NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Controlled remodeling of extracellular matrix (ECM) is essential for growth, invasion, and metastasis of malignant tumors. Matrix metalloproteinases (MMPs) are a family of secreted, zinc-dependent endopeptidases collectively capable of degrading ECM components, and there is a considerable amount of evidence that they play an important role at different steps of malignant tumor growth. Recent observations also suggest that MMPs play a role in cancer cell survival. In this chapter, we discuss the role of MMPs and their inhibitors in tumor cell invasion as a basis for prognostication and targeted therapeutic intervention.
Matrix Metalloproteinases
MMPs are a family of structurally related, zinc-dependent endopeptidases collectively capable of degrading essentially all components of the extracellular matrix (ECM). There is strong evidence for the role of MMPs in physiological ECM remodeling, e.g., during tissue morphogenesis, growth, uterine cycling and postpartum involution, tissue repair, and angiogenesis. In addition, MMPs play a role in pathological conditions with excessive degradation of ECM, such as rheumatoid arthritis, osteoarthritis, atherosclerotic plaque rupture, aortic aneurysms, periodontitis, autoimmune blistering disorders of the skin, dermal photoaging, tumor invasion, and tumor metastasis.14
To date, 21 human MMPs are known, and they can be divided into subgroups based on their structure and substrate specificity (Fig. 1). These subgroups include collagenases, stromelysins and stromelysin-like MMPs, matrilysins, gelatinases, MMP19-like MMPs, membrane-type MMPs (MTMMPs), and other MMPs (Fig. 1).14>
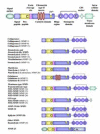
Figure 1
Domain structures of human MMPs. C, Cysteine; GPI, Glycosylphosphatidylinositol; CA, Cysteine Array.
MMPs have a multidomain structure (Fig. 1). N-terminal signal peptide directs the secretion of the proenzyme. Propeptide contains a highly conserved sequence PRCG(V/N)PD in which the cysteine forms a covalent bond with the catalytic zinc ion, the cysteine switch, which maintains the pro-MMP in latent form. The catalytic domain consists of two modules separated by a deep, active site cleft with zinc ion at the bottom.5> Three histidine residues coordinate the binding of catalytic zinc at the active site. This zinc-binding motif HE××H××G××H, together with the zinc ion, is essential for the proteolytic activity of MMPs and is conserved among all MMPs. There is also a structural zinc and at least one calcium ion located approximately 12 Å from the catalytic zinc. Proline-rich hinge region links the catalytic domain to the C-terminal hemopexin domain, which is highly conserved and contains four repeats showing sequence similarity to hemopexin, a plasma protein. A disulfide bridge connects the ends of the domain, which plays a functional role in substrate binding and in interactions with the tissue inhibitors of metalloproteinases (TIMPs). In addition to these domains, some MMPs possess additional domains described here.
Collagenases
Collagenase-1 (MMP-1), collagenase-2 (MMP-8), and collagenase-3 (MMP-13), cleave the triple helix of fibrillar collagens of types I, II, III, and V. They all cleave native type I collagen between Gly775Ile776 of α1 chain, or Gly775Leu776 of α2 chain results in 3/4 N-terminal and 1/4 C-terminal triple-helical fragments, which then denature spontaneously in 37°C into gelatin and are further degraded by other MMPs, such as gelatinases.14 In addition, MMP-13 cleaves type I collagen at N-terminal nonhelical telopeptide.6 MMP-1 cleaves preferentially type III collagen over other fibrillar collagens, and MMP-8 cleaves type I collagen most efficiently.79 MMP-13 preferentially cleaves type II collagen and also gelatin 40-fold more effectively than MMP-1 and MMP-8.79
Human MMP-1 is secreted as major 52-kDa and minor glycosylated 57-kDa proenzymes, and cleavage of propeptide produces active forms of 42 kDa and 47 kDa, respectively.10 The nine residues RWTNNFREY(183-191) in catalytic domain together with the C-terminal hemopexin domain are essential for collagenolytic activity, but additional structural elements in the catalytic domain are also required.11 MMP-1 substrates include type I, II, III, VII, VIII, and X collagens, aggrecan, serine proteinase inhibitors, and α2-macroglobulin. In contrast to many other MMPs, MMP-1 can not cleave BM components. MMP-1 expression is detected in vivo in many physiological situations, such as embryonal development and tissue repair, but also in pathological conditions, including chronic cutaneous ulcers and malignant tumors.12,13 Production of MMP-1 is induced by growth factors and cytokines. In incision wounds and chronic ulcers, MMP-1 is expressed by basal keratinocytes bordering the sites of active reepithelialization14 and is needed for keratinocyte migration on type I collagen.15
Collagenase-2 (MMP-8) is synthesized by polymorphonuclear leukocytes maturing in bone marrow, stored in intracellular granules, and released in response to extracellular stimuli.16 It is also expressed by human articular cartilage chondrocytes in vivo and by mononuclear fibroblast-like cells in rheumatoid synovium.17,18
Collagenase-3 (MMP-13) has a wide substrate specificity.79,1922 Physiological expression of MMP-13 is limited to fetal bone development, postnatal bone remodeling, gingival wound repair, and fetal cutaneous wound repair,2326 suggesting a role for MMP-13 in rapid and effective remodeling of collagenous ECM in these situations. MMP-13 expression in vivo is detected in inflammatory conditions, e.g., osteoarthritis,9 rheumatoid arthritis,25 chronic cutaneous ulcers,27 and chronic periodontitis,28 and in invasive malignant tumors, such as breast carcinomas,7 squamous cell carcinomas (SCCs) of the head and neck29 and vulva,30 primary and metastatic melanomas,31,32 and transitional cell carcinoma of the urinary bladder.33 Accordingly, recent observations show that expression of MMP-13 enhances the invasion capacity of HT1080 fibrosarcoma cells.34
Stromelysins and Stromelysin-like MMPs
Stromelysin-1 (MMP-3) and stromelysin-2 (MMP-10), have similar structure and substrate specificity. As stromelysin-3 (MMP-11) and macrophage metalloelastase (MMP-12) differ in their structure from stromelysins, they are included in this subfamily as a subgroup of stromelysin-like MMPs.14 MMP-3 and MMP-10 are expressed by keratinocytes and fibroblasts in culture and in vivo. MMP-3 is expressed by stromal cells during mammary gland development and is strongly up-regulated during postlactational mammary involution, when considerable ECM remodeling and alveolar apoptosis occur.35,36 MMP-3 can induce apoptosis or promote proliferation, depending on the differentiation status of the target cell.37 It also triggers angiogenesis and can act as a natural tumor promoter.36,37 MMP-3 is a potent activator of latent MMP-1.38 Deletion of MMP-3 impairs early dermal wound contraction, suggesting a role for MMP-3 in the organization of a multicellular actin network.39
Stromelysin-3 (MMP-11) is expressed in many invasive human tumors,40 and high expression levels correlate with poor clinical outcome in breast cancers patients.40 MMP-11 is expressed by stromal fibroblasts adjacent to tumor cells,40 but also by breast carcinoma cells that have undergone a degree of epithelial-to-mesenchymal transition.41 MMP-11 is important in the early stages of tumorigenesis by favoring cancer cell survival in a tissue environment initially not permissive for tumor growth.42 MMP-11-deficient mice have reduced chemical-induced tumorigenesis.43 MMP-11 is expressed by fibroblasts in basal cell carcinomas, squamous cell carcinomas, and benign dermatofibromas,44,45 MMP-11 prodomain contains a furin cleavage site, and the proenzyme is processed intracellularly and released as a mature enzyme.46 MMP-11 degrades α1-proteinase inhibitor (α1PI),47 but the ECM substrates have not been identified, although the existence of such substrates has been suggested.48
Macrophage metalloelastase (MMP-12) is constantly expressed by macrophages49 and in spindle-shaped stromal cells of placenta.49 MMP-12 is expressed by macrophages in atherosclerotic lesions,50 abdominal aortic aneurysms,51 and intestinal ulcerations.52 In skin, MMP-12 is expressed by tumor cells in cutaneous SCCs,53 and by macrophages in areas devoid of normal elastic fibers or with disrupted basement membrane.54 Deletion of MMP-12 in mice leads to impaired macrophage recruitment and protects from cigarette smoke-induced emphysema.55
Matrilysins
Matrilysin (MMP-7) and matrilysin-2 (endometase, MMP26) are the smallest MMPs, both lacking the hinge region and the hemopexin domain.56,57 MMP-7 has a wide substrate specificity.50,58 Unlike most MMPs, it is constitutively expressed by many epithelial cell types, often ductal epithelium of adult exocrine glands in skin, salivary glands, pancreas, liver, and breast, and by glandular epithelium of the intestine and reproductive organs.59,60 MMP-7 is also expressed in the lumenal surface of dysplastic glands in the early-stage human colorectal tumors.61 As MMP-7 activates antibacterial peptides, defensins, in intestinal mucosa, it may function similarly also in the other epithelial sites of expression.62 Mice deficient of MMP-7 have reduced intestinal mucosal defence, but also reduced intestinal tumorigenesis.61,62 MMP-7 is required for repair of airway epithelial injuries.63
The prodomain of MMP-26 has a unique cysteine switch sequence, PHCGVPDGSD.56 MMP-26 is most homologous to MMP-12. MMP-26 is expressed in human uterus and placenta, and in endometrial, lung, and prostate adenocarcinomas.56,57,64 MMP-26 may be involved in tissue-remodeling events associated with tumor progression or reproductive processes including implantation and menstruation.57
Gelatinases
GelatinaseA (72-kDa gelatinase, MMP-2) and gelatinase B (92-kDa gelatinase, MMP-9) have three tandem repeats of 58 amino acid residue long fibronectin type II-like modules in the catalytic domain. As gelatinases degrade components of basement membranes, they are believed to play a crucial role in processes requiring basement membrane disruption, such as tumor invasion and tissue infiltration of T lymphocytes.6567 MMP-2 is also thought to be important in malignancies, as its activation correlates with tumor spread and poor prognosis.68 MMP-2-deficient mice show reduced angiogenesis and tumor progression,69 and MMP-9-deficient mice show impaired metastasis formation and tumor growth.70
Membrane-type MMPs
To date, six MT-MMPs have been described: MT1-MMP (MMP-14), MT2-MMP (MMP-15), MT3-MMP (MMP-16), MT4-MMP (MMP-17), MT5-MMP (MMP-24), and MT6-MMP (MMP-25). They have a R×KR motif between the propeptide and the catalytic domain, which can be cleaved intracellularly in the trans-Golgi network by members of the proprotein convertase family, such as furin resulting in activation of MT-MMPs.71,72 They are bound to cell membrane with a stem/transmembrane/cytosolic domain of approximately 25 amino acids at the C-terminal end, except for MT4-MMP and MT6-MMP, which are glycosylphosphatidylinositol (GPI)-anchored. Localization of MT-MMPs at the cell surface implies that they play a role in cell-matrix interactions, and in cell invasion.73,74 MT1-, MT3-, MT5-, and MT6-MMP activate pro-MMP-2, and MT1- and MT2-MMP also activate pro-MMP-13.75,76 The activation of pro-MMP-13 is enhanced in the presence of a latent form of MMP-2.77 Thus, in the degradation of ECM, a proprotein convertase/MT-MMP/MMP cascade may play an important role at the level of zymogen activation.78
MT1-MMP (MMP-14) is widely expressed in normal tissues.79,80 In addition, MT1-MMP expression has been detected in tumor cells and adjacent stromal cells in a large variety of tumors.80,81 MT1-MMP expression in stromal cells is thought to represent a tumor-induced host response similar to that in wound healing.82 MT1-MMP can cleave several ECM components (Table 1).22,73,83 Expression of MT1-MMP in human fetal membranes and in early human placenta suggests a role for MT1-MMP in trophoblast invasion.84,85 MT1-MMP-deficient mice have severe defects in skeletal development and angiogenesis,86,87 and activation of pro-MMP-2 is also deficient in these mice,87 emphasizing the importance of MMP-2 activation by MT1-MMP in tumor growth and metastasis,88 and in the cartilage destruction of rheumatoid arthritis.89
Table 1
Human MMPs, their expression profile, and substrates.
