by displacing angiotensin II from its receptor. The main advantage of ARBs over ACE inhibitors is the lower incidence of cough and angioedema with their use. The ONTARGET trial compared the ARB telmisartan to the ACE inhibitor Ramipril and the combined use of these drugs. This trial established general non-inferiority of telmisartan compared to ramipril with regards to BP control as judged by outcomes such as cardiovascular deaths, myocardial infarction, stroke, and hospitalization for heart failure. Additionally, the telmisartan arm had substantially lower rates of cough and angioedema. Data from the ONTARGET trial also showed that although both telmisartan and ramipril offered equivalent renal protection, the combined use of these two drugs led to inferior renal outcomes (35). A combination of ACE inhibitors and ARBs is therefore not recommended at this time. As with ACE inhibitors, hyperkalemia remains a potential adverse effect. The risk of hyperkalemia can be attenuated by combining these agents with other medications like thiazide or loop diuretics which promote urinary potassium loss. ARBs used to cost substantially more than ACE inhibitors but the advent of generic ARB’s have addressed this concern. Today ARBs are a popular choice for treatment of hypertension, and for prevention of renal complications in patients with diabetes, and are the preferred treatment in patients who develop a cough with ACE inhibitors (34).
Diuretics
Diuretics transiently decrease blood pressure by boosting renal sodium excretion and consequently lowering plasma volume. Overtime, these changes in volume status revert back to normal, but the antihypertensive effect persists due to a decrease in peripheral vascular resistance. Hydrochlorothiazide (HCTZ) and related sulfonamide compounds (chlorthalidone) are effective for blood pressure management in patients with mild to moderate hypertension and eGFR >50. In patients with eGFR <30, loop diuretics or a combination of loop diuretics and thiazides are more efficacious (34).
Data from the Swedish Trial in Old Patients with Hypertension-2 (STOP Hypertension-2) trial demonstrated that diuretics were as efficacious as ACE inhibitors or calcium channel blockers (CCBs) in lowering BP and reducing cardiovascular mortality in patients with diabetes (36).
Use of diuretics is associated with metabolic derangements like hypokalemia, hyperglycemia, and hyperuricemia. Once again, the risk of hypokalemia associated with diuretic use can be mitigated by combining a diuretic with medications, like an ACE inhibitor, ARB, potassium-sparing diuretic, or aldosterone antagonist (37). Patients with T2DM and concomitant hypertension also demonstrate impaired nocturnal BP dipping compared to patients without diabetes. Chlorthalidone, with its longer duration of action and higher potency might be a better choice to treat hypertension in this subgroup of patients (38).
Calcium Channel Blockers (CCBs)
CCBs are sub-classified as Dihydropyridines (DHPs) (amlodipine, felodipine, isradipine, nicardipine, nifedipine) and non-DHPs (NDHPs) (verapamil, diltiazem). DHPs exert their antihypertensive activity through peripheral vasodilatation, without significantly affecting cardiac conduction and contractility. NDHPs also have a modest antihypertensive effect, but they affect cardiac automaticity and conduction, and hence are primarily used for management of arrhythmias (34).
The strongest evidence for CCB use over other classes of antihypertensive drugs comes from the Avoiding Cardiovascular Events through Combination Therapy in Patients Living with Systolic Hypertension (ACCOMPLISH) trial. This trial was designed to compare benazepril plus amlodipine to benazepril plus hydrochlorothiazide in subjects with hypertension and a high risk of cardiovascular events, and showed fewer cardiovascular events in the CCB/ACE combination arm when compared to the ACE/Diuretic combination arm (39). These results are not in line with those of ALLHAT trial which found that ACE inhibitors, CCBs and alpha-blockers were not superior to thiazide diuretics for either BP control or improvement of cardiovascular or renal outcomes (40). Regardless of these conflicting results, the available evidence positions calcium channel blockers in line with ACE/ARBs and thiazides for treatment of hypertension in patients at high risk for cardiovascular events. CCB’s are well tolerated by most patients. Common side effects include headache, peripheral edema, and flushing (41).
Adrenergic Receptor Antagonist
The adrenergic receptor antagonists have been sub-classified into three categories: beta-blockers, alpha-blockers, and combined alpha and beta-blockers. Alpha-beta blockers like carvedilol and labetalol produce greater reductions in BP compared to pure beta blockers (34).
Beta-blockers have gained popularity due to mortality benefits in patients with heart failure and in patients who have sustained a myocardial infarction. Despite lack of robust evidence, beta- blockers are widely used for primary prevention of myocardial infarction as well. Use of beta-blockers can be associated with precipitation of bronchospasm, worsening peripheral arterial disease, sexual dysfunction, and worsening of glycemic control. Of particular concern is the decreased perception of hypoglycemia symptoms in patients with diabetes (42).
