NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-.
ABSTRACT
Metabolic bone disease (MBD) encompasses a heterogeneous group of disorders having a diverse spectrum of manifestations varying from asymptomatic to florid. MBD is prevalent globally, but certain unique features characterize those occurring in the tropics. Dietary deviations, including malnutrition, environmental influences, genetic factors, and limited access to healthcare, modify the tropical presentation of MBD. Osteoporosis remains the most prevalent MBD in the tropics. Anti-osteoporotic agents are widely available, but the compliance and follow-up are poor. Fracture liaison services are gaining importance to address the low rates of patient work-up and treatment following a fracture. Though the tropics have long been plagued with communicable diseases, there is a recent increase in noncommunicable lifestyle diseases and obesity, which are major risk factors for sarcopenia. A significant subset of adults over the age of 65 years have sarcopenia complicated by obesity and are at risk of synergistic complications from both obesity and sarcopenia. "Thin-fat obesity" or "sarcopenic obesity," also known as normal weight obesity, is a recognized phenotype in South Asia, with a comparable risk for cardiometabolic disease as obesity. In addition to osteosarcopenia, other MBDs, such as rickets and osteomalacia, still prevail in tropical countries. Symptomatic hyperparathyroidism is still seen in tropical countries, unlike the West, where asymptomatic hyperparathyroidism is more common. Skeletal fluorosis, an MBD caused by chronically ingesting excess fluoride, can be asymptomatic, but a broad range of manifestations can occur, such as diffuse skeletal pain, limited mobility, osteopenia, and ossification of ligaments and interosseous membranes. The oral cavity can be a window to other MBDs like dental fluorosis in the tropics. Several infective disorders that can affect bones and joints, such as tuberculosis, leprosy, treponemal and fungal infections are prevalent in the tropics and should be considered in the differential diagnosis of tropical MBD. For complete coverage of all related areas of Endocrinology, please visit our on-line FREE web-text, WWW.ENDOTEXT.ORG.
INTRODUCTION
Metabolic bone disease (MBD) encompasses a diverse variety of bone and mineral metabolism disorders that often pose diagnostic and therapeutic challenges. The manifestations might differ in the tropics because of nutritional, environmental, and genetic factors. The presentation is often late and severe because of limited access to healthcare facilities and a lack of routine screening practices. Osteoporosis, the most prevalent MBD globally, is also widespread in the tropics. Despite ample sunlight exposure, vitamin D deficiency continues to be common. Certain conditions, such as fluorosis, lead, and cadmium toxicity, and infective skeletal disorders e.g., tuberculosis, syphilis, and leprosy, are unique to these regions. The altered presentation of the globally prevalent MBDs and conditions specific to the tropics have been highlighted here.
OSTEOPOROSIS IN THE TROPICS
Osteoporosis is the most common MBD globally and in tropical countries. Different studies in the South Asian region have suggested the prevalence of osteoporosis to be 30 to 50% among postmenopausal women and up to 20% in men above 50 years (1, 2). With increasing life expectancy in tropical countries, this prevalence will likely increase in the coming years. Several factors contribute to the high frequency and unique features of osteoporosis in tropical countries. These are summarized in table 1.
Table 1.
Unique Features of Osteoporosis in the Tropical Region (3)
| 1. | Lower peak bone mass |
| 2. | Poor dietary intake of calcium |
| 3. | A large proportion of individuals with vitamin D deficiency |
| 4. | Paucity of dual-energy X-ray absorptiometry scanners leading to delayed diagnosis |
| 5. | Relatively earlier age of menopause |
| 6. | Limited food fortification for calcium and vitamin D |
| 7. | Lower bone mineral density threshold for developing a fragility fracture |
| 8. | Less awareness and knowledge about osteoporosis |
Despite the high mortality from hip fractures and the immense costs associated with its management, awareness about osteoporosis and its screening is poor among patients and physicians. In a study from southern India, 60% of postmenopausal women (n =302, mean age of 58 years) lacked knowledge of osteoporosis. In another study among general practitioners (n = 220), the total mean score on awareness was only 22% (1, 4).
Given the limitation of the diagnostic facilities, simple, cost-effective screening tools have been validated for use in the tropics. These may range from simple clinical findings like dental health assessment to more comprehensive evaluation through the FRAX tool. Easy-to-follow clinical practice guidelines have been developed that help manage patients with osteoporosis despite the above-mentioned constraints (5, 6).
Given poor dietary calcium intake and low levels of 25-hydroxyvitamin D [25(OH)D] despite abundant sunlight, adequate calcium and vitamin D is an essential component of osteoporosis management in these patients. Anti-osteoporotic agents are widely available, but compliance and follow-up are poor. The usage of bone turnover markers is still emerging and may evolve as a cost-effective tool. Moreover, strategies such as the fracture liaison service (FLS) will be helpful to enhance care for secondary prevention of fragility fractures (7).
DISORDERS OF BONE MINERAL METABOLISM IN THE TROPICS
Rickets And Osteomalacia
Rickets and osteomalacia are also prevalent in tropical countries. In children, the prevalence of nutritional calcipenic rickets has decreased significantly but remains the usual cause of a rachitic presentation. It’s a bit paradoxical for tropical countries with abundant sunlight to have a high occurrence of vitamin D deficiency. Though rare, the genetic causes of calcipenic and phosphopenic rickets may be more frequent in specific pockets where consanguinity is practiced.
Like children, dietary deficiency is the usual etiology of osteomalacia in adults. However, certain acquired causes are common and must be ruled out. These include renal tubular dysfunction caused by heavy metal exposure, often from native medications. Moreover, Fanconi syndrome can result from certain drugs used to treat infectious disorders. For example, tenofovir used to treat hepatitis B can lead to proximal renal tubular acidosis. The rampant use of glucocorticoids in different over-the-counter products enhances the risk of glucocorticoid-induced osteomalacia. Furthermore, a gradual increase in chronic kidney disease (CKD) in tropical countries, is leading to a higher incidence of renal osteodystrophy (8).
Vitamin D deficiency in the tropics is widespread and ranges from 40 to 80%. Several postulates have been proposed to explain the lower vitamin D levels despite adequate sunlight. These include increased melanin in the skin, predominant indoor habits, and lack of vitamin D fortification. The relatively higher amount of fat for a given body mass index affects the redistribution of vitamin D. Lastly, air pollution in certain metropolitan cities reduces exposure to sunlight. Other causes of osteomalacia in tropical countries include renal tubular dysfunction and acquired hypophosphatemic disorders, covered in the “Diseases of Bone and Mineral Metabolism” section in Endotext.
Hyperparathyroidism
Parathyroid disorders could be classified as primary – wherein the parathyroid gland is diseased and secretes excessive parathyroid hormone (PTH), or secondary – where excess PTH secretion occurs as a compensatory response to systemic conditions such as vitamin D deficiency, chronic kidney disease, etc. The third variety, tertiary hyperparathyroidism, is characterized by the autonomous transformation of the parathyroid gland during prolonged secondary PTH hypersecretion (9).
Hyperparathyroidism differs in clinical presentation in tropical countries and often manifests as florid disease. Parathyroid disorders are usually slowly evolving, and overt skeletal defects are declining as more facilities start to screen for calcium in the tropics routinely. Thus, the prevalence of asymptomatic disease is gradually increasing (10). The salient differences in tropical countries are summarized in table 2.
Table 2.
Difference in Parathyroid Disorders in Tropical Countries
| 1. | Symptomatic hyperparathyroidism is still seen in tropical countries, unlike the West, where asymptomatic hyperparathyroidism is more common |
| 2. | Higher calcium and parathyroid hormone levels are found in tropics |
| 3. | Higher prevalence of vitamin D deficiency in the tropics |
| 4. | Intraoperatively a larger tumor/gland size in the tropics is usually observed |
| 5. | Brown tumors and severe bone diseases seen in the tropics are rare in the West |
Scurvy
Nutritional deficiencies are rare in the developed world but are observed in tropical countries. Vitamin C deficiency is a rare cause of MBD but should be considered while evaluating patients with suggestive symptoms and radiological features. It may affect the bone and joints, but initial diagnosis is often missed due to nonspecific symptoms and signs. Initial manifestations include irritability, decreased appetite, and delayed development followed by a pseudo-paralysis-like state wherein the patient lies still with little movement because of generalized pain, most apparent in bones due to subperiosteal hemorrhages. Swelling may be noted along the shaft of long bones.
