NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-.
ABSTRACT
Craniopharyngiomas are rare intracranial tumors that mainly arise in the sellar/parasellar (particularly suprasellar) region. They present in both children and adults with a wide range of clinical manifestations. Histologically, they are benign tumors with distinct adamantinomatous and papillary subtypes. Beta-catenin gene mutations have been identified in the adamantinomatous subtype and activating mutations in BRAF (V600E) in the papillary variant, opening further avenues in our understanding of their pathogenesis. Despite their benign classification, management is challenging due to unpredictable growth and the involvement of adjacent critical structures particularly for vision and hypothalamo-pituitary function. Surgery with or without external irradiation currently represents the mainstay of therapy for most patients; however, the optimal protocol for the management of these tumors has not yet been established. Further management options include intracystic irradiation or bleomycin, stereotactic radiosurgery, systemic chemotherapy, or targeted BRAF inhibitors (for the papillary subtype); however, the outcomes of these approaches have not yet been validated with large scale clinical trials. Following treatment, patients face a high burden of morbidity due to visual, endocrine, hypothalamic, and neuropsychological dysfunction, and long-term mortality rates are substantially elevated compared with the general population. For complete coverage of all related areas of Endocrinology, please visit our on-line FREE web-text, WWW.ENDOTEXT.ORG.
EPIDEMIOLOGY
Craniopharyngiomas account for 2–5% of all primary intracranial neoplasms and for up to 15% of intracranial tumors in children (1). Their annual incidence is reported as around 0.18 cases per 100,000 people (2), and genetic susceptibility seems unlikely. Craniopharyngiomas may be detected at any age, even in the prenatal and neonatal periods (3) and a bimodal age distribution with peak incidence rates at ages 5–14 and 50–74 years has been proposed (2,4). In population-based studies from the USA and Finland, no gender differences have been found (4,5).
PATHOLOGY AND PATHOGENESIS
Craniopharyngiomas are epithelial tumors arising along the path of the craniopharyngeal duct (the canal connecting the stomodeal ectoderm with the evaginated Rathke’s pouch). Based on the WHO classification, they are assigned grade I. Rare cases of malignant transformation (possibly triggered by previous irradiation) have been described (1). Two main pathological subtypes have been reported: the adamantinomatous variant and the papillary variant (1,6).
The adamantinomatous variant is the most common subtype and may occur at any age (Figure 1). Macroscopically, adamantinomatous craniopharyngiomas have cystic and/or solid components, necrotic debris, fibrous tissue and calcification. The cysts may be multiloculated and contain liquid ranging from “machinery oil” to shimmering cholesterol-laden fluid consisting of desquamated squamous epithelial cells, rich in membrane lipids and cytoskeleton keratin. They tend to have sharp and irregular margins, often merging into a peripheral zone of dense reactive gliosis, with abundant Rosenthal fiber formation (consisting of irregular masses of granular deposits within astrocytic processes) in the surrounding brain tissue and vascular structures. The epithelium of the adamantinomatous type is composed of three layers of cells: a distinct palisaded basal layer of small cells with darkly staining nuclei and little cytoplasm (somewhat resembling the basal cells of the epidermis of the skin); an intermediate layer of variable thickness composed of loose aggregates of stellate cells (termed stellate reticulum), with processes traversing empty intercellular spaces; and a top layer facing into the cyst lumen with abruptly enlarged, flattened and keratinized to flat plate-like squamous cells. The flat squames are desquamated singly or in distinctive stacked clusters and form nodules of “wet” keratin, which are often heavily calcified and appear grossly as white flecks. The keratinous debris may elicit an inflammatory and foreign body giant cell reaction. The presence of the typical adamantinomatous epithelium or of the “wet” keratin alone are diagnostic, whereas features only suggestive of the diagnosis in small or non-representative specimens include fibrohistiocytic reaction, necrotic debris, calcification and cholesterol clefts (1).
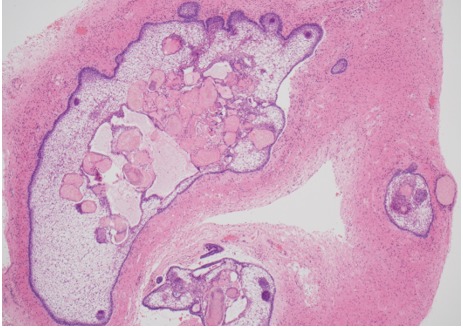
Figure 1A.
Histology of adamantinomatous craniopharyngioma. Islands of tumor with finger-like protrusions into surrounding brain tissue with central accumulation of keratin nodules; HE x40 magnification.
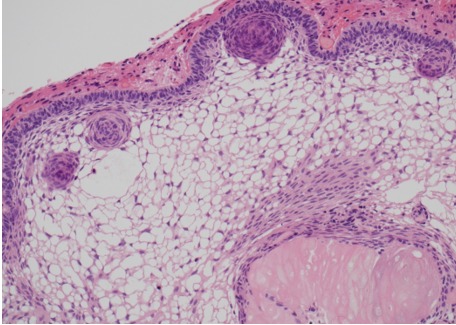
Figure 1B.
Histology of adamantinomatous craniopharyngioma. Well-differentiated epithelium with peripheral palisading, nodular whorls, and pale, microcystic areas termed ‘stellate reticulum’, as well as pale eosinophilic ‘wet keratin’ nodule (right bottom); HE x200 magnification.
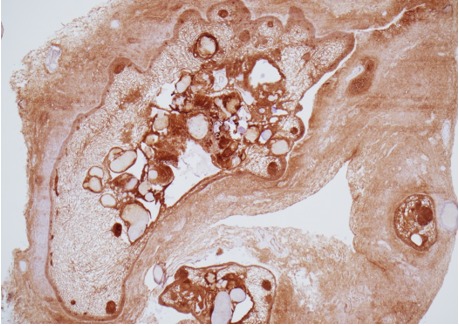
Figure 1C.
Histology of adamantinomatous craniopharyngioma. Beta-catenin immunohistochemistry of area shown in A, highlighting dark staining nodular whorls; x40 magnification
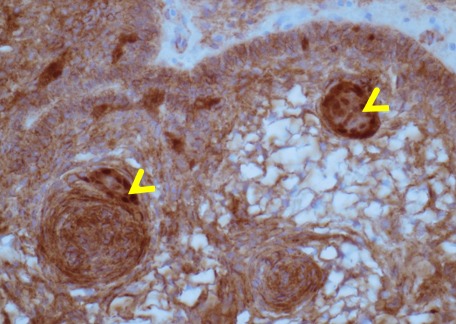
Figure 1D.
Histology of adamantinomatous craniopharyngioma. Basal epithelium demonstrating the aberrant nuclear accumulation of beta-catenin in nodular whorls (arrowheads) due to beta-catenin mutation; Anti-beta-catenin, x400 magnification.
The papillary variant has been almost exclusively described in adult populations (accounting for 14-50% of adult cases but only around 2% of pediatric cases) (Figure 2). It consists of mature squamous epithelium forming pseudopapillae and of an anastomosing fibrovascular stroma without the presence of peripheral palisading of cells or stellate reticulin, and with membranous beta-catenin immunoreactivity only. The differential diagnosis between a papillary craniopharyngioma and a Rathke’s cleft cyst may be difficult, particularly in small biopsy specimens, as the epithelial lining of the Rathke’s cysts may undergo squamous differentiation; however, the lack of a solid component and the presence of extensive ciliation and/or mucin production are suggestive of Rathke’s (1,6).
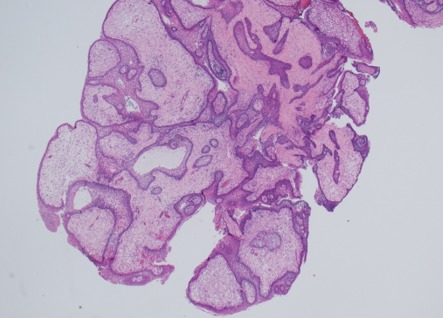
Figure 2A.
Histology of papillary craniopharyngioma. Papillae lined by non-keratinizing squamous epithelium and containing loosely structured connective tissue; HE x20 magnification.
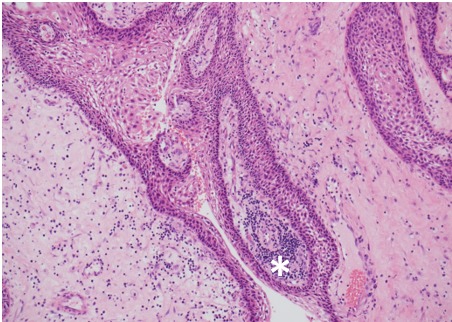
Figure 2B.
Histology of papillary craniopharyngioma. Connective tissue harbors a patchy lymphocytic infiltrate (asterix); HE x100 magnification.
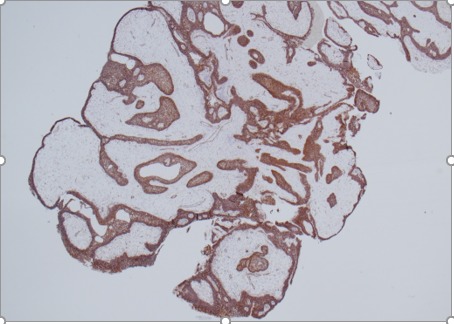
Figure 2C.
Histology of papillary craniopharyngioma. Non-keratinizing squamous epithelium highlighted by beta-catenin immunostain, x20 magnification.
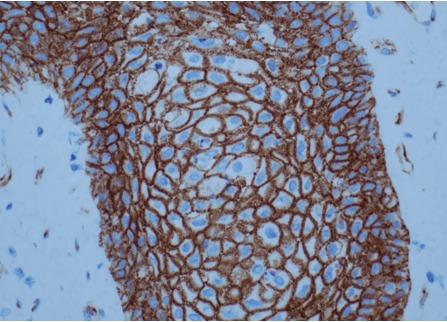
Figure 2D.
Histology of papillary craniopharyngioma. Squamous epithelium showing membranous immunoreactivity of beta-catenin, lacking clusters with aberrant nuclear accumulation, x400 magnification.
