NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-.
ABSTRACT
In tropical countries like India, there are several reports of a unique form of diabetes called fibrocalculous pancreatic diabetes (FCPD). In majority of the cases, FCPD occurs in young, lean individuals with diabetes, abdominal pain, and steatorrhea. Diabetes is typically ketosis resistant. Recent studies have shown that a proportion of the cases may have genetic factors and gene mutations that confer the risk of developing the disease. Recent studies have suggested a changing profile of the disease which could also be present in older individuals having a normal body mass index and better survival perhaps attributable to better exocrine and endocrine (diabetes) care being offered to people with FCPD. The management of exocrine insufficiency is by the same standards as in any other cause of chronic pancreatitis, and the same is true for diabetes management except for the need of insulin therapy in most cases. The most distinguishing and worrying feature of FCPD is the higher risk of developing pancreatic cancer for which vigilance is paramount. For complete coverage of all related areas of Endocrinology, please visit our on-line FREE web-text, WWW.ENDOTEXT.ORG.
INTRODUCTION
It is well known that diabetes in tropical and developing countries is different from that seen in the Western World. For example, people with diabetes in India are leaner, develop diabetes earlier than their counterparts, making the distinction between insulin deficient type 1 and insulin resistant type 2 diabetes challenging (1,2). In addition, some people with lean type diabetes in India could have a latent autoimmune diabetes of the adult (3). In earlier times, the leanness was attributable to factors like malnutrition (4), which has become less frequent over the years with increasing improvement in nutritional status in the Indian population, though not uniform (5). Type 2 diabetes in India has become associated with the “thin-fat” Indian concept, which describes the presence of visceral adiposity in thinly built persons, although not all lean diabetes in India conforms to this phenotype (2, 6,).
Another distinct subtype of diabetes seen with leanness in this population was eventually termed fibrocalculous pancreatic diabetes (FCPD) (7). It is included in the current WHO classification of Diabetes Mellitus of 2019 as a subtype within ‘other specific types of Diabetes’ (8). This form of diabetes typically occurs in young people, in people with a low body mass index (BMI), and with clinical features of malnutrition. It is unique because of structural changes identified in the pancreas described below and the associated pancreatic dysfunction. Before the onset of diabetes, there is a pre-diabetic phase of pancreatic damage referred to as tropical chronic pancreatitis, a term indicating its geographical association with tropical countries such as India.
This article will discuss the unique nature of FCPD, its pathogenesis, the changing clinical spectrum and aspects of managing the condition.
EPIDEMIOLOGY AND HISTORICAL ASPECTS
In 1959, Zudeima reported a series of patients, particularly in the lower socioeconomic groups, with features of undernutrition and presence of pancreatic calculi (9). However, it was the disease-characterization work of Dr Geevarghese a decade later that the disease became known worldwide as such (10), with several Indian states adding to the description over subsequent decades (11,12,13). India, Brazil, Thailand, and other tropical countries published observations similar to those reported by Zudeima over the next four decades (14,15).
Early reports indicated FCPD to be an important cause of diabetes in young, lean individuals with features of malnutrition. In 2008, a national prospective study involving 32 Centers across various regions of India established a comprehensive database of patients with chronic pancreatitis which yielded significant insight into the clinical profile FCPD at the time (16). The disease profile of FCPD appeared to overlap with idiopathic pancreatitis, and that leanness and malnutrition were not integral to chronic pancreatitis, perhaps reflecting improved treatment of the condition. These changes were substantiated in subsequent reports (17, 18). More recent reports indicate that FCPD accounts for only a small proportion of people with young-onset diabetes (19).
The life expectancy of people with chronic pancreatitis has increased compared to that in the past, though not yet returned to normal (20). It is universal knowledge that longer duration of, particularly uncontrolled, hyperglycemia leads to microvascular complications. Hence, with improved overall care, diabetes complications have been known to occur in patients with FCPD (21), though macrovascular complications are thought to be rare.
PATHOGENESIS
The exact pathogenesis of FCPD is unclear with oxidative stress, micronutrient deficiency, dietary toxins, and autoimmunity implicated inconclusively.
A disbalance between the oxidative stress and antioxidant responses in the body has been postulated in FCPD based on reduced levels of antioxidant markers found with heightened oxidative stress markers (22). The consumption of cassava being linked pathogenetically to FCPD (23) was later proved to be of doubtful significance (24). While malnutrition is associated with FCPD, later reports indicate it to be a consequence rather than cause of FCPD (13).
