NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Introduction
Over the last decade, ceramide has received considerable notoriety as a lipid second messenger that mediates a variety of cell stress responses induced by numerous agonists and environmental stimuli. Although ceramide signaling continues to garner the greatest interest due to its contribution to apoptosis, ceramide production may also stimulate mitogenic signals, promote the activity of growth arrest pathways and induce or inhibit cell differentiation. Not surprisingly, studies on the role of ceramide signaling in the central and peripheral nervous systems have revealed a similar myriad of complex responses. For example, ceramide may induce neuronal apoptosis or protect from apoptosis induced by excitotoxins or growth factor withdrawal,1inhibit axonal growth or promote axonal or dendritic growth,2 inhibit the inwardly rectifying Kcurrent3 or enhance the outward delayed rectifier K current4 and in response to cannabinoids, control metabolic processes or induce apoptosis of glial cells.5
The factors that influence how a cell may respond to ceramide production are slowly being identified. In general, these factors may be classified into specific protein targets,6,7potential influence of ceramide production on lipid domain formation at the site of generation,8–11 and the integrated interaction between enzymes producing ceramide with those controlling its degradation.12 These categories are not mutually exclusive since activation of ceramide-metabolizing enzymes may not only inactivate ceramide, but also activate a “sphingolipid biostat” by enhancing the production of sphingosine-1-phosphate, promoting cell growth and survival.13 Moreover, the site of ceramide production may affect its ability to interact with other lipid constituents and impart biophysical properties specific for particular ceramide-protein interactions.
It is now more or less accepted that most cell types, including neurons and glia, contain specific lipid microdomains that are relatively depleted of phospholipids but enriched in SM, cholesterol, ceramide and glycosphingolipids.14 These domains have a lipid phase behavior, called the liquid-ordered phase, that is intermediate to the more fluid liquid-crystalline or more solid gel phases.15 Although similarities exist in the extension and packing of acyl chains between the gel and liquid-ordered phase, the latter has a greater degree of lateral mobility.16 Two biophysical properties of liquid-ordered domains are their insolubility in non-ionic detergents at low temperature and low buoyant density. Accordingly, isolation of these domains following detergent extraction of cells and centrifugation through sucrose gradients has facilitated the characterization of lipid raft domains.17 Although numerous names have been given to liquid-ordered domains, lipid rafts is a general descriptor that has some consensus. However, specialized detergent-insoluble raft domains also exist. For example, caveolae share a similar lipid composition with lipid rafts but also incorporate the protein caveolin-1.18 In contrast, glycosphingolipid signaling domains have been described that are deficient in SM but still remain insoluble in detergent.19
Importantly, biophysical studies support that ceramide may become concentrated in lipid rafts and actually stabilize domain formation.9,11 These biophysical interactions may underlie the role of ceramide-rich lipid rafts in controlling some aspects of cell signaling.20,21 Further, ceramides can form pores in phospholipid-rich planar membranes.10 This raises the possibility that, in certain membranes in vivo, ceramide may form channels.10
The broad goal of this brief retrospective is to examine if knowledge of ceramide domain/pore formation may help explain or predict the outcome of some aspects of neurotrophin-induced ceramide signaling in axonal growth and cell death. To this end, we will present an overview of recent findings on how the compartmentalized production of ceramide may promote distinct ceramide-lipid, ceramide-protein interactions at the plasma membrane versus internal membranes. However, the reader is also directed to additional reviews on how the biophysical properties of ceramide in phospholipid bilayers may regulate signaling.22,23
Influence of Ceramide on Lipid Raft Domains
The bulk of biological membranes are composed of phospholipids containing relatively unsaturated acyl chains with multiple cis double bonds. In general, phospholipids do not undergo tight packing within the membrane, do not undergo extensive intermolecular hydrogen bonding and are likely to be in the typical liquid-crystalline state.24 In contrast, sphingolipids primarily contain saturated acyl chains or long acyl chains (C22-C24) that contain one double bond. These saturated acyl chains promote tight packing and the presence of both the amide linkage and allylic hydroxyl group as components of the sphingoid backbone may facilitate extensive hydrogen bonding between sphingolipids.25 Although these properties of sphingolipids are critical for contributing to formation of liquid-ordered domains, they are not sufficient.
