NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-.
ABSTRACT
Protozoa are parasitic organisms that are among the most important pathogens worldwide. They are classified into four groups and in infected persons, the disease process may be asymptomatic. Of the four groups, Flagellates and Sporozoa are implicated in the causation of endocrine dysfunction and metabolic abnormalities. The disease condition caused by the flagellates include Giardiasis, Leishmaniasis, and Trypanosomiasis and all cause endocrine abnormalities that range from growth retardation, hypogonadism, adrenal insufficiency, thyroid dysfunction which is largely a resultant effect of the sick euthyroid syndrome, and the syndrome of inappropriate ADH secretion. The Sporozoan disease that notably give rise to metabolic abnormalities is malaria especially severe malaria which is commonly caused by P Falciparum infection. Hypoglycemia is one of the defining criteria for severe malaria and in Africa where malaria is endemic the reported prevalence rate of hypoglycemia in children is as high as 60%. Other abnormalities which are infrequently reported requiring treatment albeit temporarily are hyperglycemia, hypocalcemia, and diabetes insipidus. Adrenal insufficiency when is present is a poor prognostic factor in severe malaria. Toxoplasmosis is acquired or vertically transmitted and may present with neuroendocrine manifestations and adrenal insufficiency resulting from infiltration of the affected endocrine organs with the parasites. For complete coverage of all related areas of Endocrinology, please visit our on-line FREE web-text, WWW.ENDOTEXT.ORG.
PROTOZOA
A parasite is an organism that lives on or in a host organism and gets its food from or at the expense of its host. The three main classes of parasites that can cause disease in humans include helminths, protozoa, and ectoparasites.
Protozoa are microscopic, one-celled organisms that can be free-living or parasitic in nature. Their ability to multiply in humans contributes to their survival and also permits serious infections to develop from just a single organism. Transmission of protozoa that live in a human’s intestine to another human typically occurs through a fecal-oral route and for those that live in the blood or tissue of human’s transmission to other humans is via an arthropod vector (1). Protozoa that are infectious to humans are classified into four groups based on their mode of movement;
Mastigophora – the flagellates, e.g., Giardia, Leishmania, Trypanosoma
Sporozoa – organisms whose adult stage is not motile e.g., Plasmodium, Cryptosporidium, Toxoplasma gondii,
Ciliophora – the ciliates, e.g., Balantidium
Sarcodina – the ameba, e.g., Entamoeba
MASTIGOPHORA
Mastigophora is a phylum of protozoans of the Kingdom Protista, consisting mainly of free-living flagellated unicellular organisms that reproduce by binary fission and whose habitat includes fresh and marine waters. Leishmania and Trypanosoma live in the blood, lymph, and tissue spaces and are typically transmitted from one host to another by blood feeding arthropods.
Giardiasis
This is a diarrheal illness that is caused by Giardia (also known as Giardia intestinalis, Giardia lamblia, or Giardia duodenalis). It is a microscopic parasite and is the most common cause of protozoa associated diarrhea worldwide. The prevalence rate of Giardiasis is higher in developing countries than in the developed countries of the world with Giardia species being endemic in areas of the world that have poor sanitation and high-risk groups including immunocompromised individuals (2-3).
MODE OF TRANSMISSION
The parasite is found on surfaces or in soil, food, or water that has been contaminated with feces from infected humans or animals. Infection is transmitted commonly through ingestion of infectious G lamblia cysts. In the intestine, excystation occurs and trophozoites are released into the feces. A summary of the transmission of the parasite is shown in Figure 1.
Giardia infrequently is transmitted sexually specifically through oral-anal practices. The incubation period is 3-35 days but 7-10 days on the average.
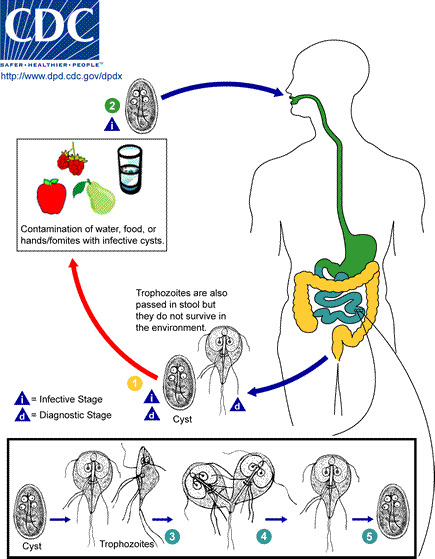
Figure 1.
Life Cycle of Giardia. Source- Centers for Disease Control and Prevention
CLINICAL PRESENTATION
The clinical manifestations include acute or chronic diarrheal disease, but the infection may be asymptomatic even in children. In a Nigeria Study (3) that evaluated stool samples of children aged between 0-5 years, about half-(41%) were positive for G. lamblia. The extraintestinal manifestations of Giardiasis include allergic presentations resulting from immune system activation, and long-term consequences such as ocular pathologies, arthritis, allergies, growth failure, muscular, and metabolic complications (4-5).
ENDOCRINE AND METABOLIC ABNORMALITIES
The complications relating to endocrine and metabolic dysfunction include growth failure and hypothyroidism. The Nigerian Report described above showed a positive association between asymptomatic giardiasis and malnutrition. The relation between Giardiasis and growth failure is explained mainly by malabsorption leading to protein energy malnutrition and micronutrient deficiencies. Other than nutritional status, other contributory factors to growth stunting in children include sanitary and socio-economic conditions, loss of intestinal surface area, and maldigestion (4-5).
Giardia infection has no direct effect or impact on thyroid function but has been reported to indirectly affect thyroid status. Worsening of hypothyroidism related to malabsorption of levothyroxine tablets occasioned by the presence of Giardia infection has been documented in the literature (6-7).
