NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-.
ABSTRACT
Type 1A diabetes (T1D) represents an autoimmune disorder that can affect individuals from within a year of birth until age 60. A number of genes strongly influence the development of disease, including genes found within the human lymphocyte antigen (HLA) complex. The role of non-HLA genes is being defined in recent studies, and we are beginning to identify pathways that lead to autoimmunity and eventually pancreatic islet cell destruction. Although genes can predispose one to type 1A diabetes, environmental factors may also play a significant role in the pathogenesis. These as-yet-undefined factors appear to have accelerated the onset and markedly increased the frequency of disease in many populations around the world over the last 30 years. The development of ever more sophisticated immunoassays to detect antibodies directed against pancreatic antigens have helped define the autoimmune nature of the disorder, but as importantly have also provided an opportunity to identify those individuals with prediabetes and to stratify their risk of developing overt hyperglycemia. Immunologic assays as well as intervention trials are allowing us to learn more about the immune pathways that are disordered and offer hope for future therapeutic approaches to prevent and reverse type 1A diabetes. For extensive review of all related areas of Endocrinology, visit WWW.ENDOTEXT.ORG.
INTRODUCTION
Type 1A diabetes mellitus is defined as immune mediated diabetes mellitus.(1-6). It can become manifest with hyperglycemia presenting in the first days of life or in adults over the age of 60. Current estimates indicate that immune mediated diabetes represents approximately 5 to 10% of the diabetes developing in adults and that approximately as many individuals develop this form of diabetes as adults as do children(7-9). In the United States the great majority (>90%) of Caucasian children developing diabetes have type 1A diabetes; whereas, approximately 50% of African American and Hispanic American children developing diabetes lack the autoantibody and immunogenetic markers of typical type 1A diabetes(10-12). Most of these latter children appear to have variants of type 2 diabetes with a small number having specific characteristic genetic syndromes (e.g. MODY: Maturity Onset Diabetes of Youth) with identified mutations of genes such as glucokinase and HNF (Hepatic Nuclear Factors)(13). In addition, studies of the pathology of the pancreas of Hispanic and African American children who lack islet autoantibodies show that all islets have some beta cells, but in decreased numbers(11). In contrast, in the pancreas of patients with type 1A diabetes, there is lobular loss of beta cells (termed pseudoatrophic islets)(11).
When an individual presents with type 1A diabetes it indicates that they and their relatives have an increased risk of having or developing a series of autoimmune disorders(12). Celiac disease, hypothyroidism, hyperthyroidism, Addison's disease and pernicious anemia are some of the most prominent associated diseases. For example approximately 1/20 patients with type 1 diabetes have celiac disease(14,15). Most of these patients are asymptomatic and the disorder is only discovered if anti-transglutaminase autoantibodies are measured and individuals with positive antibodies biopsied. In that the therapy for celiac disease, namely gluten avoidance, is highly effective, at the Barbara Davis Center we routinely screen all type 1 diabetic patients. We also screen for thyroid disease, which has an incidence of approximately 20% in type 1 diabetes, with yearly TSH measurements and for Addison's disease (21-hydroxylase autoantibodies)(16).
Studies of the pathogenesis of type 1 diabetes have blossomed during the past two decades and there are now complete books devoted to this subject (e.g., Immunology of Type 1 Diabetes available at www.barbaradaviscenter.org with appended "Teaching" slides). In terms of summarizing the pathogenesis of this disorder it is convenient to divide the disease into a series of stages (Figure 1) beginning with genetic susceptibility and ending (from an immunologic standpoint) with complete islet beta cell destruction(16). This is only a general description of the disease process. It is likely for instance that genetic determinants influence many of the stages of disease progression and are important determinants of individuals who express anti-islet autoantibodies but do not progress to diabetes (e.g., a major subset of those with anti-islet autoantibodies and the protective HLA allele DQB1*0602 allele)(17).

Figure 1
Hypothetical stages and loss of beta cells in an individual progressing to type 1A diabetes. From Eisenbarth, NEJM, 1986 (18)
STAGE I. GENETIC SUSCEPTIBILITY
An expert committee of the American Diabetes Association has divided type 1 diabetes into type 1A (immune mediated) and 1B (not immune mediated but with profound loss of insulin secretion)(19). The great majority of patients with insulin dependent diabetes have type 1A, and firm examples of type 1B are either lacking or controversial. One recent example is a description from Japan of patients who extremely rapidly developed type 1 diabetes (fulminant diabetes) such that they had marked hyperglycemia, but at diabetes presentation their HbA1c was near normal, suggesting very recent onset of hyperglycemia(20). These patients lacked anti-islet autoantibodies, a subset had elevated serum pancreatic enzymes, but many had HLA alleles associated with type 1A diabetes. On biopsy beta cells were destroyed, there were lymphocytes in the acinar pancreas, but no typical insulitis (invasion of islets by lymphocytes). Whether this is truly type 1B or an extremely rapid variant of type 1A is currently debated.

Figure 2
Genetic heterogeneity of immune mediated type 1 diabetes with monogenic, polygenic, and oligogenic forms. Modified from teaching slides www.barbaradaviscenter.org
Type 1A diabetes is itself heterogeneous, with several forms of immune mediated diabetes with known genetic causes as parts of autoimmune syndromes (thus likely to be classified as other Specific Forms of Diabetes). In particular, patients develop immune mediated diabetes when they have mutations of the AIRE (Autoimmune Regulator) gene(21) and the human gene homologous to the mutated gene causing Scurfy in mice (22)(figure 2: monogenic disorders). Mutations of the AIRE gene result in Autoimmune Polyendocrine Syndrome Type I (23,24). Mutations of the Scurfy homologue lead to overwhelming neonatal autoimmunity (IPEX syndrome: Immune dysregulation, Polyendocrinoptahy, Enteropathy, X-linked)(21). A mutation of the FoxP3 genes, an essential transcription factor for CD4+CD25+ regulatory T cells, is the cause of the IPEX syndrome(25,26). These children can develop type 1 diabetes in the first days of life and illustrate the importance of T cell regulation. Most other forms of type 1A diabetes are polygenic in etiology, and polymorphisms of genes within the major histocompatibility complex (HLA genes) play a major role in determining disease susceptibility(27,28). Such heterogeneity is also apparent in three spontaneous animal models of type 1A diabetes, the BB rat (Biobreeding), the NOD (Non-obese diabetic), and the Tokushima rat (Figure 3). For all three strains polymorphisms of genes homologous to HLA DR and DQ of man are essential for disease(29). In addition, more than 15 other loci play an important role in diabetes susceptibility of the NOD mouse, but each locus contributes relatively little (polygenic inheritance) by itself.

Figure 3
Three rodent strains that develop immune mediated diabetes. Modified from teaching slides www.barbaradaviscenter.org
For the BB rat and Tokushima rat there are major loci outside of the MHC contributing to disease (Oligogenic inheritance)(30,31). It is not likely that human type 1 diabetes is less heterogeneous than these few animal models.