Beta-blockers are also known to alter insulin resistance and lipid metabolism – properties that are especially relevant in diabetic individuals. However, these effects vary across individual drugs and are more often seen with older non-vasodilatory beta-blockers such as atenolol, metoprolol and propranolol. For instance, a randomized control trial comparing metoprolol and carvedilol in patients with T2DM demonstrated that metoprolol was associated with worsened glycemic control compared to carvedilol at doses titrated to achieve comparable BP control (43). The same study also revealed that carvedilol had beneficial impacts on lipid profile with lowering of total cholesterol, triglycerides and non-HDL cholesterol. In contrast, metoprolol use was associated with increased need for lipid lowering therapy with statins (44). Similarly, labetalol and nebivolol, highly selective beta-1-blockers with nitric oxide dependent vasodilatory properties have been shown to improve insulin resistance (45). Unopposed activation of the alpha-adrenergic system has been proposed as a putative mechanism (46). Nebivolol also decreases cellular stiffness and stimulates endothelial cell growth causing improved endothelial function (47).
Mineralocorticoid Receptor Antagonists
Steroidal mineralocorticoid receptor (MR) antagonists (spironolactone and eplerenone) and new non-steroidal antagonists such as finerenone are particularly efficacious in those with resistant hypertension, which is more common in persons with obesity and diabetes (7). They also lower mortality in patients with heart failure by blocking the deleterious effects of aldosterone on cardiac remodeling. Addition of finerenone to patients receiving ACE inhibitors or ARB reduced urinary albumin excretion compared to placebo. The FIDELIO-DKD trial also showed improved cardiovascular outcomes and reduced progression of kidney disease with finerenone (48).
Hyperkalemia is a common side effect of steroidal MR antagonists, and monitoring for hyperkalemia is of particular importance, as MR antagonists are often added to an ACE inhibitor or an ARB. This is less of a problem with the newer non-steroidal MR antagonists (49). Gynecomastia and menstrual irregularities are other potential adverse effects seen with spironolactone. Eplerenone is a more selective aldosterone antagonist and it seldom causes anti-androgenic effects. It is likely that the newer non-steroidal MR antagonist will negate many of these concerns, and they will likely be increasingly used for treatment of hypertension in patients with diabetes.
Direct Renin Inhibitors
Aliskiren, a first in class direct renin inhibitor was approved by FDA in 2007. It is an effective antihypertensive agent and provides end-organ protection, but its exact place in the hypertension treatment algorithm remains uncertain. Aliskiren improves left ventricular hypertrophy, and shows synergism when used in combination with ARB. Its side effect profile is similar to ARBs and monitoring of potassium levels is recommended (34). The Aliskiren Trial in Type 2 Diabetes Using Cardiovascular and Renal Disease Endpoints (ALTITUDE) trial was a randomized control trial evaluating the efficacy of Aliskiren in combination with ACE inhibitors or ARBs in patients with T2DM. It was prematurely halted because of increased cardiovascular events and safety concerns. Additionally, there was more hyperkalemia and hypotension with the combination (50). It is possible that these adverse events were related to use of combination therapy analogous to the ONTARGET trial. At this time, Aliskiren should not be used in combination with ACE inhibitors or ARBs for management of hypertension in patients with T2DM. Aliskiren may be used for its antiproteinuric effect in patients who are intolerant of both ACE inhibitors and ARBs.
DIABETES MEDICATIONS WITH ANTIHYPERTENSIVE EFFECTS
Several anti-diabetic medications possess modest antihypertensive properties. These should be kept in mind especially in patients concurrently receiving antihypertensive drugs who may experience hypotensive symptoms if caution is not exercised. On the other hand, several of these drugs provide cardiovascular protection, likely in part from their antihypertensive effects that make them attractive options for patients at increased cardiovascular risk. Anti-diabetic medications with these properties include thiazolidinediones, dipeptidyl diphosphatase (DPP-4) inhibitors, glucagon-like peptide 1 (GLP-1) receptor agonists, and sodium glucose cotransporter 2 (SGLT 2) inhibitors. Of these classes, GLP-1 receptor agonists appear to exert the largest effect on blood pressure (51).
In a metanalysis of 16 randomized control trials comparing the GLP-1 agonists exenatide and liraglutide to placebo as well as other antihyperglycemic agents, BP reduction was seen. Against placebo, exenatide lowered systolic BP by approximately 6 mm Hg. Similarly, a mean reduction of about 5 mm Hg in systolic BP was seen with liraglutide versus placebo (52). A randomized control trial studying the hemodynamic effects of dulaglutide also showed a reduction in systolic BP regardless of baseline readings (53). The other classes of anti-hyperglycemic medications have shown reductions in systolic BP of less than 5 mm Hg (51).
Due to their sodium and volume lowering properties, the SGLT2 inhibitors were looked at early on for effects on BP. In phase 2 and 3 trials, canagliflozin and dapagliflozin showed modest reductions in BP, just under 4 mm Hg (54,55). A recent meta-analysis confirmed this finding across all other major SGLT2 inhibitors currently on the market with a mean reduction of 3.6/1.7 mm Hg in blood pressure as compared to placebo. This reduction is comparable to that seen with low dose hydrochlorothiazide (56). The exact mechanism of BP reduction is not completely understood but is postulated to be mediated by osmotic diuresis, natriuresis, and weight loss (57,58). Importantly, these agents have been shown to have CVD and renal disease reducing properties in patients with diabetes (54,59,60).