Detergent-resistant lipid rafts isolated from plasma membranes may contain about 15–33 mol% cholesterol depending upon the cell and developmental stage.11,17,26 Importantly, the detergent-insolubility of lipid rafts is attributed solely to the lipid composition of these domains and is independent of protein components.27 The presence of cholesterol is a critical component for formation of liquid-ordered domains since it selectively promotes tight packing with saturated lipids.9,27–29 It is also important to note that the liquid-ordered phase may form between cholesterol and disaturated molecular species of phosphatidylcholine.30 However, SM and glycosphingolipids are the primary source of these saturated acyl chains in plasma membranes. Interestingly, some sterols such as coprostanol, androstenol and cholesterol sulfate inhibit formation of liquid-ordered domains.29 Consistent with this observation, 4-cholesten-3β-one, an oxidation product of cholesterol, can disrupt the formation of lipid rafts and decrease receptor-linked tyrosine kinase signaling in caveolae by interfering with the ability of the activated receptor to couple to downstream signaling partners.31
Ceramide is also a constitutive component of lipid raft domains. Several studies have reported that the basal amount of ceramide associated with lipid rafts may account for 50–60% of total cellular ceramide.26,32–35Not surprisingly, agonists such as interleukin-1β and tumor necrosis factor-α (TNF) that increase cellular ceramide levels at the plasma membrane may also increase the ceramide content of raft domains.35,36 However, TNF can also induce the hydrolysis of cholesterol-poor pools of SM located outside of liquid-ordered (lipid raft) domains,32 leading to potential differences in the properties of ceramide produced in these distinct membrane regions.
Biophysical studies strongly support that ceramide can form microdomains and/or alter the physical properties of lipid rafts (see Chapter 2). The effects of ceramide, however, are influenced by its acyl chain length, and the presence or absence of cholesterol and phospholipids in raft domains.25,37,38In the absence of cholesterol, ceramide-SM complexes may readily form due to similarities in acyl chain lengths and formation of a hydrogen bond network.11 Indeed, the presence of SM augments the formation of ceramide-enriched microdomains.25 Given that naturally occurring long-chain ceramides have a phase transition temperature above that of SM, a localized change in the SM:cermide ratio may dramatically stabilize the gel state of the local region and induce partitioning from the more fluid and surrounding phospholipids.11 Although ceramide-SM interactions promote ordered phase formation, the properties of these domains are likely to be distinct from those formed in a liquid-ordered phase in the presence of cholesterol.9,29
A recent study of cerebellar granule cells differentiated in vitro for 2–17 days indicates that lipid rafts contain about 1–1.5 mol% ceramide relative to total lipid (phospholipid+sphingolipid+sterol).26,33 Typically, cellular levels of ceramide rise from 2–5 fold depending upon the type of stimulus.39–41 Thus, it would not be unreasonable to expect ceramide levels to range from at least 2–7.5 mol% relative to total lipid in this compartment (this is probably a conservative estimate). Interestingly, addition of 10 mol% bovine brain ceramides to SM-rich membranes containing 33 mol% cholesterol had no effect in increasing acyl chain order of the membrane, i.e., no formation of ceramide-SM complexes.11 In contrast, addition of as little as 3 mol% of non-hydroxylated ceramides significantly stabilized domain formation in SM-phospholipid rich membranes containing 15 mol% cholesterol.9 Thus, the presence of phospholipids within raft domains may increase the ability of ceramide to stabilize domain formation. Indeed, lipid rafts do contain some phospholipid,26,32,33and are important sites for agonist-induced hydrolysis of phosphatidylinositol 4,5-bisphosphate.42,43 Taken together, these data strongly support that 1) ceramide production in lipid rafts can affect the stability of liquid-ordered domains and 2) ceramide can produce its own microdomains (not a liquid-ordered phase) with distinct biophysical properties from the surrounding plasma membrane.