MT2-MMP is expressed in vivo in liver, placenta, testis, colon, intestine, pancreas, kidney, lung, heart, and skeletal muscle.90 Human cell-associated, but not soluble, MT2-MMP is defective in pro-MMP-2 activation due to substitution of seven amino acids within the catalytic domain, as compared to mouse MT2-MMP capable of activating pro-MMP-2.91
MT3-MMP (MMP-16) is expressed in brain, heart, and placenta, in oral malignant melanoma,80 and brain microglial cells.92 MT3-MMP is also expressed as an alternatively spliced soluble variant that activates pro-MMP-2 and cleaves type III collagen and fibronectin (Table 1).93
MT4-MMP (MMP-17) has the least degree of sequence identity to other MT-MMPs.94 Its lacks the cytoplasmic tail, and instead of a transmembrane domain, it is attached to the cell membrane with a GPI anchor.95 MT4-MMP can be shed from the cell membrane by TIMP-insensitive metalloproteinases, possibly by certain ADAM family members.95 MT4-MMP does not activate pro-MMP-2, but sheds proform of tumor necrosis factor-a.(TNFa).96 MT4-MMP mRNA is expressed in vivo in brain, leukocytes, colon, ovary, testis, and in breast carcinomas.94
MT5-MMP (MMP-24) is predominantly expressed in kidney, pancreas, lung, and brain tissues, especially in brain tumors.78,97 MT5-MMP can activate MMP-2 in a TIMP-2-sensitive fashion. MT5-MMP may have a role in synaptic plasticity during nervous system development,98 whereas activation of pro-MMP-2 in tumor tissues may facilitate tumor progression.97
MT6-MMP (MMP-25, leucolysin), is specifically expressed by peripheral blood leucocytes,99 and in lung and spleen tissue.100 It has also been found in anaplastic astrocytomas and glioblastomas.100 It shares sequence similarity to MT4-MMP and is also GPI anchored.100 MT6-MMP may serve as a potent proteolytic tool for leukocytes during inflammatory responses,99,101 and may also facilitate tumor progression through its ability to activate pro-MMP-2 at the colon carcinoma and brain tumor cell membrane.100
Matrix Metalloproteinase 19-like MMPs
Human MMP-19 is expressed in injured and acutely inflamed synovium, especially in capillary endothelial cells.102104 The substrate specificity of MMP-19 (Table 1) also suggests a role for MMP-19 in angiogenesis.104,105
Epilysin (MMP-28) is most closely related to MMP-19, with which it shares 46% amino acid identity in the catalytic domain.106,107 MMP-28 gene contains 8 exons in contrast to other MMPs, which usually have 10 exons. Exon 4 is alternatively spliced to a transcript that does not encode the N-terminal half of the catalytic domain. MMP-28 has a furin activation sequence (RRKKR) but has no transmembrane sequence. MMP-28 is expressed in testis and lung and at lower levels in heart, colon, intestine, and brain.106,107 MMP-28 can be detected in the basal and suprabasal epidermis of intact skin, and in wounded skin, MMP-28 is seen in basal keratinocytes both at and some distance from the wound edge.107
Other MMPs
Enamelysin (MMP-20) has an expression pattern restricted to ameloblasts and odontoblasts of developing teeth. It is able to degrade amelogenin, the major protein component of the enamel matrix, aggrecan, and cartilage oligomeric matrix protein (COMP).108 Enamelysin has been suggested to play a central role in matrix remodeling during tooth development and enamel maturation.
MMP23 lacks the signal sequence, the cysteine switch, and the C-terminal domain lacks any similarity with hemopexin. N-terminal signal anchor localizes MMP-23 to the cell membrane. A single proteolytic cleavage on the RRRR motif activates MMP-23 prior to secretion.109 Although MMP-23 mRNA is found in heart, intestine, colon, placenta, lung, and pancreas, the predominant expression in ovary, testis, and prostate suggests a specialized role in reproductive processes, e.g., during ovarian follicle development.110
Regulation of MMP Activity
In general, MMP production and activity is strictly regulated in vivo. Cells within intact tissues usually do not store MMPs, and constitutive expression is minimal. Neutrophils are an exception, as they store MMP-8 and MMP-9 in secretory granules for rapid release. In addition, expression of MMP-7 is constitutive in ductal epithelium of adult exocrine glands. Activity of MMPs is regulated at multiple levels including transcription, modulation of mRNA half-life, secretion, localization, activation, and inhibition. The natural inhibitors include tissue inhibitors of metalloproteinases (TIMPs 14) and nonspecific proteinase inhibitors.
Regulation at the Transcriptional Level
The expression of MMPs in general is regulated by growth factors, cytokines, chemical agents like phorbol esters, physical stress, oncogenic transformation, cell-cell and cell-ECM interactions.14 MMP genes responsive to extracellular stimuli (MMP-1, MMP-13, MMP-3, MMP-10, MMP-7, MMP-12, MMP-9, and MMP-19) contain an AP-1 (activator protein-1) binding site in the proximal promoter approximately at position −70 with respect to the transcription initiation site. Another distal AP-1 or related element is found in the promoters of MMP-1, MMP-3, and MMP-9. Jun and Fos bind to the AP-1 cis-element and activate the transcription of the MMP gene. The proximal or distal AP-1 site is often accompanied by another cis-element, PEA-3 (polyomavirus enhancer A binding protein-3) site, that binds ETS transcription factors. AP-1 and PEA-3 together confer responsiveness to a variety of growth factors, oncogene products, and tumor promoters.111 Transforming growth factor-β (TGF-β) inhibitory element is found in MMP-1, MMP-7, and MMP-13 promoters.
Promoter regions of MMP-2, MMP-11, and MT1-MMP genes do not contain a conserved AP-1 element.4,112,113 The promoter region of human MT1-MMP has an Sp1 site crucial for maintaining MT1-MMP transcription, four CCAAT boxes, and additional unidentified positive and negative regulatory sequences.113 It lacks the typical MMP promoter regulatory sites TATA box and AP-1 and TGF-β-responsive elements, as does its murine counterpart.114
Zymogen Activation
Most MMPs are secreted as inactive proenzymes, and their proteolytic activity is regulated by zymogen activation and enzyme inhibition. MT-MMPs, MMP-11, and MMP-28 are activated intracellularly by Golgia-ssociated, furin-like proteases. For MMP-23, a single cleavage both activates it and releases it from the cell surface, where it is anchored.109 The latency of pro-MMPs is maintained by the so-called cysteine switch,115 i.e., a covalent bond between the cysteine residue in the prodomain and the Zn2+ in the catalytic domain. This interaction is disrupted by activation of the pro-MMP by proteinases such as plasmin, trypsin, kallikrein, chymase, and mast cell tryptase.14 Pro-MMPs can also be activated by mercurial compounds (aminophenyl mercuric acetate), SH-reactive agents, reactive oxygen, and detergents.3,115 Many MMPs can activate other MMPs, forming a complex network regulating the tissue proteolysis. TIMP-2 N-terminal domain and the active site of MT1-MMP can associate to form a pro-MMP-2 receptor at the cell surface, leaving the C-terminus of TIMP-2 free to bind pro-MMP-2. This allows efficient activation of pro-MMP-2 by adjacent TIMP-2-free, active MT1-MMP and may be a common mechanism for pro-MMP-2 activation by MT-MMPs.76,116,117 Thus, at low concentrations TIMP-2 enhances the activation process by concentrating MMP-2 to the site where the activator is available and in high concentrations, TIMP-2 inhibits MMP-2 activation.116,117 In tissues, physiological MMP activators are likely to include tissue or plasma proteinases or opportunistic bacterial proteinases. The plasminogen activator/plasmin system is an important activator of pro-MMPs in pathological situations.118
Localization and Trafficking
An important aspect of the regulation of MMP activity is localizing the proteolytic activity to the pericellular space.119 Anchoring MMPs to the cell surface prevents them from rapidly diffusing away and also keeps them under close regulatory control. Binding to cell surface also allows positioning of MMPs for activation, their interaction with cell surface adhesion molecules or receptors, regulation of their turnover, and focused pericellular proteolysis, as with MMP-14 localized in invadopodia.120 Accordingly, heparan sulfate proteoglycan has been shown to anchor MMP-7 on cell surface and possibly in the basement membrane in vivo.121 Similarly, MMP-2 interacts with integrin avb3,122 MMP-9 binds to cell surface hyaluronan receptor CD44123, and integrin avb6124, and pro-MMP-1 interacts with collagen receptor α2b1.125
Inhibition of MMP Activity
MMP activity can be inhibited by tissue inhibitors of metalloproteinases (TIMPs), by serine proteinase inhibitors (serpins), and by nonspecific serum proteinase inhibitors, such as α2-macroglobulin, which is important in blocking MMP activity in the synovial fluid, serum, and other body fluids.14 Serpins are glycoproteins of 50–100 kDa, abundant in all human tissues, and involved in controlling the general proteolytic activity in several tissues. Serpins include α1-antitrypsin (α1-proteinase inhibitor) and plasminogen activator inhibitor (PAI)1 and PAI2.