Radiological changes in the long bones, particularly around the knee, are peculiar to scurvy. The bones are often fragile, with bone mineral density (BMD) in the osteopenic range. Fracture healing is often associated with large callus formation. Moreover, the epiphyses and periosteum are easily detachable due to the sub-periosteal bleeding. A classical feature on radiography known as the Wimberger ring is a circular, opaque radiologic shadow surrounded by a white line, seen in the growth centers around the epiphysis, as shown in Figure 1. The cortical bone in vitamin C deficiency is characterized by thinning, often described as a “pencil-point” cortex.
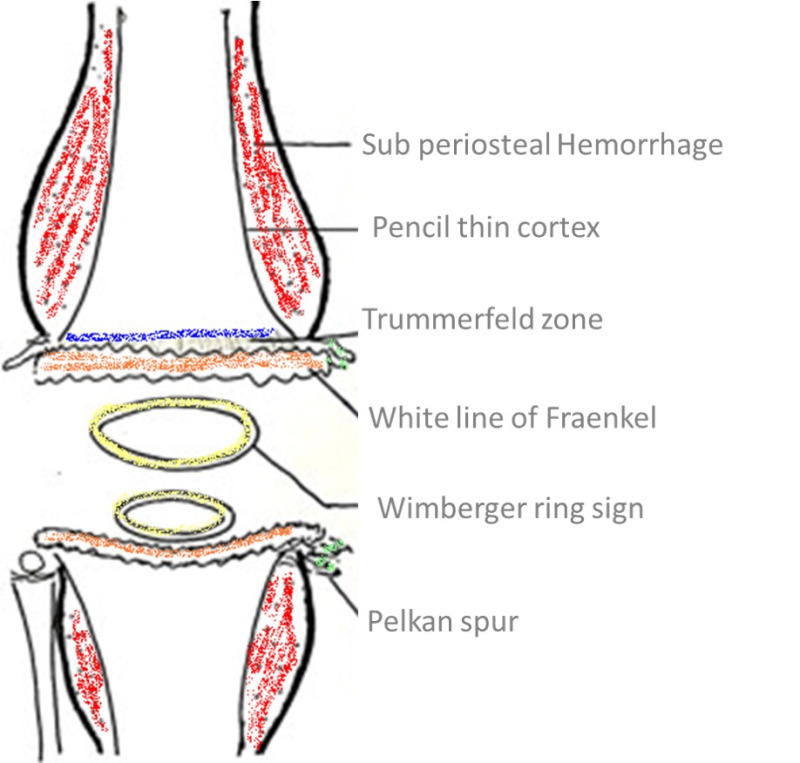
Figure 1.
Illustration of the radiological features of scurvy.
The physis exhibits the Frankel line, characterized by thickening and sclerosis accompanied by a subjacent zone of lucency. The physeal thickening is known as a Frankel line, and the adjacent lucent zone on its diaphyseal side is the Trümmerfeld zone or the scurvy line. Metaphyseal “beaks” and transverse lines of increased or decreased opacity may be seen in scurvy. The “beaks,” known as Pelkan spurs, are associated with healing fractures of the Trümmerfeld zone at the periphery of the site of calcification. Costochondral junctions of the first six or eight thoracic ribs may be expanded; this change may be related to fracturing of the zone of provisional calcification during normal respiration. The costochondral junctions are rounded and appear smooth, knobby, and steplike. The enlargement of the costochondral junctions simulates findings seen in rickets but is often painful.
The diagnosis of scurvy is based on a combination of clinical and radiographic findings. A dietary history suggestive of poor vitamin C intake for at least one to three months is required for the appearance of clinical symptoms. Unlike other MBDs, accurate laboratory measurement of vitamin C levels is unreliable as it does not reflect the tissue levels. Healing occurs rapidly with the oral administration of 100 to 200 mg/d of vitamin C (11).
Paget’s Disease Of The Bone
Paget’s disease of the bone is a rare disorder in the tropics compared to the temperate regions but has been reported in certain pockets, especially in southern India. The overall global prevalence seems to be declining. Several viruses, such as paramyxoviridae, respiratory syncytial virus, canine distemper virus, and measles virus, have been linked to its etiopathogenesis. Given the high prevalence of many of these viruses in the tropics, the tropical connection of Paget’s disease has received renewed attention.
An underlying genetic etiology also predisposes to a higher occurrence in consanguineous populations. Due to the nonspecific symptoms, poor awareness among treating physicians, and limited availability of bone scans, the diagnosis is often missed. Thus, its true prevalence is unlikely to be determined. In a study from southern India, the mean age was 60 years in a cohort of 48 patients. It was reported predominantly in men (65%), and about one-fifth of them were asymptomatic. Among the symptomatic patients, 87% have polyostotic involvement. Sixty-nine percent of them were treated with zoledronic acid, and all achieved remission immediately after therapy (12).
Renal Tubular Disorders (Acquired & Genetic)
Different varieties of genetic and acquired renal tubular acidosis (RTA) are known to occur in the tropics. Specifically, the SLC4A1 (solute carrier family 4 A1) gene mutation-induced dysfunction of the erythroid and kidney isoforms of anion exchanger 1 (AE1 or band 3) causes distal RTA in some areas of the tropics. The mutation is prevalent in Thailand, Malaysia, the Philippines, and Papua New Guinea. The inheritance is autosomal recessive and can result from either homozygous or compound heterozygous SLC4A1 mutations (13). Wilson’s disease, sickle cell anemia, and medullary sponge kidney are the other genetic causes of RTA more frequently encountered in regions where consanguinity is common.
Among the acquired causes, several drugs have been implicated in the pathogenesis of RTA. Some of these are used in managing common infective disorders in tropical countries, for example, tenofovir, for treating hepatitis B. One study reported lower bone mass (12.3%) in patients with hepatitis B on tenofovir compared to those not on the drug or those without hepatitis B (8). Among other acquired causes of RTA, amphotericin B, analgesic abuse, recurrent urinary tract infection, and hyperparathyroidism have been commonly reported in some tropical countries.
INFECTIVE DISORDERS ASSOCIATED WITH METABOLIC BONE DISEASE
Several infective disorders affect bones and joints, causing debilitating disease. Given the high incidence of infectious conditions in the tropical countries, the concurrence of bone lesions is expected.
Tuberculosis
Tuberculosis is an unusual cause of bony lesions, but immunocompromised states such as human immunodeficiency virus (HIV) infection and diabetes increase the risk. Spine involvement can present insidiously, with progressive back pain in endemic areas, especially when consecutive thoracic vertebrae are affected with relative disc preservation. Paravertebral and epidural soft tissue lesions may present as a cold abscess. Atypical tubercular osteoarticular manifestations involving the extraspinal skeleton, a prosthetic joint, or the trochanteric area, and nontuberculous mycobacterial infections should raise suspicion for immunocompromised states. Surgery combined with prolonged antitubercular therapy is indicated for neurological manifestations or deformities and provides satisfactory results in most cases. Because of the increasing resistance to antitubercular treatment, appropriate culture and sensitivity testing should be ordered whenever indicated (14).
Leprosy
Mycobacterium leprae primarily affects the skin and nerves. Effective antimicrobial therapy has improved outcome in the acute stage of infection. However, long-term sequelae such as foot drop are not unusual if the diagnosis is delayed. Ineffective penetration of antimycobacterial therapy into neural tissue can predispose to continued nerve damage and result in Charcot’s neuroarthropathy. Other chronic effects include male osteoporosis and hypogonadism. Testicular atrophy following invasion by Mycobacterium leprae have caused osteoporosis in a few cases (15).