Although the pathogenesis of craniopharyngiomas has not been fully elucidated, our understanding in this field has increased significantly in recent years. Beta-catenin gene (CTNNB1) mutations have been identified in the adamantinomatous subtype affecting exon 3 which encodes the degradation targeting box of beta-catenin; the mutant form is resistant to degradation leading to accumulation of nuclear beta-catenin protein (a transcriptional activator of the Wnt signaling pathway) (Figure 1D). Furthermore, strong beta-catenin expression has been shown in the adamantinomatous subtype indicating re-activation of the Wnt signaling pathway and subsequent de-regulation of several downstream pathways (7-10). Molecular analysis also implicates the immune response in the pathogenesis of adamantinomatous craniopharyngiomas. Cells within this subtype show signs of inflammation (in both cystic and solid components), and increased levels of cytokines including Interleukin-6 (IL-6), IL-8 and IL-10 have been identified(10-12). Furthermore, the expression of immune related genes is increased, and the immune check point proteins Programmed Death Ligand 1 (PD-L1), and Programmed Cell Death Protein 1 (PD-1) are expressed in both subtypes of craniopharyngioma(10,13). For papillary craniopharyngiomas specifically, a number of studies using whole exome sequencing, next-generation panel sequencing, pyrosequencing and Sanger sequencing have shown the presence of activating mutations in the BRAF (V600E) gene; the prevalence of which varies according to the sequencing method, generally being between 81 and 100% (8). BRAF mutations can lead to activation of the MAPK/ERK (Mitogen Activated Protein Kinase / Extracellular signal Regulated Kinases) pathway, which ultimately results to increased cell growth, proliferation, and cell survival(10). Whilst BRAF mutations are found in numerous cells within papillary craniopharyngiomas, only a small cluster of progenitor cells expressing the SOX2/SOX9 (Sex Region Y Box 2 and 9) transcription factors are believed to be involved in their tumorigenesis(14). It has also been suggested that the two pathological subtypes have different epigenomic and transcriptomic signatures, and that the cell clusters in the adamantinomatous subtype may have a functional role in the promotion of tumor invasion (8).
DIAGNOSIS
Location/Imaging
Most craniopharyngiomas are located in the sellar/parasellar region and the majority (94-95%) have a suprasellar component. Other rare locations include the nasopharynx, the paranasal area, the sphenoid bone, the ethmoid sinus, the intrachiasmatic area, the temporal lobe, the pineal gland, the posterior cranial fossa, the cerebellopontine angle, the midportion of the midbrain or, mainly relating to the papillary variant, within the 3rd ventricle(1). Plain skull X-rays, although seldom used nowadays, may show calcification and an abnormal sella. CT is helpful for evaluation of the bony anatomy, the identification of calcification and the discrimination of the solid and cystic components. They are usually of mixed attenuation; the cyst fluid has low density and the contrast medium enhances any solid portion, including the cyst capsule (1). MRI is particularly useful for the topographic and structural analysis of the tumor. The radiological appearance depends on the proportion of the solid and cystic components, the content of the cyst(s) (cholesterol, keratin, hemorrhage), and the amount of calcification present. A solid lesion appears as iso- or hypointense relative to the brain. On pre-contrast T1-weighted images, it shows enhancement following gadolinium administration, and is usually of mixed hypo- or hyperintensity on T2-weighted images. Large amounts of calcification may be visualized as areas of low signal on both T1- and T2-weighted images. A cystic element is usually hypointense on T1- and hyperintense on T2-weighted sequences, and a thin peripheral contrast-enhancing rim of the cyst can be shown on T1-weighted images. Protein, cholesterol, and methemoglobin may cause high signal on T1-weighted images, while very concentrated protein and various blood products may be associated with low T2-weighted signal (1). Imaging examples from cystic, solid, and mixed solid-cystic craniopharyngiomas are shown in Figure 3.
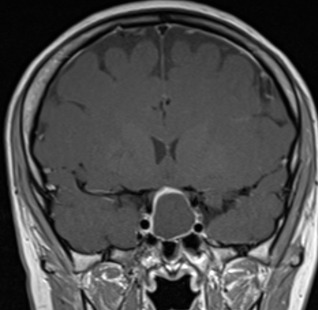
Figure 3A.
MRI images of craniopharyngiomas. Coronal section showing cystic craniopharyngioma on post-contrast T1-weighted MRI. The cyst contents are isointense and the cyst rim enhances following contrast.
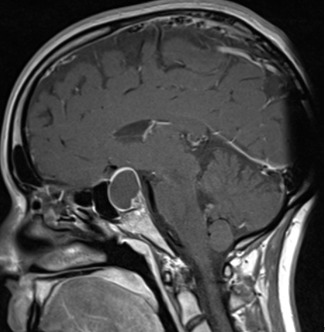
Figure 3B.
MRI images of craniopharyngiomas. Sagittal section of 3A.
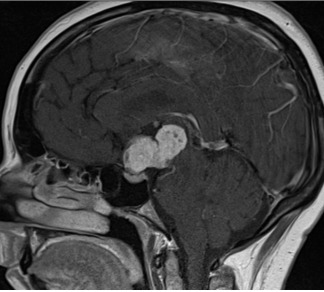
Figure 3C.
MRI images of craniopharyngiomas. Sagittal section showing a solid craniopharyngioma on T1-weighted imaging which enhances after contrast.
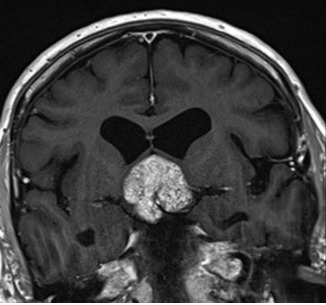
Figure 3D.
MRI images of craniopharyngiomas. Coronal section showing a solid craniopharyngioma on T1-weighted imaging which enhances after contrast.
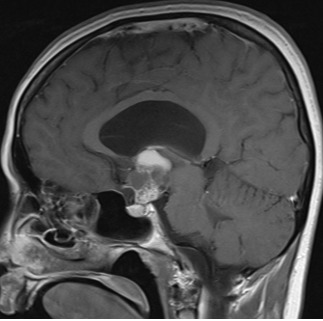
Figure 3E.
MRI images of craniopharyngiomas. Sagittal section showing a craniopharyngioma with mixed solid and cystic components on post-contrast T1-weighted imaging.
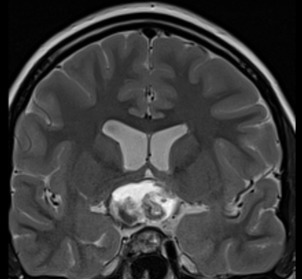
Figure 3F.
MRI images of craniopharyngiomas. Coronal section showing a mixed solid and cystic craniopharyngioma with mixed signal intensities on T2-weighted imaging.
The consistency of craniopharyngiomas can be purely or predominantly cystic, purely or predominantly solid, and mixed. When present, the calcification patterns vary from solid lumps to popcorn-like foci or, less commonly, to an eggshell pattern lining the cyst wall. Hydrocephalus has been reported in 20-38% and is probably more frequent in childhood-diagnosed disease (41-54%).
The differential diagnosis includes a number of sellar or parasellar lesions, including Rathke’s cleft cyst, dermoid cyst, epidermoid cyst, pituitary adenoma, germinoma, hamartoma, suprasellar aneurysm, arachnoid cyst, suprasellar abscess, glioma, meningioma, sarcoidosis, tuberculosis and Langerhans cell histiocytosis. Differentiation from a Rathke’s cleft cyst (typically small, round, purely cystic lesions lacking calcification), or from a pituitary adenoma (in the rare case of a homogeneously enhancing solid craniopharyngioma), may be particularly difficult (1,15).
Clinical and Hormonal Manifestations at Presentation
Patients with craniopharyngioma may present with a variety of clinical manifestations attributed to pressure effects on vital structures of the brain (visual pathways, brain parenchyma, ventricular system, major blood vessels and hypothalamo-pituitary system) (15-17). Their severity depends on the location, size, and growth potential of the tumor. The duration of the symptoms until diagnosis ranges between 1 week to 372 months(1). The presenting clinical manifestations (neurological, visual, hypothalamo-pituitary) are shown in Table 1. Headaches, nausea/vomiting, visual disturbances, growth failure (in children) and hypogonadism (in adults) are the most frequently reported. Other less common or rare features include motor disorders, such as hemi- or monoparesis, seizures, psychiatric symptoms such as emotional lability, hallucinations and paranoid delusions, autonomic disturbances, precocious puberty, the syndrome of inappropriate secretion of antidiuretic hormone, chemical meningitis due to spontaneous cyst rupture, hearing loss, anosmia, nasal obstruction, epistaxis, photophobia, emaciation, Weber’s syndrome (ipsilateral III cranial nerve palsy with contralateral hemiplegia due to midbrain infarction), and Wallenberg’s syndrome (signs due to occlusion of the posterior inferior cerebellar artery) (1). It has been proposed that in cases of craniopharyngioma diagnosed in childhood, compromised growth rate is already evident in early infancy, whereas an increase in weight tends to present later and is a predictor of obesity (18).
The hypothalamo-pituitary function at presentation may be severely affected; a summary of the results of various studies using different diagnostic tests and criteria shows that GH deficiency is present in 35–100% of the evaluated patients, FSH/LH deficiency in 38–91%, ACTH deficiency in 21–68%, TSH deficiency in 20–42% and diabetes insipidus in 6–38%.
Table 1.
Presenting Clinical Features in Children and Adults with Craniopharyngioma (10)
| Children | Adults | Total | |
|---|---|---|---|
| Headaches | 78% | 56% | 64% |
| Menstrual disorders | 57% | ||
| Visual field defects | 46% | 60% | 55% |
| Decreased visual acuity | 39% | 40% | 39% |
| Nausea/vomiting | 54% | 26% | 35% |
| Growth failure | 32% | ||
| Poor energy | 22% | 32% | 29% |
| Impaired sexual function | 28% | ||
| Impaired secondary sexual characteristics (pts aged ≥13 years) | 24% | ||
| Lethargy | 17% | 26% | 23% |
| Other cranial nerves palsies | 27% | 9% | 15% |
| Polyuria/polydipsia | 15% | 15% | 15% |
| Papilledema | 29% | 6% | 14% |
| Cognitive impairment (memory, concentration) | 10% | 17% | 14% |
| Anorexia/weight loss | 20% | 8% | 12% |
| Optic atrophy | 5% | 14% | 10% |
| Hyperphagia/excessive weight gain | 5% | 13% | 10% |
| Psychiatric symptoms/change in behavior | 10% | 8% | 8% |
| Somnolence | 5% | 10% | 8% |
| Galactorrhea | 8% | ||
| Decreased consciousness/coma | 10% | 4% | 6% |
| Cold intolerance | 0% | 8% | 5% |
| Unsteadiness/ataxia | 7% | 3% | 4% |
| Hemiparesis | 7% | 1% | 3% |
| Blindness | 3% | 3% | 3% |
| Meningitis | 0% | 3% | 2% |
MANAGEMENT
Surgery Combined or Not with External Irradiation
Surgery combined or not with adjuvant external beam irradiation is currently one of the most widely used first therapeutic approaches for craniopharyngiomas. These tumors pose challenges mainly due to their sharp, irregular borders and to their tendency to adhere to vital neurovascular structures, making surgical manipulation potentially hazardous to vital brain areas. When large cystic components are present, fluid aspiration provides relief of the obstructive manifestations and facilitates the consecutive removal of the solid portion, which should not be delayed for more than a few weeks due to the significant risk of cyst refilling (15,19). The attempted extent of excision has been a subject of significant debate and depends on the size (achieved in 0% of lesions >4 cm) and location of the tumor, the presence of hydrocephalus (particularly difficult for retrochiasmatic or within the 3rd ventricle), >10% calcification, tumor adherence to the hypothalamus, brain invasion, as well as the experience, individual judgment during the operation, and general treatment policy (aggressive or not) adopted by each neurosurgeon (1,20-22). In recent years, many tertiary centers have adopted a more conservative surgical approach, electing for partial or limited resection with radiotherapy over complete resection, when possible, with aim of hypothalamic sparing and reducing subsequent morbidity (23). In microsurgical series, post-operative mortality ranges between 0 and 5.4% (24), while in a meta- analysis including 2,955 patients early mortality of 2.6% after transsphenoidal and 3.1% after transcranial surgery were reported (25).