Tissue Inhibitors of Metalloproteinases (TIMPs)
Tissue inhibitors of metalloproteinases (TIMPs) -1, -2, -3, and -4 are important endogenous regulators of MMP activity in tissue (see George et al., in this book). TIMPs inhibit the MMP activity through noncovalent binding of the active zinc-binding sites of MMPs at molar equivalence.126 Although the primary amino acid sequence identity between the TIMPs is low, about 30%, their tertiary structure is remarkably similar. They have 12 conserved cysteine residues required for the formation of six disulfide bonds, which hold the two domains in a rigid conformation127. N-terminal domain, that contains the TIMP consensus sequence VIRAK, is necessary for MMP inhibition.128130 The C-terminal domains are more divergent and appear to be important in forming differences in specificity to each TIMP family member.131133
In general, TIMPs can inhibit the activity of all MMPs in vitro, except for MT1-MMP and MT3-MMP, which are not inhibited by TIMP-1.76,134 Due to structural similarities in the active site of MMPs and ADAMs, some ADAMs are also inhibited by TIMPs. TIMP-1 and TIMP-3 inhibit ADAM10, whereas TNF-α convertase (TACE, ADAM17) is only inhibited by TIMP-3.135,136 TIMP-1 and TIMP-3 inhibit aggrecanase-1 (ADAMTS4) (aggrecanase-1), and TIMP-3 also inhibits aggrecanase-2 (ADAMTS5).137,138 TIMP-1 has a role in tissue remodeling during embryonal growth and tumor progression, in gonadal steroidogenesis, ovulation, pregnancy, and parturition, and in inhibiting migration of vascular smooth muscle cells and endothelial cells, angiogenesis, tumor cell invasion and metastasis.127,128,139,141 It also possesses growth factor activity142 and inhibits shedding of heparin-binding epidermal growth factor and its receptor HER2.143,144
TIMP-2 is expressed in a constitutive manner by cells in culture. TIMP-2 can inhibit the shedding of TNF-α receptors (TNF-αRI and II).145 Mice with an inactivating mutation of TIMP-2 gene have normal phenotypes, in spite of severe impairment of pro-MMP-2 activation in vivo.146 In human tumors, high levels of TIMP-2 at the interface between malignant cells and stromal cells correlate with poor prognosis, suggesting that overexpression of TIMP-2 reflects a host reaction against highly invasive cells.147
Whereas other TIMPs are present in soluble form, TIMP-3 is insoluble, bound to the ECM.148 TIMP-3 promotes the detachment of transformed cells from the ECM and accelerates morphological changes associated with cell transformation.141 In addition, up-regulation of TIMP-3 has been associated with a block in the G1 phase of the cell cycle during differentiation of HL-60 leukemia cells.149 TIMP-3 can block the cellular shedding of pro-TNF-α, L-selectin and IL-6 receptor.135,136,150,151 Adenovirus-mediated gene delivery of TIMP-3 inhibits invasion and induces apoptosis in various normal and malignant cells.141,152,153 TIMP-4 is mostly expressed in the adult human heart, though very low levels of mRNA and protein are found in many tissues.154
MMPs in Tumor Growth and Invasion
MMPs have a dual role in tumor growth and metastasis processes. They promote tumor growth by degrading matrix barriers and by enhancing angiogenesis.4> On the other hand, MMPs can limit tumor neovascularization. Angiostatin is a specific inhibitor of endothelial cell proliferation and one the most effective and specific natural inhibitors of angiogenesis. It is cleaved from plasminogen by the action of plasmin and plasmin reductase,155> and by MMPs, the most efficient being MMP-12, followed by MMP-9, MMP-3, and MMP-7, with collagenases exhibiting practically no activity.156>,157> In α1 integrin knockout mice, which lack the α1b1 collagen receptor, the synthesis of MMP-7 and MMP-9 is markedly increased. This increased the plasma levels of angiostatin, leading to markedly decreased vascularization of implanted tumors.158> Endostatin, another natural angiogenesis inhibitor, is a fragment of type XVIII collagen.159> Although MMPs are not required for generation of endostatin, they are involved in the processing of collagen XVIII.160>
MMPs have also other functions.161> They can release active growth factors and angiogenic factors from the cell surface and ECM.162> They can cleave growth factor-binding proteins163> and cell surface growth factor receptors.164> They can generate an α1-antitrypsin cleavage product that promotes tumor growth and invasion.165> They may alter cell cycle checkpoint control and promote genomic instability by affecting cell adhesion.166> MMPs can also induce programmed cell death in anchorage-dependent cells.36> This can either inhibit tumor progression or promote it by enhancing selection of anchorage-independent and apoptosis-resistant subpopulations.37>
Tumor growth involves alterations in the stromal ECM and malignant tumors often induce a fibroproliferative response in the adjacent stroma, characterized by increased expression of type I and III procollagens.167> During metastasis formation, malignant cells detach from the primary tumor, invade through stromal tissue, enter the circulation, arrest at the peripheral vascular bed, and extravasate, invade the target organ, and form a metastatic colony.4>,163> Tumor cells must escape the host immune surveillance, and only a fraction of circulating tumor cells establish metastatic colonies.168> Tumor-induced angiogenesis is essential for growth of the primary tumor and metastases, and new blood vessels are sites for entry of tumor cell entry into the circulation. It is conceivable that proteolytic degradation of ECM plays a crucial role in all the above-mentioned aspects of tumor development.
A considerable body of evidence is available implicating MMPs in cancer spread. A number of studies have demonstrated a positive correlation between MMP expression and invasive and metastatic potential of malignant tumors including colon, lung, head and neck, basal cell, breast, thyroid, prostate, ovarian, and gastric carcinomas.4> For example, expression of MMP-1 correlates with poor prognosis in colorectal cancer and oesophageal cancer169>,170> and MMP-2 and MMP-3 expression is associated with lymph node metastasis and vascular invasion in SCC of esophagus.171> Similarly, high expression of MMP-13 in SCCs of the head and neck and vulva is associated with their metastasis capacity.29>,30> MMP-11 expression correlates with increased local invasiveness in head and neck SCCs172> and the level of MMP2 expression with poor prognosis of cervical SCCs.173> In general, all MMPs, the expression of which has been documented in malignant tumors, can also be expressed by nonneoplastic cells. However, MMP-13, MMP-7, MMP-12, and MMP-14 are expressed by malignantly transformed keratinocytes in SCCs but not in normal keratinocytes, indicating that their expression serves as a marker for transformation.29>,30> In addition, MMP-2 expression serves as a marker for malignant transformation of cervical epithelial cells.173>,174>
MMPs are mainly produced by nonmalignant stromal cells in malignant tumors. Tumor cells also secrete factors, such as extracellular MMP inducer (EMMPRIN), which enhance the expression of MMPs by stromal fibroblasts (see Toole, in this book). In addition, growth factors and cytokines secreted by tumor-infiltrating inflammatory cells as well as by tumor or stromal cells modulate MMP expression. Tumor invasion involves interaction between tumor cells, adjacent stromal cells, and infiltrating inflammatory cells, and it is likely that all these cells express distinct MMPs, which may complement each other's substrate specificity and form a network of MMP cascades in which one MMP cleaves a particular native or partially degraded ECM component and activates other MMPs.
TIMPs in Tumor Growth and Invasion
A number of studies have demonstrated the expression of TIMPs in tumor stroma and tumor tissue. In general, there is convincing evidence that overexpression of TIMPs by cancer cells or by the host reduces invasive and metastatic capacity of tumor cells. In cutaneous and oral SCCs, expression of TIMP-1, TIMP-2, and TIMP-3 is detected in stromal cells adjacent to the tumor,175>177> suggesting that their expression represents a host attempt to limit tumor invasion and tumor-induced angiogenesis. This notion is supported by observations indicating that the presence of TIMP-1 and TIMP-2 in SCCs correlates with less aggressive growth.178> However, in breast cancer TIMP-2 expression correlates with tumor recurrence,179> and in cervical carcinomas TIMP-2 expression correlates with poor prognosis.180> Similarly, in malignant breast cancer TIMP-1 expression is enhanced, as compared to nonmalignant breast tumor.181> However, the MMP:TIMP ratio is elevated in cervical carcinomas with poor prognosis, indicating that evaluation of either MMP or TIMP expression alone is not sufficient for prognostication of malignancies.180>
Cancer cell invasion can be inhibited by recombinant TIMPs or by overexpression of either TIMPs using a variety of gene-delivery vehicles. TIMP-2 inhibited the invasion of HT1080 fibrosarcoma cells in vitro,182>,183> but had no effect on tumor cell growth. Overexpression of TIMP-1 reduced metastasis of gastric carcinoma cells.184> TIMP-1 also reduced the growth rate and invasion of astrocytoma and mammary carcinoma cells,185>,186> and prevented metastasis of gastric carcinoma cells.187> The ability of TIMP-1 to inhibit tumor development at different stages has been demonstrated by transgenic mouse models. Constitutive overexpression of TIMP-1 in the liver suppressed tumor initiation, growth, and angiogenesis in transgenic mice, which develop hepatocellular carcinomas as a result of SV40 T antigen expression.188> These observations were also supported by a recent study in which TIMP-1 overexpression in the brain prevented tumor formation.189>
Overexpression of TIMP-2 reduced the MMP activity and suppressed growth of melanomas in the skin of immunodeficient mice,190> and melanoma cells overexpressing TIMP-2 showed a reduced metastatic capacity.191>Melanoma cells overexpressing TIMP-1 were shown to have reduced metastasis capacity due to inhibition of tumor growth following extravasation.192> TIMP-4 overexpression in breast carcinoma cells also inhibits invasion in vitro and tumor growth in vivo and results in reduction in lymph node and lung metastasis.193> Together these studies highlight both similar and diverse effects of overexpression of individual TIMPs on tumor cell phenotype in vivo. Adenoviral delivery of TIMP-2 has been shown to inhibit growth of liver metastases.194> In contrast, systemic delivery of TIMP-4 enhances mammary tumorigenesis but inhibits growth of Wilms' tumor in vivo.195>,196>
Further studies have demonstrated distinct effects of individual TIMPs on cell survival. Overexpression of TIMP-2 reduced invasion and angiogenesis but also protected the melanoma cells from apoptosis, although it increased necrosis.197> TIMP-1 has also been shown to promote survival of B cells through modulation of CD40 levels.198> However, we and others have shown that adenoviral-mediated gene delivery of TIMP-3 promotes apoptosis of a number of malignant cell types associated with reduced capacity of TIMP-3-transduced cells to bind to ECM components.141>,152>,153> TIMP-3 expressing stable colon carcinoma cell lines display reduced tumor growth,199> and in these cells TIMP-3 overexpression resulted in apoptosis through stabilization of TNF-α receptors on the cell surface.200> This suggests that individual TIMPs may modulate the levels of death proteins from the cell surface, as demonstrated by findings that shedding of FAS from the cell surface is mediated by MMP-induced cleavage and is inhibited by synthetic MMP inhibitors.201> Furthermore, TIMP-3 inhibits activity of TNF-α convertase (ADAM17), providing further evidence for complex regulation of death ligands and receptors by MMPs and TIMPs,202> which may play an important role in survival, growth, and invasion of malignant cells.