DIAGNOSIS AND MANAGEMENT
The diagnosis is made by demonstration of cysts or trophozoites in stool samples. Other means of diagnosis include small bowel biopsy and stool ELISA. The mainstay of treatment is metronidazole. Patients with stunted growth often experience catch- up growth following treatment of giardiasis.
Leishmaniasis
Leishmaniasis is a poverty related protozoal disease caused by the Leishmania donovani complex. There are 3 main forms of leishmaniases – visceral (also known as kala-azar, the most serious form of the disease), cutaneous (the most common), and mucocutaneous. The clinical spectrum of leishmaniasis ranges from a self-resolving cutaneous ulcer to a mutilating mucocutaneous disease and even to a lethal systemic illness. In Nigeria cutaneous leishmaniasis is the commonly occurring type and has been noted to occur in Northern Nigeria especially in areas bordering the Niger Republic.
MODE OF TRANSMISSION
Leishmania spp. is a parasite with a dimorphic life cycle that is controlled by the passage from vector to host .All three forms of Leishmaniasis are transmitted by the bite of infected female sand fly. The vector phase of the life cycle begins when the vector ingests blood containing the parasites with the parasites undergoing differentiation and ultimately becoming promastigotes and pass into the proboscis where where they can inoculate the host during feeding of the vector (8). These human and vector stages of Leishmania are shown in Figure 2. Infiltration of the reticuloendothelial system by amastigotes leads to the biochemical and clinical features of the disease.
Vertical transmission of visceral leishmaniasis may occur during pregnancy while cutaneous leishmaniasis may be transmitted through physical contact. Transmission via contaminated needles, blood transfusion, sexual intercourse and vertical transmission are modes of transmission that are documented albeit infrequently (8).

Figure 2.
Life cycle of Leishmania. Source -CDC
CLINICAL PRESENTATION
Visceral Leishmaniasis (VL) may present with fever, malnutrition, weight loss , hepatosplenomegaly and death if treatment is delayed or sub-optimal. Cutaneous Leishmaniasis presents with skin ulceration and nodules and the Mucocutaneous form of Leishmaniasis with skin and mucous involvement.
ENDOCRINE AND METABOLIC ABNORMALITIES
Evidence of involvement of several endocrine organs- pituitary, adrenal, thyroid, and sex glands- via histopathologic studies have been documented in VL (9). However abnormal endocrine function tests in some instances without clinical manifestations have been documented.
Reports from Africa and Europe show that thyroid function may be affected in VL but the abnormalities detected are most likely a result of euthyroid sick syndrome (ESS) or non-thyroidal illness (NTI) with no clinical presentation of thyroid disease (10-12). VL has been reported to present with primary adrenal insufficiency in with clinically overt features and in some cases biochemical evidence without clinical features may occur. Primary adrenal insufficiency (AI) was reported in VL in a patient without HIV infection and a patient with concomitant HIV infection and was attributable to parasitic infiltration of the organ (13-14). A Brazilian Series reported a prevalence rate of AI to be about 50% in persons with VL (12). In the Brazilian Report (12) that compared hormonal parameters between persons with chronic leishmaniasis and control subjects, the following were noted in some of the subjects with leishmaniasis; a) features of pituitary dysfunction characterized by low TSH, low T4, and low T3 levels (ESS/NTI), b) elevated ACTH with normal cortisol levels, c) high FSH, LH, and low testosterone levels. Other endocrine abnormalities include low parathyroid hormone (PTH) and decreased total and ionized calcium levels.
An unusual presentation of VL is adrenal cysts which has been reported to come to clinical attention because of the mass effect (15-16). The Syndrome of inappropriate ADH secretion (SIADH) is often reported in VL with hyponatremia documented as a significant contributory factor to mortality of the disease (17). The endocrine and metabolic abnormalities of Leishmaniasis and potential mechanisms underlying their occurrence are shown in Table 1.
Table 1.
Endocrine and Metabolic Dysfunction in Visceral Leishmaniasis
| Endocrine Gland | Hormonal Status | The Level of Abnormality | Underlying Mechanism | Presentations |
|---|---|---|---|---|
| Thyroid Gland | Low TSH, Low T3, Low T4 High TSH, Low T4, Low T3 | Hypothalamic/Pituitary Axis | ESS/NTI Malnutrition in VL may lead to suppressed TSH and subsequent low TH production. Parasitism of the pituitary gland. | No clinical features of thyroid disease |
| Low T4, High TSH | Primary hypothyroidism /Thyroid gland | Primary thyroid insufficiency due to the infiltration of the thyroid gland causing thyroiditis | ||
| Adrenal Gland | Low Cortisol, High ACTH | Primary adrenal insufficiency | Parasitic Infiltration of the adrenal cortex | Mucosal pigmentation, chronic diarrhea, weight loss |
| High ACTH, Normal Cortisol | Primary adrenal insufficiency | Parasitic Infiltration of the adrenal cortex | These patients have normal cortisol levels but were unable to mount an adequate response to stress | |
| High ACTH, high cortisol | Hypothalamus, Pituitary | Stress Response | ||
| Parathyroid gland | Low PTH, Hypocalcemia | Renal (interstitial nephritis) and GIT | Hypomagnesaemia resulting from increased renal loss. GIT loss from possible malabsorption and frequent diarrhea. | |
| Posterior pituitary gland | Syndrome of inappropriate ADH secretion (SIADH) | Hypothalamo-Pituitary Axis | Volume depletion from vomiting leading to increased serum osmolality and resultant Vasopressin release from the PPG. Intense inflammatory response from multiple organ involvement leads to activation of HPA axis and Vasopressin release. | Hyponatremia, elevated urinary osmolality and reduced serum osmolality |
| Gonads | High FSH, High LH and low testosterone | Primary hypogonadism | Parasitism of the testes, reduced testicular size with fewer Sertoli and Leydig cells. Malnutrition may be contributory to the low testosterone level | Delayed Puberty Erectile dysfunction |
DIAGNOSIS AND TREATMENT
A combination of clinical symptoms and laboratory parameters clinches the diagnosis. The laboratory tests involve the detection of the parasites in samples taken from the base of the ulcer and dermal scrapings- Wright ‘s stain detects round or ovoid parasite in the cytoplasm of macrophages.