Interestingly, until 1937, when Carpenter et al. (26) first described the beneficial effects of radiotherapy following aspiration of cyst contents in 4 cases, craniopharyngiomas were considered radioresistant. Historically, the role of irradiation started being established almost two decades later, following the report of the favorable outcome of the combination of minimal surgery and high-dose supervoltage irradiation in a series of 10 patients by Kramer et al. (27). The irradiation of cystic craniopharyngiomas carries the risk of cyst enlargement, arising during or within 6 months after radiotherapy, reported in 10-60% of patients (28-31). Whilst urgent surgical decompression may be needed in some cases, enlargement is transient, and does not represent tumor recurrence (15,30,32).
Recurrence Following Surgery
Recurrent tumors may arise even from small islets of craniopharyngioma cells in the gliotic brain adjacent to the tumor, which can remain even after gross total removal. The mean interval for diagnosis of recurrence following various primary treatment modalities ranges between 1 and 4.3 years. Remote recurrences as late as 30 years after initial therapy have been reported; possible mechanisms include transplantation during the surgical procedures and dissemination by meningeal seeding or CSF spreading (1). Series with radiological confirmation of the radicality of resection show that recurrence rates following gross total removal range between 0 and 62% at 10 years follow-up. These are significantly lower than those reported after partial or subtotal resection (25-100% at 10 years follow-up). In cases of limited surgery, adjuvant radiotherapy significantly improves the local control rates (recurrence rates 10-63% at 10 years follow-up). Finally, radiotherapy alone provides 10 years recurrence rates ranging between 0 and 23% (1). These results were based on the use of conventional fractionated external beam radiotherapy; tumor control rates with newer higher precision techniques, such as fractionated stereotactic conformal radiotherapy, have remained optimal with 5-year progression free survival exceeding 90% (33,34). Tumor control rates achieved by proton beam therapy in patients with craniopharyngioma are promising (35), but studies with long-term follow-up are needed. Studies with statistical comparisons of the local control rates achieved by gross total removal or the combination of surgery and radiotherapy have not provided consistent results. The interpretation of data regarding effectiveness of each therapeutic modality must be done with caution, since the published studies are retrospective, non-randomized and often specialty-biased.
The growth rate of craniopharyngiomas varies considerably and reliable clinical, radiological, and pathological criteria predicting their behavior are lacking. Thus, apart from significant impact of the treatment modality as mentioned above, attempts to identify other prognostic factors for recurrence (age, group at diagnosis, sex, imaging features, pathological subtypes) have not provided consistent results (1).
The management of recurrent tumors remains challenging, as scarring/adhesions from previous operations or irradiation make successful removal difficult. In such cases, total removal is achieved in a substantially lower rate when compared with primary surgery (0-25%). Perioperative mortality is increased following recurrence, occurring in as many as 11-24% (22). The beneficial effect of radiotherapy (proceeded or not by second surgery) in recurrent lesions has been clearly shown(15,36). Recurrent lesions with significant cystic component not amenable to total extirpation may be treated by repetitive aspirations through an indwelling Ommaya reservoir apparatus. In a small series of 11 adult patients with cystic craniopharyngiomas treated with Ommaya reservoirs, local control was achieved in 8 patients (72.7%) without the need for additional treatment over a follow up period of 41.4 months (37).
Intracystic Irradiation
Intracavitary irradiation (brachytherapy) involves stereotactically guided instillation of beta-emitting isotopes into cystic craniopharyngiomas. It delivers a higher radiation dose to the cyst than external beam radiotherapy, resulting in damage of the secretory epithelial lining, elimination of fluid production, and cyst shrinkage. The efficacy of various beta and gamma-emitting isotopes (mainly 32phosphate, 90yttrium, 186 rhenium, 198gold) has been investigated in a number of studies, but given that none of them has the ideal physical and biological profile, there is no consensus on which is the most suitable therapeutic agent. In a systematic review which included 66 children treated with brachytherapy, a reduction in tumor size was reported in 89% of children with cystic only craniopharyngiomas, and in 58% in those with mixed cystic and solid components (38). In series with mean or median follow-up between 3.1 and 11.9 years providing intracavitary irradiation (mainly with 90yttrium or 32phosphorus) at doses of 200-270 Gy, complete or partial cyst resolution was seen in 71-88%, stabilization in 3-19%, and increases in 5-10% of cases (39-44). New cyst formation or increase in the solid component of the tumor were observed in between 6.5 and 20% of cases. Although beta emitters have short range tissue penetrance, lesions in close proximity to the optic apparatus should be approached with caution (39-44). Deterioration of vision has been reported in 10-58% of cases and has been attributed to failure of cyst collapse, formation of new cysts, increase in the solid tumor, or possibly radiation damage. The reported control rates combined with low surgical morbidity and mortality render brachytherapy an attractive option for predominantly cystic tumors, particularly those that are monocystic.
Intracystic Bleomycin
Intracystic installation of the anti-neoplasmatic agent bleomycin has been used in the management of craniopharyngiomas. The drug is administered through an Ommaya reservoir connected to a catheter. In published reports the tumor control rates range between 0 and 100%. However, evidence supporting its efficacy is limited mostly to case reports or non-randomized retrospective studies, and a Cochrane review (45) exploring the effects of bleomycin in children could not recommend its use. Direct leakage of the drug to surrounding tissues during the installation procedure, diffusion though the cyst wall, or high drug doses have been associated with various toxic (hypothalamic damage, blindness, hearing loss, ischemic attacks, peritumoral edema) or even fatal effects (1,46,47). The value of this treatment option in tumor control or even in delaying surgery and/or radiotherapy, as well as the optimal protocol and the clear-cut criteria predicting the long-term outcome, remain to be established in large series with sufficient follow-up.
Intracystic Interferon-Alpha
Intracystic interferon-alpha is not neurotoxic and is therefore associated with a lower risk of adverse events when compared to other intracystic treatments. Despite encouraging results in a number of studies with short follow up, a large multicenter study demonstrated tumor progression in 75% of patients by a median of just 14 months (48,49).
Stereotactic Radiosurgery
Stereotactic radiosurgery delivers a single or small number of fraction(s) of high dose ionizing radiation to precisely mapped targets, keeping the exposure of adjacent structures to a minimum. Tumor volume and close attachment to critical structures (e.g., optic apparatus) are limiting factors for its application. Risk of optic neuropathy is <1% when the optic chiasm is constrained to a maximal dose of 10 Gy, 20 Gy, and 25 Gy, for single-fraction SRS, 3-fraction SRS, and 5-fraction SRS respectively (50). SRS achieves tumor control in a substantial number of patients with small volume lesions and reported 5-year progression free survival ranges between 61% and 90.3% (51-55). Rate of tumor control following SRS is negatively associated with tumor volume (56), thus it is particularly useful for well-defined residual disease following surgery or for the treatment of small, solid recurrent tumors situated at least 3-5mm away from the optic chiasm (49). Increasing margin dose and maximum dose >35Gy have been associated with increased risk of neurologic deficit following SRS (57). Studies with long-term follow-up evaluating the optimal marginal dose, its role in the prevention of tumor growth, and its effects on neurocognitive and neuroendocrine function, are needed.
Systemic Chemotherapy/Interferon-Alpha
The potential benefit of systemic chemotherapy in craniopharyngiomas has been investigated in a very limited number of patients. Thus, Bremer et al. (58) reported a case of successful management of a recurrent cystic tumor with the combination of vincristine, carmustine (BCNU) and procarbazine. Lippens et al. (59), after administration of five courses of doxorubicin and lomustin in 4 children with multiple or very rapid recurrences, achieved local control in 75% of them after 3-12 years follow-up. Jakacki et al. (60), in a series of 12 patients aged <21 years with progressive or recurrent craniopharyngiomas, showed that after 12 months of treatment with interferon-alpha, tumor reduction of at least 25% was observed in 3 cases. However, during the first weeks of therapy 6 patients experienced an increase in the size of the cystic component, which was finally considered as progressive disease in half of them. Interestingly, 67% of patients that completed one year of therapy without progressive disease had an increase in the size of their tumor at a median period of 11 months after discontinuation of the drug. The cytotoxicity (predominantly hepatic, neurological and cutaneous), requiring temporary discontinuation and/or dose reduction within the first 8 weeks of therapy, was significant (in up to 60% of the cases). In 2012, the same group explored the use of pegylated interferon (a derivative of interferon-alpha with a longer half-life) in five patients; all demonstrated a radiological response to treatment and two of them had a complete response (61). A subsequent phase two multi-center study gave disappointing results. Of 18 adults and children with recurrent craniopharyngiomas who were given systemic pegylated interferon, only one attained a sustained response beyond 3 months (62).
Targeted Therapy
The finding that most papillary craniopharyngiomas harbor a BRAF (V600E) mutation has opened avenues for use of pharmacological agents specifically targeting and inhibiting mutant BRAF in cases resistant to other treatments. A number of case reports and small case series have demonstrated a significant reduction in tumor size (used alone or in combination with MEK inhibitors), applied neoadjuvantly or after surgery, with or without prior radiotherapy (8,49,63-73). Common side effects associated with BRAF and MEK inhibitors seen from their use in other diseases (such as metastatic melanoma and papillary thyroid cancer) include rash, fever, diarrhea, arthralgia, and liver dysfunction (74). Cases of adamantinomatous craniopharyngiomas responding to MEK inhibitors (75), or controlled with IL-6 inhibitors used alone or in conjunction with VEGR inhibitors (12), have also been reported. The pros and cons of these new treatment modalities, particularly for aggressive tumors, warrant further assessment by trials with large number of patients and adequate follow-up. Two clinical trials (BRAF and MEK inhibitors for papillary craniopharyngiomas, and IL-6 inhibitors for children with adamantinomatous craniopharyngiomas) are currently ongoing (76,77). Initial results from one of these trials – a phase two study which included sixteen patients with papillary craniopharyngiomas harboring the BRAF V600E mutation – have been presented. All patients were treated with oral vemurafenib (BRAF inhibitor) and cobimetinib (MEK inhibitor) in 28-day cycles. Median age and follow up duration were 49.5 years and 1.8 years respectively. In those where volumetric imaging data was available, 14 (93.3%) had a radiological response to treatment, with a median tumor reduction of 83%. Grade 3 (severe) toxicities occurred in 12 patients, whilst grade 4 (potentially life threatening) toxicities occurred in two patients. Three patients stopped treatment due to adverse events (78).