At present, several synthetic MMP inhibitors are in clinical trials evaluating their ability to inhibit growth and invasion of malignant tumors in vivo (see Turpeenniemi-Hujanen, in this book). Gene delivery of TIMP-1, -2, -3, and -4 into malignant cells may also be a potent way of inhibiting tumor growth and invasion.145>,152>,153>,196> Furthermore, an effective way of inhibiting MMP expression may be blocking signaling pathways mediating activation of MMP gene expression.4> The ongoing clinical trials are expected to show whether synthetic MMP inhibitors have a place in the therapeutic arsenal aimed at inhibiting growth, invasion, and metastasis of malignant tumors.
Acknowledgments
The original work of authors has been supported by grants from the Academy of Finland, Sigrid Jusélius Foundation, the Cancer Foundation of Finland, and Turku University Central Hospital, and by research contract with Finnish Life and Pension Insurance Companies.
References
- 1.
- Cawston T. Matrix metalloproteinases and TIMPs: properties and implications for the rheumatic diseases. Mol Med Today. 1998;4:130–137. [PubMed: 9575496]
- 2.
- Nagase H, Woessner JF Jr. Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. [PubMed: 10419448]
- 3.
- Murphy G, Knäuper V. Relating matrix metalloproteinase structure to function: why the “hemopexin” domain? Matrix Biol. 1997;15:511–518. [PubMed: 9138283]
- 4.
- Westermarck J, Kähäri VM. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J. 1999;13:781–792. [PubMed: 10224222]
- 5.
- Borkakoti N. Structural studies of matrix metalloproteinases. J Mol Med. 2000;78:261–268. [PubMed: 10954198]
- 6.
- Krane SM, Byrne MH, Lemaître V. et al. Different collagenase gene products have different roles in degradation of type I collagen. J Biol Chem. 1996;271:28509–28515. [PubMed: 8910479]
- 7.
- Freije JM, Diez-Itza I, Balbín M. et al. Molecular cloning and expression of collagenase-3, a novel human matrix metalloproteinase produced by breast carcinomas. J Biol Chem. 1994;269:16766–16773. [PubMed: 8207000]
- 8.
- Knäuper V, Löpez-Otín C, Smith B. et al. Biochemical characterization of human collagenase-3. J Biol Chem. 1996;271:1544–1550. [PubMed: 8576151]
- 9.
- Mitchell PG, Magna HA, Reeves LM. et al. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest. 1996;97:761–768. [PMC free article: PMC507114] [PubMed: 8609233]
- 10.
- Wilhelm SM, Eisen AZ, Teter M. et al. Human fibroblast collagenase: glycosylation and tissue-specific levels of enzyme synthesis. Proc Natl Acad Sci U S A. 1986;83:3756–3760. [PMC free article: PMC323602] [PubMed: 3012533]
- 11.
- Chung L, Shimokawa KI, Dinakarpandian D. et al. Identification of the 183RWTNNFREY191 region as a critical segment of matrix metalloproteinase 1 for the expression of collagenolytic activity. J Biol Chem. 2000;275:29610–29617. [PubMed: 10871619]
- 12.
- McGowan KA, Bauer EA, Smith LT. Localization of type I human skin collagenase in developing embryonic and fetal skin. J Invest Dermatol. 1994;102:951–957. [PubMed: 7516399]
- 13.
- Saarialho-Kere UK. Patterns of matrix metalloproteinase and TIMP expression in chronic ulcers. Arch Dermatol Res. 1998;290 Suppl:S47–S54. [PubMed: 9710383]
- 14.
- Saarialho-Kere UK, Chang ES, Welgus HG. et al. Distinct localization of collagenase and tissue inhibitor of metalloproteinases expression in wound healing associated with ulcerative pyogenic granuloma. J Clin Invest. 1992;90:1952–1957. [PMC free article: PMC443257] [PubMed: 1430217]
- 15.
- Pilcher BK, Dumin JA, Sudbeck BD. et al. The activity of collagenase-1 is required for keratinocyte migration on a type I collagen matrix. J Cell Biol. 1997;137:1445–1457. [PMC free article: PMC2132537] [PubMed: 9182674]
- 16.
- Hasty KA, Pourmotabbed TF, Goldberg GI. et al. Human neutrophil collagenase. A distinct gene product with homology to other matrix metalloproteinases. J Biol Chem. 1990;265:11421–11424. [PubMed: 2164002]
- 17.
- Cole AA, Chubinskaya S, Schumacher B. et al. Chondrocyte matrix metalloproteinase-8. Human articular chondrocytes express neutrophil collagenase. J Biol Chem. 1996;271:11023–11026. [PubMed: 8631924]
- 18.
- Hanemaaijer R, Sorsa T, Konttinen YT. et al. Matrix metalloproteinase-8 is expressed in rheumatoid synovial fibroblasts and endothelial cells. Regulation by tumor necrosis factor-alpha and doxycycline. J Biol Chem. 1997;272:31504–31509. [PubMed: 9395486]
- 19.
- Fosang AJ, Last K, Knauper V. et al. Degradation of cartilage aggrecan by collagenase3 (MMP13). FEBS Lett. 1996;380:17–20. [PubMed: 8603731]
- 20.
- Knauper V, Cowell S, Smith B. et al. The role of the C-terminal domain of human collagenase-3 (MMP-13) in the activation of procollagenase-3, substrate specificity, and tissue inhibitor of metalloproteinase interaction. J Biol Chem. 1997;272:7608–7616. [PubMed: 9065415]
- 21.
- Sasaki T, Gohring W, Mann K. et al. Limited cleavage of extracellular matrix protein BM-40 by matrix metalloproteinases increases its affinity for collagens. J Biol Chem. 1997;272:9237–9243. [PubMed: 9083057]
- 22.
- Ashworth JL, Murphy G, Rock MJ. et al. Fibrillin degradation by matrix metalloproteinases: implications for connective tissue remodelling. Biochem J. 1999;340:171–181. [PMC free article: PMC1220235] [PubMed: 10229672]
- 23.
- Ravanti L, Häkkinen L, Larjava H. et al. Transforming growth factor-β induces collagenase-3 expression by human gingival fibroblasts via p38 mitogen-activated protein kinase. J Biol Chem. 1999;274:37292–37300. [PubMed: 10601295]
- 24.
- Johansson N, Saarialho-Kere U, Airola K. et al. Collagenase-3 (MMP-13) is expressed by hypertrophic chondrocytes, periosteal cells, and osteoblasts during human fetal bone development. Dev Dyn. 1997;208:387–397. [PubMed: 9056642]
- 25.
- Stahle-Backdahl M, Sandstedt B, Bruce K. et al. Collagenase-3 (MMP-13) is expressed during human fetal ossification and re-expressed in postnatal bone remodeling and in rheumatoid arthritis. Lab Invest. 1997;76:717–728. [PubMed: 9166290]
- 26.
- Ravanti L, Toriseva M, Penttinen R. et al. Expression of human collagenase-3 (MMP-13) by fetal skin fibroblasts is induced by transforming growth factor beta via p38 mitogen-activated protein kinase. FASEB J. 2001;15:1098–1100. [PubMed: 11292680]
- 27.
- Vaalamo M, Mattila L, Johansson N. et al. Distinct populations of stromal cells express collagenase-3 (MMP-13) and collagenase-1 (MMP-1) in chronic ulcers but not in normally healing wounds. J Invest Dermatol. 1997;109:96–101. [PubMed: 9204962]
- 28.
- Uitto VJ, Airola K, Vaalamo M. et al. Collagenase-3 (matrix metalloproteinase-13) expression is induced in oral mucosal epithelium during chronic inflammation. Am J Pathol. 1998;152:1489–1499. [PMC free article: PMC1858431] [PubMed: 9626053]
- 29.
- Johansson N, Airola K, Grénman R. et al. Expression of collagenase-3 (matrix metalloproteinase-13) in squamous cell carcinomas of the head and neck. Am J Pathol. 1997;151:499–508. [PMC free article: PMC1857999] [PubMed: 9250162]
- 30.
- Johansson N, Vaalamo M, Grénman S. et al. Collagenase-3 (MMP-13) is expressed by tumor cells in invasive vulvar squamous cell carcinomas. Am J Pathol. 1999;154:469–480. [PMC free article: PMC1849989] [PubMed: 10027405]
- 31.
- Airola K, Karonen T, Vaalamo M. et al. Expression of collagenases-1 and -3 and their inhibitors TIMP-1 and -3 correlates with the level of invasion in malignant melanomas. Br J Cancer. 1999;80:733–743. [PMC free article: PMC2362286] [PubMed: 10360651]
- 32.
- Nikkola J, Vihinen P, Vlaykova T. et al. High collagenase-1 expression correlates with a favourable chemoimmunotherapy response in human metastatic melanoma. Melanoma Res. 2001;11:157–166. [PubMed: 11333126]
- 33.
- Boström PJ, Ravanti L, Reunanen N. et al. Expression of collagenase-3 (matrix metalloproteinase-13) in transitional-cell carcinoma of the urinary bladder. Int J Cancer. 2000;88:417–423. [PubMed: 11054671]
- 34.
- Ala-Aho R, Johansson N, Baker AH. et al. Expression of collagenase-3 (MMP-13) enhances invasion of human fibrosarcoma HT-1080 cells. Int J Cancer. 2002;97:283–289. [PubMed: 11774278]
- 35.
- Lund LR, Romer J, Thomasset N. et al. Two distinct phases of apoptosis in mammary gland involution: proteinase-independent and -dependent pathways. Development. 1996;122:181–193. [PMC free article: PMC2933211] [PubMed: 8565829]
- 36.
- Thomasset N, Lochter A, Sympson CJ. et al. Expression of autoactivated stromelysin-1 in mammary glands of transgenic mice leads to a reactive stroma during early development. Am J Pathol. 1998;153:457–467. [PMC free article: PMC1852990] [PubMed: 9708806]
- 37.
- Sternlicht MD, Bissell MJ, Werb Z. The matrix metalloproteinase stromelysin-1 acts as a natural mammary tumor promoter. Oncogene. 2000;19:1102–1113. [PMC free article: PMC2933206] [PubMed: 10713697]
- 38.
- Unemori EN, Bair MJ, Bauer EA. et al. Stromelysin expression regulates collagenase activation in human fibroblasts. Dissociable control of two metalloproteinases by interferon-γ J Biol Chem. 1991;266:23477–23482. [PubMed: 1660474]
- 39.