IMPACT OF COMORBIDITIES ON CHOICE OF ANTIHYPERTENSIVE REGIMEN
Despite advances in diagnosis and management, a significant proportion of diabetic individuals develop microvascular and macrovascular complications throughout their lifetime. Indeed, many patients present with advanced complications at diagnosis. These comorbidities must be considered when choosing an antihypertensive regimen because of ancillary benefits and potential for harm. Proteinuria and chronic kidney disease are most responsive to ACE inhibitors and ARBs and these agents are considered standard of care for such patients. On the other hand, beta-blockers have demonstrated benefit in the settings of established coronary artery disease and heart failure with reduced ejection fraction (HFrEF) but have no proven mortality benefit in their absence. Their use should therefore be restricted to the appropriate settings. Beta-blockers may exacerbate peripheral arterial disease due to reflex vasoconstriction and are best avoided in such patients. Beta-blockers should also be avoided in patients with a history of brittle diabetes and frequent hypoglycemia because of their ability to mask symptoms of hypoglycemia and thus contribute to hypoglycemia unawareness. Mineralocorticoid antagonists have shown proven benefits in HFrEF and should be included in antihypertensive regimens for diabetics with heart failure.
RESISTANT HYPERTENSION
Resistant hypertension is defined as BP greater than 140/90 mm Hg despite a therapeutic strategy that includes appropriate lifestyle modifications along with a diuretic and two other antihypertensive drugs from different classes, administered at optimal doses. It poses a special therapeutic challenge for endocrinologists. It is important to keep in mind that a number of other conditions need to be excluded before diagnosing resistant hypertension. Medication non-adherence must always be ruled out and barriers such as cost and side effects should be addressed. White coat hypertension can be remarkably resistant to therapy or alternatively be associated with intolerable side effects at home, leading to medication non-adherence and can be assessed by means of ABPM. Finally, secondary causes of hypertension should be looked for. The list of causes for secondary hypertension is extensive and includes such diverse disorders as renal artery stenosis, hyperaldosteronism, obstructive sleep apnea, and illicit drug use. Of special interest to the consulting endocrinologist are the various endocrine disorders that manifest with hypertension including primary hyperaldosteronism, pheochromocytomas and paragangliomas, Cushing’s syndrome, and hypo- and hyperthyroidism. Many of these disorders are characterized by distinct clinical presentations, and an exhaustive and expensive evaluation should be discouraged in the absence of supportive signs and symptoms. Obstructive sleep apnea deserves special mention because of its close association with diabetes and obesity and must always be considered in patients with resistant hypertension. Once diagnosed, secondary hypertension is often amenable to specific therapies with immediate improvement in BP.
After confirming a diagnosis of resistant hypertension and excluding possible secondary causes, pharmacological therapy with addition of mineralocorticoid receptor antagonists is typically the most effective intervention. These agents are effective in patients with T2DM when added to existing treatment with an ACE inhibitor or ARB, diuretic and calcium channel blocker. Mineralocorticoid receptor antagonists also reduce proteinuria and have additional cardiovascular benefits as noted above. However, adding a mineralocorticoid receptor antagonist to a regimen that already includes an ACE inhibitor or ARB increases the risk for hyperkalemia. Therefore, these patients need regular monitoring of serum creatinine and potassium.
SPECIAL CONSIDERATIONS IN TYPE 1 DIABETES
Patients with type 1 diabetes currently make up about 5% to 8% of the total diabetes population in the US (1). In contrast to patients with T2DM, patients with type 1 diabetes typically develop renal disease before developing hypertension. Longitudinal studies of type 1 diabetics consistently show development of proteinuria prior to onset of hypertension (13). However, once hypertension has developed, it accelerates the course of microvascular and macrovascular disease similar to patients with T2DM. Unfortunately, there is limited data on type 1 diabetics. A randomized trial has demonstrated that an ACE inhibitor protects against deterioration in renal function in insulin-dependent diabetic nephropathy and is significantly more effective than blood-pressure control alone (69). Therefore, guidelines for antihypertensive therapy in these patients are extrapolated from patients with T2DM, such as a preference for therapy with an ACE inhibitor or ARB. Furthermore, as tight glycemic control with insulin is the cornerstone of management of these patients, beta-blockers should be avoided because of their propensity to promote hypoglycemia and their ability to mask symptoms of hypoglycemia (61).
Perhaps the most distinctive aspect of hypertension in type 1 diabetes relates to the role of glycemic control in its prevention. Data from the Diabetes Control and Complications Trial (DCCT) and the Epidemiology of Diabetes Intervention and Complications (EDIC) trials showed that intensive therapy reduced incident hypertension by 24% over a 15 year follow up period (62). Interestingly the reduction in incident hypertension was not seen while the subjects were actually on intensive control but only appeared years later, suggesting that the connection between hyperglycemia and hypertension is not direct but rather is mediated through chronic complications of diabetes such as diabetic nephropathy.
COVID-19 AND ANTIHYPERTENSIVE THERAPY IN INDIVIDUALS WITH DIABETES
The ongoing novel SARS-CoV-2 coronavirus (COVID-19) pandemic has disproportionately affected individuals with multiple medical comorbidities. For instance, a large observational study from China showed that up to 23.7% of patients with severe infection had hypertension and 16.2% had diabetes compared to just 13.4% and 5.7% respectively, of patients with non-severe infection (63). The higher incidence of adverse outcomes seen with COVID-19 in patients with hypertension and diabetes is now believed to be related not only to the direct immunosuppressive effects of these comorbidities but also to common underlying socioeconomic themes such as lack of access to quality healthcare and healthy foods (64).