Treponemal Diseases
The spirochete Treponema pallidum causes syphilis. Earlier published series have described three broad types of skeletal malformations. They include - group I: metaphyseal dystrophy, group II: osteitis-like dystrophy, and group III: periosteal dystrophy. Advances in case detection, treatment, and prevention have significantly lowered the incidence of the disease and its associated complications (16). Contrary to the earlier view that the malformations are inflammatory, newer evidence suggests that dystrophic changes are responsible. In a series of 55 cases, the most frequent osseous findings were metaphysitis, zone of rarefaction, periostitis, disorganized metaphysis, bone erosion, and Wimberger sign. The bones commonly affected were the long bones such as radius, ulna, tibia, femur, humerus, and fibula (17).
Fungal Infection
Osteoarticular mycoses are uncommon in clinical practice and Aspergillus and Candida are the organisms involved usually. Dimorphic fungi such as Histoplasma, Blastomyces, Coccidiosis, and Paracoccidiodes can affect the bones in endemic areas. They occur predominantly in immunocompetent hosts and are characterized by hematogenous dissemination. The natural history is typically indolent but occasionally the organisms behave virulently.
While mucormycosis is highly aggressive and destructive in the lung, sinuses, and brain, it is relatively indolent in the bone. Mucormycosis can affect any bones and joints without specific predilection for any particular site. Most cases occur from direct inoculation of the bone rather than through systemic seeding. Osteoarticular mucormycosis can sometimes necessitate bony amputation to prevent the nidus from spreading through the systemic circulation. A high index of suspicion, early diagnosis, and intensive treatment are the key to successful management (18).
CHEMICAL AND TOXIN-RELATED DISORDERS IN TROPICAL COUNTRIES
Skeletal Fluorosis
Skeletal fluorosis is caused by chronically ingesting excess fluoride, usually from natural sources. Skeletal fluorosis can be asymptomatic, but a broad range of manifestations, including diffuse skeletal pain, limited mobility, osteopenia, and ossification of ligaments and interosseous membranes, are known to occur. The severity of the disease depends on the amount and duration of fluoride exposure. The portal of entry is oral, and after absorption from the gastrointestinal tract, it is deposited in the skeleton, where it has a half-life of more than seven years. Incorporation of the fluoride in the hydroxyapatite crystal affects bone strength, and influences bone remodeling through the Runt-related transcription factor 2 (Runx2) and receptor activator of nuclear factor kappa-В ligand (RANKL). This alters the expression of osteocalcin and osteoprotegerin with resultant increased osteoblastic activity (19).
The usual clinical features are dental mottling, bony pains, chronic fatigue, joint stiffness with restricted range of motion, flexion contractures, radiculo-myelopathy, and increased fracture risk. The diagnosis is based on a high index of clinical suspicion and confirmed by a 24-hour estimation of the urinary fluoride level. There is no effective treatment for established skeletal fluorosis. Management consists of symptomatic therapy with analgesics and provision of adequate calcium and vitamin D. Decompressive laminectomy may be performed to relieve neurological deficits due to spine involvement. Identifying the source of high fluoride intake, defluoridation, or changing the water source helps prevent worsening.
Lead Toxicity And Its Effect On Bone
Lead toxicity remains a substantial public health problem globally and in tropical countries. Bone is a major reservoir of lead in both adults and children, accounting for 75-90% of the total body lead. The accumulated lead can gradually be released to other soft tissues and pathological sites. Bone lead accrual occurs after both environmental and occupational exposure. It is a marker of past lead exposure with a half-life of 20 years and thus increases gradually with age. (20)
Lead exerts a detrimental effect by reducing osteocalcin production and inhibiting alkaline phosphatase activity in osteoblasts. Lead suppresses type II and type X collagen expression in chondrocytes and alters growth factors and second messenger signaling responses during chondrocyte maturation. Lead stimulates the osteoclasts to enhance bone resorption. Strict vigilance and robust policy decisions to decrease lead exposure will improve skeletal health in the tropics.
Cadmium Toxicity And Metabolic Bone Disease
Exposure to cadmium is associated with kidney, bone, and cardiovascular disorders. Like lead, cadmium is stored in the renal tissue for many years (half-life 10–30 years), which could result in renal tubular dysfunction, glomerular damage, and renal failure. A population-based study among postmenopausal women showed a clear link between a high burden of cadmium and low BMD (21). There are two proposed mechanisms for bone loss; a direct action on bone cells and an indirect action on the kidney resulting in phosphate and calcium excretion. In vitro studies have demonstrated that cadmium can increase the RANKL expression, tartarate-resistant acid phosphatase (TRAP) activity, and formation of TRAP-positive cells in the presence of RANKL, resulting in increased osteoclastic activity.
In tropical countries, silversmiths have exposure to cadmium in the absence of personal protective equipment. Case reports of exposure to cadmium and consequent renal osteodystrophy have been reported (22).
Alcohol And Metabolic Bone Disease
Alcohol consumption can, directly and indirectly, affect bone health. The mechanisms are summarized in Figure 2. In a study by Peris et al., vertebral fractures were observed in 36% of those consuming alcohol chronically, but only 6.5% had BMD below the fracture threshold. Thus chronic alcohol consumption may lead to a higher propensity for vertebral fractures without impacting the BMD (23). The evaluation and treatment would depend upon the clinical profile.
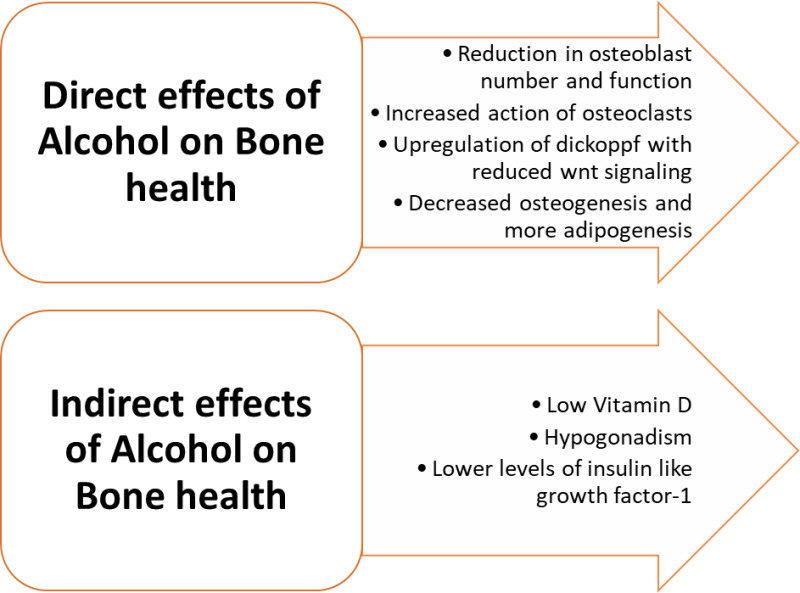
Figure 2.
Impact of chronic alcohol consumption on metabolic bone disease.
NEPHROLITHIASIS IN TROPICS
Renal stone disease is a common problem worldwide. The prevalence and composition of renal stones differ according to country, climate, and culture (24). Conforming to that trend, renal stone diseases in tropical countries exhibit some unique features. The usual constituents of stones include calcium oxalate, calcium phosphate, uric acid, cysteine, struvite, or a mixture of these. In many tropical countries, local factors influence the composition of the stone. Nephrolithiasis, in general, has been reviewed elsewhere in endotext.org (25).
The prevalence of renal stone disease in older reports ranged from 7 to 13% in North America, 5–9% in Europe, and 1–5% in Asia (26). The lower frequency in Asia could be partially from underreporting. Recent findings demonstrate that the incidence of renal stones has increased by 48.5% in the last three decades across the globe (26, 27). The age-standardized incidence rate (ASIR) was greater in countries with high, middle, and low-middle sociodemographic index (SDI) than in low SDI regions. Some African countries, such as Madagascar, South Sudan, and Burundi, had a lower incidence of renal stones (27). An increased oxalate-degrading bacteria count in the gut of black South Africans could be a possible explanation (28).
The tropical region of Asia, spanning West Asia, South Asia, and Southeast Asia, constitutes a stone-forming belt with high prevalence (5% to 19.1%) (29). Higher temperatures and sunlight exposure increase the risk in these regions (30). Similar trends are also described in Latin America (31). Globally, the peak incidence of nephrolithiasis occurs between 50 to 70 years, though in Asians, the maximum incidence is around 30 years. Males are affected more often globally and in Asia (27, 29).