Polymerase chain reaction for detection of the parasite in peripheral blood and bone marrow samples IS diagnostic. Other tests to support the diagnosis is the Leishman test, which essentially refers to observation of a delayed tuberculin type of reaction following an Intradermal injection of leishmanial antigen.
The mainstay of treatment is the pentavalent antimony compounds. Other pharmacotherapies include amphotericin B, oral miltefosine, pentamidine, and antibiotics. Treatment is individualized, thus persons with associated endocrine/metabolic dysfunction are treated on a case-by-case basis.
Trypanosomiasis
This is an anthropozoonosis caused by a protozoan hemoflagellate. Trypanosoma cruzi in American trypanosomiasis, also known as Chagas disease, is transmitted to human host by a tick. Human African trypanosomiasis (HAT) also known as sleeping sickness is caused by Trypanosoma brucei gambiense in West and Central Africa and Trypanosoma brucei rhodosiense in East Africa is transmitted to human hosts by bites of infected tsetse flies.
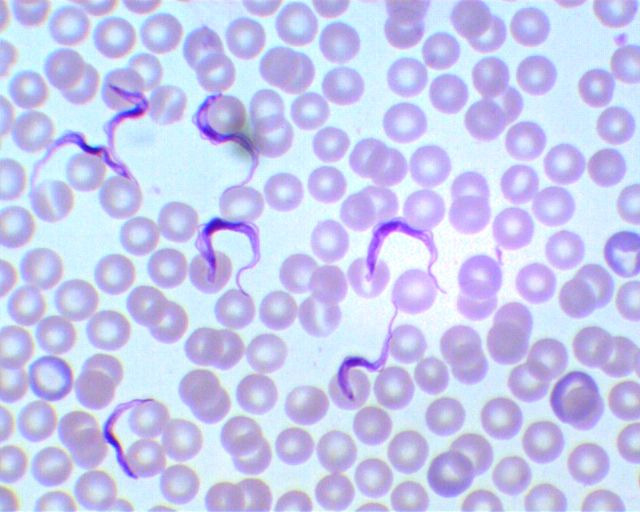
Figure 3.
The pathogen in human African trypanosomiasis
MODE OF TRANSMISSION
In HAT the tsesefly (glossina specie) injects metacyclic trypomastogotes into the skin and these pass into the blood stream and are subsequently carried to other parts of the body and body fluids. The tsetsefly becomes infected when it bites an infected person. Trypanosoma brucei gambiense may also be acquired congenitally from an infected mother.
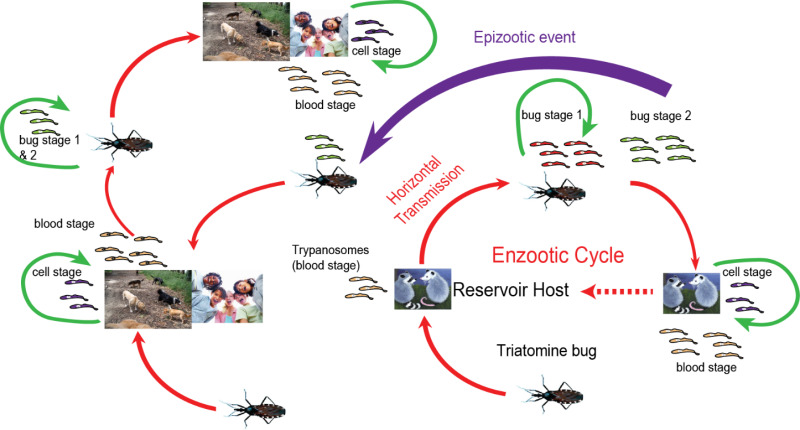
Chagas disease is transmitted by a group of blood-feeding insects known as kissing bugs or triatomid bugs. The pathogen normally circulates between bugs and wild animals in sylvatic habitats; infected bugs in domestic habitats can transmit Chagas to humans and domestic animals (dogs, guinea pigs). Details of the life cycle and transmission of the pathogen is shown in Figure 4.
CLINICAL PRESENTATION
Trypanosomiasis may cause acute illness but on the other hand the infection may be asymptomatic. Chagas disease (CD) presents in three phases: acute, indeterminate, and chronic. The acute phase occurs immediately following infection and is usually asymptomatic in most people but when symptomatic, presentation is essentially those of malaise and skin lesions. The indeterminate phase is usually asymptomatic but may progress to a chronic phase where organ systems mainly the heart and sometimes the gastrointestinal tract are affected.
HAT is characterized by an early hyper-hemolytic phase in which the trypanosomes are restricted to the blood and the lymphatic system and also characterized by organomegaly and lymphadenopathy. This is followed by a late phase or the meningo-encephalitic stage characterized by neuro-psychiatric and endocrinal disorders.
ENDOCRINE AND METABOLIC ABNORMALITIES
These occur infrequently and includes systemic neuroendocrine manifestations in the sympathetic and parasympathetic ganglia. In HAT, documented endocrine abnormalities include adrenal insufficiency, hypothyroidism, and hypogonadism in the absence of autoantibodies (18). Some authors have reported thyroid dysfunction specifically hypothyroidism in untreated HAT (19).