Identical twins of patients with type 1A diabetes have an overall risk of developing type 1 diabetes approaching seventy percent.(32) Consistent with heterogeneity, that risk varies dramatically with the diabetic twin's age of diabetes onset. If one identical twin develops diabetes prior to age 5, the risk for the other twin exceeds 50%. In contrast if the twin develops diabetes after age 25, the other twin’s risk is less than 10%(29). The risk of developing diabetes is approximately 1/20 for a sibling or offspring of a patient with type 1A diabetes. The U.S. population risk for type 1A diabetes is approximately 1/300 and the country with the highest incidence in the world (Finland) has a risk of approximately 1/100. As will be discussed subsequently, genetic polymorphisms greatly influence disease risk. A sibling of a patient with type 1 diabetes who has the highest risk genotype DR3/4 DQB1*0302 has an almost 50% risk of developing anti-islet autoantibodies and progressing to diabetes(33).
Polymorphisms of genes within the major histocompatibility complex contribute to disease of both humans and rodent models.

Figure 4
Schematic of the human major histocompatibility complex approximately 4 million base pairs in length. Redondo MJ, et al. Recent Prog Horm Res, 56: 69-89, 2001.(34) Copyright The Endocrine Society.
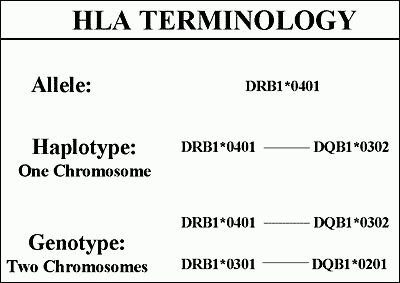
Figure 5
Hierarchy of HLA terminology with specific alleles, groups of alleles of different genes on the same chromosome, and finally genotype. Modified from teaching slides www.barbaradaviscenter.org
The alleles of different HLA genes (e.g., DRB1 and DQB1) are non-randomly associated with each other, such that with DRB1*0401 one usually finds one of three DQ alleles (e.g., DQB1*0301, DQB1*0302, DQB1*0303) rather than any one of more than forty different DQB molecules. Such non-random association of alleles of different genes on the same chromosome is termed linkage dysequilibrium.The histocompatibility complex is divided into three regions, class II, class III and class I (Figure 4). The most important determinants of type 1 diabetes are the HLA DQ and DR alleles. These molecules on the surface of antigen presenting cells (e.g., macrophages) bind and present short peptides that are recognized by T cell receptors of T lymphocytes(27,35,36). They are termed immune response genes in that the specific amino acid sequence of these molecules determines which peptides will be bound and to a large extent determine which peptides an individual will respond to. Each different amino acid sequence is given a number. For the DQ molecules both its alpha and beta chain gene are polymorphic, and thus to specify a DQ molecule one must specify both chains. For DR molecules only the DRB chain is polymorphic and thus only this chain is specified. Each number after the star indicates a specific amino acid sequence of the HLA allele (Figure 5) and the letters and first number the gene (e.g., DRB1*0401, DR B chain gene number 1, allele 0401).

Figure 6
Hierarchy of diabetes risk with examples of haplotypes that lead to diabetes susceptibility, are neutral, or protective. Modified from teaching slides www.barbaradaviscenter.org
There is a tremendous spectrum of diabetes risk associated with different DR and DQ genotypes(37-39)(Figure 6). For Caucasians with type 1A diabetes the most common diabetes-associated haplotypes are DR3 and DR4. More than 90% of patients with type 1A diabetes have one or both of these alleles versus approximately 40% of the general U.S. population. With the finer sequence information that is now available, DR4 haplotypes are subdivided based on specific variants of DRB1 and DQB1. The highest risk DR4 haplotypes have DRB1*0401, DRB1*0402, DRB1*0405, while DRB1*0403 is moderately protective. The highest risk DR4 haplotypes have DQB1*0302, with DQB1*0301 and DQB1*0303 of lower risk. Thus both DR and DQ alleles contribute to diabetes risk. DR3 haplotypes are almost always conserved with DRB1*03 combined with DQA1*0501, DQB1*0201(40). The highest risk genotype have both DR4/DR3 DQB1*0302/DQB1*0201. This genotype occurs in 2.4% of newborns in Denver, Colorado, and between 30 and 50% of children developing type 1A diabetes. Approximately 50% of children developing type 1A diabetes early (i.e., less than age 5) are DR3/4 heterozygotes versus 30% of young adults presenting with type 1A diabetes.
There are three HLA molecules that provide dominant protection. The most common is DQB1*0602 that occurs in approximately 20% of U.S. individuals(41-43). Protection is not absolute, but less than 1% of children with type 1A diabetes have this molecule. DQA1*0201 with DQB1*0303 and DRB1*1401 also provide dramatic protection, rarely being found in patients with type 1 diabetes and rarely transmitted from a parent with the alleles to their diabetic offspring(38,39). It is noteworthy that both DR and DQ alleles can protect. The specific mechanism underlying both susceptibility and protection are not fully understood. One attractive hypothesis is that protective alleles when expressed within the thymus lead to deletion of T cells with receptors that recognize a critical islet peptide(44). With deletion of such T cells, the risk of diabetes would be reduced. In addition it is likely that high-risk HLA alleles present specific peptides of target islet molecules to T lymphocytes(28).

Figure 7
Summary of subset of confirmed loci from whole genome screens associated with type 1A diabetes (from Teaching Slides www.barbaradaviscenter.org) www.barbaradaviscenter.org. Modified from Todd et al. Robust Association of four new chromosomes regions from genome-wide analyses of type 1 diabetes, Nature Genetics, June 6, 2007. (45)
Multiple additional loci (Figure 7) have been implicated with estimates that approximately 50% of the familial aggregation of type 1 diabetes is attributable to the HLA region, perhaps 10% to the insulin locus, with all other loci contributing much less, though in aggregate their contribution is important. In the Cox analysis (Figure 7) of approximately 700 sibling pairs the only significant LOD score was for a locus on chromosome 16q that was not given an iddm designation with earlier genome screens. Several areas implicated in the past had suggestive scores, but there is overlap with the families from which the original evidence was generated. It is likely that contributing loci may differ between populations contributing to the initial difficulty of replicating putative loci in different studies(56,57).More than 40 genetic loci contributing to diabetes risk have been implicated (Figure 7). Polymorphisms of the insulin gene are well established as contributing to risk. A repeat sequence upstream (5') of the insulin gene termed a Variable nucleotide tandem repeat or VNTR, is divided into three general repeat sizes with the longest set of repeats associated with protection from diabetes(46-48). This set of alleles is also associated with greater thymic production of insulin messenger RNA(49), leading to the hypothesis that greater thymic message and presumably greater proinsulin production dampens anti-insulin autoimmunity(49-51). A functional polymorphism of the LYP gene (Lymphocyte Specific Phosphatase; PTPN22- Protein Tyrosine Phosphatase) has been associated with type 1 diabetes, rheumatoid arthritis, and lupus erythematosus(52-54). The R620W missense mutation (tryptophan replacing arginine) disrupts the binding of the phosphatase to the molecule Csk and this blocks its ability to down-regulate T cell receptor signaling. With an odds ratio of between 1.7 and 2.0 of the "autoimmunity” allele which is relatively common (5-10% allele frequency) there is a large genetic effect that is much greater than CTLA-4 polymorphisms associated with diabetes risk(55). Combining known diabetogenic polymorphisms of LYP, the insulin gene, alleles of DP, DQ, and DR class II immune response genes, as well all of the new loci account for approximately 48% of the familial aggregation of type 1A diabetes, with DR and DQ loci accounting for 41% of this 48%(45). A recent study suggests that for a major subset of individuals with the highest risk HLA genotype (DR3/4-DQ2/DQ8 heterozygotes) who share both HLA haplotypes with a diabetic sibling, risk of activating anti-islet autoimmunity is as high as 80%(33).