Human leukocyte antigen (HLA) associations as part of genetic susceptibility studies were inconclusive. Altered expression of genes such as serum protease inhibitor Kazal type 1 (SPINK1), cationic trypsinogen (PRSS1), anionic trypsinogen (PRSS2), and chymotrypsinogen C have been noted in FCPD and in the Indian population, the N34S variant of the SPINK gene appears to confer susceptibility in 33% (25). Two hit model with the first step being alteration of genes leading to supertrypsin formation in the acinar cells of pancreas, followed by a second hit involving unidentified genes leading to gross structural changes within the gland and/or manifestation of disease was proposed as a more plausible phenomenon over a decade ago (26).
In summary, a multitude of pathogenetic mechanisms for FCPD has been proposed based mostly on earlier evidence from a time when the disease was reportedly more prevalent, neither one of which alone may play a singular role and that a complex interaction, most likely of gene-environment, underlies disease evolution.
TABLE 1.
PATHOPHYSIOLOGICAL FACTORS POSTULATED IN THE ETIO-PATHOGENESIS OF FCPD
| Genes Identified In FCPD Serum protease inhibitor Kazal type 1 (SPINK1)- especially N34S mutation Cationic trypsinogen (PRSS1) Anionic trypsinogen (PRSS2) Chymotrypsinogen C | Factors Implicated With Inconclusive Evidence Toxins e.g. Cassava consumption Imbalance of oxidative stress and antioxidants Malnutrition |
| Proposed Two-Hit Model First hit: genetic mutation leading to alteration in pancreatic acinar cells Second hit: unidentified genes leading to disease manifestation | |
PATHOLOGY OF DIABETES IN FCPD
While early evidence supported reduced mass and insulin secretion by beta cells, insulin resistance has been recognized to play a role in the pathogenesis of diabetes in FCPD.
Both basal insulin secretion and stimulated insulin levels in response to a glucose tolerance test are reduced in people with FCPD compared to controls (11,13). Preservation of some beta cell function is supported clinically by the relatively rare instances of ketoacidosis following withdrawal of insulin (27). Ketoacidosis may be less common due to not only some residual insulin secretory activity but also due to reduced glucagon reserve secondary to alpha cell destruction and reduced availability of non-esterified fatty acid substrate for ketogenesis due to lack of visceral fat (28). Studies have also suggested that exocrine function could correlate with endocrine dysfunction. While it is simple and logical to presume that any pancreatic pathology would reduce insulin production, the situation is more complex than meets the eye.
There is evidence of insulin resistance (IR) in people with FCPD when measured by mean glucose disposal rates and homeostasis model assessment-IR, independent of BMI (13,29). Deficiency of pancreatic polypeptide (PP) has been postulated to underlie IR in FCPD, particularly at the level of the liver where defects in the internalization of liver glucose transporter-2 as well as altered bioavailability/ function of hepatic insulin receptor have been observed. The role of glucagon and thereby preservation or otherwise of alpha cells is debatable. Reduced fat store in people with FCPD may lead to the storage of triglyceride in the liver, which could predispose to insulin resistance.
This century has seen groundbreaking work in understanding the pathophysiology of endocrine dysfunction following non-necrotizing pancreatitis, including prediabetes and diabetes (29). New mediators and their associations include increased interleukin-6 driving insulin resistance and reduced pro-glucagon gut peptides oxyntomodulin [capable of signaling through glucagon like peptide 1 (GLP-1) and glucagon receptors] and glicentin with the former showing potential as a biomarker to distinguish post-pancreatic diabetes from T2DM. These pathways may well be implicated in FCPD, at least in part.
CLINICAL FEATURES AND NATURAL HISTORY OF FCPD
The first symptom is abdominal pain, noted in the upper abdomen radiating to the back and relieved by stooping forward or lying in a prone position, typical of pancreatitis pain. The pain abates, both in frequency and severity, which is followed by development of oily and frothy stool due to fat malabsorption indicating exocrine pancreatic insufficiency, as in pancreatic involvement from other chronic pathologies. Rise in blood glucose and eventually diabetes results usually one to two decades after the onset of abdominal pain. Tropical chronic pancreatitis is the pre-diabetes phase of the disease, whereas FCPD is the term used once diabetes has been diagnosed by universal diagnostic criteria.
FCPD has been classically described in young, lean, malnourished people of low socioeconomic background residing in tropical countries. The onset of pancreatic disease is in childhood with diabetes developing between 15 and 35 years of age, requiring treatment with large doses of insulin for moderate to severe hyperglycemia, and usually no ketosis despite insulin withdrawal (7).