Unique Aspects In Tropics
TROPICAL CLIMATE AND STONE FORMATION
Higher ambient temperature and low humidity in the tropics increase fluid loss through sweating. Urinary concentration increases as compensation, and relatively insoluble salts such as calcium oxalate and urates tend to precipitate and serve as foci of stone formation. Sweating-induced reduction in urinary pH favors the crystallization of uric acid and further enhances the risk of urate stone (32). Renal colic is thus more common during warmer months (33). Additionally, studies suggest that males are more susceptible to developing kidney stones due to increased temperature. However, whether this is related to the total cumulative heat exposure or differential pathophysiological response is unclear (34, 35).
METABOLIC SYNDROME AND NEPHROLITHIASIS
The prevalence of metabolic syndrome in topical countries is on the rise (36-38). The upsurge in the frequency of nephrolithiasis could be attributed somewhat to the increasing burden of metabolic disorders. A consistent association between the prevalence of metabolic syndrome and renal stones has been demonstrated (39). Obesity, diabetes, hypertension, and insulin resistance, all components of metabolic syndrome, are risk factors for stone formation (40). Hyperuricemia, another manifestation of insulin resistance, is a recognized pathophysiologic link. Insulin resistance decreases urinary ammonium production leading to the formation of acidic urine, augmenting the potential for lithogenesis (41, 42). However, the predominant stone type in metabolic syndrome is oxalate. Hyperoxaluria in metabolic syndrome is multifactorial in etiology and could be related to changes in gut flora, increased renal oxidative stress, and alteration in the balance between promoters and inhibitors of lithogenesis (43, 44).
CHILDHOOD ENDEMIC BLADDER STONES
The incidence of vesical calculus has declined significantly over the last few decades in developed nations but is still prevalent in the tropics. Reliance on carbohydrate-rich food and lack of protein early in life results in a relative deficiency of phosphates and leads to the formation of insoluble urinary salts. The boys are predisposed as their long tortuous urethra is a hindrance to clearing the debris (45, 46).
DENTAL DISORDERS IN THE TROPICS
Tropical oral disorders include a wide range of conditions that may be manifestations of systemic diseases. Osteoporosis continues to be one of the most underdiagnosed and under-reported conditions in the tropics. Though the diagnosis of osteoporosis is based on DXA, it has been suggested that signs in the oral cavity and dental X-rays can be used for primary screening in resource-limited settings. Several radiographic indices, such as mandibular cortical index, mandibular cortical width, antegonial Index, gonial index, panoramic mandibular index, and alveolar crest resorption degree (M/M ratio), have been explored as screening tools for osteoporosis (49).
The oral cavity can be a window to many other MBDs in the tropics. Vitamin D is crucial for the mineralization of bones and teeth. Low vitamin D levels lead to malformed, hypomineralized teeth that are brittle and vulnerable to decay, also known as “rachitic teeth (50). Dental abscesses are characteristic of vitamin D-resistant rickets (VDRR). Other characteristic manifestations of VDRR include dentin defects, unusually large pulp chambers, enlarged pulp horns, and enamel hypoplasia (51). Brown tumor, loss of bone density, soft tissue calcification, and dental abnormalities, such as developmental defects and changes in tooth eruption, are common oral symptoms in hyperparathyroidism. Malocclusion due to the drifting of teeth and spacing of the teeth may be the first signs of hyperparathyroidism (52).
Tropical infective diseases such as tuberculosis can atypically present with oral manifestations. Lesions in the jaw in the form of osteomyelitis or simple bone radiolucency, as well as superficial ulcers, patches, and indurated soft tissue lesions, are described (53). Dental fluorosis is caused by excessive fluoride ingestion during tooth development. Dental fluorosis is common in the tropics and is described in the previous section (54, 55). Clinically, mild cases of dental fluorosis manifest as an opaque white appearance of the enamel from increased subsurface porosity. Moderate dental fluorosis manifests as yellow to light brown staining in the areas of enamel damage. Severe dental fluorosis results in a porous enamel that is poorly mineralized and stains brown (56). Preventive strategies involve control of fluoride levels in drinking water (57).
SARCOPENIA AND BONE – A TROPICAL PERSPECTIVE
Sarcopenia, defined as the loss of muscle mass and strength, is an emerging global health problem. In a recent meta-analysis on the worldwide prevalence of sarcopenia, using different classifications and cut-off points, the prevalence of sarcopenia varied between 10 to 27% (58). Multi-center research from nine nations (Finland, Poland, Spain, China, Ghana, India, Mexico, Russia, and South Africa) across three continents revealed an overall frequency of 15.2% (59).
Types Of Sarcopenia
When no other etiology other than aging is apparent, the condition is called "primary" (or age-related) sarcopenia. The term "secondary" sarcopenia refers to sarcopenia from one more additional cause (60). Many tropical countries are seeing a rapid increase in the aging population owing to improved healthcare facilities (61, 62). Hence, primary sarcopenia can potentially become a serious health concern globally, particularly in the developing world. Secondary sarcopenia could be activity related (resulting from bed rest, a sedentary lifestyle), disease-related (associated with endocrine diseases, advanced organ failure, malignancy, inflammatory disease), or nutrition-related (inadequate dietary intake of energy/protein, malabsorption, use of medications that cause anorexia, etc.) (60).
Risk Factors For Sarcopenia In The Tropics
Though the tropics have been plagued with communicable diseases for a long time, there is a recent increase in noncommunicable lifestyle diseases and obesity, major risk factors for sarcopenia (63, 64). A significant subset of adults over 65 years have sarcopenia complicated by obesity and are at risk of synergistic complications from obesity and sarcopenia (65). "Thin-fat obesity" or "sarcopenic obesity," also known as normal-weight obesity, is a recognized phenotype in South Asia with a comparable risk for cardiometabolic disease to conventional obesity. It is described as a condition in which a person has a normal body mass index (BMI) but a higher body fat percentage (based on ethnicity and gender-specific cut-offs) (66).
Muscle mass and strength are lower in South Asians than in Caucasians (67). The South Asian Working Action Group on Sarcopenia (SWAG-SARCO) consensus has been developed for diagnosing sarcopenia in South Asian nations while considering these ethnic characteristics (64). Malnutrition is still a significant public health issue in underdeveloped nations, notwithstanding the rise in obesity (68-70). In addition to the traditional risk factors, HIV-associated sarcopenia is prevalent in Africa. In a study from Brazil, HIV-infected patients had a 4.95 higher risk for sarcopenia than the controls, which persisted even following adjustments for age and BMI (71).
Osteosarcopenia
Numerous studies support the concept of a bone-muscle unit, in which molecules released by the skeletal muscle secretome influence bone, and the osteokines secreted by osteoblasts and osteocytes modulate the muscle cells (72). Duque et al. originally used the term "osteosarcopenia" to refer to an older population subgroup having both sarcopenia and osteoporosis. Clinically, unfavorable outcomes such as falls, fractures, loss of function, and frailty arise when both illnesses coexist. Resistance training, adequate protein and calcium consumption, and maintenance of optimum levels of vitamin D are simple therapies that have a dual favorable effect on bone and muscle and decrease falls, fractures, and disability (73).
CHALLENGES IN THE DIAGNOSIS AND MANAGEMENT OF MBD IN THE TROPICS
Prevalence Of Vitamin D Deficiency
Since skin exposure to ultraviolet radiation is the major source of vitamin D, it has long been believed that residing in tropical countries ensures adequate vitamin D levels. However, there is overwhelming evidence for widespread vitamin D deficiency in the tropics. Several other factors which could affect vitamin D levels, such as adiposity, skin pigmentation, genetic factors, clothing habits, sun avoidance, regular use of sunscreen, cloud cover and pollution, have been implicated (74).
Most studies have defined vitamin D insufficiency as serum 25(OH)D less than 50 nmol/L (20 ng/mL). Using this diagnostic cut-off, South Asia has a prevalence of vitamin D insufficiency of 70% or more, and the prevalence in Southeast Asia ranges from 6-70% (75).