STAGE II: TRIGGERING
Anti-islet autoimmunity (i.e., insulin autoantibodies), insulitis, and immune mediated diabetes can be triggered in a number of animal models by a large number of immunologic and genetic manipulations(58-60). Perhaps the most relevant of these manipulations is the administration of poly-IC (poly inosinic cytodylic acid) to normal rat strains that have the diabetes susceptible major histocompatibility RT1-U haplotype(61). Administration of poly-IC in some of these normal strains leads to insulitis while in others it leads to overt diabetes with islet beta cell destruction. Poly-IC interacts with Toll 3 receptors of the innate immune system leading to a cascade of intracellular and cytokine mediated events. Infection of Diabetes Resistant BB rats, a strain of rats that does not develop diabetes and lacks the lymphopenia gene of the spontaneously diabetic BB rat strain, leads to diabetes probably by a pathogenic mechanism similar to the effects of poly-IC(62). Poly-IC is a mimic of viral double stranded RNA and thus it is easy to envision that many common RNA viral infections may induce diabetes in genetically susceptible patients. Normal mouse strains such as Balb/c mice rapidly develop insulin autoantibodies if challenged with an insulin peptide (B chain peptide, amino acids 9 to 23)(58). If the peptide is administered with poly-IC, insulitis is induced and in genetically susceptible mice diabetes can be induced. These and many other studies in animal models indicate that normal animals harbor autoreactive B and T lymphocytes that can be expanded and activated, with resultant diabetes. Though these strains are "normal” they have variants of MHC molecules that determine disease susceptibility, by influencing T cell responses to relevant peptides. The class II MHC molecules (equivalent of DR and DQ of man) appear to be most important, and these molecules probably influence disease either by the peptides they bind and present to T cells within islets and draining lymph nodes and by their influence on the thymic T cell repertoire(63). It has recently been appreciated that the position or “register” of insulin B chain amino acids 9-23 presented by the class II MHC molecule activates insulin specific T cells towards islet autoimmunity in a spontaneous mouse model of autoimmune diabetes. With the remarkable homology between mouse and humans (e.g., similar MHC class II molecules contributing genetic risk, identical amino acid sequences for insulin B chain amino acids 9-23, and similar T cell receptors responding to insulin), the underlying biology of insulin presentation may be identical in mouse and humans(64,65).
In humans, environmental factors that trigger anti-islet autoimmunity are largely unknown. Congenital rubella infection is associated with a risk of type 1 diabetes that exceeds 1/5(66,67). It is however only congenital infection that increases the risk of diabetes, and leads to a series of autoimmune disorders (e.g., thyroid autoimmunity). It is thus likely that the congenital infection damages the developing immune system leading to relatively broad disease susceptibility(68).
Figure 8 lists a number of additional environmental factors that may impact on the development of type 1A diabetes. The enteroviruses are probably the most extensively studied(69,70). Investigators have associated for instance antibodies to Coxsackie viruses and Coxsackie viral RNA with type 1A diabetes(71). An important study from Finland has provided

Figure 8
Environmental factors that may increase or decrease diabetes risk. Congenital rubella infection is bolded as it is the only clearly defined factor.
evidence that enterovirus infection may be associated with the activation of anti-islet autoimmunity as measured by the appearance of anti-islet autoantibodies. Such studies have been difficult to replicate, and the DAISY study from Denver, Colorado, that also follows newborns from birth for evidence of anti-islet autoimmunity has not found an association with the enteroviral infection(70).

Figure 9
Secular trend in the incidence of type 1A diabetes in Finland subdivided by the age of appearance of diabetes. Modified from teaching slides www.barbaradaviscenter.org
Dietary factors that may contribute to diabetes are being extensively studied. One hypothesis is that bovine milk ingestion, particularly in the first months of life, is associated with development of diabetes(72). Again there is conflicting data with reports that bovine milk ingestion increases the development of anti-islet autoantibodies, and reports from Denver, Munich, and Melbourne, that it does not(73). Recently two studies (Germany and Denver) have implicated early (<3 months) introduction of cereals as a risk factor for type 1 diabetes(74,75). In the Denver study, late introduction (>7 months) also increased risk. At present it is my view that the data do not allow any firm recommendation in terms of changing infant diet, except to emphasize the current standard recommendation for introduction of cereals between 3 and 7 months.
Environmental factors might not only increase the development of diabetes but may also provide protection. There is a very wide range in the risk of type 1 diabetes ranging from an annual incidence of less than 1/100,000 in China to approximately 50/100,000 in Finland(76). Much of this difference may relate to genetic factors, but there is strong evidence that environmental factors are influencing diabetes risk. The strongest evidence comes from a marked secular trend in terms of increasing diabetes incidence in multiple populations(77). As shown in figure 9, the incidence has increased dramatically particularly for children developing diabetes prior to age 5, increasing more than 3 fold over the past three decades. Such a rapid change in disease incidence cannot be due to changes in gene pool. Something that increases risk has been added, or more likely an environmental factor that decreases risk, has been removed from the population. With increasing public health, a "hygiene” hypothesis has been advanced, particularly directed at asthma and type 1 diabetes(78). It is hypothesized that as the environment becomes "cleaner” the normal development of the immune system is disrupted (e.g., regulatory T cell development is subnormal) resulting in increases of both presumed Th2 (asthma) and Th1 (Type 1 diabetes) mediated diseases. For instance, one review discusses decreasing pinworm infection as a potential factor(78).
STAGE III AUTOIMMUNITY
The assays for anti-islet autoantibodies have improved remarkably over the past three decades(79). A series of anti-islet autoantibody workshops where sera is sent blinded to multiple laboratories throughout the world has stimulated assay improvements and standardization(69). Such workshops have evaluated not only anti-islet autoantibodies of humans but also of the NOD mouse model of type 1 diabetes. In the mouse model the only specific autoantibody detected reacted with insulin(80) and similar to studies in humans, fluid phase radioassays provided better sensitivity and specificity compared to ELISA. Newer assays using electrochemiluminescence to detect islet autoantibodies has allowed for higher sensitivity and specificity and the potential to multiplex each individual assay.(81,82)
The autoantibodies that are primarily measured in humans react with insulin, glutamic acid decarboxylase 65, and ICA512 (IA-2) and ZnT8 (zinc transporter 8) (Figure 10). GAD67 autoantibodies are primarily a subset of antibodies that cross-react with GAD65, and similarly IA-2beta antibodies are predominantly a subset of ICA512 autoantibodies.