The clinical profile of FCPD and previously described criteria may no longer be strictly applicable as reported in a nationwide study of pancreatitis conducted in 32 centers in different regions of India, including a total of 1033 patients (16). According to that survey, which used original criteria including a low BMI, the disease-affected people had normal BMI and presentation was later in life, contrary to the original descriptions of this entity. In this study, tropical pancreatitis accounted for only 3.8% of chronic pancreatitis, suggesting a downward trend in its incidence or at least the diagnosis. Classical signs such as cyanotic hue, distended abdomen, and parotid gland swelling may also no longer be seen (12).
With appropriate diabetes care and good pancreatic enzyme supplementation, malnutrition is rare, as is death due to diabetes in adolescence (30). As is true in patients with chronic pancreatitis, people with FCPD are also now likely to have a relatively ‘normal’ BMI and not necessarily be ‘lean’ as per the original description of this entity. Severity of diabetes appears to be higher in lean individuals. Thus, in addition to the typical, young and malnourished patient with FCPD, it may be important to consider an “extended spectrum” of FCPD, with people being older, higher BMI, and at risk of diabetes vascular complications also being considered in the spectrum.
More research is needed to characterize FCPD beyond simple clinical criteria. These criteria alone may yield a disproportionately large number of these cases, and those that are limited to a geographical region. Hence, we call for development of newer criteria for FCPD, perhaps including a biological approach.
The natural history of FCPD begins with abdominal pain and progresses through a subclinical e.g. pancreatic calcification phase followed by exocrine and later endocrine pancreatic insufficiency. This has undergone a shift from earlier days of abdominal pain in childhood, diabetes in adolescence and death in early adulthood. Unlike the reports of early mortality in the past, it is well known that people with FCPD now live longer, with 80% living beyond 35 years since the onset of abdominal pain (21).
People with FCPD appear to be at risk of microvascular diabetes-related complications like retinopathy, nephropathy, and neuropathy as in other patients with diabetes, perhaps secondary to the longer life span that these patients enjoy (11). Macrovascular complications like stroke and peripheral vascular diseases are however relatively rare likely due to relative youth, lower cholesterol levels, and leanness at least in the majority of patients (3,12).
Modifiable mortality attributable to the standard vascular and non-vascular complications in FCPD remains the same as in T2DM and T1DM. The risk of death from pancreatic cancer is disproportionately high, particularly for women (31).
DIAGNOSIS AND WORK UP OF FCPD
As per the currently established criteria, FCPD should be suspected in young, lean people from tropical countries who have a history of abdominal pain and steatorrhea, particularly if they have features suggesting malnutrition along with diabetes and especially if the diabetes is ketosis resistant (30). Conventionally, the disease is thought to occur only in tropical countries, however currently, there is no evidence to link any geographical factor with the etiology of the disease. The absence of a single detectable secondary cause of chronic pancreatitis is important. Also, given the changing profile of the disease discussed above, it is not limited to people with a lower BMI and in an adolescent age group. FCPD is an important but increasingly less frequent cause of diabetes in the young (32).
The diagnosis of FCPD is made when diabetes mellitus is associated with characteristic abnormalities in the pancreas including large intraductal calculi, fibrosis, or ductal changes. The presence of large and discrete pancreatic stones is a classical feature of the diagnosis of FCPD (15). Computerized tomography, magnetic resonance imaging, or endoscopic retrograde cholangiopancreatography may be required to clinch the diagnosis if typical calculi are absent on standard modalities such as x ray or ultrasound.
Thorough diagnostic workup should include a gastroenterological and an endocrinological consultation, with the latter ensuring standard tests in the management of all types of diabetes, including regular measurement of glycosylated hemoglobin, renal function, lipid profile, urine albumin creatinine ratio, foot neurovascular assessment, retinal screening, and when indicated, screening for cardiovascular disease.
Endocrine tests, like the measurement of C-peptide, may help clinical management, when a low C-peptide level would signal the need for insulin therapy, but they have no specific role in the diagnosis of FCPD. However, CA-19.9 for the early diagnosis of pancreatic cancer assumes a more important role from the point of view of long-term prognosis. Exocrine pancreatic dysfunction may be assessed by several tests of which low fecal elastase-1 assay is of practical significance, as well as low fecal chymotrypsin. The secretin pancreozymin test, which is a more dynamic assessment of pancreatic secretory function in response to the hormone secretin, is more cumbersome involving an endoscope.
TABLE 2.
DIAGNOSTIC CRITERIA FOR FCPD
| Occurrence in a tropical country |
| Diabetes as per standard diagnostic criteria |
| Evidence of chronic pancreatitis: Pancreatic calculi on X-ray or At least 3 of the following: Abnormal pancreatic morphology by imaging Chronic abdominal pain since childhood Steatorrhea Abnormal pancreatic function test |
| Absence of other causes of pancreatitis like alcoholism, hyperparathyroidism, marked hypertriglyceridemia, hepatobiliary disease etc. |

Figure 1.