Diagnosis Of Osteoporosis: Pitfalls And Challenges
The diagnosis and management of MBD in the tropics is fraught with challenges. The gold standard tool for measuring BMD is the dual-energy X-ray absorptiometry (DXA) scan (76). However, the limited availability of DXA and the associated costs continue to be a major hurdle (77). The World Health Organization (WHO) defined T-score of ≤-2.5 SD, originally designed as an epidemiological tool, has been widely adopted as both a diagnostic and intervention threshold for osteoporosis (78). The (National Health and Nutrition Examination (NHANES III) Survey was employed to create the Caucasian reference database. NHANES III is a nationally representative sample of 14,646 women and men in the United States. The data from this survey is used as the reference database in most DXA machines (79, 80). There is a need to have country-specific reference ranges appropriate for the representative population (81, 82).
Utility Of Cost-Effective Screening Tools
To circumvent the challenges associated with the cost and availability of DXA machines, a number of cost-effective screening tools for osteoporosis have been explored. FRAX®, developed by the former WHO Collaborating Centre at the University of Sheffield, is the most widely validated and used fracture risk assessment tool (83). The risk of hip fracture and other osteoporotic fractures varies greatly globally. The FRAX models are calibrated to countries where fracture and death epidemiology is known to adjust for these fluctuations (84). Currently, FRAX is available in 65 countries, including two Asian, 35 European, nine Middle East and African, two North American, seven Latin American, and two Oceanian. However, country-specific FRAX thresholds are unavailable in many tropical countries (85, 86). Another screening tool of note, the Osteoporosis Self-Assessment Tool for Asians (OSTA), has been validated in multiethnic population in Asian countries (87,88).These screening tools identify patients at high risk for osteoporosis and optimize use of DXA in resource-limited settings (89).
Osteoporosis Awareness
Studies in multiethnic populations across Asia have consistently demonstrated poor knowledge of osteoporosis among women (90-92). Similarly, poor awareness among at-risk populations is a major concern across regions of Africa and South America (93-95). Low awareness among physicians and healthcare authorities contributes to the enormous treatment gap in osteoporosis (93,96-98). Though osteoporosis and osteoporosis-related fractures have consumed significant health resources, it is not recognized as a health priority in tropical countries due to the ‘more’ essential diseases such as tuberculosis, malaria, and human immunodeficiency virus (94). Supporting research, raising awareness and establishing public health services will go a long way in preventing the enormous morbidity and mortality associated with fractures in these regions.
Fracture Liaison Services
FLS are gaining importance to address the low rates of patient work-up and treatment following a fracture. The FLS model attempts to avoid future fractures because there is at least a two-fold risk of refracture (99). The International Osteoporosis Foundation recommends the establishment of FLS to identify and treat patients with fractures properly. Universal FLS implementation in Brazil, Mexico, Colombia, and Argentina was predicted to prevent 31,400 fractures, avoid 292,281 bed days, and save 58.4 million USD in 2019 (100). However, FLS programs are yet to be universally established (100-102). Educating and engaging both private and public sectors in the efficient delivery of FLS could be a pragmatic solution to prevent osteoporotic fractures.
CONCLUSION
MBD is widely prevalent but often ignored in the tropics. Understanding the spectrum of MBD in the tropics will not only address the gap but may also throw light on unique pathophysiological aspects of bone metabolism. Nutrition, environment, infections, and genetic traits are responsible for the development of a diverse array of MBD in the tropics. Optimum utilization of resources is a key to tackle this challenge.
REFERENCES
- 1.
- Senthilraja M, Cherian KE, Jebasingh FK, Kapoor N, Paul TV, Asha HS. Osteoporosis knowledge and beliefs among postmenopausal women: A cross-sectional study from a teaching hospital in southern India. J Family Med Prim Care. 2019;8(4):1374–8. [PMC free article: PMC6510091] [PubMed: 31143724]
- 2.
- Shetty S, Kapoor N, Naik D, Asha HS, Prabu S, Thomas N, et al. Osteoporosis in healthy South Indian males and the influence of life style factors and vitamin d status on bone mineral density. J Osteoporos. 2014;2014:723238. [PMC free article: PMC4244976] [PubMed: 25478284]
- 3.
- Malhotra N, Mithal A. Osteoporosis in Indians. Indian J Med Res. 2008;127(3):263–8. [PubMed: 18497441]
- 4.
- Thakur P, Kuriakose C, Cherian KE, Asha HS, Kapoor N, Paul TV. Knowledge gap regarding osteoporosis among medical professionals in Southern India. J Eval Clin Pract. 2020;26(1):272–80. [PubMed: 31062414]
- 5.
- Cherian KE, Kapoor N, Shetty S, Naik D, Thomas N, Paul TV. Evaluation of Different Screening Tools for Predicting Femoral Neck Osteoporosis in Rural South Indian Postmenopausal Women. J Clin Densitom. 2018;21(1):119–24. [PubMed: 28958825]
- 6.
- Cherian KE, Kapoor N, Meeta M, Paul TV. Screening Tools for Osteoporosis in India: Where Do We Place Them in Current Clinical Care? J Midlife Health. 2021;12(4):257–62. [PMC free article: PMC8849153] [PubMed: 35264830]
- 7.
- Bhadada SK, Chadha M, Sriram U, Pal R, Paul TV, Khadgawat R, et al. The Indian Society for Bone and Mineral Research (ISBMR) position statement for the diagnosis and treatment of osteoporosis in adults. Arch Osteoporos. 2021;16(1):102. [PubMed: 34176015]
- 8.
- Sajith KG, Kapoor N, Shetty S, Goel A, Zachariah U, Eapen CE, et al. Bone Health and Impact of Tenofovir Treatment in Men with Hepatitis-B Related Chronic Liver Disease. J Clin Exp Hepatol. 2018;8(1):23–7. [PMC free article: PMC5938523] [PubMed: 29743793]
- 9.
- Mukherjee S, Arya AK, Bhadada SK, Pal R, Lohani S, Gupta A, et al. Characterization of primary hyperparathyroidism based on target organ involvement: An analysis from the Indian PHPT registry. Clin Endocrinol (Oxf). 2023 [PubMed: 36998119]
- 10.
- Shetty S, Cherian KE, Shetty S, Kapoor N, Jebasingh FK, Cherian A, et al. Does Baseline PTH Influence Recovery of Bone Mineral Density, Trabecular Bone Score and Bone Turnover Markers? A Prospective Study Following Curative PArathyroidectomy in Primary Hyperparathyroidism. Endocr Pract. 2020;26(12):1442–50. [PubMed: 33471736]
- 11.
- Fain O. Musculoskeletal manifestations of scurvy. Joint Bone Spine. 2005;72(2):124–8. [PubMed: 15797491]
- 12.
- Cherian KE, Kapoor N, Shetty S, Jebasingh FK, Asha HS, Hephzibah J, et al. Paget's Disease of Bone: An Entity Still Exists in India. Indian J Endocrinol Metab. 2018;22(3):368–72. [PMC free article: PMC6063169] [PubMed: 30090729]
- 13.
- Khositseth S, Bruce LJ, Walsh SB, Bawazir WM, Ogle GD, Unwin RJ, et al. Tropical distal renal tubular acidosis: clinical and epidemiological studies in 78 patients. Qjm. 2012;105(9):861–77. [PubMed: 22919024]
- 14.
- Pigrau-Serrallach C, Rodríguez-Pardo D. Bone and joint tuberculosis. Eur Spine J. 2013;22 Suppl 4(Suppl 4):556-66. [PMC free article: PMC3691411] [PubMed: 22711012]
- 15.
- Ishikawa S, Ishikawa A, Yoh K, Tanaka H, Fujiwara M. Osteoporosis in Male and Female Leprosy Patients. Calcified Tissue International. 1999;64(2):144–7. [PubMed: 9914322]
- 16.
- Koliou M, Chatzicharalampous E, Charalambous M, Aristeidou K. Congenital syphilis as the cause of multiple bone fractures in a young infant case report. BMC Pediatr. 2022;22(1):728. [PMC free article: PMC9768959] [PubMed: 36539748]
- 17.