MORBIDITY AND MORTALITY
Craniopharyngiomas are associated with significant long-term morbidity (mainly involving endocrine, visual, hypothalamic, neurobehavioral, and cognitive sequelae), which is attributed to the damage of critical structures by the primary or recurrent tumor and/or to the adverse effects of the therapeutic interventions. Notably, the severity of the radiation-induced late toxicity is affected by the total and per fraction doses, the volume of the exposed normal tissue, and the young age in childhood populations (1).
Endocrine
Long-term endocrine morbidity is significant. At last assessment, the rates of individual hormone deficits range between 88−100% for GH, 80−95% for FSH/LH, 55−88% for ACTH, 39−95% for TSH and 25−86% for ADH (1). Restoration of pre-existing hormone deficits following surgical removal is rare, and aggressive surgery leads to more frequent pituitary dysfunction (1,23,79).
The phenomenon of «growth without growth hormone» has been reported in some children with craniopharyngioma who show normal or even accelerated linear growth, despite their untreated GH deficiency. The pathophysiological mechanism has not been clarified; the obesity-associated hyperinsulinemia has been proposed as a factor stimulating growth by affecting serum concentrations of IGF-I or by binding directly to the IGF-I receptor (80,81). Review of adult patients with craniopharyngioma and severe GH deficiency but no recent GH treatment (from the KIMS database: Pfizer International Metabolic Database) has shown that those with childhood-onset disease were shorter than those with adult-onset disease, and obesity was more common in the adult-onset patients. Furthermore, quality of life, assessed by Quality of Life-Assessment of Growth Hormone Deficiency in Adults (QoL-AGHDA) and the Nottingham Health Profile, was markedly reduced with no significant differences between those with childhood-onset and those with adult-onset disease (82). A 3-year longitudinal analysis of the changes in height, weight, and body mass index (BMI) SDS in 199 GH-treated pre-pubertal children with post-surgical and/or post-irradiated craniopharyngioma showed that GH therapy induced excellent linear growth compared with children with other forms of organic GH deficiency. Still, the children with craniopharyngioma had a higher BMI; GH had no salutary effect on weight SDS and caused only a mild improvement in BMI SDS (83). A study of 351 patients with adult-onset craniopharyngioma compared with 370 patients with non-functioning pituitary adenomas matched for age and sex (all GH deficient) demonstrated that, after two years of GH replacement, there were significant similar improvements in both groups in free-fat mass, total and low-density lipoprotein and Quality of Life Assessment in the GH-deficient score compared with baseline. Results from a 12-year prospective study showed children with craniopharyngioma treated with GH had improved weight and quality of life outcomes compared to those who were not replaced, or in those who only received GH as adults (84). Observational studies have also shown that growth hormone replacement is not associated with increased risk of tumor recurrence.
Diabetes insipidus with an absent or impaired sense of thirst confers a significant risk of serious electrolyte imbalance, and is one of the most difficult complications to manage. In this group of patients, the maintenance of osmotic balance has been shown to be precarious with recurrent episodes of hyper- or hyponatremia contributing to morbidity and mortality. Careful fluid balance with close monitoring of intake/output and daily weights is crucial.
Vision
Visual outcome is adversely affected by the presence of visual symptoms at diagnosis and by daily irradiation doses >2 Gy (1). Radiation optic neuropathy occurs in 1–2% patients receiving doses to 50 Gy and this is mostly confined to those with pre-radiotherapy visual impairment, with the risk being higher with doses of 55 Gy and above (1,28).
Hypothalamic
Hypothalamic damage may result in hyperphagia and uncontrollable obesity, disorders of thirst and water/electrolyte balance, behavioral and cognitive impairment, loss of temperature control and disordered sleep pattern. Among these, obesity is the most frequent (reported in 26-61% of the patients treated by surgery combined or not with radiotherapy) and is a consequence of the disruption of the mechanisms controlling satiety, hunger, and energy balance (1,15,85-88). Possible contributing mechanisms include lack of sensitivity to endogenous leptin (89,90) and reduced energy expenditure, and is exaggerated by comorbidities including neurological defects, visual failure, somnolence (91), sleep disturbance, hypopituitarism and psychosocial disorders (92). In a study of 63 survivors of childhood craniopharyngioma, all those with marked obesity after surgery had evidence of significant alterations of the normal hypothalamic anatomy, with their MRI showing either complete deficiency or extensive destruction of the floor of the 3rd ventricle (93). Several image grading systems, used pre- or post-operatively, have been proposed to help predict hypothalamic sequalae and hypothalamic morbidity by defining hypothalamic involvement on imaging and severity of tumor adherence to the hypothalamus (94-98). Furthermore, it has been reported that the basal metabolic rate adjusted to total body weight is significantly lower in adults with craniopharyngioma compared with controls, and that the energy intake/basal metabolic rate ratio is significantly lower in subjects with tumor growth into the 3rd ventricle (99). Children with surgically-treated craniopharyngioma were found to have decreased aerobic capacity during an exercise test, which was most pronounced in those with hypothalamic involvement. Interestingly, in this study, GH treatment was associated with significant positive effect on aerobic capacity only in the absence of hypothalamic involvement (100). Finally, high levels of the orexigenic gastric hormone ghrelin have not been found in these patients (101). Factors proposed to be associated with significant hypothalamic morbidity are young age at presentation, hypothalamic disturbance at diagnosis, hypothalamic invasion, attempts to remove adherent tumor from the region of hypothalamus, multiple operations for recurrence, and hypothalamic radiation doses >51 Gy (1,102,103). Interestingly, in a retrospective study including 45 adults with craniopharyngioma followed for a median of 26 months, a lower BMI pre-operatively was predictive of greater post-operative weight gain(104). In contrast, a higher pre-operative BMI has been found to be associated with severe post-operative obesity in children(18). Hypothalamic obesity often results in devastating metabolic and psychosocial complications, necessitating provision of dietary and behavioral modifications, encouragement of regular physical activity, psychological counselling, and anti-obesity drugs. Based on a limited number of published cases, gastric bypass surgery results in weight loss; in a systematic review and meta-analysis including 21 cases of bariatric surgery for hypothalamic obesity in patients with craniopharyngioma (6 with adjustable gastric banding, 8 with sleeve gastrectomy, 6 with Roux-en-Y gastric bypass and 1 with biliopancreatic diversion), it was shown that the maximal mean weight loss was achieved in the gastric bypass group after 12 months (105). Furthermore, Weismann et al. (106) in a series of 7 patients with morbid obesity after surgery for craniopharyngioma, who underwent laparoscopic gastric banding or laparoscopic sleeve gastrectomy, reported no significant loss of body weight. A case control study suggested that Roux-en-Y surgery, but not sleeve gastrectomy, yielded equivalent weight loss in craniopharyngioma patients to those with “common” obesity and resulted in significant reductions to BMI after one year (107). The same group subsequently conducted a larger, multi-center case control study, with a median follow up of 5.2 years (108). Obese patients with craniopharyngioma had a mean weight loss of 22% at 5 years after bariatric surgery; irrespective of type of procedure. In contrast to their original findings (107), obese controls lost more weight after Roux-en-Y gastric bypass, whereas sleeve gastrectomy led to similar results in both groups (108). Medical therapies including dextroamphetamine, the combination of diazoxide and metformin (aiming to reduce the hyperinsulinemia), octreotide (aiming to reduce hyperinsulinemia and simultaneously enhance the insulin action), glucagon-like peptide-1 analogues, and a novel methionine aminopeptidase 2 inhibitor, have all been proposed as potential approaches to this significant problem (92). However, outcomes following these therapies are variable and long-term benefits have not yet been established. Studies with large number of patients and longer follow-up are needed to establish the efficacy and safety of these surgical and medical management options.
Neuropsychological and Cognitive
The compromised neuropsychological and cognitive function in patients with craniopharyngioma after surgery and radiation therapy contributes significantly to poor academic and work performance, disrupted family and social relationships, disrupted body image, and impaired quality of life. Gross total resection, radiotherapy, pre-operative hypothalamic involvement, or intra-operative hypothalamic injury have been associated with a lower quality of life in adults and children with craniopharyngioma (109). It has also been proposed that visual, neurological, and endocrine morbidities negatively impact neuropsychological outcomes (110,111). Areas particularly affected (especially in childhood-onset disease) include memory, attention, executive function, and motivation (110,112-114), with hypothalamic involvement being a risk factor for poorer outcomes (113). In a series of 121 patients followed-up for a mean period of 10 years, Duff et al.(115) found that 40% had poor functional neuropsychiatric outcome. Karavitaki et al. (15), in a series of 121 patients, found cumulative probabilities for permanent motor deficits, epilepsy, psychological disorders necessitating treatment, and complete dependency for basal daily activities at 10-year follow-up of 11%, 12%, 15% and 9%, respectively. There is no consensus on the therapeutic option with the least unfavorable impact on the neurobehavioral outcome, necessitating prospective studies with formal neuropsychological testing and specific behavioral assessment before and after any intervention. Such data will be particularly important for young children, as there are uncertainties including whether delaying irradiation is a reasonable policy in this age group.
Long-Term Mortality
The mortality rates of patients with craniopharyngioma have been described to be 3-6 times higher than that of the general population and reported 10-years survival rates range between 83% and 93% (1,87). Qiao et al.(116) reported a significant fall in the SMR from 6.2 (95% CI 4.1-9.4) to 2.9 (95% CI 2.2-3.8) for studies published before 2010, and after 2010, respectively (116). Apart from the deaths directly attributed to the tumor (pressure effects to critical structures) and to the surgical interventions, the risk of cardio-/cerebrovascular and respiratory mortality is increased (1,117,118). In one study which included 244 patients with childhood onset craniopharyngioma, 11% of patients developed a cerebral infarction. Hydrocephalus and gross total resection were identified as risk factors, and none were attributable to radiotherapy (119). The increased cardiovascular mortality in this population may be driven in part by hypothalamic obesity and related metabolic complications. Long-term follow-up of adult patients with craniopharyngiomas has demonstrated increased prevalence of the metabolic syndrome compared with the general population (120), and hypothalamic involvement has been shown to have negative impact on mortality (121). It has been suggested that in childhood populations the hypoadrenalism and the associated hypoglycemia, as well as the metabolic consequences of ADH deficiency and absent thirst, may also contribute to the excessive mortality. The impact of tumor recurrence on the long-term mortality is widely accepted and the 10-year survival rates in such cases range between 29% and 70%, depending on the subsequent treatment modalities (15).