- Bullard KM, Lund L, Mudgett JS. et al. Impaired wound contraction in stromelysin-1-deficient mice. Ann Surg. 1999;230:260–265. [PMC free article: PMC1420869] [PubMed: 10450741]
- 40.
- Rouyer N, Wolf C, Chenard MP. et al. Stromelysin-3 gene expression in human cancer: an overview. Invasion Metastasis. 1994;14:269–275. [PubMed: 7657519]
- 41.
- Ahmad A, Hanby A, Dublin E. et al. Stromelysin 3: an independent prognostic factor for relapse-free survival in node-positive breast cancer and demonstration of novel breast carcinoma cell expression. Am J Pathol. 1998;152:721–728. [PMC free article: PMC1858384] [PubMed: 9502414]
- 42.
- Noël AC, Lefebvre O, Maquoi E. et al. Stromelysin-3 expression promotes tumor take in nude mice. J Clin Invest. 1996;97:1924–1930. [PMC free article: PMC507262] [PubMed: 8621777]
- 43.
- Masson R, Lefebvre O, Noël A. et al. In vivo evidence that the stromelysin-3 metalloproteinase contributes in a paracrine manner to epithelial cell malignancy. J Cell Biol. 1998;140:1535–1541. [PMC free article: PMC2132679] [PubMed: 9508784]
- 44.
- Unden AB, Sandstedt B, Bruce K. et al. Stromelysin-3 mRNA associated with myofibroblasts is overexpressed in aggressive basal cell carcinoma and in dermatofibroma but not in dermatofibrosarcoma. J Invest Dermatol. 1996;107:147–153. [PubMed: 8757754]
- 45.
- Thewes M, Worret WI, Engst R. et al. Stromelysin-3 (ST-3): immunohistochemical characterization of the matrix metalloproteinase (MMP)-11 in benign and malignant skin tumours and other skin disorders. Clin Exp Dermatol. 1999;24:122–126. [PubMed: 10233668]
- 46.
- Pei D, Weiss SJ. Furin-dependent intracellular activation of the human stromelysin-3 zymogen. Nature. 1995;375:244–247. [PubMed: 7746327]
- 47.
- Pei D, Majmudar G, Weiss SJ. Hydrolytic inactivation of a breast carcinoma cell-derived serpin by human stromelysin-3. J Biol Chem. 1994;269:25849–25855. [PubMed: 7523394]
- 48.
- Noël A, Boulay A, Kebers F. et al. Demonstration in vivo that stromelysin-3 functions through its proteolytic activity. Oncogene. 2000;19:1605–1612. [PubMed: 10734321]
- 49.
- Belaaouaj A, Shipley JM, Kobayashi DK. et al. Human macrophage metalloelastase. Genomic organization, chromosomal location, gene linkage, and tissue-specific expression. J Biol Chem. 1995;270:14568–14575. [PubMed: 7782320]
- 50.
- Halpert I, Sires UI, Roby JD. et al. Matrilysin is expressed by lipid-laden macrophages at sites of potential rupture in atherosclerotic lesions and localizes to areas of versican deposition, a proteoglycan substrate for the enzyme. Proc Natl Acad Sci U S A. 1996;93:9748–9753. [PMC free article: PMC38500] [PubMed: 8790402]
- 51.
- Curci JA, Liao S, Huffman MD. et al. Expression and localization of macrophage elastase (matrix metalloproteinase-12) in abdominal aortic aneurysms. J Clin Invest. 1998;102:1900–1910. [PMC free article: PMC509141] [PubMed: 9835614]
- 52.
- Vaalamo M, Karjalainen-Lindsberg ML, Puolakkainen P. et al. Distinct expression profiles of stromelysin-2 (MMP-10), collagenase-3 (MMP-13), macrophage metalloelastase (MMP-12), and tissue inhibitor of metalloproteinases-3 (TIMP-3) in intestinal ulcerations. Am J Pathol. 1998;152:1005–1014. [PMC free article: PMC1858229] [PubMed: 9546361]
- 53.
- Kerkelä E, Ala-Aho R, Jeskanen L. et al. Expression of human macrophage metalloelastase (MMP-12) by tumor cells in skin cancer. J Invest Dermatol. 2000;114:1113–1119. [PubMed: 10844553]
- 54.
- Vaalamo M, Kariniemi AL, Shapiro SD. et al. Enhanced expression of human metalloelastase (MMP-12) in cutaneous granulomas and macrophage migration. J Invest Dermatol. 1999;112:499–505. [PubMed: 10201535]
- 55.
- Hautamaki RD, Kobayashi DK, Senior RM. et al. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 1997;277:2002–2004. [PubMed: 9302297]
- 56.
- Park HI, Ni J, Gerkema FE. et al. Identification and characterization of human endometase (matrix metalloproteinase-26) from endometrial tumor. J Biol Chem. 2000;275:20540–20544. [PubMed: 10801841]
- 57.
- Uría JA, López-Otín C. Matrilysin-2, a new matrix metalloproteinase expressed in human tumors and showing the minimal domain organization required for secretion, latency, and activity. Cancer Res. 2000;60:4745–4751. [PubMed: 10987280]
- 58.
- Sires UI, Murphy G, Baragi VM. et al. Matrilysin is much more efficient than other matrix metalloproteinases in the proteolytic inactivation of α1-antitrypsin. Biochem Biophys Res Commun. 1994;204:613–620. [PubMed: 7980522]
- 59.
- Saarialho-Kere U, Crouch EC, Parks WC. Matrix metalloproteinase matrilysin is constitutively expressed in adult human exocrine epithelium. J Invest Dermatol. 1995;105:190–196. [PubMed: 7636300]
- 60.
- Wilson CL, Heppner KJ, Rudolph LA. et al. The metalloproteinase matrilysin is preferentially expressed by epithelial cells in a tissue-restricted pattern in the mouse. Mol Biol Cell. 1995;6:851–869. [PMC free article: PMC301245] [PubMed: 7579699]
- 61.
- Wilson CL, Heppner KJ, Labosky PA. et al. Intestinal tumorigenesis is suppressed in mice lacking the metalloproteinase matrilysin. Proc Natl Acad Sci U S A. 1997;94:1402–1407. [PMC free article: PMC19803] [PubMed: 9037065]
- 62.
- Wilson CL, Ouellette AJ, Satchell DP. et al. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science. 1999;286:113–117. [PubMed: 10506557]
- 63.
- Dunsmore SE, Saarialho-Kere UK, Roby JD. et al. Matrilysin expression and function in airway epithelium. J Clin Invest. 1998;102:1321–1331. [PMC free article: PMC508979] [PubMed: 9769324]
- 64.
- De Coignac AB, Elson G. et al. Cloning of MMP-26. A novel matrilysin-like proteinase. Eur J Biochem. 2000;267:3323–3329. [PubMed: 10824119]
- 65.
- Aimes RT, Quigley JP. Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4- and 1/4-length fragments. J Biol Chem. 1995;270:5872–5876. [PubMed: 7890717]
- 66.
- Fridman R, Toth M, Pena D. et al. Activation of progelatinase B (MMP-9) by gelatinase A (MMP-2). Cancer Res. 1995;55:2548–2555. [PubMed: 7780967]
- 67.
- Esparza J, Vilardell C, Calvo J. et al. Fibronectin upregulates gelatinase B (MMP-9) and induces coordinated expression of gelatinase A (MMP-2) and its activator MT1-MMP (MMP-14) by human T lymphocyte cell lines. A process repressed through RAS/MAP kinase signaling pathways. Blood. 1999;94:2754–2766. [PubMed: 10515879]
- 68.
- Yu AE, Hewitt RE, Kleiner DE. et al. Molecular regulation of cellular invasion-role of gelatinase A and TIMP-2. Biochem Cell Biol. 1996;74:823–831. [PubMed: 9164651]
- 69.
- Itoh T, Tanioka M, Yoshida H. et al. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 1998;58:1048–1051. [PubMed: 9500469]
- 70.
- Itoh T, Tanioka M, Yoshida H. et al. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 1998;58:1048–1051. [PubMed: 9500469]
- 71.
- Sato H, Kinoshita T, Takino T. et al. Activation of a recombinant membrane type 1-matrix metalloproteinase (MT1-MMP) by furin and its interaction with tissue inhibitor of metalloproteinases (TIMP)-2. FEBS Lett. 1996;393:101–104. [PubMed: 8804434]
- 72.
- Steiner DF. The proprotein convertases. Curr Opin Chem Biol. 1998;2:31–39. [PubMed: 9667917]
- 73.
- d'Ortho MP, Will H, Atkinson S. et al. Membrane-type matrix metalloproteinases 1 and 2 exhibit broad-spectrum proteolytic capacities comparable to many matrix metalloproteinases. Eur J Biochem. 1997;250:751–757. [PubMed: 9461298]
- 74.
- Hotary K, Allen E, Punturieri A. et al. Regulation of cell invasion and morphogenesis in a three-dimensional type I collagen matrix by membrane-type matrix metalloproteinases 1, 2, and 3. J Cell Biol. 2000;149:1309–1323. [PMC free article: PMC2175112] [PubMed: 10851027]
- 75.
- Knäuper V, Will H, López-Otín C. et al. Cellular mechanisms for human procollagenase-3 (MMP-13) activation. Evidence that MT1-MMP (MMP-14) and gelatinase A (MMP-2) are able to generate active enzyme. J Biol Chem. 1996;271:17124–17131. [PubMed: 8663255]
- 76.
- Will H, Atkinson SJ, Butler GS. et al. The soluble catalytic domain of membrane type 1 matrix metalloproteinase cleaves the propeptide of progelatinase A and initiates autoproteolytic activation. Regulation by TIMP-2 and TIMP-3. J Biol Chem. 1996;271:17119–17123. [PubMed: 8663332]
- 77.
- Cowell S, Knäuper V, Stewart ML. et al. Induction of matrix metalloproteinase activation cascades based on membrane-type 1 matrix metalloproteinase: associated activation of gelatinase A, gelatinase B and collagenase 3. Biochem J. 1998;331:453–458. [PMC free article: PMC1219375] [PubMed: 9531484]
- 78.
- Pei D. Identification and characterization of the fifth membrane-type matrix metalloproteinase MT5-MMP. J Biol Chem. 1999;274:8925–8932. [PubMed: 10085137]
- 79.
- Sato H, Takino T, Okada Y. et al. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994;370:61–65. [PubMed: 8015608]
- 80.
- Takino T, Sato H, Shinagawa A. et al. Identification of the second membrane-type matrix metalloproteinase (MT-MMP-2) gene from a human placenta cDNA library. MT-MMPs form a unique membrane-type subclass in the MMP family. J Biol Chem. 1995;270:23013–23020. [PubMed: 7559440]
- 81.