Adrenal insufficiency in trypanosomiasis may be primary or secondary and this is seen especially in untreated cases (20). The adrenal may serve as a reservoir for the T. cruzi infection and some investigators have noted a correlation between Chagasic myocarditis and infection within the central vein of the adrenal gland (21).
Secondary hypogonadism in both sexes had also been demonstrated in some reports which clearly showed that in the majority of the cases, the pathology was not at the level of the pituitary gland but rather an extra pituitary origin (22). Clearly, primary hypogonadism was not demonstrated to be a contributory factor to cases of hypogonadism in persons with trypanosomiasis.
Metabolic abnormalities are infrequently reported however a case of spurious hypoglycemia (23) has been documented in which hypoglycemia was attributable to invitro utilization of glucose by the parasite. The metabolic and endocrine abnormalities are shown in Table 2.
Table 2.
Endocrine and Metabolic Dysfunction in Trypanosomiasis
| Endocrine Gland | Hormone /Hormonal status | The level of abnormality | Possible reason | Clinical Presentation |
|---|---|---|---|---|
| Thyroid Gland | Low TSH, Low T3 Low T4 High TSH, Low T4, Low T3 | Secondary hypothyroidism Primary hypothyroidism | Elevated plasma cytokines related to untreated HAT Parasitic thyroiditis | |
| HPA Axis | Subnormal Cortisol response to ACTH Subnormal cortisol response to ACTH Subnormal ACTH and cortisol response to (CRT) test | Primary Adrenal Insufficiency Secondary adrenal insufficiency | Parasitic invasion of the adrenal gland. Iatrogenic: Suramin in doses exceeding the quantity employed in the treatment of trypanosomiasis inhibits adrenocortical hormone synthesis. Adaptation of the HPA state to the cytokines released due to inflammatory status of the underlying disease | |
| Glucose metabolism | Low glucose levels | Spurious Hypoglycemia Clinical hypoglycemia | Invitro utilization of glucose by trypanosome Iatrogenic: Pentamidine | |
| HPG Axis | In Men: Low Testosterone, Low LH and FSH Positive response of testosterone to GnRH/LHRH stimulation In women: Low Estradiol, low basal LH and FSH levels and positive response to GnRH/ LHRH | Secondary hypogonadism Tertiary hypogonadism Tertiary hypogonadism | Most likely due to inflammatory status of the underlying disease Mechanism not known but may be due to cytokine release Mechanism not known but may be due to cytokine release | Loss of libido, Impotence Impotence Amenorrhea |
DIAGNOSIS
Diagnosis of HAT involves a three-tiered approach for infections due to T.b. Gambiense and a two-tiered approach for that due to T.b. rhodosiense. The three steps for gambiense HAT include a screening test, diagnostic confirmation, and stage determination while that for rhodesiense HAT are diagnostic confirmation and stage determination. Screening is for gambiense HAT involves serology - CATT (Card Agglutination Test for Trypanosomiasis), which detects the presence of specific antibodies in the patient’s blood or serum. Diagnostic confirmation is done to detect the presence of trypanosomes in lymph node aspirates, chancre smear, or in blood. Stage determination is via the detection of trypanosomes (after centrifugation) and white cell count in the cerebrospinal fluid (lumbar puncture): Hemolymphatic stage is characterized by the absence of trypanosomes AND ≤ 5 white cells/mm3 and the Meningoencephalitic stage is defined by evidence of trypanosomes OR > 5 white cells/mm3.
Diagnosis of Chagas disease is via identification of Trypanosoma cruzi by direct microscopy of fresh blood or blood concentrated by the microhematocrit method. Serological tests for anti-Trypanosoma cruzi antibodies are performed for cases of suspected disease but no definitive diagnosis by microscopy.
PHARMACOTHERAPY FOR TRYPANOSMIASIS
Acute or chronic Chagas disease can be treated with either benznidazole or nifurtimox for those without cardiac or GIT complications. Drugs employed in the management of HAT include Nifurtimox, Eflornithine, Melarsoprol, Pentamidine, and Prednisolone (24).
SPOROZOANS
Sporozoans are a group of non-flagellated, non-ciliated and non-amoeboid protists that are responsible for diseases such as malaria and toxoplasmosis.
Malaria
Malaria is an infection caused by single-celled parasites that enter the blood through the bite of an Anopheles mosquito. These parasites, called plasmodia, belong to at least five species; P falciparum, P vivax, P ovale, P malariae and P knowlesi. Plasmodium parasites spend several parts of their life cycle inside humans and another part inside mosquitoes. During the human part of their life cycle, Plasmodium parasites infect and multiply inside liver cells and red blood cells.
Malaria infection begins when an infected female Anopheles mosquito bites a person, injecting Plasmodium parasites, in the form of sporozoites, into the bloodstream and then to the liver. In the liver, asexual multiplication of the sporozoites take place and these are released from the liver as merozoites which invade the red blood cells and multiply within the red cells until the cells burst and the released merozoites invade more red cells with the cycle repeating itself and causing fever. Some of the merozoites leave the cycle of asexual multiplication and instead of replicating within the cells develop into sexual forms of the parasites known as gametocytes. Following the bite of an infected person, gametocytes are ingested by the mosquito and develop into gametes which ultimately become sporozoites which travel to the mosquito’s salivary glands (25).
CLINICAL PRESENTATION
Uncomplicated malaria presents with fever, headache, and generalized malaise. Severe malaria refers to the demonstration of asexual forms of the malaria parasites (commonly P falciparum) in a patient with a potentially fatal manifestations or complications of malaria. Severe malaria is characterized by altered mentation, severe hemolysis, some metabolic abnormalities, and organ complications.