- Sachdev M, Bery K, Chawla S. Osseous manifestations in congenital syphilis: a study of 55 cases. Clin Radiol. 1982;33(3):319–23. [PubMed: 7075138]
- 18.
- Taj-Aldeen SJ, Gamaletsou MN, Rammaert B, Sipsas NV, Zeller V, Roilides E, et al. Bone and joint infections caused by mucormycetes: A challenging osteoarticular mycosis of the twenty-first century. Med Mycol. 2017;55(7):691–704. [PMC free article: PMC6251651] [PubMed: 28053147]
- 19.
- Joseph A, Rajan R, Paul J, Cherian KE, Kapoor N, Jebasingh F, et al. The continuing crippling challenge of skeletal fluorosis – Case series and review of literature. Journal of Clinical and Translational Endocrinology: Case Reports. 2022;24:100114.
- 20.
- Monir AU, Gundberg CM, Yagerman SE, van der Meulen MC, Budell WC, Boskey AL, et al. The effect of lead on bone mineral properties from female adult C57/BL6 mice. Bone. 2010;47(5):888–94. [PMC free article: PMC3386851] [PubMed: 20643234]
- 21.
- Akesson A, Bjellerup P, Lundh T, Lidfeldt J, Nerbrand C, Samsioe G, et al. Cadmium-induced effects on bone in a population-based study of women. Environ Health Perspect. 2006;114(6):830–4. [PMC free article: PMC1480481] [PubMed: 16759980]
- 22.
- Paul J, Cherian KE, Thomas N, Paul TV. Hypophosphataemic osteomalacia due to cadmium exposure in the silver industry. Occupational Medicine. 2020;70(3):207–10. [PubMed: 31974582]
- 23.
- Peris P, Guañabens N, Parés A, Pons F, del Rio L, Monegal A, et al. Vertebral fractures and osteopenia in chronic alcoholic patients. Calcif Tissue Int. 1995;57(2):111–4. [PubMed: 7584870]
- 24.
- Amato M, Lusini ML, Nelli F. Epidemiology of nephrolithiasis today. Urol Int. 2004;72 Suppl 1:1–5. [PubMed: 15133324]
- 25.
- Song L, Maalouf NM. Nephrolithiasis. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, et al., editors. Endotext. South Dartmouth (MA): MDText.com, Inc. Copyright © 2000-2023, MDText.com, Inc.; 2000.
- 26.
- Sorokin I, Mamoulakis C, Miyazawa K, Rodgers A, Talati J, Lotan Y. Epidemiology of stone disease across the world. World J Urol. 2017;35(9):1301–20. [PubMed: 28213860]
- 27.
- Qian X, Wan J, Xu J, Liu C, Zhong M, Zhang J, et al. Epidemiological Trends of Urolithiasis at the Global, Regional, and National Levels: A Population-Based Study. Int J Clin Pract. 2022;2022:6807203. [PMC free article: PMC9159214] [PubMed: 35685546]
- 28.
- Magwira CA, Kullin B, Lewandowski S, Rodgers A, Reid SJ, Abratt VR. Diversity of faecal oxalate-degrading bacteria in black and white South African study groups: insights into understanding the rarity of urolithiasis in the black group. J Appl Microbiol. 2012;113(2):418–28. [PubMed: 22616725]
- 29.
- Liu Y, Chen Y, Liao B, Luo D, Wang K, Li H, et al. Epidemiology of urolithiasis in Asia. Asian J Urol. 2018;5(4):205–14. [PMC free article: PMC6197415] [PubMed: 30364478]
- 30.
- Yanagawa M, Kawamura J, Onishi T, Soga N, Kameda K, Sriboonlue P, et al. Incidence of urolithiasis in northeast Thailand. Int J Urol. 1997;4(6):537–40. [PubMed: 9477179]
- 31.
- Ahmad F, Nada MO, Farid AB, Haleem MA, Razack SM. Epidemiology of urolithiasis with emphasis on ultrasound detection: a retrospective analysis of 5371 cases in Saudi Arabia. Saudi J Kidney Dis Transpl. 2015;26(2):386–91. [PubMed: 25758899]
- 32.
- Fakheri RJ, Goldfarb DS. Ambient temperature as a contributor to kidney stone formation: implications of global warming. Kidney Int. 2011;79(11):1178–85. [PubMed: 21451456]
- 33.
- Basiri A, Moghaddam SM, Khoddam R, Nejad ST, Hakimi A. Monthly variations of urinary stone colic in Iran and its relationship to the fasting month of Ramadan. J Pak Med Assoc. 2004;54(1):6–8. [PubMed: 15058633]
- 34.
- Stamatelou K, Goldfarb DS. Epidemiology of Kidney Stones. Healthcare (Basel). 2023;11(3) [PMC free article: PMC9914194] [PubMed: 36766999]
- 35.
- Vicedo-Cabrera AM, Goldfarb DS, Kopp RE, Song L, Tasian GE. Sex differences in the temperature dependence of kidney stone presentations: a population-based aggregated case-crossover study. Urolithiasis. 2020;48(1):37–46. [PMC free article: PMC7357996] [PubMed: 30900001]
- 36.
- Cuevas A, Alvarez V, Carrasco F. Epidemic of metabolic syndrome in Latin America. Curr Opin Endocrinol Diabetes Obes. 2011;18(2):134–8. [PubMed: 21358406]
- 37.
- Okafor CI. The metabolic syndrome in Africa: Current trends. Indian J Endocrinol Metab. 2012;16(1):56–66. [PMC free article: PMC3263198] [PubMed: 22276253]
- 38.
- Pandit K, Goswami S, Ghosh S, Mukhopadhyay P, Chowdhury S. Metabolic syndrome in South Asians. Indian J Endocrinol Metab. 2012;16(1):44–55. [PMC free article: PMC3263197] [PubMed: 22276252]
- 39.
- Soligo M, Morlacco A, Zattoni F, Valotto C. G DEG, Beltrami P. Metabolic syndrome and stone disease. Panminerva Med. 2022;64(3):344–58. [PubMed: 34609121]
- 40.
- Khan SR, Pearle MS, Robertson WG, Gambaro G, Canales BK, Doizi S, et al. Kidney stones. Nat Rev Dis Primers. 2016;2:16008. [PMC free article: PMC5685519] [PubMed: 27188687]
- 41.
- Abate N, Chandalia M, Cabo-Chan AV Jr, Moe OW, Sakhaee K. The metabolic syndrome and uric acid nephrolithiasis: novel features of renal manifestation of insulin resistance. Kidney Int. 2004;65(2):386–92. [PubMed: 14717908]
- 42.
- Fuster DG, Bobulescu IA, Zhang J, Wade J, Moe OW. Characterization of the regulation of renal Na+/H+ exchanger NHE3 by insulin. Am J Physiol Renal Physiol. 2007;292(2):F577–85. [PMC free article: PMC2861556] [PubMed: 17018843]
- 43.
- Domingos F, Serra A. Metabolic syndrome: a multifaceted risk factor for kidney stones. Scand J Urol. 2014;48(5):414–9. [PubMed: 24708398]
- 44.
- Boyd C, Wood K, Whitaker D, Assimos DG. The influence of metabolic syndrome and its components on the development of nephrolithiasis. Asian J Urol. 2018;5(4):215–22. [PMC free article: PMC6197366] [PubMed: 30364536]
- 45.
- Halstead SB. Epidemiology of bladder stone of children: precipitating events. Urolithiasis. 2016;44(2):101–8. [PubMed: 26559057]
- 46.
- Ashworth M. Endemic bladder stones. Bmj. 1990;301(6756):826–7. [PMC free article: PMC1663964] [PubMed: 2282417]
- 47.
- Liu CC, Wu CF, Chen BH, Huang SP, Goggins W, Lee HH, et al. Low exposure to melamine increases the risk of urolithiasis in adults. Kidney Int. 2011;80(7):746–52. [PubMed: 21633410]
- 48.
- Chang CK, Lee JI, Chang CF, Lee YC, Jhan JH, Wang HS, et al. Betel Nut Chewing Is Associated with the Risk of Kidney Stone Disease. J Pers Med. 2022;12(2) [PMC free article: PMC8879579] [PubMed: 35207614]
- 49.