- Okada A, Bellocq JP, Rouyer N. et al. Membrane-type matrix metalloproteinase (MT-MMP) gene is expressed in stromal cells of human colon, breast, and head and neck carcinomas. Proc Natl Acad Sci U S A. 1995;92:2730–2734. [PMC free article: PMC42292] [PubMed: 7708715]
- 82.
- Okada A, Tomasetto C, Lutz Y. et al. Expression of matrix metalloproteinases during rat skin wound healing: evidence that membrane type-1 matrix metalloproteinase is a stromal activator of pro-gelatinase A. J Cell Biol. 1997;137:67–77. [PMC free article: PMC2139851] [PubMed: 9105037]
- 83.
- Ohuchi E, Imai K, Fujii Y. et al. Membrane type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J Biol Chem. 1997;272:2446–2451. [PubMed: 8999957]
- 84.
- Fortunato SJ, Menon R, Lombardi SJ. Expression of a progelatinase activator (MT1-MMP) in human fetal membranes. Am J Reprod Immunol. 1998;39:316–322. [PubMed: 9602249]
- 85.
- Hurskainen T, Seiki M, Apte SS. et al. Production of membrane-type matrix metalloproteinase-1 (MT-MMP1) in early human placenta: a possible role in placental implantation? J Histochem Cytochem. 1998;46:221–229. [PubMed: 9446829]
- 86.
- Holmbeck K, Bianco P, Caterina J. et al. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. [PubMed: 10520996]
- 87.
- Zhou Z, Apte SS, Soininen R. et al. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc Natl Acad Sci U S A. 2000;97:4052–4057. [PMC free article: PMC18145] [PubMed: 10737763]
- 88.
- Nabeshima K, Inoue T, Shimao Y. et al. Front-cell-specific expression of membrane-type 1 matrix metalloproteinase and gelatinase A during cohort migration of colon carcinoma cells induced by hepatocyte growth factor/scatter factor. Cancer Res. 2000;60:3364–3369. [PubMed: 10910039]
- 89.
- Yamanaka H, Makino K, Takizawa M. et al. Expression and tissue localization of membrane-types 1, 2, and 3 matrix metalloproteinases in rheumatoid synovium. Lab Invest. 2000;80:677–687. [PubMed: 10830778]
- 90.
- Therét N, Musso O, L'Helgoualc'h A. et al. Differential expression and origin of membrane-type 1 and 2 matrix metalloproteinases (MT-MMPs) in association with MMP2 activation in injured human livers. Am J Pathol. 1998;153:945–954. [PMC free article: PMC1853032] [PubMed: 9736043]
- 91.
- Miyamori H, Takino T, Seiki M. et al. Human membrane type-2 matrix metalloproteinase is defective in cell-associated activation of progelatinase A. Biochem Biophys Res Commun. 2000;267:796–800. [PubMed: 10673371]
- 92.
- Yoshiyama Y, Sato H, Seiki M. et al. Expression of the membrane-type 3 matrix metalloproteinase (MT3-MMP) in human brain tissues. Acta Neuropathol (Berl). 1998;96:347–350. [PubMed: 9796998]
- 93.
- Matsumoto S, Katoh M, Saito S. et al. Identification of soluble type of membrane-type matrix metalloproteinase-3 formed by alternatively spliced mRNA. Biochim Biophys Acta. 1997;1354:159–170. [PubMed: 9396633]
- 94.
- Puente XS, Pendás AM, Llano E. et al. Molecular cloning of a novel membrane-type matrix metalloproteinase from a human breast carcinoma. Cancer Res. 1996;56:944–949. [PubMed: 8640782]
- 95.
- Itoh Y, Kajita M, Kinoh H. et al. Membrane type 4 matrix metalloproteinase (MT4-MMP, MMP-17) is a glycosylphosphatidylinositol-anchored proteinase. J Biol Chem. 1999;274:34260–34266. [PubMed: 10567400]
- 96.
- English WR, Puente XS, Freije JM. et al. Membrane type 4 matrix metalloproteinase (MMP-17) has tumor necrosis factor-α convertase activity but does not activate pro-MMP2. J Biol Chem. 2000;275:14046–14055. [PubMed: 10799478]
- 97.
- Llano E, Pendás AM, Freije JP. et al. Identification and characterization of human MT5-MMP, a new membrane-bound activator of progelatinase A overexpressed in brain tumors. Cancer Res. 1999;59:2570–2576. [PubMed: 10363975]
- 98.
- Jaworski DM. Developmental regulation of membrane type-5 matrix metalloproteinase (MT5-MMP) expression in the rat nervous system. Brain Res. 2000;860:174–177. [PubMed: 10727639]
- 99.
- Pei D. Leukolysin/MMP25/MT6-MMP: a novel matrix metalloproteinase specifically expressed in the leukocyte lineage. Cell Res. 1999;9:291–303. [PubMed: 10628838]
- 100.
- Velasco G, Cal S, Merlos-Suarez A. et al. Human MT6-matrix metalloproteinase: identification, progelatinase A activation, and expression in brain tumors. Cancer Res. 2000;60:877–882. [PubMed: 10706098]
- 101.
- English WR, Velasco G, Stracke JO. et al. Catalytic activities of membrane-type 6 matrix metalloproteinase (MMP25). FEBS Lett. 2001;491:137–142. [PubMed: 11226436]
- 102.
- Pendás AM, Knäuper V, Puente XS. et al. Identification and characterization of a novel human matrix metalloproteinase with unique structural characteristics, chromosomal location, and tissue distribution. J Biol Chem. 1997;272:4281–4286. [PubMed: 9020145]
- 103.
- Sedlacek R, Mauch S, Kolb B. et al. Matrix metalloproteinase MMP-19 (RASI-1) is expressed on the surface of activated peripheral blood mononuclear cells and is detected as an autoantigen in rheumatoid arthritis. Immunobiology. 1998;198:408–423. [PubMed: 9562866]
- 104.
- Kolb C, Mauch S, Krawinkel U. et al. Matrix metalloproteinase-19 in capillary endothelial cells: expression in acutely, but not in chronically, inflamed synovium. Exp Cell Res. 1999;250:122–130. [PubMed: 10388526]
- 105.
- Stracke JO, Hutton M, Stewart M. et al. Biochemical characterization of the catalytic domain of human matrix metalloproteinase 19. Evidence for a role as a potent basement membrane degrading enzyme. J Biol Chem. 2000;275:14809–14816. [PubMed: 10809722]
- 106.
- Lohi JL, Wilson CL, Roby JD. et al. Epilysin, a novel human matrix metalloproteinase (MMP28) expressed in testis and keratinocytes and in response to injury. J Biol Chem. 2001;276:10134–10144. [PubMed: 11121398]
- 107.
- Marchenko GN, Strongin AY. MMP-28, a new human matrix metalloproteinase with an unusual cysteine-switch sequence is widely expressed in tumors. Gene. 2001;265:87–93. [PubMed: 11255011]
- 108.
- Llano E, Pendás AM, Knäuper V. et al. Identification and structural and functional characterization of human enamelysin (MMP-20). Biochemistry. 1997;36:15101–15108. [PubMed: 9398237]
- 109.
- Pei D, Kang T, Qi H. Cysteine array matrix metalloproteinase (CA-MMP)/MMP23 is a type II transmembrane matrix metalloproteinase regulated by a single cleavage for both secretion and activation. J Biol Chem. 2000;275:33988–97. [PubMed: 10945999]
- 110.
- Velasco G, Pendás AM, Fueyo A. et al. Cloning and characterization of human MMP-23, a new matrix metalloproteinase predominantly expressed in reproductive tissues and lacking conserved domains in other family members. J Biol Chem. 1999;274:4570–4576. [PubMed: 9988691]
- 111.
- Gutman A, Wasylyk B. The collagenase gene promoter contains a TPA and oncogene-responsive unit encompassing the PEA3 and AP-1 binding sites. EMBO J. 1990;9:2241–2246. [PMC free article: PMC551948] [PubMed: 2162765]
- 112.
- Benbow U, Brinckerhoff CE. The AP-1 site and MMP gene regulation: What is all the fuss about? Matrix Biol. 1997;15:519–526. [PubMed: 9138284]
- 113.
- Lohi J, Lehti K, Valtanen H. et al. Structural analysis and promoter characterization of the human membrane-type matrix metalloproteinase-1 (MT1-MMP) gene. Gene. 2000;242:75–86. [PubMed: 10721699]
- 114.
- Haas TL, Stitelman D, Davis SJ. et al. Egr-1 mediates extracellular matrix-driven transcription of membrane type 1 matrix metalloproteinase in endothelium. J Biol Chem. 1999;274:22679–22685. [PubMed: 10428849]
- 115.
- Springman EB, Angleton EL, Birkedal-Hansen H. et al. Multiple modes of activation of latent human fibroblast collagenase: evidence for the role of a Cys73 active-site zinc complex in latency and a “cysteine switch” mechanism for activation. Proc Natl Acad Sci U S A. 1990;87:364–368. [PMC free article: PMC53264] [PubMed: 2153297]
- 116.
- Butler GS, Butler MJ, Atkinson SJ. et al. The TIMP-2 membrane type 1 metalloproteinase “receptor” regulates the concentration and efficient activation of progelatinase A. A kinetic study. J Biol Chem. 1998;273:871–880. [PubMed: 9422744]
- 117.
- Zucker S, Drews M, Conner C. et al. Tissue inhibitor of metalloproteinase-2 (TIMP-2) binds to the catalytic domain of the cell surface receptor, membrane type 1-matrix metalloproteinase 1 (MT1-MMP). J Biol Chem. 1998;273:1216–1222. [PubMed: 9422789]
- 118.
- Carmeliet P, Moons L, Lijnen R. et al. Urokinase-generated plasmin activates matrix metalloproteinases during aneurysm formation. Nat Genet. 1997;17:439–444. [PubMed: 9398846]
- 119.
- Murphy G, Gavrilovic J. Proteolysis and cell migration: creating a path? Curr Opin Cell Biol 1999. Oct;11(5):614–21. [PubMed: 10508651]
- 120.
- Nakahara H, Howard L, Thompson EW. et al. Transmembrane/cytoplasmic domain-mediated membrane type 1-matrix metalloprotease docking to invadopodia is required for cell invasion. Proc Natl Acad Sci U S A. 1997;94:7959–7964. [PMC free article: PMC21537] [PubMed: 9223295]
- 121.
- Yu WH, Woessner JF Jr. Heparan sulfate proteoglycans as extracellular docking molecules for matrilysin (matrix metalloproteinase 7). J Biol Chem. 2000;275:4183–4191. [PubMed: 10660581]
- 122.
- Brooks PC, Stromblad S, Sanders LC. et al. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin avb3. Cell. 1996;85(5):683–93. [PubMed: 8646777]
- 123.
- Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–76. [PMC free article: PMC316345] [PubMed: 10652271]
- 124.
- Thomas GJ, Lewis MP, Hart IR. et al. alpha v beta 6 integrin promotes invasion of squamous carcinoma cells through up-regulation of matrix metalloproteinase-9. Int J Cancer. 2001;92:641–50. [PubMed: 11340566]
- 125.
- Dumin JA, Dickeson SK, Stricker TP. et al. Pro-collagenase-1 (matrix metalloproteinase-1) binds the α2β1 integrin upon release from keratinocytes migrating on type I collagen. J Biol Chem. 2001;276:29368–29374. [PubMed: 11359786]
- 126.
- Gomis-Ruth FX, Maskos K, Betz M. et al. Mechanism of inhibition of the human matrix metalloproteinase stromelysin-1 by TIMP-1. Nature. 1997;389:77–81. [PubMed: 9288970]
- 127.
- Gomez DE, Alonso DF, Yoshiji H. et al. Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur J Cell Biol. 1997;74:111–122. [PubMed: 9352216]
- 128.
- Murphy G, Houbrechts A, Cockett MI. et al. The N-terminal domain of tissue inhibitor of metalloproteinases retains metalloproteinase inhibitory activity. Biochemistry. 1991;30:8097–8102. [PubMed: 1868085]
- 129.
- DeClerck YA, Yean TD, Lee Y. et al. Characterization of the functional domain of tissue inhibitor of metalloproteinases-2 (TIMP-2). Biochem J. 1993;289:65–69. [PMC free article: PMC1132131] [PubMed: 8424773]
- 130.
- Langton KP, Barker MD, McKie N. Localization of the functional domains of human tissue inhibitor of metalloproteinases-3 and the effects of a Sorsby's fundus dystrophy mutation. J Biol Chem. 1998;273:16778–16781. [PubMed: 9642234]
- 131.
- Goldberg GI, Strongin A, Collier IE. et al. Interaction of 92-kDa type IV collagenase with the tissue inhibitor of metalloproteinases prevents dimerization, complex formation with interstitial collagenase, and activation of the proenzyme with stromelysin. J Biol Chem. 1992;267:4583–4591. [PubMed: 1311314]
- 132.
- Ogata Y, Itoh Y, Nagase H. Steps involved in activation of the pro-matrix metalloproteinase 9 (progelatinase B)-tissue inhibitor of metalloproteinases-1 complex by 4-aminophenylmercuric acetate and proteinases. J Biol Chem. 1995;270:18506–18511. [PubMed: 7629179]
- 133.
- Bigg HF, Shi YE, Liu YE. et al. Specific, high affinity binding of tissue inhibitor of metalloproteinases-4 (TIMP-4) to the COOH-terminal hemopexin-like domain of human gelatinase A. TIMP-4 binds progelatinase A and the COOH terminal domain in a similar manner to TIMP-2. J Biol Chem. 1997;272:15496–15500. [PubMed: 9182583]
- 134.
- Shimada T, Nakamura H, Ohuchi E. et al. Characterization of a truncated recombinant form of human membrane type 3 matrix metalloproteinase. Eur J Biochem. 1999;262:907–914. [PubMed: 10411655]
- 135.
- Amour A, Slocombe PM, Webster A. et al. TNF-alpha converting enzyme (TACE) is inhibited by TIMP-3. FEBS Lett. 1998;435:39–44. [PubMed: 9755855]
- 136.
- Amour A, Knight CG, Webster A. et al. The in vitro activity of ADAM-10 is inhibited by TIMP-1 and TIMP-3. FEBS Lett. 2000;473:275–279. [PubMed: 10818225]
- 137.
- Tortorella MD, Burn TC, Pratta MA. et al. Purification and cloning of aggrecanase-1: a member of the ADAMTS family of proteins. Science. 1999;284:1664–1666. [PubMed: 10356395]
- 138.
- Kashiwagi M, Tortorella M, Nagase H. et al. TIMP-3 is a potent inhibitor of aggrecanase-1 (ADAM-TS4) and aggrecanase-2 (ADAM-TS5). J Biol Chem. 2001;276:12501–12504. [PubMed: 11278243]
- 139.
- George SJ, Johnson JL, Angelini GD. et al. Adenovirus-mediated gene transfer of the human TIMP-1 gene inhibits smooth muscle cell migration and neointimal formation in human saphenous vein. Hum Gene Ther. 1998;9:867–877. [PubMed: 9581909]
- 140.
- Fernandez HA, Kallenbach K, Seghezzi G. et al. Inhibition of endothelial cell migration by gene transfer of tissue inhibitor of metalloproteinases-1. J Surg Res. 1999;82:156–162. [PubMed: 10090824]
- 141.
- Ahonen M, Baker AH, Kähäri VM. Adenovirus-mediated gene delivery of tissue inhibitor of metalloproteinases-3 inhibits invasion and induces apoptosis in melanoma cells. Cancer Res. 1998;58:2310–2315. [PubMed: 9622064]
- 142.
- Hayakawa T, Yamashita K, Tanzawa K. et al. Growth-promoting activity of tissue inhibitor of metalloproteinases-1 (TIMP-1) for a wide range of cells. A possible new growth factor in serum. FEBS Lett. 1992;298:29–32. [PubMed: 1544418]
- 143.
- Dethlefsen SM, Raab G, Moses MA. et al. Extracellular calcium influx stimulates metalloproteinase cleavage and secretion of heparin-binding EGF-like growth factor independently of protein kinase C. J Cell Biochem. 1998;69:143–153. [PubMed: 9548562]
- 144.
- Codony-Servat J, Albanell J, Lopez-Talavera JC. et al. Cleavage of the HER2 ectodomain is a pervanadate-activable process that is inhibited by the tissue inhibitor of metalloproteases-1 in breast cancer cells. Cancer Res. 1999;59:1196–1201. [PubMed: 10096547]
- 145.
- Lombard MA, Wallace TL, Kubicek MF. et al. Synthetic matrix metalloproteinase inhibitors and tissue inhibitor of metalloproteinase (TIMP)-2, but not TIMP-1, inhibit shedding of tumor necrosis factor-alpha receptors in a human colon adenocarcinoma (Colo 205) cell line. Cancer Res. 1998;58:4001–4007. [PubMed: 9731514]
- 146.
- Caterina JJ, Yamada S, Caterina NC. et al. Inactivating mutation of the mouse tissue inhibitor of metalloproteinases-2(Timp-2) gene alters proMMP-2 activation. J Biol Chem. 2000;275:26416–26422. [PubMed: 10827176]
- 147.
- Henriet P, Blavier L, Declerck YA. Tissue inhibitors of metalloproteinases (TIMP) in invasion and proliferation. APMIS. 1999;107:111–119. [PubMed: 10190287]
- 148.
- Leco KJ, Khokha R, Pavloff N. et al. Tissue inhibitor of metalloproteinases-3 (TIMP-3) is an extracellular matrix-associated protein with a distinctive pattern of expression in mouse cells and tissues. J Biol Chem. 1994;269:9352–9360. [PubMed: 8132674]
- 149.
- Wick M, Burger C, Brusselbach S. et al. A novel member of human tissue inhibitor of metalloproteinases (TIMP) gene family is regulated during G1 progression, mitogenic stimulation, differentiation, and senescence. J Biol Chem. 1994;269:18953–18960. [PubMed: 8034652]
- 150.
- Hargreaves PG, Wang F, Antcliff J. et al. Human myeloma cells shed the interleukin-6 receptor: inhibition by tissue inhibitor of metalloproteinase-3 and a hydroxamate-based metalloproteinase inhibitor. Br J Haematol. 1998;101:694–702. [PubMed: 9674743]
- 151.
- Borland G, Murphy G, Ager A. Tissue inhibitor of metalloproteinases-3 inhibits shedding of L-selectin from leukocytes. J Biol Chem. 1999;274:2810–2815. [PubMed: 9915814]
- 152.
- Baker AH, Zaltsman AB, George SJ. et al. Divergent effects of tissue inhibitor of metalloproteinase-1, -2, or -3 overexpression on rat vascular smooth muscle cell invasion, proliferation, and death in vitro. TIMP-3 promotes apoptosis. J Clin Invest. 1998;101:1478–1487. [PMC free article: PMC508704] [PubMed: 9502791]
- 153.
- Baker AH, George SJ, Zaltsman AB. et al. Inhibition of invasion and induction of apoptotic cell death of cancer cell lines by overexpression of TIMP-3. Br J Cancer. 1999;79:1347–1355. [PMC free article: PMC2362728] [PubMed: 10188875]
- 154.
- Greene J, Wang M, Liu YE. et al. Molecular cloning and characterization of human tissue inhibitor of metalloproteinase 4. J Biol Chem. 1996;271:30375–30380. [PubMed: 8939999]
- 155.
- Gately S, Twardowski P, Stack MS. et al. The mechanism of cancer-mediated conversion of plasminogen to the angiogenesis inhibitor angiostatin. Proc Natl Acad Sci U S A. 1997;94:10868–10872. [PMC free article: PMC23512] [PubMed: 9380726]
- 156.
- Cornelius LA, Nehring LC, Harding E. et al. Matrix metalloproteinases generate angiostatin: effects on neovascularization. J Immunol. 1998;161:6845–6852. [PubMed: 9862716]
- 157.
- Patterson BC, Sang QA. Angiostatin-converting enzyme activities of human matrilysin (MMP-7) and gelatinase B/type IV collagenase (MMP-9). J Biol Chem. 1997;272:28823–28825. [PubMed: 9360944]
- 158.
- Pozzi A, Moberg PE, Miles LA. et al. Elevated matrix metalloprotease and angiostatin levels in integrin α1 knockout mice cause reduced tumor vascularization. Proc Natl Acad Sci U S A. 2000;97:2202–2207. [PMC free article: PMC15778] [PubMed: 10681423]
- 159.
- O'Reilly MS, Boehm T, Shing Y. et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. [PubMed: 9008168]
- 160.
- Felbor U, Dreier L, Bryant RA. et al. Secreted cathepsin L generates endostatin from collagen XVIII. EMBO J. 2000;19:1187–1194. [PMC free article: PMC305660] [PubMed: 10716919]
- 161.
- Lukashev ME, Werb Z. ECM signalling: orchestrating cell behaviour and misbehaviour. Trends Cell Biol. 1998;8:437–441. [PubMed: 9854310]
- 162.
- Suzuki M, Raab G, Moses MA. et al. Matrix metalloproteinase-3 releases active heparin-binding EGF-like growth factor by cleavage at a specific juxtamembrane site. J Biol Chem. 1997;272:31730–31737. [PubMed: 9395517]
- 163.