X-ray abdomen showing multiple tiny opacities in epigastric region, suggestive of pancreatic calcification.

Figure 2.
Ultrasound of abdomen showing atrophic pancreas, with dilated main pancreatic duct and multiple calculi in main pancreatic duct.

Figure 3.
CT scan of abdomen showing atrophic pancreas with few parenchymal foci of calcification. Main pancreatic duct is dilated along with hyper dense calculi.

Figure 4.
MRI scan of abdomen (axial -T2 weight HASTE sequence) showing atrophic pancreas with dilated main pancreatic duct and few filling defects within, suggestive of calculi.

Figure 5.
MRI scan of abdomen (coronal - T2 weight HASTE sequence): Atrophic pancreas with dilated main pancreatic duct and few filling defects within, suggestive of calculi.
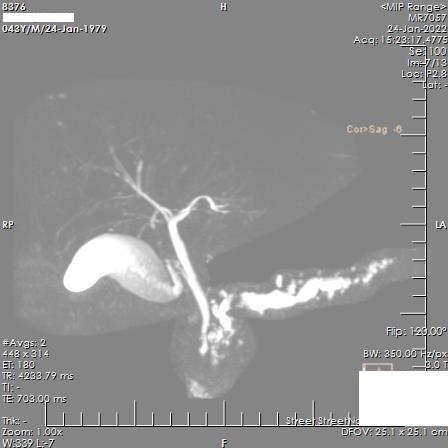
Figure 6.
Maximum Intensity Projection Image showing irregular dilatation of main pancreatic duct with calculi within.
MANAGEMENT
The management of FCPD includes management of diabetes, with special consideration for aspects of chronic pancreatitis. While diabetes can be managed with lifestyle changes and oral drugs in a few cases, insulin is required in many cases.
Nutritional Management
As in other form of diabetes, medical nutrition therapy is the cornerstone of FCPD management. As most FCPD patients are lean, calorie restriction should be avoided. A balanced diet with adequate carbohydrates, fat, and proteins must be ensured. A low-fat diet helps in the management of steatorrhea (33). A gastrointestinal consultation to assess correlation of steatorrhea and other gastrointestinal symptoms with various foods should help in planning appropriate meals. Foods with potential for toxic effect on the pancreas, such as cassava (tapioca) should be avoided, though no conclusive link between the two has been proven and the damage to the pancreas is advanced. Fat soluble vitamins should be replaced as their absorption may be limited in FCPD. If available, sublingual or parenteral formulations of vitamin D may be preferred, to guard against pancreatic osteodystrophy. Smoking and alcohol must be avoided altogether.
Pain Management
Pain is a common symptom of FCPD and can occur due to acute inflammation of the pancreas, increased pressure within the parenchyma and the ductal system, ischemia of the gland, microvascular complications such as neuritis, and due to the space-occupying effect of pseudocysts. Abdominal pain is ideally managed by non-opioid analgesic, though in later stages their judicious use may be necessary (34). Subcutaneous octreotide, a synthetic somatostatin analogue, has been used to reduce the pain of chronic pancreatitis. It inhibits pancreatic secretion and increases the contractility of the sphincter of oddi.
Multiple antioxidants have proven benefit in pain reduction when used together, also in combination with micronutrients (34). Pancreatic enzyme replacement therapy (PERT – discussed in detail in the next section), while important from the exocrine therapy perspective, may benefit pain only if high protease containing (>25,000 USP units per tablet) preparation, in an uncoated formulation, is prescribed multiple times a day. PERT degrades cholecystokinin-releasing peptide in the duodenum and facilitates inhibition of endogenous pancreatic activity and thus may help with pain reduction. Endoscopic interventions to remove stones and lithotripsy may be helpful (35). Severe pain may require surgical procedures such as ductal decompression, drainage procedures like pancreatojejunostomy, and ablative procedures like subtotal pancreatectomy in refractory cases. Coeliac blockage has also been tried as a pain-relieving intervention.
Management of Pancreatic Exocrine Insufficiency
Steatorrhea responds to pancreatic enzyme supplements, which also helps improve glucose control. PERT is necessary to ensure adequate nutrient absorption and prevent down- stream effects such as protein-energy and vitamin malnutrition. It is important to prescribe an optimal dose of PERT, at the right time. Enteric coated PERT is the preferred option, unless being used also for pain relief, as it prevents the contents from being denatured by the acidic medium of the stomach prior to reaching the duodenum (34). Standard approach includes initial doses of 500-1000 lipase units/kg of body weight with each meal, up to maximum of 2,500 lipase units/kg per meal. While doses may vary with meal content and portion, in practice, 10,000-20,000IU for a small meal with 40,000 to 50,000 for a main meal often suffice in persons with diabetes who may need lower doses when following low-fat diet (36).