Figure 10
Five characterized islet autoantigens with specific/sensitive autoantibody assays. Modified from teaching slides www.barbaradaviscenter.org
Insulin autoantibodies are usually the first autoantibody to appear in children followed from birth for the development of type 1A diabetes(84,85). These autoantibodies can appear in the first six months of life. Once insulin autoantibodies appear in such young children there is a high risk of development of additional anti-islet autoantibodies and progression to diabetes. More than 90% of children developing type 1A diabetes prior to age 5 have insulin autoantibodies while less than 50% of children developing diabetes after age 12 have such autoantibodies(86). Therapy with human insulin induces insulin antibodies that cannot at present be distinguished from insulin autoantibodies. Thus if an individual has been treated with insulin for more than several weeks, positive insulin autoantibodies are not interpretable. For all autoantibodies measured in the first 9 months of life, the antibodies may be transplacental in origin, a particular problem if a mother has type 1 diabetes and is treated with insulin.There are a number of important caveats in the utilization of anti-islet autoantibody assays. The field developed from the initial observation that patient's sera "stained” islets of cut sections of human pancreas, the cytoplasmic islet cell antibody (ICA) assay(83). This assay, given its utilization of human pancreas from cadaveric donation and subjective reading of slides, has proven the most difficult to standardize(69). The assay predominantly detects antibodies reacting with GAD65, IA-2 and ZnT8, but does not detect anti-insulin autoantibodies. Given the difficulty in standardization, reliability over time, and major overlap with defined autoantibody assays, a number of investigators no longer utilize this assay. For research purposes and potentially in older adults with what has been termed LADA (latent autoimmune diabetes of adults) the ICA assay may have utility in that there is evidence of one or more additional autoantibodies detected with this assay and not with GAD65, IA-2, ZnT8 and insulin autoantibody determination.

Figure 11
Progression to diabetes of first-degree relatives of patients with type 1 diabetes subdivided by the number of autoantibodies expressed (of GAD65, IA-2, and insulin). Adapted from Verge et al, Diabetes 1996 (87)
A single autoantibody, even when present on multiple occasions, is associated with only a modest risk of progression to diabetes: approximately 10%(87,88). Once two or more anti-islet autoantibodies are present in children, progression to diabetes is very high, approaching almost 100% after 15 years of follow up(89) (Figure 11). In addition, once multiple autoantibodies are present it is very unusual for an individual to lose all expression of autoantibodies prior to the development of overt diabetes. Following the development of diabetes, IA-2 and more slowly GAD65 (over decades) autoantibodies wane. Following islet or pancreatic transplantation expression of GAD65 and IA-2 autoantibodies can be induced in patients with long-standing diabetes(90).
The most specific of the autoantibodies react with the molecule IA-2, but IA-2 autoantibodies are usually detected following the appearance of insulin and/or GAD65 autoantibodies(84). Even with IA-2 autoantibodies, however, there are apparent "false” positives in terms of diabetes risk. We evaluated approximately 10 individuals with either transient IA-2 autoantibodies or normal controls with IA-2 autoantibodies. None of these individuals expressed an additional anti-islet autoantibody. In contrast to patients diagnosed with or developing type 1 diabetes, the ICA512/IA-2 autoantibodies of nine out of ten of these normal individuals did not recognize multiple ICA512 epitopes and did not react with the dominant ICA512 autoantigenic domain(91). This indicates that even with a highly specific radioassay, if one screens tens of thousands of sera, one can find sera that presumably by chance cross-react with some epitope of the IA-2 molecule. It is much less likely to find an individual with antibodies that by chance react with two different islet autoantigens using fluid phase radioassays set with specificity at the 99th percentile of controls.
STAGE IV. LOSS OF INSULIN SECRETION
Not all individuals with two or more autoantibodies are destined to progress to type 1 diabetes. For instance the diabetes risk is unknown for individuals with expression of two or more anti-islet autoantibodies with the protective HLA molecule DQA1*0102, DQB1*0602. Figure 12 is a life table of progression to diabetes of first-degree relatives with high titer cytoplasmic autoantibodies with a subset of relatives having the protective HLA allele DQB1*0602. Two of the DQB1*0602 relatives expressed multiple "biochemical” anti-islet autoantibodies (one with two autoantibodies and the other three) and neither of these individuals has yet progressed to diabetes. In that approximately 1% of patients with type 1 diabetes have DQB1*0602 it is possible that these individuals will eventually progress to diabetes.

Figure 12
Lack of progression to diabetes of cytoplasmic ICA positive first-degree relatives that have the protective HLA allele DQB1*0602. Modified from teaching slides www.barbaradaviscenter.org
There are three general hypotheses in terms of progression to type 1A diabetes (Figure 13). At present, beta cell mass is not readily measured over time in humans, so it is not possible to absolutely define progression of beta cell loss. There is however no doubt that measurable anti-islet autoimmunity precedes the development of diabetes in terms of anti-islet autoantibodies in humans, and autoantibodies and T cell invasion in animal models. In the NOD mouse there is evidence of some beta cell destruction and beta cell regeneration prior to the onset of diabetes (92). There is also evidence for a change in the immune system close to the time of onset of diabetes (i.e., Th2 to Th1)(93-96). This change is associated with more rapid disease progression, ability to transfer diabetes by T cells, and a time window during which a specific immunotherapy (monoclonal anti-CD3 antibodies) is effective(97). In humans the best evidence for progressive loss of beta cell function comes from studies of insulin and C-peptide secretion(98). C-peptide, the connecting peptide of proinsulin, is secreted in equimolar amount to insulin, but C-peptide is not present in insulin preparations utilized to treat diabetes. Thus C-peptide has become an important indicator of remaining beta cell function. Following the onset of diabetes it has long been appreciated that C-peptide secretion progressively declines, until for most patients with type 1 diabetes C-peptide becomes non-detectable, associated with true insulin dependence. In a similar manner, first phase insulin secretion following a bolus of glucose on intravenous glucose tolerance testing is progressively lost for relatives followed to the development of type 1 diabetes(99). Such metabolic abnormalities may result in part from functional inhibition of beta cell secretion, but pathologic studies indicate that beta cell mass is normal for identical twins of patients that have not activated anti-islet autoimmunity, and for new onset patients that bulk of beta cells are destroyed(100). Within the pancreas of a patient with type 1 diabetes there is heterogeneity of islet lesions, with most islets lacking all beta cells and with no lymphocytic infiltrates (pseudoatrophic islets), few normal islets with no infiltrates, and few islets with remaining beta cells and infiltrates. This is perhaps analogous to the progressive development of vitiligo in patients, with patches of skin with all melanocytes destroyed, whereas other skin is normal.STAGE IV. LOSS OF INSULIN SECRETION

Figure 13
Three hypotheses of progression to type 1A diabetes. Modified from teaching slides www.barbaradaviscenter.org
STAGE V. OVERT DIABETES
The development of type 1 diabetes is usually perceived as an abrupt event, and some individuals may rapidly manifest severe hyperglycemia. Now that we can follow individuals to the development of type 1A diabetes, we can see that anti-islet autoantibodies can precede hyperglycemia by years, and there is usually some deterioration in glucose tolerance more than one year prior to diabetes onset (particularly with intravenous glucose tolerance testing)(101). The majority of individuals identified to be diabetic following autoantibody testing are found to have a diabetic 2-hour glucose on oral glucose tolerance testing (>200mg/dl) rather than fasting hyperglycemia. The acute presentation with severe hyperglycemia and ketoacidosis is life threatening, and it is estimated that approximately 1/200 children die at the onset of type 1 diabetes(102,103). Such children typically have a medical history where the first health care providers have failed to make the diagnosis of diabetes; the child then presents again later and dies with cerebral edema. The classic symptoms of polyuria, polydipsia, and weight loss are usually present but the initial diagnosis is still missed. The alternative diagnosis of nausea and vomiting due to viral illness is the most common mistaken diagnosis, and with the ready availability of glucose determination from a finger or heel stick, there should be a low threshold in emergency rooms and physicians’ offices for ruling out diabetes. Though transient hyperglycemia can occur, such children obviously need close follow up. We usually arrange glucose monitoring for children thought to have transient hyperglycemia, and measure anti-islet autoantibodies(104). Of those with anti-islet autoantibodies and transient hyperglycemia, almost all progress to type 1 diabetes within several months.STAGE V. OVERT DIABETES
A report by Barker and coworkers illustrates the potential importance of screening for anti-islet autoantibodies and monitoring high-risk children. Less than 5% of children in such intensive follow-up who developed diabetes were hospitalized with ketoacidosis compared to 40% of children not being screened. Figure 14 illustrates the blood glucose for the two groups at the time of diagnosis with markedly elevated levels in the non-screened children, including values greater than 1,000 mg/dl associated with severe metabolic decompensation(105).