CONCLUSIONS AND FUTURE PERSPECTIVES
Craniopharyngiomas present many unique challenges for clinicians. Whilst controversies regarding the optimal management approach for these rare tumors still exist, the need to prevent hypothalamic morbidities associated with surgical intervention in this area is essential.
Enhanced understanding of the pathogenesis of both adamantinomatous and papillary craniopharyngiomas has led to the concept of targeted medical therapy, an area at the forefront of translational research. Optimal outcomes following the use of BRAF V600 and MEK inhibitors have been described in case reports and are a promising treatment prospect, with hope that their efficacy and safety are supported by the results of large, prospective, randomized studies.
Patients face a high burden of post-treatment morbidity due to endocrine, visual, hypothalamic, and neuropsychological complications, and mortality rates are increased compared with the general population. Obesity is one of the most significant comorbidities with often devastating sequelae; its pathogenesis is multifactorial, and its management is one of the most challenging problems clinicians have to deal with. Given the complexity of these tumors, care for these patients should be provided by an experienced multidisciplinary team.
ACKNOWLEDGEMENTS
Radiology images provided courtesy of Dr. Swarupsinh Chavda, Consultant Neuroradiologist, Queen Elizabeth Hospital Birmingham, UK.
REFERENCES
- 1.
- Karavitaki N, Cudlip S, Adams CB, Wass JA. Craniopharyngiomas. Endocrine reviews. 2006;27:371–397. [PubMed: 16543382]
- 2.
- Nielsen EH, Feldt-Rasmussen U, Poulsgaard L, Kristensen LO, Astrup J, Jorgensen JO, Bjerre P, Andersen M, Andersen C, Jorgensen J, Lindholm J, Laurberg P. Incidence of craniopharyngioma in Denmark (n = 189) and estimated world incidence of craniopharyngioma in children and adults. Journal of neuro-oncology. 2011;104:755–763. [PubMed: 21336771]
- 3.
- Scagliotti V, Avagliano L, Gualtieri A, Graziola F, Doi P, Chalker J, Righini A, Korbonits M, Bulfamante G, Jacques TS, Massa V, Gaston-Massuet C. Histopathology and molecular characterisation of intrauterine-diagnosed congenital craniopharyngioma. Pituitary. 2016;19:50–56. [PubMed: 26350256]
- 4.
- Bunin GR, Surawicz TS, Witman PA, Preston-Martin S, Davis F, Bruner JM. The descriptive epidemiology of craniopharyngioma. J Neurosurg. 1998;89:547–551. [PubMed: 9761047]
- 5.
- Sorva R, Heiskanen O. Craniopharyngioma in Finland. A study of 123 cases. Acta Neurochir (Wien). 1986;81:85–89. [PubMed: 3489356]
- 6.
- Crotty TB, Scheithauer BW, Young WF Jr, Davis DH, Shaw EG, Miller GM, Burger PC. Papillary craniopharyngioma: a clinicopathological study of 48 cases. J Neurosurg. 1995;83:206–214. [PubMed: 7616262]
- 7.
- Buslei R, Nolde M, Hofmann B, Meissner S, Eyupoglu IY, Siebzehnrübl F, Hahnen E, Kreutzer J, Fahlbusch R. Common mutations of beta-catenin in adamantinomatous craniopharyngiomas but not in other tumours originating from the sellar region. Acta neuropathologica. 2005;109:589–597. [PubMed: 15891929]
- 8.
- Larkin S, Karavitaki N. Recent advances in molecular pathology of craniopharyngioma. F1000Res. 2017;6:1202. [PMC free article: PMC5531159] [PubMed: 28781761]
- 9.
- Müller HL, Merchant TE, Puget S, Martinez-Barbera JP. New outlook on the diagnosis, treatment and follow-up of childhood-onset craniopharyngioma. Nat Rev Endocrinol. 2017;13:299–312. [PubMed: 28155902]
- 10.
- Alexandraki KI, Kaltsas GA, Karavitaki N, Grossman AB. The Medical Therapy of Craniopharyngiomas: The Way Ahead. The Journal of clinical endocrinology and metabolism. 2019;104:5751–5764. [PubMed: 31369091]
- 11.
- Donson AM, Apps J, Griesinger AM, Amani V, Witt DA, Anderson RCE, Niazi TN, Grant G, Souweidane M, Johnston JM, Jackson EM, Kleinschmidt-DeMasters BK, Handler MH, Tan AC, Gore L, Virasami A, Gonzalez-Meljem JM, Jacques TS, Martinez-Barbera JP, Foreman NK, Hankinson TC. Molecular Analyses Reveal Inflammatory Mediators in the Solid Component and Cyst Fluid of Human Adamantinomatous Craniopharyngioma. Journal of neuropathology and experimental neurology. 2017;76:779–788. [PMC free article: PMC6005018] [PubMed: 28859336]
- 12.
- Grob S, Mirsky DM, Donson AM, Dahl N, Foreman NK, Hoffman LM, Hankinson TC, Mulcahy Levy JM. Targeting IL-6 Is a Potential Treatment for Primary Cystic Craniopharyngioma. Frontiers in Oncology. 2019:9. [PMC free article: PMC6712354] [PubMed: 31497533]
- 13.
- Coy S, Rashid R, Lin JR, Du Z, Donson AM, Hankinson TC, Foreman NK, Manley PE, Kieran MW, Reardon DA, Sorger PK, Santagata S. Multiplexed immunofluorescence reveals potential PD-1/PD-L1 pathway vulnerabilities in craniopharyngioma. Neuro Oncol. 2018;20:1101–1112. [PMC free article: PMC6280314] [PubMed: 29509940]
- 14.
- Martinez-Barbera JP, Andoniadou CL. Biological Behaviour of Craniopharyngiomas. Neuroendocrinology. 2020;110:797–804. [PubMed: 32126562]
- 15.
- Karavitaki N, Brufani C, Warner JT, Adams CB, Richards P, Ansorge O, Shine B, Turner HE, Wass JA. Craniopharyngiomas in children and adults: systematic analysis of 121 cases with long-term follow-up. Clin Endocrinol (Oxf). 2005;62:397–409. [PubMed: 15807869]
- 16.
- Gautier A, Godbout A, Grosheny C, Tejedor I, Coudert M, Courtillot C, Jublanc C, De Kerdanet M, Poirier JY, Riffaud L, Sainte-Rose C, Van Effenterre R, Brassier G, Bonnet F, Touraine P. Markers of recurrence and long-term morbidity in craniopharyngioma: a systematic analysis of 171 patients. The Journal of clinical endocrinology and metabolism. 2012;97:1258–1267. [PubMed: 22319039]
- 17.
- Nielsen EH, Jørgensen JO, Bjerre P, Andersen M, Andersen C, Feldt-Rasmussen U, Poulsgaard L, Kristensen L, Astrup J, Jørgensen J, Laurberg P. Acute presentation of craniopharyngioma in children and adults in a Danish national cohort. Pituitary. 2013;16:528–535. [PubMed: 23225120]
- 18.
- Müller HL, Emser A, Faldum A, Bruhnken G, Etavard-Gorris N, Gebhardt U, Oeverink R, Kolb R, Sörensen N. Longitudinal study on growth and body mass index before and after diagnosis of childhood craniopharyngioma. The Journal of clinical endocrinology and metabolism. 2004;89:3298–3305. [PubMed: 15240606]
- 19.
- Fahlbusch R, Honegger J, Paulus W, Huk W, Buchfelder M. Surgical treatment of craniopharyngiomas: experience with 168 patients. J Neurosurg. 1999;90:237–250. [PubMed: 9950494]
- 20.
- De Vile CJ, Grant DB, Kendall BE, Neville BG, Stanhope R, Watkins KE, Hayward RD. Management of childhood craniopharyngioma: can the morbidity of radical surgery be predicted? J Neurosurg. 1996;85:73–81. [PubMed: 8683285]
- 21.
- Van Effenterre R, Boch AL. Craniopharyngioma in adults and children: a study of 122 surgical cases. J Neurosurg. 2002;97:3–11. [PubMed: 12134929]
- 22.
- Cossu G, Jouanneau E, Cavallo LM, Elbabaa SK, Giammattei L, Starnoni D, Barges-Coll J, Cappabianca P, Benes V, Baskaya MK, Bruneau M, Meling T, Schaller K, Chacko AG, Youssef AS, Mazzatenta D, Ammirati M, Dufour H, Laws E, Berhouma M, Daniel RT, Messerer M. Surgical management of craniopharyngiomas in adult patients: a systematic review and consensus statement on behalf of the EANS skull base section. Acta Neurochir (Wien). 2020;162:1159–1177. [PubMed: 32112169]
- 23.
- Tan TSE, Patel L, Gopal-Kothandapani JS, Ehtisham S, Ikazoboh EC, Hayward R, Aquilina K, Skae M, Thorp N, Pizer B, Didi M, Mallucci C, Blair JC, Gaze MN, Kamaly-Asl I, Spoudeas H, Clayton PE. The neuroendocrine sequelae of paediatric craniopharyngioma: a 40-year meta-data analysis of 185 cases from three UK centres. European journal of endocrinology. 2017;176:359–369. [PubMed: 28073908]
- 24.
- Buchfelder M, Schlaffer SM, Lin F, Kleindienst A. Surgery for craniopharyngioma. Pituitary. 2013;16:18–25. [PubMed: 22836237]
- 25.
- Elliott RE, Jane JA Jr, Wisoff JH. Surgical management of craniopharyngiomas in children: meta-analysis and comparison of transcranial and transsphenoidal approaches. Neurosurgery. 2011;69:630–643. [PubMed: 21499159]
- 26.
- Carpenter R, Chamberlin G, Frazier C. The treatment of hypophyseal stalk tumours by evacuation and irradiation. Am J Roent. 1937;38:162–167.
- 27.
- Kramer S, McKissock W, Concannon JP. Craniopharyngiomas. Treatment by combined surgery and radiation therapy. J Neurosurg. 1961;18:217–226. [PubMed: 13753942]
- 28.
- Aggarwal A, Fersht N, Brada M. Radiotherapy for craniopharyngioma. Pituitary. 2013;16:26–33. [PubMed: 22948229]
- 29.
- Lamiman K, Wong KK, Tamrazi B, Nosrati JD, Olch A, Chang EL, Kiehna EN. A quantitative analysis of craniopharyngioma cyst expansion during and after radiation therapy and surgical implications. Neurosurgical focus. 2016;41:E15. [PubMed: 27903114]
- 30.