ENDOCRINE AND METABOLIC ABNORMALITIES
Hypoglycemia is commonly documented in severe malaria and hyperglycemia although infrequently reported is seen in severe malaria as well as uncomplicated malaria. Hypocalcemia is also reported in severe malaria as well as in cases of uncomplicated malaria.
Hypoglycemia may be iatrogenic due to quinine administration or due to increased glucose turnover secondary to increased glucose uptake resulting from anaerobic glycolysis and alterations in glucose production in severe malaria (26). Nigeria Reports have hypoglycemia (blood glucose <2.2mmol/L) documented in 60% of children diagnosed with severe malaria (27). Hyperglycemia on the other hand infrequently occurs in persons with malaria and it may present in uncomplicated malaria or severe malaria due to P falciparum infection. The causes of hyperglycemia sometimes necessitate the temporary use of insulin and is multifactorial. These include release of counter-regulatory hormones in response to the stress of the underlying malaria disease condition and pro inflammatory cytokines which increase blood glucose (28). Other proposed reasons for hyperglycemia are reduced sensitivity to insulin and increased gastric and small intestine permeability for sucrose in malaria patients (29-30).
Hypocalcemia is reported in severe malaria as well as in cases of uncomplicated malaria. It has been shown that there is an inverse relationship between parasite load and calcium levels with calcium levels returning to normal following treatment and parasite clearance (31). Altered magnesium metabolism and disturbed parathyroid gland function have been documented as possible reasons for hypocalcemia in malaria (32).
The pituitary-thyroid axis may be depressed in severe malaria and this is most likely attributable to adaptations of the pituitary-thyroid axis to the underlying illness. A Report has noted suppressed T4 and TSH levels with a poorly responsive pituitary gland to TRH stimulation as evidenced by low TSH levels following stimulation (33-34). Another possible mechanism underlying the secondary hypothyroidism is parasitic sequestration within the hypothalamo-pituitary portal system (35).
Clinical and biochemical parameters of central diabetes insipidus which in some cases warranted treatment has been reported in severe malaria. The suggested mechanism is obstruction of the neurohypophyseal microvasculature (36-37).
Primary and secondary adrenal insufficiency which may present with subnormal cortisol levels and in some cases overt features of hypocortisolemia may be seen in severe malaria. Primary adrenal insufficiency may be due to necrosis or impaired circulation due to sequestration of parasites. Some reports have results suggestive of cytokines playing a role in modulating the hypothalamic-pituitary-adrenal axis in secondary adrenal insufficiency. Other potential mechanisms for secondary adrenal insufficiency are erythrocyte sequestration within the hypothalamic -pituitary portal system, altered setpoint for cortisol inhibition of ACTH, and production of a peptide like mammalian somatostatin which has been found to inhibit ACTH secretion in vitro (38-39).
DIAGNOSIS AND TREATMENT
Direct microscopy employed on thin and thick blood films enable parasite detection, species identification, quantification, and monitoring of parasitemia. Serology via the rapid diagnostic tests which detects the parasite antigen can be employed.

Figure 5:
Giemsa-stained peripheral blood smear. Arrow A showing a classic, ring-shaped trophozoite of Plasmodium falciparum. Arrow B showing a classic, headphone-shaped trophozoite of P. falciparum. Arrow C showing two trophozoites of P. falciparum within the same red blood cell. (Reproduced from BMJ Case Reports (Surce-Parikh et al. Classic image: peripheral blood smear in a case of Plasmodium falciparum cerebral malaria http://dx.doi.org/10.1136/bcr-2014-205820
Severe malaria regardless of the infecting specie is treated with intravenous Artesunate and interim oral treatment, artemether -lumefantrine. Other oral antimalarial drugs are Atovaquone-proguanil, Quinine. and Mefloquine.
Toxoplasmosis
Toxoplasmosis is an infection with Toxoplasma gondii, an obligate intracellular protozoon, which is ingested in the form oocysts in material contaminated by feces from infected cats. Oocysts may also be transported to food by flies and cockroaches.
TRANSMISSION
It is primarily an intestinal parasite in cats and has a wide host of intermediate hosts including sheep and mice and exists in three forms: oocysts, tachyzoites, and bradyzoites. Oocysts are only produced in the definitive host – the cat. When passed in the feces and then ingested, the oocysts can infect humans and other intermediate hosts where they develop into tachyzoites- rapidly multiplying trophozoite form of T. gondii. They divide rapidly in cells, causing tissue destruction and spreading the infection. The transmission of toxoplasmosis is shown in Figure 5. Tachyzoites in pregnant women are capable of infecting the fetus. Eventually tachyzoites localize to muscle tissues and the CNS where they convert to tissue cysts, or bradyzoites which is the dormant stage. This is thought to be a response to the host immune reaction. Ingestion of cysts in contaminated meat is also a source of infection, as bradyzoites transform back into tachyzoites upon entering a new host.

Figure 5.
Mode of transmission of toxoplasmosis
CLINICAL PRESENTATION
Toxoplasmosis is often asymptomatic or associated with mild self-limiting symptoms except in immunocompromised persons and sometimes in cases that are congenitally transmitted via the transplacental route (congenital toxoplasmosis).
CNS toxoplasmosis is one of the most common and important opportunistic infections in patients who are immunocompromised -the clinical manifestations are often nonspecific, with the most common presenting symptoms being headache, lethargy, fever, and focal neurologic signs. In congenital toxoplasmosis (CT), chorioretinitis is the most common manifestation and may cause seizure, hydrocephalus, and psychomotor delay.