Ideally, patients should take half the dose with first bite of a meal, and the remaining half in the middle or at the end. However, as most patients prefer a reduced pill burden, the entire dose can be taken with the first bite of food to ensure adherence and convenience. Patients should be advised to avoid copious quantities of water with the tablet to prevent rapid transit through the upper gut.
The adequacy of PERT replacement is determined clinically by enquiring about stool consistency, presence or absence of oily droplets in the stool, weight gain, muscle mass, and bone health. Fecal fat and breath tests to evaluate response to PERT are reserved for use in a research setting. Fecal elastase-1 is not impacted by PERT and cannot be used as a monitoring tool. PERT can be combined with proton pump inhibitors and/or histamine-2 blockers, as these increase the responsiveness to PERT by reducing acidic degradation of the formulations.
Diabetes Management
A balanced and healthy diet with whole grains and adequate micro-and macronutrient intake is important, with due care given to the quantity of carbohydrate intake in the presence of diabetes. Restriction of simple carbohydrates remains a key recommendation. Adequate protein intake is important. In the presence of steatorrhea, a low-fat diet is important. Adequate physical activity, like brisk walking, and stress reduction are beneficial lifestyle measures advisable for all patients (36).
Insulin levels have been noted to be lower in majority of FCPD patients and so insulin remains the drug of choice. Long-acting basal insulin and multiple doses of rapid acting insulin at mealtime is the ideal insulin regime in FCPD (Figure 7). Glycemic targets to be attained with anti-diabetes therapy are similar to those recommended for all types of diabetes. Cardio-metabolic risk factors, such as hypertension and dyslipidemia, should be approached in a standard manner. Very high triglyceride levels > 500 mg/dl put people with FCPD at risk of pancreatitis, and therefore may need medications such as fibrates (33).

FIGURE 7:
Representative Ambulatory Glucose Profile of a patient with FCPD well controlled on basal-bolus insulin therapy (Time in range 87%; time above range 10% and time below range 3%; glucose management indicator 6.4%).
Oral anti-diabetes agents are useful only in a minority of patients with FCPD, as insulin is the medication of choice (36). Among secretagogues, short acting drugs like repaglinide, which stimulate insulin secretion may be considered. Metformin has the benefit of glucose control without hypoglycemia and may be beneficial in countering insulin resistance in some patients. In addition, there is evidence that metformin could protect against pancreatic cancer (37). However, metformin may worsen gastrointestinal symptoms and lead to diarrhea and undesirable weight loss, and hence cautious use is advised.
Oral antidiabetic agents that seem less favorable in FCPD include alpha glucosidase inhibitors due to gastrointestinal symptoms, thiazolidinediones due to the link with osteoporosis, and oral sodium glucose co-transporters due to the associated weight loss until more research indicating benefit becomes available. Incretin based drugs, including GLP-1 receptor agonists or dipeptidyl peptidase 4 inhibitors should not be prescribed in FCPD, given the link between incretin-based therapies and pancreatitis. In fact, there is data from India that GLP-1 increases two-fold in people with FCPD compared to people with type 2 diabetes and controls (38). Gastric inhibitory peptide receptor antagonist and oxyntomodulin antagonism are emerging concepts in the management of post-pancreatic diabetes, and may well apply to FCPD (39).
SUMMARY
FCPD, a subtype of type 3c diabetes of pancreatic origin, that is unique to tropical regions of the world. There is a need for further research into etiology. The clinical phenotype is changing, and is not limited to the classical young, malnourished subject to an “extended spectrum” including older people with higher BMI without malnutrition and predilection to both exocrine and endocrine (diabetes-related) complications. Approach to its management includes standard care for pancreatic exocrine insufficiency and for diabetes, with a preference for insulin therapy for most patients. Judicious use of oral anti-diabetes agents may be appropriate for a few due to the presence of insulin resistance independent of intra-abdominal adipose tissue, a fascinating area of future research. A critical aspect of managing FCPD is early detection of pancreatic cancer, for which no specific guidance is available to date other than vigilance towards it. Future research on the genetic and environmental aspects of FCPD causation will help uncover new therapeutic approaches that may hold promise for further improving lives of people with FCPD.
ACKNOWLEDGEMENT
We thank Dr. Abhishek B. Yashod, MD (Radio-Diagnosis), Chellaram Hospital: Diabetes Care & Multispecialty, Pune, for providing the CT scan and MRI images.
REFERENCES
- 1.