- Shi Z, Esiashvili N, Janss AJ, Mazewski CM, MacDonald TJ, Wrubel DM, Brahma B, Schwaibold FP, Marcus RB, Crocker IR, Shu HK. Transient enlargement of craniopharyngioma after radiation therapy: pattern of magnetic resonance imaging response following radiation. Journal of neuro-oncology. 2012;109:349–355. [PubMed: 22692563]
- 31.
- Rutenberg MS, Rotondo RL, Rao D, Holtzman AL, Indelicato DJ, Huh S, Morris CG, Mendenhall WM. Clinical outcomes following proton therapy for adult craniopharyngioma: a single-institution cohort study. Journal of neuro-oncology. 2020;147:387–395. [PubMed: 32086697]
- 32.
- Rajan B, Ashley S, Thomas DG, Marsh H, Britton J, Brada M. Craniopharyngioma: improving outcome by early recognition and treatment of acute complications. International journal of radiation oncology, biology, physics. 1997;37:517–521. [PubMed: 9112447]
- 33.
- Minniti G, Saran F, Traish D, Soomal R, Sardell S, Gonsalves A, Ashley S, Warrington J, Burke K, Mosleh-Shirazi A, Brada M. Fractionated stereotactic conformal radiotherapy following conservative surgery in the control of craniopharyngiomas. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2007;82:90–95. [PubMed: 17161483]
- 34.
- Combs SE, Thilmann C, Huber PE, Hoess A, Debus J, Schulz-Ertner D. Achievement of long-term local control in patients with craniopharyngiomas using high precision stereotactic radiotherapy. Cancer. 2007;109:2308–2314. [PubMed: 17469176]
- 35.
- Luu QT, Loredo LN, Archambeau JO, Yonemoto LT, Slater JM, Slater JD. Fractionated proton radiation treatment for pediatric craniopharyngioma: preliminary report. Cancer J. 2006;12:155–159. [PubMed: 16630407]
- 36.
- Jose CC, Rajan B, Ashley S, Marsh H, Brada M. Radiotherapy for the treatment of recurrent craniopharyngioma. Clinical oncology (Royal College of Radiologists (Great Britain)) 1992; 4:287-289. [PubMed: 1390343]
- 37.
- Frio F, Solari D, Cavallo LM, Cappabianca P, Raverot G, Jouanneau E. Ommaya Reservoir System for the Treatment of Cystic Craniopharyngiomas: Surgical Results in a Series of 11 Adult Patients and Review of the Literature. World Neurosurg. 2019;132:e869–e877. [PubMed: 31400528]
- 38.
- Guimarães MM, Cardeal DD, Teixeira MJ, Lucio JEDC, Sanders FH, Kuromoto RK, Matushita H. Brachytherapy in paediatric craniopharyngiomas: a systematic review and meta-analysis of recent literature. Child's Nervous System. 2022;38:253–262. [PubMed: 34618201]
- 39.
- Pollock BE, Lunsford LD, Kondziolka D, Levine G, Flickinger JC. Phosphorus-32 intracavitary irradiation of cystic craniopharyngiomas: current technique and long-term results. International journal of radiation oncology, biology, physics. 1995;33:437–446. [PubMed: 7673031]
- 40.
- Voges J, Sturm V, Lehrke R, Treuer H, Gauss C, Berthold F. Cystic craniopharyngioma: long-term results after intracavitary irradiation with stereotactically applied colloidal beta-emitting radioactive sources. Neurosurgery. 1997;40:263–269. [PubMed: 9007857]
- 41.
- Van den Berge JH, Blaauw G, Breeman WA, Rahmy A, Wijngaarde R. Intracavitary brachytherapy of cystic craniopharyngiomas. J Neurosurg. 1992;77:545–550. [PubMed: 1527612]
- 42.
- Hasegawa T, Kondziolka D, Hadjipanayis CG, Lunsford LD. Management of cystic craniopharyngiomas with phosphorus-32 intracavitary irradiation. Neurosurgery. 2004;54:813–820. [PubMed: 15046646]
- 43.
- Julow JV. Intracystic irradiation for craniopharyngiomas. Pituitary. 2013;16:34–45. [PubMed: 23196808]
- 44.
- Kickingereder P, Maarouf M, El Majdoub F, Fuetsch M, Lehrke R, Wirths J, Luyken K, Schomaecker K, Treuer H, Voges J, Sturm V. Intracavitary brachytherapy using stereotactically applied phosphorus-32 colloid for treatment of cystic craniopharyngiomas in 53 patients. Journal of neuro-oncology. 2012;109:365–374. [PubMed: 22717668]
- 45.
- Zhang S, Fang Y, Cai BW, Xu JG, You C. Intracystic bleomycin for cystic craniopharyngiomas in children. Cochrane Database Syst Rev. 2016;7:Cd008890. [PMC free article: PMC6457977] [PubMed: 27416004]
- 46.
- Takahashi H, Yamaguchi F, Teramoto A. Long-term outcome and reconsideration of intracystic chemotherapy with bleomycin for craniopharyngioma in children. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery 2005; 21:701-704. [PubMed: 15928963]
- 47.
- Hukin J, Steinbok P, Lafay-Cousin L, Hendson G, Strother D, Mercier C, Samson Y, Howes W, Bouffet E. Intracystic bleomycin therapy for craniopharyngioma in children: the Canadian experience. Cancer. 2007;109:2124–2131. [PubMed: 17407137]
- 48.
- Kilday JP, Caldarelli M, Massimi L, Chen RH, Lee YY, Liang ML, Parkes J, Naiker T, van Veelen ML, Michiels E, Mallucci C, Pettorini B, Meijer L, Dorfer C, Czech T, Diezi M, Schouten-van Meeteren AYN, Holm S, Gustavsson B, Benesch M, Müller HL, Hoffmann A, Rutkowski S, Flitsch J, Escherich G, Grotzer M, Spoudeas HA, Azquikina K, Capra M, Jiménez-Guerra R, MacDonald P, Johnston DL, Dvir R, Constantini S, Kuo MF, Yang SH, Bartels U. Intracystic interferon-alpha in pediatric craniopharyngioma patients: an international multicenter assessment on behalf of SIOPE and ISPN. Neuro Oncol. 2017;19:1398–1407. [PMC free article: PMC5596165] [PubMed: 28499018]
- 49.
- Hamblin R, Tsermoulas G, Karavitaki N. Craniopharyngiomas. La Presse Médicale. 2021;50:104078.
- 50.
- Albano L, Losa M, Flickinger J, Mortini P, Minniti G. Radiotherapy of Parasellar Tumours. Neuroendocrinology. 2020;110:848–858. [PubMed: 32126559]
- 51.
- Xu Z, Yen CP, Schlesinger D, Sheehan J. Outcomes of Gamma Knife surgery for craniopharyngiomas. Journal of neuro-oncology. 2011;104:305–313. [PubMed: 21153860]
- 52.
- Losa M, Pieri V, Bailo M, Gagliardi F, Barzaghi LR, Gioia L, Del Vecchio A, Bolognesi A, Mortini P. Single fraction and multisession Gamma Knife radiosurgery for craniopharyngioma. Pituitary. 2018;21:499–506. [PubMed: 30043097]
- 53.
- Tsugawa T, Kobayashi T, Hasegawa T, Iwai Y, Matsunaga S, Yamamoto M, Hayashi M, Kenai H, Kano T, Mori H, Nagano O, Hasegawa S, Inoue A, Nagatomo Y, Onoue S, Sato M, Yasuda S. Gamma Knife Surgery for Residual or Recurrent Craniopharyngioma After Surgical Resection: A Multi-institutional Retrospective Study in Japan. Cureus. 2020;12:e6973–e6973. [PMC free article: PMC7075476] [PubMed: 32201653]
- 54.
- Niranjan A, Kano H, Mathieu D, Kondziolka D, Flickinger JC, Lunsford LD. Radiosurgery for craniopharyngioma. International journal of radiation oncology, biology, physics. 2010;78:64–71. [PubMed: 20005637]
- 55.
- Kobayashi T, Kida Y, Mori Y, Hasegawa T. Long-term results of gamma knife surgery for the treatment of craniopharyngioma in 98 consecutive cases. J Neurosurg. 2005;103:482–488. [PubMed: 16383245]
- 56.
- Lee CC, Yang HC, Chen CJ, Hung YC, Wu HM, Shiau CY, Guo WY, Pan DH, Chung WY, Liu KD. Gamma Knife surgery for craniopharyngioma: report on a 20-year experience. J Neurosurg. 2014;121 Suppl:167–178. [PubMed: 25434950]
- 57.
- Pikis S, Mantziaris G, Lavezzo K, Dabhi N, Sheehan J. Stereotactic radiosurgery for craniopharyngiomas. Acta Neurochirurgica. 2021;163:3201–3207. [PubMed: 34518903]
- 58.
- Bremer AM, Nguyen TQ, Balsys R. Therapeutic benefits of combination chemotherapy with vincristine, BCNU, and procarbazine on recurrent cystic craniopharyngioma. A case report. Journal of neuro-oncology. 1984;2:47–51. [PubMed: 6470759]
- 59.
- Lippens RJ, Rotteveel JJ, Otten BJ, Merx H. Chemotherapy with Adriamycin (doxorubicin) and CCNU (lomustine) in four children with recurrent craniopharyngioma. Eur J Paediatr Neurol. 1998;2:263–268. [PubMed: 10726829]
- 60.
- Jakacki RI, Cohen BH, Jamison C, Mathews VP, Arenson E, Longee DC, Hilden J, Cornelius A, Needle M, Heilman D, Boaz JC, Luerssen TG. Phase II evaluation of interferon-alpha-2a for progressive or recurrent craniopharyngiomas. J Neurosurg. 2000;92:255–260. [PubMed: 10659012]
- 61.
- Yeung JT, Pollack IF, Panigrahy A, Jakacki RI. Pegylated interferon-α-2b for children with recurrent craniopharyngioma. Journal of neurosurgery Pediatrics. 2012;10:498–503. [PubMed: 23061825]
- 62.
- Goldman S, Pollack IF, Jakacki RI, Billups CA, Poussaint TY, Adesina AM, Panigrahy A, Parsons DW, Broniscer A, Robinson GW, Robison NJ, Partap S, Kilburn LB, Onar-Thomas A, Dunkel IJ, Fouladi M. Phase II study of peginterferon alpha-2b for patients with unresectable or recurrent craniopharyngiomas: a Pediatric Brain Tumor Consortium report. Neuro Oncol. 2020;22:1696–1704. [PMC free article: PMC7690365] [PubMed: 32393959]
- 63.