Further research on COVID-19 infection in this subset of patients led to questions on the role of ACE inhibitors and ARBs in its pathogenesis. Specifically, the observation that the novel coronavirus binds to human cells via the angiotensin converting enzyme 2 raised concerns that medications like ACE inhibitors and ARBs that increase levels of this enzyme might accelerate infection with the novel coronavirus. However, at this time there are no clinical data to support this hypothesis and the European Society of Cardiology Council on Hypertension, the American College of Cardiology (ACC)/ American Heart Association (AHA)/ Heart Failure Society of America (HFSA) and the American Society of Hypertension have all released policy statements strongly recommending that patients continue treatment with their usual antihypertensive regimen. Therefore, at this time, recognizing the multiple benefits obtained with these classes of medications in patients with diabetes or hypertension, it is not advisable to discontinue therapy simply because of COVID-19 infection (65).
RECENT GUIDELINES
The high blood pressure clinical practice guidelines released by the ACC/AHA Task Force in 2017 redefined hypertension as a blood pressure greater than 130/80 mm Hg and eliminated the category of pre-hypertension altogether. By lowering the threshold for diagnosis, this new definition immediately reclassifies a large proportion of individuals with diabetes as hypertensive, and consequently raises the incidence and prevalence of hypertension in the diabetic population. These guidelines recommend that pharmacologic therapy be initiated in patients with diabetes who have a blood pressure of greater than 130/80 mm Hg as it is assumed that they have an increased risk of cardiovascular disease. In the general population it is recommended that the 10-year atherosclerotic cardiovascular disease (ASCVD) risk be calculated. Pharmacotherapy should be initiated in those with an ASCVD risk of greater than ten percent when the blood pressure is greater than 130/80 mm Hg while the remainder can be treated with lifestyle modification alone (66).
The position statement on cardiovascular risk management in diabetes released by the American Diabetes Association (ADA) in 2021 retains the traditional cut off of 140/90 mm Hg for diagnosis of hypertension among individuals with diabetes. Just like the ACC/AHA guidelines, the ADA guidelines incorporate the ASCVD risk calculator in their treatment algorithm. The ADA guidelines differ however, in that the score is used to determine the target blood pressure. Thus, individuals with diabetes who have a score below fifteen percent have a target blood pressure of less than 140/90 mm Hg while individuals with a score greater than fifteen percent should aim for less than 130/80 mm Hg if such a goal can be safely achieved. This approached is based on observations from the SPRINT and other trials that the absolute benefit from BP reduction correlated with absolute baseline cardiovascular risk. These guidelines also emphasize the importance of individualized treatment targets and considering patient preferences and provider judgement when setting blood pressure goals (67).
Both the ACC/AHA guidelines and the ADA guidelines recommend pharmacologic therapy with two drugs belonging to different classes in patients with stage 2 hypertension, defined as a blood pressure greater than 160/100 mm Hg. This recommendation is based on evidence from multiple trials showing that combination therapy is safe and more efficacious than monotherapy in achieving blood pressure control. Combination therapy also leads to faster lowering of blood pressure and accelerates achievement of target levels, minimizing target organ damage in patients with stage 2 hypertension. The ADA guidelines also support use of single pill fixed dose combinations to maximize patient adherence. However, it should be noted that single pill combinations are often difficult to titrate, leading to suboptimal dosing of one component because of intolerance to maximal dosing of the other. This is especially relevant for ACE-inhibitors, ARBs and beta-blockers that show dose dependent benefits and should always be up titrated to maximally tolerated doses.
SUMMARY
Adequate treatment of hypertension in patients with diabetes is critical for prevention of end-organ damage and limiting the massive socioeconomic burden imposed by these disorders.
However, despite an abundance of evidence supporting tight control of blood pressure in diabetic individuals, it is sobering to note that BP targets are not met in the majority. Indeed, a larger retrospective registry-based study showed that as recently as 2018 only 48% of adults with diabetes were able to achieve a blood pressure of less than 130/80 mm Hg (68). Barriers to achieving good control include poor access to quality healthcare, lack of awareness among patients and providers, and concerns about side effects of tight control especially among older and frail individuals.
Judicious selection of therapy and consideration of relevant side-effect profiles is paramount. The potential for both beneficial and detrimental drug interactions should be kept in mind and drug combinations should be chosen after due deliberation. ACE inhibitors and ARBs continue to enjoy a special place in the management of hypertension in patients with diabetes and remain the preferred agents in this population subgroup. Combined use of these agents, however, is not recommended due to poor renal outcomes and hyperkalemia. The ancillary antihypertensive effects of antidiabetic medications should also be considered when designing an optimal regimen.
Goal blood pressure in patients with diabetes remains a subject of active discussion. This is reflected in the divergent recommendations offered by major organizations as noted above. While the evidence for lowering of blood pressure to a target of 140/90 mm Hg is unequivocal, the benefits of further intensification of therapy are less clear and must be balanced against the risk of adverse events such as falls, electrolyte abnormalities, and renal failure. Moreover, BP measurement protocols applied in trial settings can yield lower readings than comparable measurements in real world clinic settings, raising questions of whether such tight control is truly needed. A nuanced approach based on cardiovascular risk factors, comorbidities and patient preferences is encouraged.