- Unnikrishnan R, Anjana RM, Mohan V. Diabetes mellitus and its complications in India. Nat Rev Endocrinol. 2016 Jun;12(6):357-70. [PubMed: 27080137]
- 2.
- Lontchi-Yimagou E, Dasgupta R, Anoop S, Kehlenbrink S, Koppaka S, Goyal A, Venkatesan P, Livingstone R, Ye K, Chapla A, Carey M, Jose A, Rebekah G, Wickramanayake A, Joseph M, Mathias P, Manavalan A, Kurian ME, Inbakumari M, Christina F, Stein D, Thomas N, Hawkins M. An Atypical Form of Diabetes Among Individuals With Low BMI. Diabetes Care. 2022 Jun 2;45(6):1428-1437. [PMC free article: PMC9184261] [PubMed: 35522035]
- 3.
- Unnikrishnan AG, Singh SK, Sanjeevi CB. Prevalence of GAD65 Antibodies in Lean Subjects with Type 2 Diabetes. Annals of the New York Academy of Sciences 2004; 1037: 118–121. [PubMed: 15699503]
- 4.
- Samal KC, Kanungo A, Sanjeevi CB. Clinicoepidemiological and Biochemical Profile of Malnutrition-Modulated Diabetes Mellitus. Annals of the New York Academy of Sciences 2006; 958: 131–137. [PubMed: 12021092]
- 5.
- Nguyen PH, Scott S, Headey D, Singh N, Tran LM, Menon P, Ruel MT. The double burden of malnutrition in India: Trends and inequalities (2006-2016). PLoS One. 2021 Feb 25;16(2):e0247856. [PMC free article: PMC7906302] [PubMed: 33630964]
- 6.
- Wells JC, Pomeroy E, Walimbe SR, Popkin BM, Yajnik CS. The Elevated Susceptibility to Diabetes in India: An Evolutionary Perspective. Front Public Health. 2016 Jul 7;4:145. [PMC free article: PMC4935697] [PubMed: 27458578]
- 7.
- Bajaj, J.S. (1987). Malnutrition-Related Diabetes. In: Miehlke, K. (eds) Kongreß. Verhandlungen der Deutschen Gesellschaft für Innere Medizin, vol 93. J.F. Bergmann-Verlag.
- 8.
- Classification of diabetes mellitus. Geneva: World Health Organization; 2019. Licence: CC BY-NC-SA 3.0 IGO
- 9.
- Zuidema PJ. Cirrhosis and disseminated calcification of the pancreas in patients with malnutrition. Trop Geogr Med 1959; 11: 70–74. [PubMed: 13659585]
- 10.
- Geevarghese PJ, Pitchumoni CS, Nair SR. Is protein malnutrition an initiating cause of pancreatic calcification? J Assoc Physicians India 1969; 17: 417–419. [PubMed: 5348578]
- 11.
- Jyotsna VP, Singh SK, Gopal D, Unnikrishnan AG, Agrawal NK, Singh SK et al. Clinical and biochemical profiles of young diabetics in North-Eastern India. J Assoc Physicians India 2002; 50: 1130–1134. [PubMed: 12516694]
- 12.
- Unnikrishnan R, Mohan V. Fibrocalculous pancreatic diabetes (FCPD). Acta Diabetol 2015; 52: 1–9. [PubMed: 25395047]
- 13.
- Dasgupta R, Naik D, Thomas N. Emerging concepts in the pathogenesis of diabetes in fibrocalculous pancreatic diabetes: J Diabetes 2015; 7: 754–761. [PubMed: 25707547]
- 14.
- Dani R, Penna FJ, Nogueira CED. Etiology of chronic calcifying pancreatitis in Brazil: a report of 329 consecutive cases. International Journal of Pancreatology 1986; 1: 399–406. [PubMed: 3681031]
- 15.
- Barman KK, Premalatha G, Mohan V. Tropical chronic pancreatitis. Postgraduate Medical Journal 2003; 79: 606–615. [PMC free article: PMC1742869] [PubMed: 14654569]
- 16.
- Balakrishnan V, Unnikrishnan AG, Thomas V, Choudhuri G, Veeraraju P, Singh SP et al. Chronic pancreatitis. A prospective nationwide study of 1,086 subjects from India. JOP 2008; 9: 593–600. [PubMed: 18762690]
- 17.
- Papita R, Nazir A, Anbalagan VP, Anjana RM, Pitchumoni C, Chari S et al. Secular trends of fibrocalculous pancreatic diabetes and diabetes secondary to alcoholic chronic pancreatitis at a tertiary care diabetes centre in South India. JOP 2012; 13: 205–209. [PubMed: 22406602]
- 18.