- Rajendra Santosh AB, Jones T. Tropical Oral Disease: Analysing Barriers, Burden, Nutrition, Economic Impact, and Inequalities. Front Nutr. 2021;8:729234. [PMC free article: PMC8647765] [PubMed: 34881277]
- 50.
- Botelho J, Machado V, Proença L, Delgado AS, Mendes JJ, Vitamin D. Deficiency and Oral Health: A Comprehensive Review. Nutrients. 2020;12(5) [PMC free article: PMC7285165] [PubMed: 32438644]
- 51.
- Souza AP, Kobayashi TY, Lourenço Neto N, Silva SM, Machado MA, Oliveira TM. Dental manifestations of patient with vitamin D-resistant rickets. J Appl Oral Sci. 2013;21(6):601–6. [PMC free article: PMC3891287] [PubMed: 24473729]
- 52.
- Mittal S, Gupta D, Sekhri S, Goyal S. Oral manifestations of parathyroid disorders and its dental management. Journal of Dental and Allied Sciences. 2014;3(1):34–8.
- 53.
- Jain P, Jain I. Oral Manifestations of Tuberculosis: Step towards Early Diagnosis. J Clin Diagn Res. 2014;8(12):Ze18–21. [PMC free article: PMC4316362] [PubMed: 25654056]
- 54.
- Unde MP, Patil RU, Dastoor PP. The Untold Story of Fluoridation: Revisiting the Changing Perspectives. Indian J Occup Environ Med. 2018;22(3):121–7. [PMC free article: PMC6309358] [PubMed: 30647513]
- 55.
- Dissanayake CB, Chandrajith R. Medical geology in tropical countries with special reference to Sri Lanka. Environ Geochem Health. 2007;29(2):155–62. [PubMed: 17256098]
- 56.
- DenBesten P, Li W. Chronic fluoride toxicity: dental fluorosis. Monogr Oral Sci. 2011;22:81–96. [PMC free article: PMC3433161] [PubMed: 21701193]
- 57.
- Niazi FC, Pepper T. Dental fluorosis. StatPearls [Internet]: StatPearls Publishing; 2022. [PubMed: 36251814]
- 58.
- Petermann-Rocha F, Balntzi V, Gray SR, Lara J, Ho FK, Pell JP, et al. Global prevalence of sarcopenia and severe sarcopenia: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2022;13(1):86–99. [PMC free article: PMC8818604] [PubMed: 34816624]
- 59.
- Tyrovolas S, Koyanagi A, Olaya B, Ayuso-Mateos JL, Miret M, Chatterji S, et al. Factors associated with skeletal muscle mass, sarcopenia, and sarcopenic obesity in older adults: a multi-continent study. J Cachexia Sarcopenia Muscle. 2016;7(3):312–21. [PMC free article: PMC4864288] [PubMed: 27239412]
- 60.
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–23. [PMC free article: PMC2886201] [PubMed: 20392703]
- 61.
- Organization WH. Noncommunicable diseases in the South-East Asia Region, 2011: situation and response. 2012.
- 62.
- Chidakel A, Eb C, Child B. The comparative financial and economic performance of protected areas in the Greater Kruger National Park, South Africa: Functional diversity and resilience in the socio-economics of a landscape-scale reserve network. Journal of Sustainable Tourism. 2020;28(8):1100–19.
- 63.
- Nyirenda MJ. Noncommunicable diseases in sub-Saharan Africa: understanding the drivers of the epidemic to inform intervention strategies. Int Health. 2016;8(3):157–8. [PubMed: 27178673]
- 64.
- Dhar M, Kapoor N, Suastika K, Khamseh ME, Selim S, Kumar V, et al. South Asian Working Action Group on SARCOpenia (SWAG-SARCO) - A consensus document. Osteoporos Sarcopenia. 2022;8(2):35–57. [PMC free article: PMC9263178] [PubMed: 35832416]
- 65.
- Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol. 2018;14(9):513–37. [PMC free article: PMC6241236] [PubMed: 30065268]
- 66.
- Kapoor N. Thin Fat Obesity: The Tropical Phenotype of Obesity. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, et al., editors. Endotext. South Dartmouth (MA): MDText.com, Inc. Copyright © 2000-2023, MDText.com, Inc.; 2000.
- 67.
- Pal R, Bhadada SK, Aggarwal A, Singh T. The prevalence of sarcopenic obesity in community-dwelling healthy Indian adults - The Sarcopenic Obesity-Chandigarh Urban Bone Epidemiological Study (SO-CUBES). Osteoporos Sarcopenia. 2021;7(1):24–9. [PMC free article: PMC8044592] [PubMed: 33869802]
- 68.
- Akhtar S. Malnutrition in South Asia-A Critical Reappraisal. Crit Rev Food Sci Nutr. 2016;56(14):2320–30. [PubMed: 25830938]
- 69.
- Modjadji P, Madiba S. The double burden of malnutrition in a rural health and demographic surveillance system site in South Africa: a study of primary schoolchildren and their mothers. BMC Public Health. 2019;19(1):1087. [PMC free article: PMC6689169] [PubMed: 31399048]
- 70.
- Gassmann F, de Groot R, Dietrich S, Timar E, Jaccoud F, Giuberti L, et al. Determinants and drivers of young children’s diets in Latin America and the Caribbean: Findings from a regional analysis. PLOS Global Public Health. 2022;2(7):e0000260. [PMC free article: PMC10021987] [PubMed: 36962164]
- 71.
- Pinto Neto LF, Sales MC, Scaramussa ES, da Paz CJ, Morelato RL. Human immunodeficiency virus infection and its association with sarcopenia. Braz J Infect Dis. 2016;20(1):99–102. [PMC free article: PMC9425396] [PubMed: 26626165]
- 72.
- Reginster JY, Beaudart C, Buckinx F, Bruyère O. Osteoporosis and sarcopenia: two diseases or one? Curr Opin Clin Nutr Metab Care. 2016;19(1):31–6. [PMC free article: PMC4888925] [PubMed: 26418824]
- 73.
- Hirschfeld HP, Kinsella R, Duque G. Osteosarcopenia: where bone, muscle, and fat collide. Osteoporos Int. 2017;28(10):2781–90. [PubMed: 28733716]
- 74.
- Mendes MM, Hart K, Botelho P, Lanham‐New S. Vitamin D status in the tropics: is sunlight exposure the main determinant? : Wiley Online Library; 2018.
- 75.
- Nimitphong H, Holick MF. Vitamin D status and sun exposure in southeast Asia. Dermatoendocrinol. 2013;5(1):34–7. [PMC free article: PMC3897596] [PubMed: 24494040]
- 76.
- Consensus development conference. diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1993;94(6):646–50. [PubMed: 8506892]
- 77.
- Varthakavi PK, Joshi AS, Bhagwat NM, Chadha MD. Osteoporosis treatment in India: Call for action. Indian J Endocrinol Metab. 2014;18(4):441–2. [PMC free article: PMC4138894] [PubMed: 25143895]
- 78.
- Mithal A, Bansal B, Kyer CS, Ebeling P. The Asia-Pacific Regional Audit-Epidemiology, Costs, and Burden of Osteoporosis in India 2013: A report of International Osteoporosis Foundation. Indian J Endocrinol Metab. 2014;18(4):449–54. [PMC free article: PMC4138897] [PubMed: 25143898]
- 79.
- Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, et al. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8(5):468–89. [PubMed: 9850356]
- 80.
- Looker AC, Orwoll ES, Johnston CC Jr, Lindsay RL, Wahner HW, Dunn WL, et al. Prevalence of low femoral bone density in older U.S. adults from NHANES III. J Bone Miner Res. 1997;12(11):1761–8. [PubMed: 9383679]
- 81.
- Cherian KE, Kapoor N, Asha HS, Thomas N, Paul TV. Influence of Different Reference Databases on Categorization of Bone Mineral Density: A Study on Rural Postmenopausal Women from Southern India. Indian J Endocrinol Metab. 2018;22(5):579–83. [PMC free article: PMC6166560] [PubMed: 30294563]
- 82.
