NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Auerbach S, Casey W, Chang D, et al. Standard Methods for Development of EPA Transcriptomic Assessment Products (ETAPs). Washington (DC): U.S. Environmental Protection Agency; 2024 Mar.

Standard Methods for Development of EPA Transcriptomic Assessment Products (ETAPs).
Show detailsThe ETAP consists of three primary components with associated processes and decision points within each component. The three primary components consist of: 1) initial database searches and systematic evidence map development; 2) short-term in vivo transcriptomic study for POD identification; and 3) assessment development and reporting (Fig. 2-1). The main concepts of the ETAP are that the underlying methods and data analysis procedures are highly standardized and structured, and the decision context is narrowly focused on substances with no existing or publicly accessible repeated dose toxicity studies or human evidence suitable for use as a POD and reference value derivation. Due to the standardized methods, the ETAP includes a streamlined review process that is intended to facilitate the rapid development, execution, and release of the human health assessments.
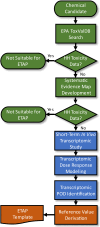
Figure 2-1
Flow chart depicting the three main components and associated processes in developing an ETAP. The green-colored processes and decision points are associated with the initial database searches and systematic search of the literature (documented in the (more...)
The first component of an ETAP is identifying potentially relevant toxicological studies. Candidate substances for ETAP are screened for publicly available repeated dose toxicity data using the US EPA ToxVal database (ToxValDB). If no suitable studies are identified in the ToxValDB, then systematic evidence map (SEM) methods are used to identify and organize the research available on a specific substance (Thayer et al. 2022a; Thayer et al. 2022b). For the ETAP, a SEM is developed to identify and evaluate the literature base associated with the candidate substance for mammalian in vivo repeated dose toxicity studies or suitable human evidence. Resources searched include databases of published research (e.g., PubMed, Web of Science, ProQuest) as well as repositories of studies that may not have been peer-reviewed, such as those summarized in European Chemicals Agency (ECHA) registration dossiers or EPA’s ChemView database. In addition, searches may be conducted to discern whether studies exist in such regulatory reporting databases but are classified as confidential business information (CBI). If such studies exist, then inquiries are made to determine whether they can be made available to the public. Based on the SEM, chemicals confirmed to have no publicly available mammalian in vivo repeated dose toxicity studies or suitable human studies may be eligible for development of an ETAP.
The next component of an ETAP is performing a 5-day in vivo rat study and identification of the POD using transcriptomics. Transcriptomics is the characterization of gene expression changes in a cell, tissue, organ, or organism of interest. Transcriptional changes can provide a quantitative assessment of disruptions to signaling pathways, biological processes, and molecular functions by a chemical substance and the doses at which these disruptions occur (Thomas et al. 2007). The transcriptomic POD is derived from the transcriptomic benchmark dose (BMD) or more specifically from the benchmark dose lower confidence bound (BMDL) and is defined as the dose at which there were no coordinated transcriptional changes that would indicate a potential toxicity of concern. The coordinated transcriptional changes used to identify the POD do not necessarily discriminate between specific hazards, adverse or adaptive effects, nor are they used to infer a mechanism or mode of action. Multiple studies have demonstrated good concordance between short-term transcriptomic BMD values (when grouped by gene sets based on pathway, biological process, or molecular function) and phenotypic apical2 effect BMD values from traditional, rodent toxicity studies [reviewed in (EPA 2024)]. For in vivo repeated dose studies of 5-day duration, gene set-based transcriptomic BMD values have been demonstrated to be concordant with both non-cancer phenotypic responses in subchronic and chronic toxicity studies in rodent models. The concordance between transcriptional and apical responses was robust across different exposure durations, exposure routes, species, sex, target tissues, physicochemical properties, toxicokinetic half-lives, and technology platforms. The concordance between the transcriptomic BMD values with non-cancer apical BMD values was approximately equivalent to the observed inter-study variability in the repeated dose toxicity studies (EPA 2024).
In the ETAP, a 5-day repeated dose design in both male and female rats is used as the basis for the transcriptomic study. Transcriptomic measurements are performed using targeted RNA sequencing in kidney, liver, adrenal gland, brain, heart, lung, ovary (females), spleen, testis (males), thyroid, thymus, and uterus (females). Transcriptomic BMD modeling is performed consistent with the National Toxicology Program (NTP) Approach to Genomic Dose Response Modeling (NTP 2018), with adaptations for the targeted RNA-sequencing gene expression platform used in this method (EPA 2024). The gene ontology (GO) biological process class with the lowest median BMD value is identified across all the tissues examined in either sex. The median BMDL associated with the identified GO biological process class is selected as the transcriptomic POD. The transcriptomic POD is converted to a Human Equivalent Dose (HED) using an oral dosimetric adjustment factor (DAF) based on allometric scaling (EPA 2011a).
The final step is the development of the assessment and reporting the results. The transcriptomic POD obtained from the 5-day in vivo oral exposure study is used in the derivation of a TRV through application of uncertainty factors (UFs) that are consistent with traditional human health assessment guidelines and practice (EPA 2022). The values of the individual UFs and the overall composite value are the same across the individual assessments due to the standardized nature of the studies and data analysis procedures. The TRV is defined as an estimate of a daily oral dose to the human population that is likely to be without appreciable risk of adverse non-cancer health effects over a lifetime. The TRV is derived from a transcriptomic POD with UFs applied to reflect limitations of the data used. The results from the systematic evidence mapping, 5-day transcriptomic study, and TRV derivation are compiled and reported in a standardized ETAP reporting template.
Footnotes
- 2
An apical endpoint is an observable outcome in a whole organism, such as a clinical sign or pathologic state, that is indicative of a disease state that can result from exposure to a toxicant (NASEM 2007). In this document, apical endpoints also include other phenotypic responses (e.g., organ and body weight changes) that are commonly used as critical effects in chemical risk assessment.
- OVERVIEW AND PRINCIPLES OF THE METHOD - Standard Methods for Development of EPA ...OVERVIEW AND PRINCIPLES OF THE METHOD - Standard Methods for Development of EPA Transcriptomic Assessment Products (ETAPs)
Your browsing activity is empty.
Activity recording is turned off.
See more...