ENDOCRINE AND METABOLIC ABNORMALITIES
Endocrine defects in toxoplasmosis are usually neuroendocrine in nature and may occur in congenital toxoplasmosis as well as acquired toxoplasmosis. Endocrine manifestations of Toxoplasmosis include hypogonadotropic hypogonadism, precocious puberty, short stature, and diabetes insipidus. Documented hormonal abnormalities in persons with congenital toxoplasmosis result from hypothalamo-pituitary dysfunction and include growth hormone, gonadotropin, and ADH deficiency (40-41) (Figure 6).

Figure 6.
Computerized tomography of brain showing dilated ventricles with multiple subependymal and parenchymal calcifications (arrow). (Source: Mohammed S et al; Congenital toxoplasmosis presenting as central diabetes insipidus in an infant: a case report BMC Res Notes. 2014; 7: 184.
Hypogonadotropic hypogonadism may occur transiently as a result of the modulation of the hypothalamus-pituitary axis by cytokines. Trypanosomiasis which is seen as a ring enhancing lesions in the brain during MRI imaging is an organic cause of the neuroendocrine abnormalities noted in some series (42-44).
Toxoplasmosis has been suggested as a possible risk factor for type 2 diabetes mellitus. Some Researchers have suggested that Toxoplasma gondii directly effects pancreatic cells through beta cell destruction (45-46).
DIAGNOSIS AND TREATMENT
Diagnosis is made via direct detection of the parasites in body fluid and tissue samples.
Serology and molecular techniques are also employed in the diagnosis. Imaging preferable MRI is of use in suspected CNS involvement. Pyrimethamine, Sulfadiazine and Trimethorprin, and sulfamethoxazole are pharmacotherapies for managing toxoplasmosis.
BALANTIDIASIS
Balantidiasis is a disease caused by Balantidium coli, a ciliated protozoan and the only ciliate known to be capable of infecting humans. It is transmitted via contaminated water or food through cysts often associated with swine, the primary reservoir host. Endocrine and metabolic dysfunction or abnormalities are not documented in Balantidiasis.
SARCODINA
Amoebiasis is a diarrhea illness caused by infection with Entamoeba histolytica and is acquired by fecal-oral transmission. In some instances, extraintestinal diseases may occur but endocrine and metabolic dysfunction are not known to occur.
CONCLUSION
Protozoal infections are rare but significant causes of metabolic and endocrine abnormalities. The possibility of protozoal infections as possible causes of these abnormalities should be sought out in regions where these infections are prevalent especially after exclusion of other commonly occurring causes.
REFERENCES
- 1.
- Mousa HA. The Hidden Scene behind the High prevalence of Giardiasis and other infectious diseases in developing countries. J Infect Dis Ther. 2014;2:e106. [CrossRef]
- 2.
- Espelage Werner, an der Heiden Matthias, Stark Klaus, Alpers Katharina. Characteristics and risk factors for symptomatic Giardia lamblia infections in Germany. BMC Public Health. 2010;10:41. [PMC free article: PMC2824735] [PubMed: 20105338] [CrossRef]
- 3.
- Inabo HI, Yau B, Yakubu SE. Asymptomatic Giardiasis and Nutritional Status of Children in Two Local Government Areas in Kaduna State, Nigeria. Sierra Leone Journal of Medical Research. 2011;3:157–162.
- 4.
- Jeffrey R. Donowitz, Masud Alam, Mamun Kabir, Jennie Z. Ma, Forida Nazib, James A. Platts-Mills, Luther A. Bartelt, Rashidul Haque, William A. Petri, Jr, A Prospective Longitudinal Cohort to Investigate the Effects of Early Life Giardiasis on Growth and All Cause Diarrhea. Clinical Infectious Diseases. 2016;63(6):792–797. [PMC free article: PMC4996141] [PubMed: 27313261]
- 5.
- Simsek Z, Zeyrek FY, Kurcer MA. Effect of Giardia infection on growth and psychomotor development of children aged 0-5 years. Journal of Tropical Pediatrics. 2004;50(2):90–3. [PubMed: 15088797]
- 6.
- Radaeli Rde F, Diehl LA. Increased levothyroxine requirement in a woman with previously well-controlled hypothyroidism and intestinal giardiasis. Arq Bras Endocrinol Metabol. 2011;55:81–4. [PubMed: 21468525]
- 7.
- Seppel T, Rose F, Schlaghecke R. Chronic intestinal giardiasis with isolated levothyroxine malabsorption as reason for severe hypothyroidism - implications for localization of thyroid hormone absorption in the gut. Exp Clin Endocrinol Diabetes. 1996;104:180–2. [PubMed: 8740944]
- 8.
- Miroslava Avila-García, Javier Mancilla, Enrique Segura-Cervantes and Norma Galindo-Sevilla (March 19th 2014). Transmission to Humans, Leishmaniasis - Trends in Epidemiology, Diagnosis and Treatment, David M. Claborn, IntechOpen, DOI: 10.5772/57271. https://www
.intechopen .com/books/leishmaniasis-trends-in-epidemiology-diagnosis-and-treatment /transmission-to-humans. [CrossRef] - 9.
- Meleney HE. The histopathology of kala-azar in the hamster, monkey and man. Am J Pathol. 1925;1:147–168. [PMC free article: PMC1931682] [PubMed: 19969637]
- 10.
- Nahid R. Yousif, Rimaz E. Gurashi, Mohamed A. El Tahir, Safa Wadidi. Abd El Karim A. Abdrabo. Evaluation of Thyroid Function among Visceral Leishmaniasis Sudanese Patients. African Journal of Medical Sciences. 2018;3(8)
- 11.