- Fowlkes JL, Enghild JJ, Suzuki K. et al. Matrix metalloproteinases degrade insulin-like growth factor-binding protein-3 in dermal fibroblast cultures. J Biol Chem. 1994;269:25742–25746. [PubMed: 7523391]
- 164.
- Levi E, Fridman R, Miao HQ. et al. Matrix metalloproteinase 2 releases active soluble ectodomain of fibroblast growth factor receptor 1. Proc Natl Acad Sci U S A. 1996;93:7069–7074. [PMC free article: PMC38937] [PubMed: 8692946]
- 165.
- Kataoka H, Uchino H, Iwamura T. et al. Enhanced tumor growth and invasiveness in vivo by a carboxyl-terminal fragment of α1proteinase inhibitor generated by matrix metalloproteinases: a possible modulatory role in natural killer cytotoxicity. Am J Pathol. 1999;154:457–468. [PMC free article: PMC1849991] [PubMed: 10027404]
- 166.
- Tlsty TD. Cell-adhesion-dependent influences on genomic instability and carcinogenesis. Curr Opin Cell Biol. 1998;10:647–653. [PubMed: 9818176]
- 167.
- Kauppila S, Saarela J, Stenbäck F. et al. Expression of mRNAs for type I and type III procollagens in serous ovarian cystadenomas and cystadenocarcinomas. Am J Pathol. 1996;148:539–548. [PMC free article: PMC1861689] [PubMed: 8579116]
- 168.
- Stetler-Stevenson WG, Aznavoorian S, Liotta LA. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol. 1993;9:541–573. [PubMed: 8280471]
- 169.
- Murray GI, Duncan ME, O'Neil P. et al. Matrix metalloproteinase-1 is associated with poor prognosis in colorectal cancer. Nat Med. 1996;2:461–462. [PubMed: 8597958]
- 170.
- Murray GI, Duncan ME, O'Neil P. et al. Matrix metalloproteinase-1 is associated with poor prognosis in oesophageal cancer. J Pathol. 1998;185:256–261. [PubMed: 9771478]
- 171.
- Shima I, Sasaguri Y, Kusukawa J. et al. Production of matrix metalloproteinase-2 and metalloproteinase-3 related to malignant behavior of esophageal carcinoma. A clinicopathological study. Cancer. 1992;70:2747–2753. [PubMed: 1451050]
- 172.
- Muller D, Wolf C, Abecassis J. et al. Increased stromelysin 3 gene expression is associated with increased local invasiveness in head and neck squamous cell carcinomas. Cancer Res. 1993;53:165–169. [PubMed: 7677979]
- 173.
- Davidson B, Goldberg I, Kopolovic J. et al. MMP-2 and TIMP-2 expression correlates with poor prognosis in cervical carcinoma---a clinicopathologic study using immunohistochemistry and mRNA in situ hybridization. Gynecol Oncol. 1999;73:372–382. [PubMed: 10366463]
- 174.
- Talvensaari A, Apaja-Sarkkinen M, Höyhtyä M. et al. Matrix metalloproteinase 2 immunoreactive protein appears early in cervical epithelial dedifferentiation. Gynecol Oncol. 1999;72:306–311. [PubMed: 10053100]
- 175.
- Wagner SN, Ockenfels HM, Wagner C. et al. Differential expression of tissue inhibitor of metalloproteinases-2 by cutaneous squamous and basal cell carcinomas. J Invest Dermatol. 1996;106:321–326. [PubMed: 8601735]
- 176.
- Airola K, Ahonen M, Johansson N. et al. Human TIMP-3 is expressed during fetal development, hair growth cycle, and cancer progression. J Histochem Cytochem. 1998;46:437–447. [PubMed: 9524189]
- 177.
- Sutinen M, Kainulainen T, Hurskainen T. et al. Expression of matrix metalloproteinases (MMP-1 and -2) and their inhibitors (TIMP-1, -2 and -3) in oral lichen planus, dysplasia, squamous cell carcinoma and lymph node metastasis. Br J Cancer. 1998;77:2239–2245. [PMC free article: PMC2150416] [PubMed: 9649139]
- 178.
- Polette M, Clavel C, Muller D. et al. Detection of mRNAs encoding collagenase I and stromelysin 2 in carcinomas of the head and neck by in situ hybridization. Invasion Metastasis. 1991;11:76–83. [PubMed: 1655673]
- 179.
- Visscher DW, Höyhtyä M, Ottosen SK. et al. Enhanced expression of tissue inhibitor of metalloproteinase-2 (TIMP-2) in the stroma of breast carcinomas correlates with tumor recurrence. Int J Cancer. 1994;59:339–344. [PubMed: 7927938]
- 180.
- Nuovo GJ, MacDonnell PB, Simsir A. et al. Correlation of the in situ detection of polymerase chain reactionamplified metalloproteinase complementary DNAs and their inhibitors with prognosis in cervical carcinoma. Cancer Res. 1995;55:267–275. [PubMed: 7812956]
- 181.
- Yoshiji H, Gomez DE, Thorgeirsson UP. Enhanced RNA expression of tissue inhibitor of metalloproteinases-1 (TIMP-1) in human breast cancer. Int J Cancer. 1996;69:131–134. [PubMed: 8608981]
- 182.
- Albini A, Melchiori A, Santi L. et al. Tumor cell invasion inhibited by TIMP-2. J Natl Cancer Inst. 1991;83:775–779. [PubMed: 1645772]
- 183.
- DeClerck YA, Yean TD, Chan D. et al. Inhibition of tumor invasion of smooth muscle cell layers by recombinant human metalloproteinase inhibitor. Cancer Res. 1991;51:2151–2157. [PubMed: 2009533]
- 184.
- Tsuchiya Y, Sato H, Endo Y. et al. Tissue inhibitor of metalloproteinase 1 is a negative regulator of the metastatic ability of a human gastric cancer cell line, KKLS, in the chick embryo. Cancer Res. 1993;53:1397–1402. [PubMed: 8443819]
- 185.
- Matsuzawa K, Fukuyama K, Hubbard SL. et al. Transfection of an invasive human astrocytoma cell line with a TIMP-1 cDNA: Modulation of astrocytoma invasive potential. J Neuropathol Exp Neurol. 1996;55:88–96. [PubMed: 8558175]
- 186.
- Alonso DF, Skilton G, DeLorenzo MS. et al. Histopathological findings in a highly invasive mouse mammary carcinoma transfected with human tissue inhibitor of metalloproteinases-1. Oncol Rep. 1998;5:1083–1087. [PubMed: 9683813]
- 187.
- Watanabe M, Takahashi Y, Ohta T. et al. Inhibition of metastasis in human gastric cancer cells transfected with tissue inhibitor of metalloproteinase 1 gene in nude mice. Cancer. 1996;77:1676–1680. [PubMed: 8608561]
- 188.
- Martin DC, Ruther U, Sanchez-Sweatman OH. et al. Inhibition of SV40 T antigen-induced hepatocellular carcinoma in TIMP1 transgenic mice. Oncogene. 1996;13:569–576. [PubMed: 8760297]
- 189.
- Kruger A, Sanchez-Sweatman OH, Martin DC. et al. Host TIMP-1 overexpression confers resistance to experimental brain metastasis of a fibrosarcoma cell line. Oncogene. 1998;16:2419–2423. [PubMed: 9620561]
- 190.
- Montgomery AM, Mueller BM, Reisfeld RA. et al. Effect of tissue inhibitor of the matrix metalloproteinases-2 expression on the growth and spontaneous metastasis of a human melanoma cell line. Cancer Res. 1994;54:5467–5473. [PubMed: 7923181]
- 191.
- Oku T, Ata N, Yonezawa K. et al. Antimetastatic and antitumor effect of a recombinant human tissue inhibitor of metalloproteinases-2 in murine melanoma models. Biol Pharm Bull. 1997;20:843–849. [PubMed: 9300128]
- 192.
- Koop S, Khokha R, Schimdt EE. et al. Overexpression of metalloproteinase inhibitor in B16F10 cells does not affect extravasation but reduces tumor growth. Cancer Res. 1994;54:4791–4797. [PubMed: 8062280]
- 193.
- Wang M, Liu YE, Greene J. et al. Inhibition of tumor growth and metastasis of human breast cancer cells transfected with tissue inhibitor of metalloproteinase 4. Oncogene. 1997;14:2767–2774. [PubMed: 9190892]
- 194.
- Brand K, Baker AH, Perez-Canto A. et al. Treatment of colorectal liver metastases by adenoviral transfer of tissue inhibitor of metalloproteinases-2 into the liver tissue. Cancer Res. 2000;60:5723–5730. [PubMed: 11059766]
- 195.
- Jiang Y, Wang M, Celiker MY. et al. Stimulation of mammary tumorigenesis by systemic tissue inhibitor of matrix metalloproteinase 4 gene delivery. Cancer Res. 2001;61:2365–2370. [PubMed: 11289097]
- 196.
- Celiker MY, Wang M, Atsidaftos E. et al. Inhibition of Wilms' tumor growth by intramuscular administration of tissue inhibitor of metalloproteinases-4 plasmid DNA. Oncogene. 2001;20:4337–4343. [PubMed: 11466614]
- 197.
- Valente P, Fassina G, Melchiori A. et al. TIMP-2 over-expression reduces invasion and angiogenesis and protects B16F10 melanoma cells from apoptosis. Int J Cancer. 1998;75:246–253. [PubMed: 9462715]
- 198.
- Guedez L, Courtemanch L, Stetler-Stevenson M. Tissue inhibitor of metalloproteinase (TIMP)-1 induces differentiation and an antiapoptotic phenotype in germinal center B cells. Blood. 1998;92:1342–1349. [PubMed: 9694723]
- 199.
- Bian J, Wang Y, Smith MR. et al. Suppression of in vivo tumor growth and induction of suspension cell death by tissue inhibitor of metalloproteinases (TIMP)-3. Carcinogenesis. 1996;17:1805–11. [PubMed: 8824499]
- 200.
- Smith MR, Kung HF, Durum SK. et al. TIMP-3 induces cell death by stabilizing TNF-alpha receptors on the surface of human colon carcinoma cells. Cytokine. 1997;9:770–780. [PubMed: 9344510]
- 201.
- Kayagaki N, Kawasaki A, Ebata T. et al. Metalloproteinase-mediated release of human Fas ligand. J Exp Med. 1995;182:1777–1783. [PMC free article: PMC2192231] [PubMed: 7500022]
- 202.
- Amour A, Slocombe PM, Webster A. et al. TNF-alpha converting enzyme (TACE) is inhibited by TIMP-3. FEBS Lett. 1998;435:39–44. [PubMed: 9755855]
- Matrix Metalloproteinases in Cancer Cell Invasion - Madame Curie Bioscience Data...Matrix Metalloproteinases in Cancer Cell Invasion - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...