- Aylwin SJB, Bodi I, Beaney R. Pronounced response of papillary craniopharyngioma to treatment with vemurafenib, a BRAF inhibitor. Pituitary. 2016;19:544–546. [PMC free article: PMC4996872] [PubMed: 26115708]
- 64.
- Brastianos PK, Shankar GM, Gill CM, Taylor-Weiner A, Nayyar N, Panka DJ, Sullivan RJ, Frederick DT, Abedalthagafi M, Jones PS. Dramatic response of BRAF V600E mutant papillary craniopharyngioma to targeted therapy. JNCI: Journal of the National Cancer Institute. 2016:108. [PMC free article: PMC4862417] [PubMed: 26498373]
- 65.
- Rostami E, Witt Nyström P, Libard S, Wikström J, Casar-Borota O, Gudjonsson O. Recurrent papillary craniopharyngioma with BRAFV600E mutation treated with neoadjuvant-targeted therapy. Acta Neurochirurgica. 2017;159:2217–2221. [PMC free article: PMC5636852] [PubMed: 28918496]
- 66.
- Roque A, Odia Y. BRAF-V600E mutant papillary craniopharyngioma dramatically responds to combination BRAF and MEK inhibitors. CNS Oncology. 2017;6:95–99. [PMC free article: PMC6020871] [PubMed: 28425764]
- 67.
- Himes BT, Ruff MW, van Gompel JJ, Park SS, Galanis E, Kaufmann TJ, Uhm JH. Recurrent papillary craniopharyngioma with BRAF V600E mutation treated with dabrafenib: Case report. J Neurosurg. 2018;1:1–5. [PubMed: 29701552]
- 68.
- Rao M, Bhattacharjee M, Shepard S, Hsu S. Newly diagnosed papillary craniopharyngioma with BRAF V600E mutation treated with single-agent selective BRAF inhibitor dabrafenib: a case report. Oncotarget. 2019:10. [PMC free article: PMC6800270] [PubMed: 31666933]
- 69.
- Khaddour K, Chicoine MR, Huang J, Dahiya S, Ansstas G. Successful Use of BRAF/MEK Inhibitors as a Neoadjuvant Approach in the Definitive Treatment of Papillary Craniopharyngioma. Journal of the National Comprehensive Cancer Network J Natl Compr Canc Netw. 2020;18:1590–1595. [PubMed: 33285519]
- 70.
- Di Stefano AL, Guyon D, Sejean K, Feuvret L, Villa C, Berzero G, Desforges Bullet V, Halimi E, Boulin A, Baussart B, Gaillard S. Medical debulking with BRAF/MEK inhibitors in aggressive BRAF-mutant craniopharyngioma. Neurooncol Adv 2020; 2:vdaa141-vdaa141. [PMC free article: PMC7680180] [PubMed: 33241217]
- 71.
- Juratli TA, Jones PS, Wang N, Subramanian M, Aylwin SJB, Odia Y, Rostami E, Gudjonsson O, Shaw BL, Cahill DP, Galanis E, Barker FG 2nd, Santagata S, Brastianos PK. Targeted treatment of papillary craniopharyngiomas harboring BRAF V600E mutations. Cancer. 2019;125:2910–2914. [PMC free article: PMC7032527] [PubMed: 31314136]
- 72.
- Chik CL, van Landeghem FKH, Easaw JC, Mehta V. Aggressive Childhood-onset Papillary Craniopharyngioma Managed With Vemurafenib, a BRAF Inhibitor. Journal of the Endocrine Society 2021; 5:bvab043. [PMC free article: PMC8064044] [PubMed: 33928205]
- 73.
- Bernstein A, Mrowczynski OD, Greene A, Ryan S, Chung C, Zacharia BE, Glantz M. Dual BRAF/MEK therapy in BRAF V600E-mutated primary brain tumors: a case series showing dramatic clinical and radiographic responses and a reduction in cutaneous toxicity. J Neurosurg. 2019:1–6. [PubMed: 31675726]
- 74.
- Welsh SJ, Corrie PG. Management of BRAF and MEK inhibitor toxicities in patients with metastatic melanoma. Ther Adv Med Oncol. 2015;7:122–136. [PMC free article: PMC4346212] [PubMed: 25755684]
- 75.
- Patel K, Allen J, Zagzag D, Wisoff J, Radmanesh A, Gindin T, Nicolaides T. Radiologic response to MEK inhibition in a patient with a WNT-activated craniopharyngioma. Pediatr Blood Cancer. 2021;68:e28753. [PubMed: 33073916]
- 76.
- Vemurafenib and Cobimetinib in Treating Patients With BRAF V600E Mutation Positive Craniopharyngioma. https://ClinicalTrials.gov/show/NCT03224767.
- 77.
- Tocilizumab in Children With ACP. https://ClinicalTrials.gov/show/NCT03970226.
- 78.
- Brastianos PK, Twohy E, Geyer SM, Gerstner ER, Kaufmann TJ, Ruff M, Bota DA, Reardon DA, Cohen AL, Fuente MIDL, Lesser GJ, Campian JL, Agarwalla P, Kumthekar P, Cahill DP, Shih HA, Brown PD, Santagata S, Barker FG, Galanis E. Alliance A071601: Phase II trial of BRAF/MEK inhibition in newly diagnosed papillary craniopharyngiomas. Journal of Clinical Oncology. 2021;39:2000–2000.
- 79.
- Cohen M, Bartels U, Branson H, Kulkarni AV, Hamilton J. Trends in treatment and outcomes of pediatric craniopharyngioma, 1975-2011. Neuro Oncol. 2013;15:767–774. [PMC free article: PMC3661103] [PubMed: 23486689]
- 80.
- DeVile CJ, Grant DB, Hayward RD, Stanhope R. Growth and endocrine sequelae of craniopharyngioma. Arch Dis Child. 1996;75:108–114. [PMC free article: PMC1511642] [PubMed: 8869189]
- 81.
- Schoenle EJ, Zapf J, Prader A, Torresani T, Werder EA, Zachmann M. Replacement of growth hormone (GH) in normally growing GH-deficient patients operated for craniopharyngioma. The Journal of clinical endocrinology and metabolism. 1995;80:374–378. [PubMed: 7852493]
- 82.
- Kendall-Taylor P, Jonsson PJ, Abs R, Erfurth EM, Koltowska-Haggstrom M, Price DA, Verhelst J. The clinical, metabolic and endocrine features and the quality of life in adults with childhood-onset craniopharyngioma compared with adult-onset craniopharyngioma. European journal of endocrinology. 2005;152:557–567. [PubMed: 15817911]
- 83.
- Geffner M, Lundberg M, Koltowska-Haggstrom M, Abs R, Verhelst J, Erfurth EM, Kendall-Taylor P, Price DA, Jonsson P, Bakker B. Changes in height, weight, and body mass index in children with craniopharyngioma after three years of growth hormone therapy: analysis of KIGS (Pfizer International Growth Database). The Journal of clinical endocrinology and metabolism. 2004;89:5435–5440. [PubMed: 15531494]
- 84.
- Boekhoff S, Bogusz A, Sterkenburg AS, Eveslage M, Müller HL. Long-term Effects of Growth Hormone Replacement Therapy in Childhood-onset Craniopharyngioma: Results of the German Craniopharyngioma Registry (HIT-Endo). European journal of endocrinology. 2018;179:331–341. [PubMed: 30139824]
- 85.
- Daousi C, Dunn AJ, Foy PM, MacFarlane IA, Pinkney JH. Endocrine and neuroanatomic features associated with weight gain and obesity in adult patients with hypothalamic damage. Am J Med. 2005;118:45–50. [PubMed: 15639209]
- 86.
- Stripp DC, Maity A, Janss AJ, Belasco JB, Tochner ZA, Goldwein JW, Moshang T, Rorke LB, Phillips PC, Sutton LN, Shu HK. Surgery with or without radiation therapy in the management of craniopharyngiomas in children and young adults. International journal of radiation oncology, biology, physics. 2004;58:714–720. [PubMed: 14967425]
- 87.
- Pereira AM, Schmid EM, Schutte PJ, Voormolen JH, Biermasz NR, van Thiel SW, Corssmit EP, Smit JW, Roelfsema F, Romijn JA. High prevalence of long-term cardiovascular, neurological and psychosocial morbidity after treatment for craniopharyngioma. Clin Endocrinol (Oxf). 2005;62:197–204. [PubMed: 15670196]
- 88.
- Muller HL, Bueb K, Bartels U, Roth C, Harz K, Graf N, Korinthenberg R, Bettendorf M, Kuhl J, Gutjahr P, Sorensen N, Calaminus G. Obesity after childhood craniopharyngioma--German multicenter study on pre-operative risk factors and quality of life. Klin Padiatr. 2001;213:244–249. [PubMed: 11528558]
- 89.
- Roth C, Wilken B, Hanefeld F, Schroter W, Leonhardt U. Hyperphagia in children with craniopharyngioma is associated with hyperleptinaemia and a failure in the downregulation of appetite. European journal of endocrinology. 1998;138:89–91. [PubMed: 9461323]
- 90.
- Pinkney J, Wilding J, Williams G, MacFarlane I. Hypothalamic obesity in humans: what do we know and what can be done? Obes Rev. 2002;3:27–34. [PubMed: 12119657]
- 91.
- Harz KJ, Muller HL, Waldeck E, Pudel V, Roth C. Obesity in patients with craniopharyngioma: assessment of food intake and movement counts indicating physical activity. The Journal of clinical endocrinology and metabolism. 2003;88:5227–5231. [PubMed: 14602754]
- 92.
- van Iersel L, van Santen HM, Brokke KE, Bulthuis LCM, Adan RAH, van den Akker ELT. Pathophysiology and individualized treatment of hypothalamic obesity following craniopharyngioma and other suprasellar rumors: a systematic review. Endocrine reviews. 2018;40:193–235. [PubMed: 30247642]
- 93.
- de Vile CJ, Grant DB, Hayward RD, Kendall BE, Neville BG, Stanhope R. Obesity in childhood craniopharyngioma: relation to post-operative hypothalamic damage shown by magnetic resonance imaging. The Journal of clinical endocrinology and metabolism. 1996;81:2734–2737. [PubMed: 8675604]
- 94.
- Prieto R, Pascual JM, Barrios L. Topographic Diagnosis of Craniopharyngiomas: The Accuracy of MRI Findings Observed on Conventional T1 and T2 Images. AJNR American journal of neuroradiology. 2017;38:2073–2080. [PMC free article: PMC7963600] [PubMed: 28935625]
- 95.
- Prieto R, Pascual JM, Rosdolsky M, Barrios L. Preoperative Assessment of Craniopharyngioma Adherence: Magnetic Resonance Imaging Findings Correlated with the Severity of Tumor Attachment to the Hypothalamus. World Neurosurg. 2018;110:e404–e426. [PubMed: 29138072]
- 96.