- Al-Ezzy AI, Abood WN. Correlation of Rk39-Specific Antibodies and Thyroid Function in Patients with Visceral Leishmaniasis. Eurasian J Med. 2016;48(3):181–185. [PMC free article: PMC5268599] [PubMed: 28149142]
- 12.
- Frederico Araujo Lima Verde, Verde Francisco Agenor Araujo Lima, Neto Augusto Saboia, Almeida Paulo César, Emir Mendonça Lima Verde. Hormonal Disturbances in Visceral Leishmaniasis (Kala-Azar). Am J Trop Med Hyg. 2011;84(5):668–673. [PMC free article: PMC3083731] [PubMed: 21540373]
- 13.
- Mondain-Miton V, Toussaint-Gari M, Hofman P, Marty P, Carles M, De Salvador F, et al. Atypical leishmaniasis in a patient infected with human immunodeficiency virus. Clin Infect Dis. 1995;21:663–665. [PubMed: 8527563]
- 14.
- Kaneria MV, Jagtap S, Modi C, Kamath S. Atypical presentation of Visceral Leishmaniasis. JAPI 2005;53. [PubMed: 16121818]
- 15.
- Brandonisio O, Fumarola L, Spinelli R, Gradoni L. Unusual presentation of leishmaniasis as an adrenal cystic mass. Eur J Clin Microbiol Infect Dis. 2002;21(9):682–3. [PubMed: 12373503]
- 16.
- Brenner DS, Jacobs SC, Drachenberg CB, Papadimitriou JC. Isolated visceral leishmaniasis presenting as an adrenal cystic mass. Arch Pathol Lab Med. 2000;124(10):1553–6. [PubMed: 11035597]
- 17.
- Daher EF, Soares DS, Filho SL, et al. Hyponatremia and risk factors for death in human visceral leishmaniasis: new insights from a cross-sectional study in Brazil. BMC Infect Dis. 2017;17(1):168. [PMC free article: PMC5322621] [PubMed: 28231825]
- 18.
- Reincke M, Arlt W, Heppner C, Petzke F, Chrousos GP, Allolio B. Neuroendocrine dysfunction in African trypanosomiasis. The role of cytokines. Ann N Y Acad Sci. 1998;840:809–21. [PubMed: 9629307]
- 19.
- Reincke M, Allolio B, Petzke F, Heppner C, Mbulamber D, Vollmer D, et al. Thyroid dysfunction in African trypanosomiasis: a possible role for inflammatory cytokines. Clin Endocrinol (Oxf). 1993;39(4):455–461. [PubMed: 8287572]
- 20.
- Reincke M, Allolio B, Petzke F, Heppner C, Mbulamber D, Vollmer D, et al. Impairment of Adrenocortical Function Associated with Increased Plasma Tumor Necrosis Factor-Alpha and Interleukin-6 Concentrations in African Trypanosomiasis. Neuroimmunomodulation. 1994;1:14–22. [PubMed: 8528879]
- 21.
- Paolo WF, Nosanchuk JD. Adrenal Infections. International journal of infectious diseases. 2006;10(5):343–353. [PMC free article: PMC7110804] [PubMed: 16483815]
- 22.
- Boersma A, Noireau F, Hublart M. Gonadotropic axis and Trypanosoma brucei gambiense infection. Annales de la Société belge de médecine tropicale. 1989;69(2):127–35. [PubMed: 2802809]
- 23.
- Nieman Roger E., et al. Severe African Trypanosomiasis with Spurious Hypoglycemia. The Journal of Infectious Diseases. 1989;159:360–362. [PubMed: 2915160]
- 24.
- Pan American Health Organization. Guidelines for diagnosis and treatment of Chagas disease. Washington, D.C. 2019.
- 25.
- Baer K, Klotz C, Kappe SH, et al. Release of hepatic Plasmodium yoelii merozoites into the pulmonary microvasculature. PLoS Pathogens. 2007;(11):e171. [PMC free article: PMC2065874] [PubMed: 17997605]
- 26.
- Planche T, Dzeing A, Ngou-Milama E, Kombila M, Stacpoole PW. Metabolic complications of malaria. Curr Top Microbiol Immunol. 2005;295:105–36. [PubMed: 16265889]
- 27.
- Osonuga OA. Osonuga Ifabunmi, Derkyi-Kwarteng L. Prevalence of hypoglycemia among severe malaria children in rural Africa population. Asian Pacific Journal of Tropical Disease. 2011;1(3):192–194.
- 28.
- Kiely A, McClenaghan NH, Flatt PR, Newsholme P. Pro-inflammatory cytokines increase glucose, alanine and triacylglycerol utilization but inhibit insulin secretion in a clonal pancreatic beta-cell line. J Endocrinol. 2007;195(1):113–23. [PubMed: 17911403]
- 29.
- Singh Y, Joshi SC, Satyawali V, Gupta A. A case of severe falciparum malaria presenting with hyperglycemia. J Med Trop. 2014;16:39–41.
- 30.
- Yatendra Singh, Joshi Subhash C., Vivekanand Satyawali, Abhisek Gupta. A case of severe falciparum malaria presenting with hyperglycemia. Journal of Medicine in the Tropics. 2014;16:39–41.
- 31.
- Prabha MR, Pereira P, Chowta N, Hegde BM. Clinical implications of hypocalcemia in malaria. Indian J Med Res. 1998;108:62–5. [PubMed: 9785681]
- 32.
- Singh PS, Singh N. Tetany with Plasmodium falciparum infection. J Assoc Physicians India. 2012;60:57–8. [PubMed: 23405546]
- 33.
- Feinsod FM, Heinrich G. Malaria infection presenting with symptoms of thyroid insufficiency and amenorrhoea. Trans R Soc Trop Med Hyg. 1981;75(1):117–8. [PubMed: 7268839]
- 34.