Figure 14
Glucose at diagnosis in children screened for anti-islet autoantibodies and metabolically monitored, versus children from the general population presenting with diabetes(105). Modified from teaching slides www.barbaradaviscenter.org of Type 1 Diabetes: Molecular, Cellular and Clinical Immunology.
At the onset of type 1 diabetes, almost all individuals have residual insulin secretion, and there is convincing evidence that residual insulin secretion as measured by C-peptide secretion is of clinical benefit (less hypoglycemia, less microvascular complications, and much easier diabetes management). With many immunologic therapies it is possible to prevent diabetes in animal models, and as reviewed, the disorder is predictable in humans. At present there is no proven therapy to either prevent progression to type 1 diabetes or to halt beta cell loss after presentation with diabetes. One arm of the DPT-1 prevention trial, where low doses of subcutaneous insulin were administered, did not delay progression to diabetes(106). The DPT-1 trial of oral insulin indicated that overall, oral insulin did not delay progression to diabetes. A subset analysis of antibody positive relatives entering the trial with elevated levels of insulin autoantibodies, however, suggests a delay in progression to diabetes of approximately 4 years. A repeat trial of oral insulin for diabetes prevention is underway by TrialNet(107). Several studies of anti-CD3 monoclonal antibody modified to reduce cytokine release have shown promise in patients with new onset and recent onset diabetes(108-111), but these monoclonal antibodies only transiently delay the loss of c-peptide secretion(112-114). Other therapies include anti-CD20 monoclonal antibody (rituximab targeted to B cells). Treatment with CTLA-4 Ig and alefacept (anti-CD2) similarly transiently delays the loss of c-peptide(115-117). In contrast, trials of a GAD/alum vaccine did not delay the loss of endogenous c-peptide production nor did therapies targeting the IL-1 pathway(118-120). It is our belief that more specific therapies targeting autoreactive immune cells such as recently reported by Roep and colleagues(121) used in combination will allow for disease prevention and potentially a cure of type 1 diabetes when done in conjunction with islet replacement
.SUMMARY
The National Institutes of Health has created a cooperative trial network to develop therapies to prevent beta cell loss in prediabetics and new onset patients, termed TrialNet. Relatives of type 1 patients throughout North America can be screened for diabetes risk, and trials are underway to preserve beta cell function in new onset patients. A contact number for TrialNet is 1-800-HALT-DM1. In addition, the Immune Tolerance Network is evaluating therapies in a series of disorders designed to restore "tolerance,” including type 1A diabetes. They are seeking applications to test innovative therapies at immunetolerance.org.
Acknowledgments: Our research is supported by grants DK32083, DK095995, DK085509, DK097681, AI097346, Diabetes Endocrine Research Center (P30 DK57516), Autoimmunity Center of Excellence (UM1AI110503) and Human Islet Research Center (UC4DK104223) from the National Institute of Health, the Juvenile Diabetes Research Foundation, Brehm Coalition, Helmsley Foundation and a grant from the Children's Diabetes Foundation at Denver. We wish to acknowledge the late Dr. George Eisenbarth whose numerous contributions to this chapter and the field, have been invaluable.
References
1. Eisenbarth GS. Banting Lecture 2009: An unfinished journey: molecular pathogenesis to prevention of type 1A diabetes. Diabetes 2010; 59:759-774
2. Todd JA. Etiology of type 1 diabetes. Immunity 2010; 32:457-467
3. Stadinski B, Kappler J, Eisenbarth GS. Molecular targeting of islet autoantigens. Immunity 2010; 32:446-456
4. Skyler JS. Immunomodulation for type 1 diabetes mellitus. International journal of clinical practice Supplement 2010:59-63
5. Wong FS, Wen L. The study of HLA class II and autoimmune diabetes. Current molecular medicine 2003; 3:1-15
6. Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet 2014; 383:69-82
7. Lorenzen T, Pociot F, Hougaard P, et al. Long-term risk of IDDM in first-degree relatives of patients with IDDM. Diabetologia 1994; 37:321-327
8. Janzon L, Bergentz SE, Ericsson BF, et al. The arm-ankle pressure gradient in relation to cardiovascular risk factors in intermittent claudication. Circulation 1981; 63:1339-1341
9. Zimmet P, Turner R, McCarty D, et al. Crucial points at diagnosis. Type 2 diabetes or slow type 1 diabetes. Diabetes care 1999; 22 Suppl 2:B59-64
10. Pinhas-Hamiel O, Dolan LM, Daniels SR, et al. Increased incidence of non-insulin-dependent diabetes mellitus among adolescents. The Journal of pediatrics 1996; 128:608-615
11. Gianani R, Campbell-Thompson M, Sarkar SA, et al. Dimorphic histopathology of long-standing childhood-onset diabetes. Diabetologia 2010; 53:690-698
12. Triolo TM, Armstrong TK, McFann K, et al. Additional Autoimmune Disease Found in 33% of Patients at Type 1 Diabetes Onset. Diabetes care 2011; 34:1211-1213
13. McCarthy MI, Hattersley AT. Learning from molecular genetics: novel insights arising from the definition of genes for monogenic and type 2 diabetes. Diabetes 2008; 57:2889-2898
14. Bao F, Yu L, Babu S, et al. One third of HLA DQ2 homozygous patients with type 1 diabetes express celiac disease-associated transglutaminase autoantibodies. Journal of autoimmunity 1999; 13:143-148
15. Hoffenberg EJ, Bao F, Eisenbarth GS, et al. Transglutaminase antibodies in children with a genetic risk for celiac disease. The Journal of pediatrics 2000; 137:356-360
16. Baker PR, Baschal EE, Fain PR, et al. Dominant suppression of Addison's disease associated with HLA-B15. The Journal of clinical endocrinology and metabolism 2011; 96:2154-2162
17. Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet 2001; 358:221-229
18. Eisenbarth GS. Type I diabetes mellitus. A chronic autoimmune disease. The New England journal of medicine 1986; 314:1360-1368
19. Diagnosis and classification of diabetes mellitus. Diabetes care 2004; 27 Suppl 1:S5-S10
20. Imagawa A, Hanafusa T, Miyagawa J, et al. A novel subtype of type 1 diabetes mellitus characterized by a rapid onset and an absence of diabetes-related antibodies. Osaka IDDM Study Group. The New England journal of medicine 2000; 342:301-307
21. Robles DT, Eisenbarth GS, Ikegami H, et al. Endocrinology and metabolism clinics of North America. Philadelphia: W.B. Saunders; 2002.