- Garg PK, Narayana D. Changing phenotype and disease behaviour of chronic pancreatitis in India: evidence for gene-environment interactions. Glob Health Epidemiol Genom. 2016 Oct 18;1:e17. [PMC free article: PMC5870434] [PubMed: 29868209]
- 19.
- Unnikrishnan R, Mohan V. Fibrocalculous Pancreatic Diabetes. Curr Diab Rep. 2020 Apr 11;20(6):19. [PubMed: 32277298]
- 20.
- Beyer, Georg et al. Chronic pancreatitis. The Lancet, Volume 396, Issue 10249, 499 – 512. [PubMed: 32798493]
- 21.
- Mohan V, Premalatha G, Padma A, Chari ST, Pitchumoni CS. Fibrocalculous Pancreatic Diabetes: Long-term survival analysis. Diabetes Care 1996; 19: 1274–1278. [PubMed: 8908394]
- 22.
- Rossi L, Pfützer RH, Parvin S, Ali L, Sattar S, Kahn AKA et al. SPINK1/PSTI Mutations Are Associated with Tropical Pancreatitis in Bangladesh. Pancreatology 2001; 1: 242–245. [PubMed: 12120202]
- 23.
- McMillan DE, Geevarghese PJ. Dietary cyanide and tropical malnutrition diabetes. Diabetes Care. 1979;2:202–8. [PubMed: 574813]
- 24.
- Mathangi DC, Deepa R, Mohan V, Govindarajan M, Namasivayam A. Long-Term Ingestion of Cassava (Tapioca) Does Not Produce Diabetes or Pancreatitis in the Rat Model. IJGC 2000; 27: 203–208. [PubMed: 10952402]
- 25.
- Hassan Z, Mohan V, Ali L, Allotey R, Barakat K, Faruque MO et al. SPINK1 is a susceptibility gene for fibrocalculous pancreatic diabetes in subjects from the Indian subcontinent. Am J Hum Genet 2002; 71: 964–968. [PMC free article: PMC378551] [PubMed: 12187509]
- 26.
- Mahurkar S, Reddy DN, Rao GV, Chandak GR. Genetic mechanisms underlying the pathogenesis of tropical calcific pancreatitis. World J Gastroenterol. 2009;15 (3):264-269. [PMC free article: PMC2653322] [PubMed: 19140225]
- 27.
- Melki G, Laham L, Karim G, Komal F, Kumar V, Barham S et al. Chronic Pancreatitis Leading to Pancreatogenic Diabetes Presenting in Diabetic Ketoacidosis: A Rare Entity. Gastroenterology Res 2019; 12: 208–210. [PMC free article: PMC6731042] [PubMed: 31523331]
- 28.
- Shivaprasad C, Gautham K, Palani P, Gupta S, Shah K. Intra-abdominal fat estimation by bio-electrical impedance analysis in patients with fibrocalculous pancreatic diabetes compared with BMI matched type 2 diabetic subjects and healthy controls. Diabetes Metab Syndr. 2020 Sep-Oct;14 (5):789-795. [PubMed: 32531743]
- 29.
- Petrov, Maxim S. Panorama of mediators in postpancreatitis diabetes mellitus. Current Opinion in Gastroenterology 2020 September; 36(5):443-451. [PubMed: 32618612]
- 30.
- Praveen, G., Mohan, V. Fibrocalculous pancreatic diabetes—current scenario in developing countries. Int J Diabetes Dev Ctries 2018; 38: 131–132.
- 31.
- Cho, J., Pandol, S.J. & Petrov, M.S. Risk of cause-specific death, its sex and age differences, and life expectancy in post-pancreatitis diabetes mellitus. Acta Diabetol 2021; 58: 797–807. [PMC free article: PMC9254257] [PubMed: 33590329]
- 32.
- Unnikrishnan AG, Bhatia E, Bhatia V, Bhadada SK, Sahay RK, Kannan A, Kumaravel V, Sarma D, Ganapathy B, Thomas N, John M, Jayakumar RV, Kumar H, Nair V, Sanjeevi CB. Type 1 diabetes versus type 2 diabetes with onset in persons younger than 20 years of age. Ann N Y Acad Sci. 2008 Dec;1150:239-44. [PubMed: 19120303]
- 33.
- Kumaran S, Unnikrishnan AG. Fibrocalculous pancreatic diabetes. J Diabetes Complications. 2021 Jan;35(1):107627. doi: . Epub 2020 May 22.10.1016/j.jdiacomp.2020.107627 [PubMed: 32553576] [CrossRef]
- 34.