REFERENCES
- 1.
- Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2020.
- 2.
- Oparil S, Zaman MA, Calhoun DA. Pathogenesis of hypertension. Ann Intern Med. 2003;139(9):761–776. [PubMed: 14597461]
- 3.
- The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–86. [PubMed: 8366922]
- 4.
- Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383. [PubMed: 12556541]
- 5.
- Sowers JR, Epstein M, Frohlich ED. Diabetes, hypertension, and cardiovascular disease: an update. Hypertension. 2001;37(4):1053–9. [PubMed: 11304502]
- 6.
- Sowers JR. Diabetes mellitus and vascular disease. Hypertension. 2013;61(5):943–7. [PMC free article: PMC3648858] [PubMed: 23595139]
- 7.
- Hill M, Yanag Y, Zhang L, Sun Z. jia G, Sowers J Parrish A, Insulin resistance, cardiovascular stiffening, cardiovascular disease. Metabolism Clinical Experimental. 2021;119:154766. [PubMed: 33766485]
- 8.
- Victor RG. Braunwald’s heart disease: A textbook of cardiovascular Medicine. 9th Edition. Philadelphia PA:Elsevier, Sauders. 2012 Chapter 45.
- 9.
- Van Buren PN, Toto RD. The pathogenesis and management of hypertension in diabetic kidney disease. Med Clin North Am. 2013;97(1):31–51. [PubMed: 23290728]
- 10.
- Victor RG, Goldman's Cecil Medicine 24 th Eds: L. Goldman, AI Schafer. Vol. 1. 2012. Saunders, An Imprint of Elsevier.
- 11.
- Whaley-Connell A, Sowers JR. Aldosterone and risk for insulin resistance. Hypertension. 2011;58(6):998–1000. [PMC free article: PMC3230820] [PubMed: 22215931]
- 12.
- Sowers JR, Whaley-Connell A, Epstein M. Narrative Review: The emerging clinical implications of the role of aldosterone in the metabolic syndrome and resistant hypertension. Ann Intern Med. 2009;150:776–83. [PMC free article: PMC2824330] [PubMed: 19487712]
- 13.
- Van Buren PN, Toto R. Hypertension in diabetic nephropathy: epidemiology, mechanisms, and management. Adv Chronic Kidney Dis. 2011 Jan;18(1):28–41. [PMC free article: PMC3221014] [PubMed: 21224028]
- 14.
- Hertz R, Unger A, Cornell J, Saunders E. Racial Disparities in Hypertension prevalence, Awareness, and Management. Arch Intern Med. 2005;165(18):2098–104. [PubMed: 16216999]
- 15.
- Belanger MJ, Hill MA, Angelidi AM, Dalamaga M, Sowers JR, Mantzoros CS. Covid-19 and Disparities in Nutrition and Obesity. N Engl J Med. 2020 Sep 10;383(11):e69. [PubMed: 32668105]
- 16.
- Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Sacks FM., American Heart Association. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension. 2006 Feb;47(2):296–308. [PubMed: 16434724]
- 17.
- Thrope R, Brandon D, LaVeist T. Social context as an explanation for race disparities in hypertension: Findings from the Exploring Health Disparities in Integrated Communities (EHDIC). Study Social Science & Medicine. 2008;67(10):1604–161. [PMC free article: PMC3521570] [PubMed: 18701200]
- 18.
- Muntner P, Shimbo D, Carey RM, Charleston JB, Gaillard T, Misra S, Myers MG, Ogedegbe G, Schwartz JE, Townsend RR, Urbina EM, Viera AJ, White WB, Wright JT Jr. Measurement of Blood Pressure in Humans: A Scientific Statement From the American Heart Association. Hypertension. 2019 May;73(5):e35–e66. [PubMed: 30827125]
- 19.
- Cohen JB, Cohen DL. Integrating Out-of-Office Blood Pressure in the Diagnosis and Management of Hypertension. Curr Cardiol Rep. 2016;18:112. [PMC free article: PMC5068246] [PubMed: 27677895]
- 20.
- Leitao CB, Canani LH, Silveiro SP, Gross JL. Ambulatory Blood Pressure Monitoring and Type 2 Diabetes Mellitus. Arq Bras Cardiol. 2007;88(2):315–321. [PubMed: 18066457]
- 21.
- Parati G, Bilo G. Should 24-h ambulatory blood pressure monitoring be done in every patient with diabetes? Diabetes Care. 2009 Nov;32 Suppl 2(Suppl 2):S298-304. [PMC free article: PMC2811450] [PubMed: 19875569]
- 22.
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–53. [PubMed: 9742976]
- 23.
- Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703–13. [PMC free article: PMC28659] [PubMed: 9732337]
- 24.
- Hansson L, Zanchetti A, Carruthers SG, Dahlöf B, Elmfeldt D, Julius S, Ménard J, Rahn KH, Wedel H, Westerling S. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755–62. [PubMed: 9635947]
- 25.
- Patel A., ADVANCE Collaborative Group. MacMahon S, Chalmers J, Neal B, Woodward M, Billot L, Harrap S, Poulter N, Marre M, Cooper M, Glasziou P, Grobbee DE, Hamet P, Heller S, Liu LS, Mancia G, Mogensen CE, Pan CY, Rodgers A, Williams B. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370:829–40. [PubMed: 17765963]
- 26.