22. Patel DD. Escape from tolerance in the human X-linked autoimmunity-allergic disregulation syndrome and the Scurfy mouse. The Journal of clinical investigation 2001; 107:155-157
23. Bjorses P, Aaltonen J, Horelli-Kuitunen N, et al. Gene defect behind APECED: a new clue to autoimmunity. Human molecular genetics 1998; 7:1547-1553
24. Michels AW, Eisenbarth GS. Autoimmune polyendocrine syndrome type 1 (APS-1) as a model for understanding autoimmune polyendocrine syndrome type 2 (APS-2). Journal of internal medicine 2009; 265:530-540
25. Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nature immunology 2003; 4:330-336
26. Owen CJ, Jennings CE, Imrie H, et al. Mutational analysis of the FOXP3 gene and evidence for genetic heterogeneity in the immunodysregulation, polyendocrinopathy, enteropathy syndrome. The Journal of clinical endocrinology and metabolism 2003; 88:6034-6039
27. Klein J, Sato A. The HLA system. First of two parts. The New England journal of medicine 2000; 343:702-709
28. Lee KH, Wucherpfennig KW, Wiley DC. Structure of a human insulin peptide-HLA-DQ8 complex and susceptibility to type 1 diabetes. Nature immunology 2001; 2:501-507
29. Mordes JP, Greiner DL, Rossini AA. Animal models of autoimmune diabetes mellitus. . In: LeRoith D, Taylor SI, Olefsky JM, eds. Diabetes mellitus : a fundamental and clinical text. Philadelphia: Lippincott-Raven; 1996:349-360.
30. Martin AM, Maxson MN, Leif J, et al. Diabetes-prone and diabetes-resistant BB rats share a common major diabetes susceptibility locus, iddm4: additional evidence for a "universal autoimmunity locus" on rat chromosome 4. Diabetes 1999; 48:2138-2144
31. Yu B, Gauthier L, Hausmann DH, et al. Binding of conserved islet peptides by human and murine MHC class II molecules associated with susceptibility to type I diabetes. European journal of immunology 2000; 30:2497-2506
32. Redondo MJ, Jeffrey J, Fain PR, et al. Concordance for islet autoimmunity among monozygotic twins. The New England journal of medicine 2008; 359:2849-2850
33. Aly TA, Ide A, Jahromi MM, et al. Extreme genetic risk for type 1A diabetes. Proceedings of the National Academy of Sciences of the United States of America 2006; 103:14074-14079
34. Redondo MJ, Fain PR, Eisenbarth GS. Genetics of type 1A diabetes. Recent progress in hormone research 2001; 56:69-89
35. Kwon OJ, Brautbar C, Weintrob N, et al. Immunogenetics of HLA class II in Israeli Ashkenazi Jewish, Israeli non-Ashkenazi Jewish, and in Israeli Arab IDDM patients. Human immunology 2001; 62:85-91
36. Undlien DE, Lie BA, Thorsby E. HLA complex genes in type 1 diabetes and other autoimmune diseases. Which genes are involved? Trends in genetics : TIG 2001; 17:93-100
37. Lie BA, Ronningen KS, Akselsen HE, et al. Application and interpretation of transmission/disequilibrium tests: transmission of HLA-DQ haplotypes to unaffected siblings in 526 families with type 1 diabetes. American journal of human genetics 2000; 66:740-743
38. Redondo MJ, Kawasaki E, Mulgrew CL, et al. DR- and DQ-associated protection from type 1A diabetes: comparison of DRB1*1401 and DQA1*0102-DQB1*0602*. The Journal of clinical endocrinology and metabolism 2000; 85:3793-3797
39. Kawasaki E, Noble J, Erlich H, et al. Transmission of DQ haplotypes to patients with type 1 diabetes. Diabetes 1998; 47:1971-1973
40. Erlich H, Valdes AM, Noble J, et al. HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: analysis of the type 1 diabetes genetics consortium families. Diabetes 2008; 57:1084-1092
41. Baisch JM, Weeks T, Giles R, et al. Analysis of HLA-DQ genotypes and susceptibility in insulin-dependent diabetes mellitus. The New England journal of medicine 1990; 322:1836-1841
42. Pugliese A, Gianani R, Moromisato R, et al. HLA-DQB1*0602 is associated with dominant protection from diabetes even among islet cell antibody-positive first-degree relatives of patients with IDDM. Diabetes 1995; 44:608-613
43. Pugliese A, Kawasaki E, Zeller M, et al. Sequence analysis of the diabetes-protective human leukocyte antigen-DQB1*0602 allele in unaffected, islet cell antibody-positive first degree relatives and in rare patients with type 1 diabetes. The Journal of clinical endocrinology and metabolism 1999; 84:1722-1728
44. Barrett JC, Clayton DG, Concannon P, et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nature genetics 2009; 41:703-707
45. Todd JA, Walker NM, Cooper JD, et al. Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nature genetics 2007; 39:857-864
46. Bell GI, Horita S, Karam JH. A polymorphic locus near the human insulin gene is associated with insulin-dependent diabetes mellitus. Diabetes 1984; 33:176-183
47. Undlien DE, Bennett ST, Todd JA, et al. Insulin gene region-encoded susceptibility to IDDM maps upstream of the insulin gene. Diabetes 1995; 44:620-625
48. Barratt BJ, Payne F, Lowe CE, et al. Remapping the insulin gene/IDDM2 locus in type 1 diabetes. Diabetes 2004; 53:1884-1889
49. Pugliese A, Brown D, Garza D, et al. Self-antigen-presenting cells expressing diabetes-associated autoantigens exist in both thymus and peripheral lymphoid organs. The Journal of clinical investigation 2001; 107:555-564
50. Pugliese A, Zeller M, Fernandez A, Jr., et al. The insulin gene is transcribed in the human thymus and transcription levels correlated with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nature genetics 1997; 15:293-297
51. Bennett ST, Wilson AJ, Esposito L, et al. Insulin VNTR allele-specific effect in type 1 diabetes depends on identity of untransmitted paternal allele. The IMDIAB Group. Nature genetics 1997; 17:350-352
52. Bottini N, Musumeci L, Alonso A, et al. A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nature genetics 2004; 36:337-338
53. Kyogoku C, Langefeld CD, Ortmann WA, et al. Genetic association of the R620W polymorphism of protein tyrosine phosphatase PTPN22 with human SLE. American journal of human genetics 2004; 75:504-507
54. Begovich AB, Carlton VE, Honigberg LA, et al. A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. American journal of human genetics 2004; 75:330-337
55. Ueda H, Howson JM, Esposito L, et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature 2003; 423:506-511
56. Noble JA, Valdes AM, Varney MD, et al. HLA class I and genetic susceptibility to type 1 diabetes: results from the Type 1 Diabetes Genetics Consortium. Diabetes 2010; 59:2972-2979
57. Burren OS, Adlem EC, Achuthan P, et al. T1DBase: update 2011, organization and presentation of large-scale data sets for type 1 diabetes research. Nucleic acids research 2011; 39:D997-1001