- Drewes AM, Bouwense SAW, Campbell CM, Ceyhan GO, Delhaye M, Demir IE, Garg PK, van Goor H, Halloran C, Isaji S, Neoptolemos JP, Olesen SS, Palermo T, Pasricha PJ, Sheel A, Shimosegawa T, Szigethy E, Whitcomb DC, Yadav D; Working group for the International (IAP – APA – JPS – EPC) Consensus Guidelines for Chronic Pancreatitis. Guidelines for the understanding and management of pain in chronic pancreatitis. Pancreatology. 2017 Sep-Oct;17(5):720-731. doi: . Epub 2017 Jul 13.10.1016/j.pan.2017.07.006 [PubMed: 28734722] [CrossRef]
- 35.
- Kitano M, Gress TM, Garg PK, Itoi T, Irisawa A, Isayama H, Kanno A, Takase K, Levy M, Yasuda I, Lévy P, Isaji S, Fernandez-Del Castillo C, Drewes AM, Sheel ARG, Neoptolemos JP, Shimosegawa T, Boermeester M, Wilcox CM, Whitcomb DC. International consensus guidelines on interventional endoscopy in chronic pancreatitis. Recommendations from the working group for the international consensus guidelines for chronic pancreatitis in collaboration with the International Association of Pancreatology, the American Pancreatic Association, the Japan Pancreas Society, and European Pancreatic Club. Pancreatology. 2020 Sep; 20(6): 1045-1055. [PubMed: 32792253]
- 36.
- Johnston PC, Thompson J, Mckee A, Hamill C, Wallace I. Diabetes and Chronic Pancreatitis: Considerations in the Holistic Management of an Often Neglected Disease. J Diabetes Res. 2019 Oct 7;2019:2487804 [PMC free article: PMC6800932] [PubMed: 31687406]
- 37.
- Dong YW, Shi YQ, He LW, Cui XY, Su PZ. Effects of metformin on survival outcomes of pancreatic cancer: a meta-analysis. Oncotarget. 2017 May 26; 8(33): 55478-55488. [PMC free article: PMC5589674] [PubMed: 28903435]
- 38.
- Ghosh I, Mukhopadhyay P, Das K, Anne M B, Ali Mondal S, Basu M, Nargis T, Pandit K, Chakrabarti P, Ghosh S. Incretins in fibrocalculous pancreatic diabetes: A unique subtype of pancreatogenic diabetes. J Diabetes. 2021 Jun;13(6):506-511. doi: . Epub 2020 Dec 16.10.1111/1753-0407.13139 [PubMed: 33247879] [CrossRef]
- 39.
- Petrov MS. Post-pancreatitis diabetes mellitus: investigational drugs in preclinical and clinical development and therapeutic implications. Expert Opin Investig Drugs. 2021 Jul; 30(7): 737-747. [PubMed: 33993813]
- Review Fibrocalculous pancreatic diabetes (FCPD).[Acta Diabetol. 2015]Review Fibrocalculous pancreatic diabetes (FCPD).Unnikrishnan R, Mohan V. Acta Diabetol. 2015 Feb; 52(1):1-9. Epub 2014 Nov 14.
- Review Fibrocalculous pancreatic diabetes.[Minerva Endocrinol. 2016]Review Fibrocalculous pancreatic diabetes.Goundan P, Junqueira A, Kelleher-Yassen D, Steenkamp D. Minerva Endocrinol. 2016 Mar; 41(1):70-7. Epub 2015 Oct 16.
- Non-tropical fibrocalculous pancreatic diabetes: case reports and review of recent literature.[J Int Med Res. 2020]Non-tropical fibrocalculous pancreatic diabetes: case reports and review of recent literature.Xia F, Zhou W, Wang B, Hu Y. J Int Med Res. 2020 Jul; 48(7):300060520938967.
- Review Fibrocalculous pancreatic diabetes.[J Diabetes Complications. 2021]Review Fibrocalculous pancreatic diabetes.Kumaran S, Unnikrishnan AG. J Diabetes Complications. 2021 Jan; 35(1):107627. Epub 2020 May 22.
- Prevalence of End-Organ Damage, Beta Cell Reserve, and Exocrine Pancreas Defect in Fibrocalculous Pancreatic Diabetes: An Eastern India Perspective.[Indian J Endocrinol Metab. 2019]Prevalence of End-Organ Damage, Beta Cell Reserve, and Exocrine Pancreas Defect in Fibrocalculous Pancreatic Diabetes: An Eastern India Perspective.Anne B, Ghosh S, Ghosh I, Ray S, Chowdhury S, Dutta D. Indian J Endocrinol Metab. 2019 Jul-Aug; 23(4):438-445.
- Fibrocalculous Pancreatic Diabetes - EndotextFibrocalculous Pancreatic Diabetes - Endotext
Your browsing activity is empty.
Activity recording is turned off.
See more...