- Bakris GL, Sowers JR., American Society of Hypertension Writing Group. ASH position paper: treatment of hypertension in patients with diabetes-an update. J Clin Hypertens (Greenwich). 2008;10:707–13. [PMC free article: PMC8673265] [PubMed: 18844766]
- 27.
- Schrier RW, Estacio RO, Esler A, Mehler P. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int. 2002;61:1086–97. [PubMed: 11849464]
- 28.
- ACCORD Study Group. Cushman WC, Evans GW, Byington RP, Goff DC Jr, Grimm RH Jr, Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC, Ismail-Beigi F. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–85. [PMC free article: PMC4123215] [PubMed: 20228401]
- 29.
- The SPRINT Research Group. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373:2103–2116. [PMC free article: PMC4689591] [PubMed: 26551272]
- 30.
- Brouwer TF, Vehmeijer JT, Kalkman DN, Berger WR, van den Born BH, Peters RJ, Knops RE. Intensive Blood Pressure Lowering in Patients With and Patients Without Type 2 Diabetes: A Pooled Analysis From Two Randomized Trials. Diabetes Care. 2018 Jun;41(6):1142–1148. [PubMed: 29212825]
- 31.
- Chaturvedi N, Sjolie AK, Stephenson JM, Abrahamian H, Keipes M, Castellarin A, Rogulja-Pepeonik Z, Fuller JH. Effect of lisinopril on progression of retinopathy in normotensive people with type 1 diabetes. The EUCLID Study Group. EURODIAB Controlled Trial of Lisinopril in Insulin-Dependent Diabetes Mellitus. Lancet. 1998;351:28–31. [PubMed: 9433426]
- 32.
- Wing LM, Reid CM, Ryan P, Beilin LJ, Brown MA, Jennings GL, Johnston CI, McNeil JJ, Macdonald GJ, Marley JE, Morgan TO, West MJ. Second Australian National Blood Pressure Study Group. A comparison of outcomes with angiotensin-converting enzyme inhibitors and diuretics for hypertension in the elderly. N Eng J Med. 2003;348:583–92. [PubMed: 12584366]
- 33.
- Pfeffer MA, McMurray JJ, Velazquez EJ, Rouleau JL, Køber L, Maggioni AP, Solomon SD, Swedberg K, Van de Werf F, White H, Leimberger JD, Henis M, Edwards S, Zelenkofske S, Sellers MA, Califf RM., Valsartan in Acute Myocardial Infarction Trial Investigators. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Eng J Med. 2003;349:1893–906.
- 34.
- Kaplan NM. In Braunwald’s Heart Disease: A textbook of Cardiovascular Medicine. 9th Edition. Philadelphia, PA: Elsevier, Saunders. 2012. Chapter 46. Systemic Hypertension: Therapy; p. 955.
- 35.
- ONTARGET Investigators. Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P, Anderson C. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Eng J Med. 2008;358:1547–59. [PubMed: 18378520]
- 36.
- Lindholm LH, Hansson L, Ekbom T, Dahlöf B, Lanke J, Linjer E, Scherstén B, Wester PO, Hedner T, de Faire U. Comparison of antihypertensive treatments in preventing cardiovascular events in elderly diabetic patients: results from the Swedish Trial in Old Patients with Hypertension-2. STOP Hypetension-2 Study Group. J Hypertens. 2000;18:1671–5. [PubMed: 11081782]
- 37.
- Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, Stoyanovsky V, Antikainen RL, Nikitin Y, Anderson C, Belhani A, Forette F, Rajkumar C, Thijs L, Banya W, Bulpitt CJ., HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Eng J Med. 2008;358:1887–98. [PubMed: 18378519]
- 38.
- Ernst ME, Carter BL, Goerdt CJ, Steffensmeier JJ, Phillips BB, Zimmerman MB, Bergus GR. Comparative antihypertensive effects of hydrochlorothiazide and chlorthalidone on ambulatory and office blood pressure. Hypertension. 2006;47:352–8. [PubMed: 16432050]
- 39.
- Jamerson K, Weber MA, Bakris GL, Dahlöf B, Pitt B, Shi V, Hester A, Gupte J, Gatlin M, Velazquez EJ., ACCOMPLISH Trial Investigators. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Eng J Med. 2008;359:2417–28. [PubMed: 19052124]
- 40.
- Barzilay JI, Davis BR, Cutler JA, Pressel SL, Whelton PK, Basile J, Margolis KL, Ong ST, Sadler LS, Summerson J., ALLHAT Collaborative Research Group. Fasting glucose levels and incident diabetes mellitus in older nondiabetic adults randomized to receive 3 different classes of antihypertensive treatment: a report from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Arch Intern Med. 2006;166(20):2191–201. [PubMed: 17101936]
- 41.
- Hansson L, Hedner T, Lund-Johansen P, Kjeldsen SE, Lindholm LH, Syvertsen JO, Lanke J, de Faire U, Dahlöf B, Karlberg BE. Randomised trial of effects of calcium antagonists compared with diuretics and beta-blockers on cardiovascular morbidity and mortality in hypertension: the Nordic Diltiazem (NORDIL) study. Lancet. 2000;356:359–65. [PubMed: 10972367]
- 42.
- Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. UK Prospective Diabetes Study Group. BMJ. 1998;317:713–20. [PMC free article: PMC28660] [PubMed: 9732338]
- 43.
- Bakris GL, Fonseca V, Katholi RE, McGill JB, Messerli FH, Phillips RA, Raskin P, Wright JT Jr, Oakes R, Lukas MA, Anderson KM, Bell DS. GEMINI Investigators. Metabolic effects of carvedilol vs metoprolol in patients with type 2 diabetes mellitus and hypertension: a randomized controlled trial. JAMA. 2004 Nov 10;292(18):2227–36. [PubMed: 15536109]
- 44.
- Bell DS, Bakris GL, McGill JB. Comparison of carvedilol and metoprolol on serum lipid concentration in diabetic hypertensive patients. Diabetes Obes Metab. 2009 Mar;11(3):234–8. [PubMed: 18564334]
- 45.
- Fonseca VA. Effects of beta-blockers on glucose and lipid metabolism. Curr Med Res Opin. 2010 Mar;26(3):615–29. [PubMed: 20067434]
- 46.
- Marketou M, Gupta Y, Jain S, Vardas P. Differential Metabolic Effects of Beta-Blockers: an Updated Systematic Review of Nebivolol. Curr Hypertens Rep. 2017 Mar;19(3):22. [PubMed: 28283926]
- 47.
- Hillebrand U, Lang D, Telgmann RG, Hagedorn C, Reuter S, Kliche K, Stock CM, Oberleithner H, Pavenstädt H, Büssemaker E, Hausberg M. Nebivolol decreases endothelial cell stiffness via the estrogen receptor beta: a nano-imaging study. J Hypertens. 2009;27:517–26. [PubMed: 19330906]
- 48.
- Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, Kolkhof P, Nowack C, Schloemer P, Joseph A, Filippatos G. FIDELIO-DKD Investigators. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N Engl J Med. 2020 Dec 3;383(23):2219–2229. [PubMed: 33264825]
- 49.
- Bakris G, Aggrawal R, Anker S, Pitt B, et al. Effect of Finerenone on Chronic kidney disease outcomes in Type 2 Diabetes . N Engl J Med. 2020;383:2219–29. [PubMed: 33264825]
- 50.
- Parving HH, Brenner BM, McMurray JJ, de Zeeuw D, Haffner SM, Solomon SD, Chaturvedi N, Persson F, Desai AS, Nicolaides M, Richard A, Xiang Z, Brunel P, Pfeffer MA. ALTITUDE Investigators. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med. 2012;367(23):2204–13. [PubMed: 23121378]
- 51.
- Khangura Darshan, Kurukulasuriya L. Romayne, Sowers James R. Treatment of hypertension in diabetes: a contemporary approach with a focus on improving cardiovascular outcomes. Expert Review of Endocrinology & Metabolism. 2016;11(1):41–50. [PubMed: 30063450]
- 52.
- Wang B, Zhong J, Lin H, Zhao Z, Yan Z, He H, Ni Y, Liu D, Zhu Z. Blood pressure-lowering effects of GLP-1 receptor agonists exenatide and liraglutide: a meta-analysis of clinical trials. Diabetes Obes Metab. 2013;15(8):737–49. [PubMed: 23433305]
- 53.
- Ferdinand KC, White WB, Calhoun DA, Lonn EM, Sager PT, Brunelle R, Jiang HH, Threlkeld RJ, Robertson KE, Geiger MJ. Effects of the once-weekly glucagon-like peptide-1 receptor agonist dulaglutide on ambulatory blood pressure and heart rate in patients with type 2 diabetes mellitus. Hypertension. 2014;64(4):731–7. [PubMed: 24980665]
- 54.
- Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR., CANVAS Program Collaborative Group. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377(7):644–657. [PubMed: 28605608]
- 55.
- Sjöström CD, Johansson P, Ptaszynska A, List J, Johnsson E. Dapagliflozin lowers blood pressure in hypertensive and non-hypertensive patients with type 2 diabetes. Diab Vasc Dis Res. 2015;12(5):352–8. [PubMed: 26008804]
- 56.
- Panagiotis G, Agarwal R. SGLT-2 Inhibitors and Ambulatory BP Reduction. Diabetes Care. 2019;42:693–700. [PMC free article: PMC6429633] [PubMed: 30894383]
- 57.
- Xiang B, Zhao X, Zhou X. Cardiovascular benefits of sodium-glucose cotransporter 2 inhibitors in diabetic and nondiabetic patients. Cardiovasc Diabetol. 2021;20(1):78. [PMC free article: PMC8028072] [PubMed: 33827579]
- 58.
- Majewski C, Bakris GL. Blood pressure reduction: an added benefit of sodium-glucose cotransporter 2 inhibitors in patients with type 2 diabetes. Diabetes Care. 2015;38(3):429–30. [PMC free article: PMC4876696] [PubMed: 25715414]
- 59.
- Zinman B, Wanner C, Lachen J, Fitchett D, et al. Empagliflozin, cardiovascular outcomes, and mortality in Type 2 Diabetes. The EMPARA-REG outcome investigators N Eng. J Med. 2015;373:2117–2128. [PubMed: 26378978]
- 60.