58. Abiru N, Maniatis AK, Yu L, et al. Peptide and major histocompatibility complex-specific breaking of humoral tolerance to native insulin with the B9-23 peptide in diabetes-prone and normal mice. Diabetes 2001; 50:1274-1281
59. Heath VL, Hutchings P, Fowell DJ, et al. Peptides derived from murine insulin are diabetogenic in both rats and mice, but the disease-inducing epitopes are different: evidence against a common environmental cross-reactivity in the pathogenicity of type 1 diabetes. Diabetes 1999; 48:2157-2165
60. Wen L, Chen NY, Tang J, et al. The regulatory role of DR4 in a spontaneous diabetes DQ8 transgenic model. The Journal of clinical investigation 2001; 107:871-880
61. Ellerman KE, Like AA. Susceptibility to diabetes is widely distributed in normal class IIu haplotype rats. Diabetologia 2000; 43:890-898
62. Ellerman KE, Richards CA, Guberski DL, et al. Kilham rat triggers T-cell-dependent autoimmune diabetes in multiple strains of rat. Diabetes 1996; 45:557-562
63. Wucherpfennig KW, Eisenbarth GS. Type 1 diabetes. Nature immunology 2001; 2:767-768
64. Stadinski BD, Zhang L, Crawford F, et al. Diabetogenic T cells recognize insulin bound to IAg7 in an unexpected, weakly binding register. Proceedings of the National Academy of Sciences of the United States of America 2010; 107:10978-10983
65. Michels AW. Targeting the trimolecular complex. Clin Immunol 2013; 149:339-344
66. Ginsberg-Fellner F, Witt ME, Yagihashi S, et al. Congenital rubella syndrome as a model for type 1 (insulin-dependent) diabetes mellitus: increased prevalence of islet cell surface antibodies. Diabetologia 1984; 27 Suppl:87-89
67. Shaver KA, Boughman JA, Nance WE. Congenital rubella syndrome and diabetes: a review of epidemiologic, genetic, and immunologic factors. American annals of the deaf 1985; 130:526-532
68. Rabinowe SL, George KL, Loughlin R, et al. Congenital rubella. Monoclonal antibody-defined T cell abnormalities in young adults. The American journal of medicine 1986; 81:779-782
69. Lonnrot M, Korpela K, Knip M, et al. Enterovirus infection as a risk factor for beta-cell autoimmunity in a prospectively observed birth cohort: the Finnish Diabetes Prediction and Prevention Study. Diabetes 2000; 49:1314-1318
70. Graves PM, Norris JM, Pallansch MA, et al. The role of enteroviral infections in the development of IDDM: limitations of current approaches. Diabetes 1997; 46:161-168
71. Rewers M, Norris JM. Epidemiology of type I diabetes. In: Eisenbarth GS, Lafferty KJ, eds. Type I diabetes : molecular, cellular, and clinical immunology. New York: Oxford University Press; 1996:172-208.
72. Martin JM, Trink B, Daneman D, et al. Milk proteins in the etiology of insulin-dependent diabetes mellitus (IDDM). Annals of medicine 1991; 23:447-452
73. Norris JM, Beaty B, Klingensmith G, et al. Lack of association between early exposure to cow's milk protein and beta-cell autoimmunity. Diabetes Autoimmunity Study in the Young (DAISY). Jama 1996; 276:609-614
74. Norris JM, Barriga K, Klingensmith G, et al. Timing of initial cereal exposure in infancy and risk of islet autoimmunity. Jama 2003; 290:1713-1720
75. Ziegler AG, Schmid S, Huber D, et al. Early infant feeding and risk of developing type 1 diabetes-associated autoantibodies. Jama 2003; 290:1721-1728
76. Yang Z, Wang K, Li T, et al. Childhood diabetes in China. Enormous variation by place and ethnic group. Diabetes care 1998; 21:525-529
77. Onkamo P, Vaananen S, Karvonen M, et al. Worldwide increase in incidence of Type I diabetes--the analysis of the data on published incidence trends. Diabetologia 1999; 42:1395-1403
78. Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. The New England journal of medicine 2002; 347:911-920
79. Wenzlau JM, Moua O, Sarkar SA, et al. SlC30A8 is a major target of humoral autoimmunity in type 1 diabetes and a predictive marker in prediabetes. Annals of the New York Academy of Sciences 2008; 1150:256-259
80. Bonifacio E, Atkinson M, Eisenbarth G, et al. International Workshop on Lessons From Animal Models for Human Type 1 Diabetes: Identification of Insulin but Not Glutamic Acid Decarboxylase or IA-2 as Specific Autoantigens of Humoral Autoimmunity in Nonobese Diabetic Mice. Diabetes 2001; 50:2451-2458
81. Margolis DJ, Gupta J, Hoffstad O, et al. Lack of effectiveness of hyperbaric oxygen therapy for the treatment of diabetic foot ulcer and the prevention of amputation: a cohort study. Diabetes care 2013; 36:1961-1966
82. Miao D, Guyer KM, Dong F, et al. GAD65 autoantibodies detected by electrochemiluminescence assay identify high risk for type 1 diabetes. Diabetes 2013; 62:4174-4178
83. Bottazzo GF, Florin-Christensen A, Doniach D. Islet-cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet 1974; 2:1279-1283
84. Bonifacio E, Scirpoli M, Kredel K, et al. Early autoantibody responses in prediabetes are IgG1 dominated and suggest antigen-specific regulation. J Immunol 1999; 163:525-532
85. Yu L, Robles DT, Abiru N, et al. Early expression of antiinsulin autoantibodies of humans and the NOD mouse: evidence for early determination of subsequent diabetes. Proceedings of the National Academy of Sciences of the United States of America 2000; 97:1701-1706
86. Vardi P, Ziegler AG, Mathews JH, et al. Concentration of insulin autoantibodies at onset of type I diabetes. Inverse log-linear correlation with age. Diabetes care 1988; 11:736-739
87. Verge CF, Gianani R, Kawasaki E, et al. Prediction of type I diabetes in first-degree relatives using a combination of insulin, GAD, and ICA512bdc/IA-2 autoantibodies. Diabetes 1996; 45:926-933
88. Bingley PJ, Bonifacio E, Williams AJ, et al. Prediction of IDDM in the general population: strategies based on combinations of autoantibody markers. Diabetes 1997; 46:1701-1710
89. Ziegler AG, Rewers M, Simell O, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. Jama 2013; 309:2473-2479
90. Bosi E, Braghi S, Maffi P, et al. Autoantibody response to islet transplantation in type 1 diabetes. Diabetes 2001; 50:2464-2471
91. Clark CM, Jr. How should we respond to the worldwide diabetes epidemic? Diabetes care 1998; 21:475-476
92. Sreenan S, Pick AJ, Levisetti M, et al. Increased beta-cell proliferation and reduced mass before diabetes onset in the nonobese diabetic mouse. Diabetes 1999; 48:989-996
93. Gazda LS, Charlton B, Lafferty KJ. Diabetes results from a late change in the autoimmune response of NOD mice. Journal of autoimmunity 1997; 10:261-270
94. Dilts SM, Lafferty KJ. Autoimmune diabetes: the involvement of benign and malignant autoimmunity. Journal of autoimmunity 1999; 12:229-232