- Davis TM, Supanaranond W, Pukrittayakamee S, Krishna S, Hart GR, Burrin JR, et al. The pituitary-thyroid axis in severe falciparum malaria: evidence for depressed thyrotroph and thyroid gland function. Trans R Soc Trop Med Hyg. 1990;84(3):330–5. [PubMed: 2260159]
- 35.
- Selvaraj V. Hypopituitarism: A rare sequel of cerebral malaria – Presenting as delayed awakening from general anesthesia. Anesth Essays Res. 2015;9(2):287–289. [PMC free article: PMC4563979] [PubMed: 26417148]
- 36.
- Premji R, Roopnarinesingh N, Cohen J, Sen S. Cerebral Malaria: An Unusual Cause of Central Diabetes Insipidus 2016 |Article ID 2047410 | https://doi.org/ 10.1155/2016/2047410. [PMC free article: PMC4875973] [PubMed: 27242936] [CrossRef]
- 37.
- Grimwade K, French N, Mthembu D, Gilks C. Polyuria in association with Plasmodium falciparum malaria in a region of unstable transmission. Trans R Soc Trop Med Hyg. 2004;98(4):255–60. [PubMed: 15049465]
- 38.
- Timothy M. E. Davis, Li Thi Anh Thu, Tran Quang Binh, Ken Robertson, John R. Dyer, Phan Thi Danh, Desiree Meyer, Miles H. Beaman, Trinh Kim Anh. The hypothalamic-pituitary-adrenocortical Axis in Severe Falciparum Malaria: Effects of Cytokines. The Journal of Clinical Endocrinology & Metabolism. 1997;82(9):3029–3033. [PubMed: 9284738]
- 39.
- Mohapatra MK, Barihar PK, Mohapatra A. Adrenal insufficiency in severe falciparum malaria: its outcome and prediction by discriminant score. IJCMR. 2019;6(9):148–155.
- 40.
- Mohamed S., Osman A., Al Jurayyan N.A., et al. Congenital toxoplasmosis presenting as central diabetes insipidus in an infant: a case report. BMC Res Notes. 2014;184(7) https://doi.org. [PMC free article: PMC3986852] [PubMed: 24674575] [CrossRef]
- 41.
- Suresh Babu P.S., Nagendra K., Sarfaraz Navaz R., et al. Congenital toxoplasmosis presenting as hypogonadotropic hypogonadism. Indian J Pediatr. 2007;74:577–579. [PubMed: 17595502]
- 42.
- Massa G, Vanderschueren-Lodeweyckx M, Van Vliet G, Craen M, de Zegher F, Eggermont E. Hypothalamo-pituitary dysfunction in congenital toxoplasmosis. Eur J Pediatr. 1989;148(8):742–4. [PubMed: 2792124]
- 43.
- Oktenli C, Doganci L, Ozgurtas T, Araz RE, Tanyuksel M, Musabak U. Transient hypogonadotrophic hypogonadism in males with acute toxoplasmosis: suppressive effect of interleukin-1b on the secretion of GnRH. Human Reproduction. 2004;19(4):859–866. [PubMed: 14990538]
- 44.
- Setian N, Andrade RSF, Kuperman H, Della Manna T, Dichtchekenian V, Damiani D. Precocious Puberty: An Endocrine Manifestation in Congenital Toxoplasmosis. Journal of Pediatric Endocrinology and Metabolism. 2002;15:9. https://doi.org. [PubMed: 12503855] [CrossRef]
- 45.
- Majidiani Hamidreza, Dalvand Sahar, Daryani Ahmad, Ma de la Luz Galvan-Ramirez, Foroutan-Rad Masoud. Is chronic toxoplasmosis a risk factor for diabetes mellitus? A systematic review and meta-analysis of case–control studies. The Brazilian Journal of Infectious Diseases. 2016;20(6):605–609. [PMC free article: PMC9427549] [PubMed: 27768900]
- 46.
- Shirbazou Shahnaz, Delpisheh Ali, Mokhetari Rahim, Tavakoli Ghafor. Serologic Detection of Anti Toxoplasma gondii Infection in Diabetic Patients. Iran Red Crescent Med J. 2013;15(8):701–703. [PMC free article: PMC3918195] [PubMed: 24578838]
- Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.[Cochrane Database Syst Rev. 2022]Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, et al. Cochrane Database Syst Rev. 2022 Feb 1; 2(2022). Epub 2022 Feb 1.
- Malaria Surveillance - United States, 2016.[MMWR Surveill Summ. 2019]Malaria Surveillance - United States, 2016.Mace KE, Arguin PM, Lucchi NW, Tan KR. MMWR Surveill Summ. 2019 May 17; 68(5):1-35. Epub 2019 May 17.
- Review Adrenal Insufficiency.[Endotext. 2000]Review Adrenal Insufficiency.Alexandraki KI, Sanpawithayakul K, Grossman A. Endotext. 2000
- Review Immune Checkpoint Inhibitors Related Endocrine Adverse Events.[Endotext. 2000]Review Immune Checkpoint Inhibitors Related Endocrine Adverse Events.Elshimy G, Raj R, Akturk HK, Schriber A, Sisterna N, Ahmad I, Jacob A, Michels AW, Correa R. Endotext. 2000
- Review Infections in Endocrinology: Tuberculosis.[Endotext. 2000]Review Infections in Endocrinology: Tuberculosis.Jacob JJ, Paul PAM. Endotext. 2000
- Protozoa and Endocrine Dysfunction - EndotextProtozoa and Endocrine Dysfunction - Endotext
Your browsing activity is empty.
Activity recording is turned off.
See more...