- Balachandran K, Asirvatham AR, Mahadevan S. The Impact of GE Lunar vs ICMR Database in Diagnosis of Osteoporosis among South Indian Subjects. Indian J Endocrinol Metab. 2019;23(5):525–8. [PMC free article: PMC6873249] [PubMed: 31803591]
- 83.
- Kanis JA, McCloskey EV, Johansson H, Oden A, Ström O, Borgström F. Development and use of FRAX in osteoporosis. Osteoporos Int. 2010;21 Suppl 2:S407–13. [PubMed: 20464374]
- 84.
- Kanis JA, Odén A, McCloskey EV, Johansson H, Wahl DA, Cooper C. A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporos Int. 2012;23(9):2239–56. [PMC free article: PMC3421108] [PubMed: 22419370]
- 85.
- Lekamwasam S. The diversity of Fracture Risk Assessment Tool (FRAX)-based intervention thresholds in Asia. Osteoporos Sarcopenia. 2019;5(4):104–8. [PMC free article: PMC6953527] [PubMed: 31938728]
- 86.
- Kanis JA, Harvey NC, Cooper C, Johansson H, Odén A, McCloskey EV. A systematic review of intervention thresholds based on FRAX : A report prepared for the National Osteoporosis Guideline Group and the International Osteoporosis Foundation. Arch Osteoporos. 2016;11(1):25. [PMC free article: PMC4978487] [PubMed: 27465509]
- 87.
- Chandran M, Chin YA, Choo KS, Ang WC, Huang XF, Liu XM, et al. Comparison of the Osteoporosis Self-Assessment Tool for Asians and the fracture risk assessment tool - FRAX to identify densitometric defined osteoporosis: A discriminatory value analysis in a multi-ethnic female population in Southeast Asia. Osteoporos Sarcopenia. 2020;6(2):53–8. [PMC free article: PMC7374549] [PubMed: 32715094]
- 88.
- Agarwal K, Cherian KE, Kapoor N, Paul TV. OSTA as a screening tool to predict osteoporosis in Indian postmenopausal women - a nationwide study. Arch Osteoporos. 2022;17(1):121. [PubMed: 36087221]
- 89.
- Nagendra L, Bhavani N, Menon VU, Pavithran PV, Menon AS, Abraham N, et al. FRAX-based osteoporosis treatment guidelines for resource-poor settings in India. Arch Osteoporos. 2021;16(1):69. [PubMed: 33852082]
- 90.
- Cheung EYN, Tan KCB, Cheung CL, Kung AWC. Osteoporosis in East Asia: Current issues in assessment and management. Osteoporos Sarcopenia. 2016;2(3):118–33. [PMC free article: PMC6372753] [PubMed: 30775478]
- 91.
- Nguyen NV, Dinh TA, Ngo QV, Tran VD, Breitkopf CR. Awareness and knowledge of osteoporosis in Vietnamese women. Asia Pac J Public Health. 2015;27(2):Np95–105. [PubMed: 22087035]
- 92.
- Tan HC, Seng JJB, Low LL. Osteoporosis awareness among patients in Singapore (OASIS)-a community hospital perspective. Arch Osteoporos. 2021;16(1):151. [PMC free article: PMC8497186] [PubMed: 34623530]
- 93.
- Albergaria BH, Chalem M, Clark P, Messina OD, Pereira RMR, Vidal LF. Consensus statement: osteoporosis prevention and treatment in Latin America-current structure and future directions. Arch Osteoporos. 2018;13(1):90. [PMC free article: PMC6132387] [PubMed: 30143914]
- 94.
- Sitati FC, Obimbo MM, Gichangi P. Knowledge and Beliefs on Osteoporosis among African Postmenopausal Women in a Kenyan Semi-Rural County of Kiambu. J Bone Metab. 2021;28(1):91–8. [PMC free article: PMC7973406] [PubMed: 33730788]
- 95.
- Njeze Ngozi R, Ikechukwu O, Miriam A, Olanike AU, Akpagbula Ulugo D, Njeze Nneze C. Awareness of osteoporosis in a polytechnic in Enugu, South East Nigeria. Arch Osteoporos. 2017;12(1):51. [PubMed: 28540650]
- 96.
- Tay CL, Ng WL, Beh HC, Lim WC, Hussin N. Screening and management of osteoporosis: a survey of knowledge, attitude and practice among primary care physicians in Malaysia. Arch Osteoporos. 2022;17(1):72. [PMC free article: PMC9041673] [PubMed: 35474021]
- 97.
- Kim JH, Park YS, Oh KJ, Lee SY, Lee SY, Lee SK, et al. Perception of severe osteoporosis amongst medical doctors in South Korea: Awareness, impact, and treatment. Osteoporos Sarcopenia. 2016;2(1):45–63. [PMC free article: PMC6372755] [PubMed: 30775468]
- 98.
- Paruk F, Tsabasvi M, Kalla AA. Osteoporosis in Africa-where are we now. Clin Rheumatol. 2021;40(9):3419–28. [PubMed: 32797362]
- 99.
- Bonanni S, Sorensen AA, Dubin J, Drees B. The Role of the Fracture Liaison Service in Osteoporosis Care. Mo Med. 2017;114(4):295–8. [PMC free article: PMC6140089] [PubMed: 30228614]
- 100.
- Aziziyeh R, Perlaza JG, Saleem N, Guiang H, Szafranski K, McTavish RK. Benefits of fracture liaison services (FLS) in four Latin American countries: Brazil, Mexico, Colombia, and Argentina. J Med Econ. 2021;24(1):96–102. [PubMed: 33334205]
- 101.
- Chang Y, Huang C, Hwang J, Kuo J, Lin K, Huang H, et al. Fracture liaison services for osteoporosis in the Asia-Pacific region: current unmet needs and systematic literature review. Osteoporos Int. 2018;29(4):779–92. [PubMed: 29285627]
- 102.
- Bernstein BP, Duma MTN, Or O, Fisher-Negev T, Weil Y. Fragility fracture management and FLS models in South Africa and Israel. OTA Int. 2022;5(3) Suppl:e171. [PMC free article: PMC9359006] [PubMed: 35949497]
- ABSTRACT
- INTRODUCTION
- OSTEOPOROSIS IN THE TROPICS
- DISORDERS OF BONE MINERAL METABOLISM IN THE TROPICS
- INFECTIVE DISORDERS ASSOCIATED WITH METABOLIC BONE DISEASE
- CHEMICAL AND TOXIN-RELATED DISORDERS IN TROPICAL COUNTRIES
- NEPHROLITHIASIS IN TROPICS
- DENTAL DISORDERS IN THE TROPICS
- SARCOPENIA AND BONE – A TROPICAL PERSPECTIVE
- CHALLENGES IN THE DIAGNOSIS AND MANAGEMENT OF MBD IN THE TROPICS
- CONCLUSION
- REFERENCES
- Review Thin Fat Obesity: The Tropical Phenotype of Obesity.[Endotext. 2000]Review Thin Fat Obesity: The Tropical Phenotype of Obesity.Kapoor N. Endotext. 2000
- Review Pediatric Endocrinology- A Tropical Perspective.[Endotext. 2000]Review Pediatric Endocrinology- A Tropical Perspective.Raizada N, Nonglait PL. Endotext. 2000
- Review Adrenal Disorders in the Tropics.[Endotext. 2000]Review Adrenal Disorders in the Tropics.Mukhopadhyay P, Pandit K, Ghosh S. Endotext. 2000
- Review Disorders of Adrenal Glands and Sex Development in Children: Insights from the Tropics.[Endotext. 2000]Review Disorders of Adrenal Glands and Sex Development in Children: Insights from the Tropics.Raizada N, Nonglait PL. Endotext. 2000
- Review Pituitary Diseases in the Tropics.[Endotext. 2000]Review Pituitary Diseases in the Tropics.Kumar S, Kumar KVSH. Endotext. 2000
- Metabolic Bone Disease in the Tropics - EndotextMetabolic Bone Disease in the Tropics - Endotext
Your browsing activity is empty.
Activity recording is turned off.
See more...