95. Andre I, Gonzalez A, Wang B, et al. Checkpoints in the progression of autoimmune disease: lessons from diabetes models. Proceedings of the National Academy of Sciences of the United States of America 1996; 93:2260-2263
96. Shimada A, Charlton B, Taylor-Edwards C, et al. Beta-cell destruction may be a late consequence of the autoimmune process in nonobese diabetic mice. Diabetes 1996; 45:1063-1067
97. Chatenoud L, Primo J, Bach JF. CD3 antibody-induced dominant self tolerance in overtly diabetic NOD mice. J Immunol 1997; 158:2947-2954
98. Rosenbauer J, Herzig P, von Kries R, et al. Temporal, seasonal, and geographical incidence patterns of type I diabetes mellitus in children under 5 years of age in Germany. Diabetologia 1999; 42:1055-1059
99. Chase HP, Cuthbertson DD, Dolan LM, et al. First-phase insulin release during the intravenous glucose tolerance test as a risk factor for type 1 diabetes. The Journal of pediatrics 2001; 138:244-249
100. Foulis AK, McGill M, Farquharson MA, et al. A search for evidence of viral infection in pancreases of newly diagnosed patients with IDDM. Diabetologia 1997; 40:53-61
101. Srikanta S, Ganda OP, Rabizadeh A, et al. First-degree relatives of patients with type I diabetes mellitus. Islet-cell antibodies and abnormal insulin secretion. The New England journal of medicine 1985; 313:461-464
102. Laing SP, Swerdlow AJ, Slater SD, et al. The British Diabetic Association Cohort Study, II: cause-specific mortality in patients with insulin-treated diabetes mellitus. Diabetic medicine : a journal of the British Diabetic Association 1999; 16:466-471
103. Laing SP, Swerdlow AJ, Slater SD, et al. The British Diabetic Association Cohort Study, I: all-cause mortality in patients with insulin-treated diabetes mellitus. Diabetic medicine : a journal of the British Diabetic Association 1999; 16:459-465
104. Herskowitz RD, Wolfsdorf JI, Ricker AT, et al. Transient hyperglycemia in childhood: identification of a subgroup with imminent diabetes mellitus. Diabetes Res 1988; 9:161-167
105. Barker JM, Goehrig SH, Barriga K, et al. Clinical characteristics of children diagnosed with type 1 diabetes through intensive screening and follow-up. Diabetes care 2004; 27:1399-1404
106. Effects of insulin in relatives of patients with type 1 diabetes mellitus. The New England journal of medicine 2002; 346:1685-1691
107. Skyler JS, Krischer JP, Wolfsdorf J, et al. Effects of oral insulin in relatives of patients with type 1 diabetes: The Diabetes Prevention Trial--Type 1. Diabetes care 2005; 28:1068-1076
108. Herold KC, Hagopian W, Auger JA, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. The New England journal of medicine 2002; 346:1692-1698
109. Herold KC, Gitelman SE, Willi SM, et al. Teplizumab treatment may improve C-peptide responses in participants with type 1 diabetes after the new-onset period: a randomised controlled trial. Diabetologia 2013; 56:391-400
110. Lebastchi J, Deng S, Lebastchi AH, et al. Immune therapy and beta-cell death in type 1 diabetes. Diabetes 2013; 62:1676-1680
111. Herold KC, Gitelman SE, Ehlers MR, et al. Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes 2013; 62:3766-3774
112. Pozzilli P, Guglielmi C, Maggi D, et al. Clinical update on the use of immuno modulators (antiCD3, GAD, Diapep277, anti-IL1) in type 1 diabetes. Current pharmaceutical design 2011; 17:3224-3228
113. Sherry N, Hagopian W, Ludvigsson J, et al. Teplizumab for treatment of type 1 diabetes (Protege study): 1-year results from a randomised, placebo-controlled trial. Lancet 2011; 378:487-497
114. Bach JF. Anti-CD3 antibodies for type 1 diabetes: beyond expectations. Lancet 2011; 378:459-460
115. Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. The New England journal of medicine 2009; 361:2143-2152
116. Orban T, Bundy B, Becker DJ, et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet 2011; 378:412-419
117. Rigby MR, DiMeglio LA, Rendell MS, et al. Targeting of memory T cells with alefacept in new-onset type 1 diabetes (T1DAL study): 12 month results of a randomised, double-blind, placebo-controlled phase 2 trial. The lancet Diabetes & endocrinology 2013; 1:284-294
118. Wherrett DK, Bundy B, Becker DJ, et al. Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: a randomised double-blind trial. Lancet 2011; 378:319-327
119. Ludvigsson J, Krisky D, Casas R, et al. GAD65 antigen therapy in recently diagnosed type 1 diabetes mellitus. The New England journal of medicine 2012; 366:433-442
120. Moran A, Bundy B, Becker DJ, et al. Interleukin-1 antagonism in type 1 diabetes of recent onset: two multicentre, randomised, double-blind, placebo-controlled trials. Lancet 2013; 381:1905-1915
121. Roep BO, Solvason N, Gottlieb PA, et al. Plasmid-encoded proinsulin preserves C-peptide while specifically reducing proinsulin-specific CD8(+) T cells in type 1 diabetes. Science translational medicine 2013; 5:191ra182
- Review Type 1 Diabetes Mellitus and Celiac Disease: Distinct Autoimmune Disorders That Share Common Pathogenic Mechanisms.[Horm Res Paediatr. 2019]Review Type 1 Diabetes Mellitus and Celiac Disease: Distinct Autoimmune Disorders That Share Common Pathogenic Mechanisms.Goodwin G. Horm Res Paediatr. 2019; 92(5):285-292. Epub 2019 Oct 8.
- A Prospective Study to Evaluate the Possible Role of Cholecalciferol Supplementation on Autoimmunity in Hashimoto's Thyroiditis.[J Assoc Physicians India. 2023]A Prospective Study to Evaluate the Possible Role of Cholecalciferol Supplementation on Autoimmunity in Hashimoto's Thyroiditis.Bhakat B, Pal J, Das S, Charaborty SK, SircarMedical NR, Kolkata, RGKar, NorthBengal, Siliguri. J Assoc Physicians India. 2023 Jan; 71(1):1.
- Review Immune intervention for type 1 diabetes mellitus.[Int J Clin Pract Suppl. 2011]Review Immune intervention for type 1 diabetes mellitus.Skyler JS. Int J Clin Pract Suppl. 2011 Feb; (170):61-70.
- Seropositive Neuromyelitis Optica in a Case of Undiagnosed Ankylosing Spondylitis: A Neuro-Rheumatological Conundrum.[Qatar Med J. 2022]Seropositive Neuromyelitis Optica in a Case of Undiagnosed Ankylosing Spondylitis: A Neuro-Rheumatological Conundrum.Ghosh Md R, Roy D, León-Ruiz M, Das S, Dubey S, Benito-León J. Qatar Med J. 2022; 2022(3):29. Epub 2022 Jul 7.
- Review Immunopathogenesis and immunotherapeutic approaches to type 1A diabetes.[Expert Opin Biol Ther. 2004]Review Immunopathogenesis and immunotherapeutic approaches to type 1A diabetes.Azam A, Eisenbarth GS. Expert Opin Biol Ther. 2004 Oct; 4(10):1569-75.
- Pathogenesis of Type 1A Diabetes - EndotextPathogenesis of Type 1A Diabetes - Endotext
Your browsing activity is empty.
Activity recording is turned off.
See more...