- Wheeler DC, Stefanson BV, Jongs N, Chertow GM, et al. DAPA-CKD Trial committees and investigators. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease. Lancet Diabetes and Endocrinology. 2021;9(1):22–31. [PubMed: 33338413]
- 61.
- Sowers JR. Treatment of hypertension in patients with diabetes. Arch Intern Med. 2004 Sep 27;164(17):1850–7. [PubMed: 15451759]
- 62.
- de Boer IH, Kestenbaum B, Rue TC, Steffes MW, Cleary PA, Molitch ME, Lachin JM, Weiss NS, Brunzell JD. Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group. Insulin therapy, hyperglycemia, and hypertension in type 1 diabetes mellitus. Arch Intern Med. 2008 Sep 22;168(17):1867–73. [PMC free article: PMC2701288] [PubMed: 18809813]
- 63.
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS. China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020 Apr 30;382(18):1708–1720. [PMC free article: PMC7092819] [PubMed: 32109013]
- 64.
- Mueller M, Purnell T, Mensah G, Cooper L. Reducing Racial and Ethnic Disparities in Hypertension Prevention and Control: What Will It Take to Translate Research into Practice and Policy? American Journal of Hypertension. 2015;28(6):699–716. [PMC free article: PMC4447820] [PubMed: 25498998]
- 65.
- Hill MA, Mantzoros C, Sowers JR. Commentary: COVID-19 in patients with diabetes. Metabolism. 2020 Jun;107:154217. [PMC free article: PMC7102643] [PubMed: 32220611]
- 66.
- Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018 Jun;71(6):e13–e115. [PubMed: 29133356]
- 67.
- American Diabetes Association. 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021 ; 44 (Supplement Jan 1): S125-50. [PubMed: 33298421]
- 68.
- Wang L, Li X, Wang Z, Bancks MP, Carnethon MR, Greenland P, Feng YQ, Wang H, Zhong VW. Trends in Prevalence of Diabetes and Control of Risk Factors in Diabetes Among US Adults, 1999-2018. JAMA. 2021 Jun 25; [PMC free article: PMC8233946] [PubMed: 34170288]
- 69.
- Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993 Nov 11;329(20):1456–62. [PubMed: 8413456]
- ABSTRACT
- INTRODUCTION
- PATHOPHYSIOLOGY OF HYPERTENSION IN DIABETES
- BLOOD PRESSURE MEASUREMENT AND MONITORING
- BLOOD PRESSURE TARGETS IN PATIENTS WITH DIABETES
- TREATMENT OF HYPERTENSION
- DIABETES MEDICATIONS WITH ANTIHYPERTENSIVE EFFECTS
- IMPACT OF COMORBIDITIES ON CHOICE OF ANTIHYPERTENSIVE REGIMEN
- RESISTANT HYPERTENSION
- SPECIAL CONSIDERATIONS IN TYPE 1 DIABETES
- COVID-19 AND ANTIHYPERTENSIVE THERAPY IN INDIVIDUALS WITH DIABETES
- RECENT GUIDELINES
- SUMMARY
- REFERENCES
- Review Moderately Elevated Blood Pressure: A Systematic Review[ 2004]Review Moderately Elevated Blood Pressure: A Systematic ReviewSwedish Council on Health Technology Assessment. 2004 Oct
- Review Moderately Elevated Blood Pressure: A Systematic Review[ 2008]Review Moderately Elevated Blood Pressure: A Systematic ReviewSwedish Council on Health Technology Assessment. 2008 Sep
- Comparative Efficacy and Safety of BP-Lowering Pharmacotherapy in Patients Undergoing Maintenance Dialysis: A Network Meta-Analysis of Randomized, Controlled Trials.[Clin J Am Soc Nephrol. 2020]Comparative Efficacy and Safety of BP-Lowering Pharmacotherapy in Patients Undergoing Maintenance Dialysis: A Network Meta-Analysis of Randomized, Controlled Trials.Shaman AM, Smyth B, Arnott C, Palmer SC, Mihailidou AS, Jardine MJ, Gallagher MP, Perkovic V, Jun M. Clin J Am Soc Nephrol. 2020 Aug 7; 15(8):1129-1138. Epub 2020 Jul 16.
- Review [Individualising antihypertensive therapy in patients with diabetes. A guideline by the Austrian Diabetes Association (update 2023)].[Wien Klin Wochenschr. 2023]Review [Individualising antihypertensive therapy in patients with diabetes. A guideline by the Austrian Diabetes Association (update 2023)].Saely CH, Schernthaner GH, Brix J, Klauser-Braun R, Zitt E, Drexel H, Schernthaner G. Wien Klin Wochenschr. 2023 Jan; 135(Suppl 1):147-156. Epub 2023 Apr 20.
- First-line drugs inhibiting the renin angiotensin system versus other first-line antihypertensive drug classes for hypertension.[Cochrane Database Syst Rev. 2018]First-line drugs inhibiting the renin angiotensin system versus other first-line antihypertensive drug classes for hypertension.Chen YJ, Li LJ, Tang WL, Song JY, Qiu R, Li Q, Xue H, Wright JM. Cochrane Database Syst Rev. 2018 Nov 14; 11(11):CD008170. Epub 2018 Nov 14.
- Hypertension in Diabetes - EndotextHypertension in Diabetes - Endotext
Your browsing activity is empty.
Activity recording is turned off.
See more...