- Müller HL, Gebhardt U, Teske C, Faldum A, Zwiener I, Warmuth-Metz M, Pietsch T, Pohl F, Sörensen N, Calaminus G. Post-operative hypothalamic lesions and obesity in childhood craniopharyngioma: results of the multinational prospective trial KRANIOPHARYNGEOM 2000 after 3-year follow-up. European journal of endocrinology. 2011;165:17–24. [PubMed: 21490122]
- 97.
- Müller HL, Merchant TE, Warmuth-Metz M, Martinez-Barbera J-P, Puget S. Craniopharyngioma. Nature Reviews Disease Primers. 2019;5:75. [PubMed: 31699993]
- 98.
- Puget S, Garnett M, Wray A, Grill J, Habrand JL, Bodaert N, Zerah M, Bezerra M, Renier D, Pierre-Kahn A, Sainte-Rose C. Pediatric craniopharyngiomas: classification and treatment according to the degree of hypothalamic involvement. J Neurosurg. 2007;106:3–12. [PubMed: 17233305]
- 99.
- Holmer H, Pozarek G, Erfurth E-M, Wirfält E, Ekman B, Björk J, Popovic V. Reduced energy expenditure and impaired feeding-related signals but not high energy intake reinforces hypothalamic obesity in adults with childhood onset craniopharyngioma. The Journal of clinical endocrinology and metabolism. 2010;95:5395–5402. [PubMed: 20826582]
- 100.
- Piguel X, Abraham P, Bouhours-Nouet N, Gatelais F, Dufresne S, Rouleau S, Coutant R. Impaired aerobic exercise adaptation in children and adolescents with craniopharyngioma is associated with hypothalamic involvement. European journal of endocrinology. 2012;166:215–222. [PubMed: 22096113]
- 101.
- Goldstone AP, Patterson M, Kalingag N, Ghatei MA, Brynes AE, Bloom SR, Grossman AB, Korbonits M. Fasting and postprandial hyperghrelinemia in Prader-Willi syndrome is partially explained by hypoinsulinemia, and is not due to peptide YY3-36 deficiency or seen in hypothalamic obesity due to craniopharyngioma. The Journal of clinical endocrinology and metabolism. 2005;90:2681–2690. [PubMed: 15687345]
- 102.
- Elowe-Gruau E, Beltrand J, Brauner R, Pinto G, Samara-Boustani D, Thalassinos C, Busiah K, Laborde K, Boddaert N, Zerah M, Alapetite C, Grill J, Touraine P, Sainte-Rose C, Polak M, Puget S. Childhood craniopharyngioma: hypothalamus-sparing surgery decreases the risk of obesity. The Journal of clinical endocrinology and metabolism. 2013;98:2376–2382. [PubMed: 23633208]
- 103.
- Mortini P, Gagliardi F, Bailo M, Spina A, Parlangeli A, Falini A, Losa M. Magnetic resonance imaging as predictor of functional outcome in craniopharyngiomas. Endocrine. 2016;51:148–162. [PubMed: 26179178]
- 104.
- Duan D, Wehbeh L, Mukherjee D, Hamrahian AH, Rodriguez FJ, Gujar S, Khalafallah AM, Hage C, Caturegli P, Gallia GL, Ahima RS, Maruthur NM, Salvatori R. Preoperative BMI Predicts Postoperative Weight Gain in Adult-onset Craniopharyngioma. The Journal of clinical endocrinology and metabolism. 2021;106:e1603–e1617. [PMC free article: PMC7993568] [PubMed: 33417676]
- 105.
- Bretault M, Boillot A, Muzard L, Poitou C, Oppert JM, Barsamian C, Gatta B, Muller H, Weismann D, Rottembourg D, Inge T, Veyrie N, Carette C, Czernichow S. Clinical review: Bariatric surgery following treatment for craniopharyngioma: a systematic review and individual-level data meta-analysis. The Journal of clinical endocrinology and metabolism. 2013;98:2239–2246. [PubMed: 23533238]
- 106.
- Weismann D, Pelka T, Bender G, Jurowich C, Fassnacht M, Thalheimer A, Allolio B. Bariatric surgery for morbid obesity in craniopharyngioma. Clin Endocrinol (Oxf). 2013;78:385–390. [PubMed: 22506774]
- 107.
- Wijnen M, Olsson DS, van den Heuvel-Eibrink MM, Wallenius V, Janssen JA, Delhanty PJ, van der Lely AJ, Johannsson G, Neggers SJ. Efficacy and safety of bariatric surgery for craniopharyngioma-related hypothalamic obesity: a matched case-control study with 2 years of follow-up. Int J Obes (Lond). 2017;41:210–216. [PubMed: 27795552]
- 108.
- van Santen SS, Wolf P, Kremenevski N, Boguszewski CL, Beiglböck H, Fiocco M, Wijnen M, Wallenius VR, van den Heuvel-Eibrink MM, van der Lely AJ, Johannsson G, Luger A, Krebs M, Buchfelder M, Delhanty PJD, Neggers S, Olsson DS. Bariatric Surgery for Hypothalamic Obesity in Craniopharyngioma Patients: A Retrospective, Matched Case-Control Study. The Journal of clinical endocrinology and metabolism. 2021;106:e4734–e4745. [PMC free article: PMC8530717] [PubMed: 34265053]
- 109.
- Eveslage M, Calaminus G, Warmuth-Metz M, Kortmann RD, Pohl F, Timmermann B, Schuhmann MU, Flitsch J, Faldum A, Müller HL. The Postopera tive Quality of Life in Children and Adolescents with Craniopharyngioma. Deutsches Arzteblatt international. 2019;116:321–328. [PMC free article: PMC6620763] [PubMed: 31219033]
- 110.
- Poretti A, Grotzer MA, Ribi K, Schonle E, Boltshauser E. Outcome of craniopharyngioma in children: long-term complications and quality of life. Dev Med Child Neurol. 2004;46:220–229. [PubMed: 15077699]
- 111.
- Muller HL, Faldum A, Etavard-Gorris N, Gebhardt U, Oeverink R, Kolb R, Sorensen N. Functional capacity, obesity and hypothalamic involvement: cross-sectional study on 212 patients with childhood craniopharyngioma. Klin Padiatr. 2003;215:310–314. [PubMed: 14677094]
- 112.
- Ondruch A, Maryniak A, Kropiwnicki T, Roszkowski M, Daszkiewicz P. Cognitive and social functioning in children and adolescents after the removal of craniopharyngioma. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery 2011; 27:391-397. [PubMed: 20931204]
- 113.
- Ozyurt J, Muller HL, Thiel CM. A systematic review of cognitive performance in patients with childhood craniopharyngioma. Journal of neuro-oncology. 2015;125:9–21. [PubMed: 26369768]
- 114.
- Fjalldal S, Holmer H, Rylander L, Elfving M, Ekman B, Osterberg K, Erfurth EM. Hypothalamic involvement predicts cognitive performance and psychosocial health in long-term survivors of childhood craniopharyngioma. The Journal of clinical endocrinology and metabolism. 2013;98:3253–3262. [PubMed: 23771923]
- 115.
- Duff J, Meyer FB, Ilstrup DM, Laws ER, Schleck CD, Scheithauer BW. Long-term outcomes for surgically resected craniopharyngiomas. Neurosurgery. 2000;46:291–302. [PubMed: 10690718]
- 116.
- Qiao N. Excess mortality after craniopharyngioma treatment: are we making progress? Endocrine. 2019;64:31–37. [PubMed: 30569259]
- 117.
- Erfurth EM, Holmer H, Fjalldal SB. Mortality and morbidity in adult craniopharyngioma. Pituitary. 2013;16:46–55. [PubMed: 22961634]
- 118.
- Wijnen M, Olsson DS, van den Heuvel-Eibrink MM, Hammarstrand C, Janssen J, van der Lely AJ, Johannsson G, Neggers S. Excess morbidity and mortality in patients with craniopharyngioma: a hospital-based retrospective cohort study. European journal of endocrinology. 2018;178:93–102. [PubMed: 29046325]
- 119.
- Boekhoff S, Bison B, Genzel D, Eveslage M, Otte A, Friedrich C, Flitsch J, Müller HL. Cerebral Infarction in Childhood-Onset Craniopharyngioma Patients: Results of KRANIOPHARYNGEOM 2007. Frontiers in Oncology. 2021:11. [PMC free article: PMC8317984] [PubMed: 34336685]
- 120.
- Wijnen M, Olsson DS, van den Heuvel-Eibrink MM, Hammarstrand C, Janssen J, van der Lely AJ, Johannsson G, Neggers S. The metabolic syndrome and its components in 178 patients treated for craniopharyngioma after 16 years of follow-up. European journal of endocrinology. 2018;178:11–22. [PubMed: 28882980]
- 121.
- Daubenbuchel AM, Hoffmann A, Gebhardt U, Warmuth-Metz M, Sterkenburg AS, Muller HL. Hydrocephalus and hypothalamic involvement in pediatric patients with craniopharyngioma or cysts of Rathke's pouch: impact on long-term prognosis. European journal of endocrinology. 2015;172:561–569. [PubMed: 25650403]
- Craniopharyngioma in Pediatrics and Adults.[Adv Exp Med Biol. 2023]Craniopharyngioma in Pediatrics and Adults.Piloni M, Gagliardi F, Bailo M, Losa M, Boari N, Spina A, Mortini P. Adv Exp Med Biol. 2023; 1405:299-329.
- Review Craniopharyngiomas.[Endocr Rev. 2006]Review Craniopharyngiomas.Karavitaki N, Cudlip S, Adams CB, Wass JA. Endocr Rev. 2006 Jun; 27(4):371-97. Epub 2006 Mar 16.
- Review Craniopharyngiomas.[Presse Med. 2021]Review Craniopharyngiomas.Hamblin R, Tsermoulas G, Karavitaki N. Presse Med. 2021 Dec; 50(4):104078. Epub 2021 Oct 22.
- Review Pituitary Tumors in Childhood.[Endotext. 2000]Review Pituitary Tumors in Childhood.Colao A, Pirchio R. Endotext. 2000
- Exome sequencing identifies BRAF mutations in papillary craniopharyngiomas.[Nat Genet. 2014]Exome sequencing identifies BRAF mutations in papillary craniopharyngiomas.Brastianos PK, Taylor-Weiner A, Manley PE, Jones RT, Dias-Santagata D, Thorner AR, Lawrence MS, Rodriguez FJ, Bernardo LA, Schubert L, et al. Nat Genet. 2014 Feb; 46(2):161-5. Epub 2014 Jan 12.
- Craniopharyngiomas - EndotextCraniopharyngiomas - Endotext
Your browsing activity is empty.
Activity recording is turned off.
See more...