Effect of Ceramides on Sphingomyelin-Cholesterol Poor Membranes
The de novo metabolic route for ceramide production begins with the formation of the sphingoid backbone via the enzyme serine-palmitoyl transferase to form 3-ketosphinganine. Following reduction, sphinganine may then serve as substrate for the enzyme dihydroceramide synthase (often called ceramide synthase).12 Although agonist-induced ceramide production may occur via SM hydrolysis, Kolesnick and colleagues were the first to show that chemotherapeutics may also produce apoptogenic pools of ceramide via activation of ceramide synthase.44 Subsequently, numerous reports have ascribed a role for the de novo pathway in ceramide-mediated biologies. The enzyme ceramide synthase localizes to the endoplasmic reticulum and possibly mitochondria.45 These membranes are rather deficient in SM and cholesterol46 and do not form liquid-ordered domains.47 Depending on the species, purified mitochondrial membranes isolated from adult brain tissue contain approximately 1.9–3.7 mol% SM and about 7 mol% cholesterol48 (both relative to total lipid); plasma membrane values range from 10–20 mol% and 30–40 mol% for SM and cholesterol, respectively.47
The decreased cholesterol and increased phospholipid content of these membranes may have specific consequences on the formation of ceramide-rich domains. Indeed, addition of up to 25 mol% of bovine brain ceramides to model membranes composed of 1-palmitoyl-2-oleoyl phosphatidylcholine (POPC) or 1,2-dipalmitoyl phosphatidylcholine (DPPC) did not result in microdomain formation.11 In contrast, addition of 8–12 mol % bovine brain ceramides to DPPC bilayers resulted in the greatest cluster size (number of molecules) in the ceramide-enriched phase which decreased upon addition of 25–40 mol % ceramide.49 Similarly, addition of 10 mol% bovine brain ceramides to 1,2-dimyristoyl phosphatidylcholine (DMPC) bilayers resulted in ceramide microdomain formation.50 This was attributed to the hydrophobic mismatch between the longer chain acyl species present in bovine brain ceramides and the relatively short myristoyl chain of the DMPC.50 However, this same group further reported that ceramide-enriched microdomains were also formed by SMase treatment of POPC:N-palmitoyl-SM (C16:0 SM) bilayers.25 Although acyl chain mismatch is not as apparent in this system, increased ceramide domain formation may arise from the increased tendency of the newly produced C16:0 ceramide to form a gel phase at 37°C.11Further, a recent biophysical study using mixed lipid monolayer films containing DMPC with either N-palmitoyl-sphingosine (C16:0 ceramide) or N-nervonoyl-sphingosine (C24:1 ceramide) found that the different ceramides created very distinct “domain morphologies”.51 Although it is difficult to directly extrapolate these studies to interactions within more complex biological membranes, collectively, they support that the ability of ceramide to form a microdomain is strongly influenced by the mol% ceramide and the molecular species of the acyl chains in both ceramide and the surrounding phospholipids.
Can we now relate any of the biophysical data to what may occur during de novo synthesis of ceramide in response to an agonist in cholesterol-poor membranes? B-cell receptor crosslinking by anti-IgM antiserum leads to activation-induced cell death via an apoptotic pathway.52 In Burkitt's lymphoma Ramos cells, a critical component of this pathway is the initial de novo synthesis of C16:0 ceramide at 6 hr followed by a latter increase in the formation of a C24:1 ceramide species.53 Importantly, inhibition of de novo ceramide production with fumonisin B1 (a ceramide synthase inhibitor) blocked anti-IgM-induced ceramide production, loss of mitochondrial function and apoptosis.53 This result would suggest that de novo ceramide production within either mitochondrial or microsomal membranes is necessary to induce mitochondrial dysfunction. Based upon the biophysical data presented above, these results raise the possibility that the early production of C16:0 ceramide may initially promote more lateral phase separation leading to disruption of mitochondrial processes, i.e., changes in permeability or increased oxidation.54 However, no evidence exists to strongly support or negate a role of ceramide-domain formation in disrupting mitochondrial function. Alternatively, rapid production of C16:0 ceramide may lead to the formation of a stable channel which has been demonstrated in cholesterol poor-phospholipid rich lipid membranes.10The size of pores composed of C16:0 ceramide is estimated to be large enough to accommodate the release of cytochrome c if they form in the outer mitochondrial membrane.10 In this regard, direct addition of C2-ceramide, which can also form a stable pore,10 to isolated mitochondria induced cytochrome c release.55 A complicating point to this discussion, however, is that de novo ceramide synthesis occurs via the formation of dihydroceramide, a molecule that can not form channels.10 Formation of ceramide requires enzymatic desaturation of the dihydro precursor. Although increases in mitochondrial levels of ceramide have been demonstrated in vivo,56 it remains unclear whether ceramide formation occurs directly in mitochondria, via the action of the microsomal dihydroceramide desaturase57 upon mitochondrial pools of newly formed dihydroceramide, or as a result of transfer from the endoplasmic reticulum. A caveat to the latter possibility is that the half-time for the rate of ceramide transfer between lipid bilayers is on the orders of days.58 Finally, since acyl chain length may influence both ceramide microdomain morphology51 and microdomain formation in phospholipid-rich membranes,11 it would not be unreasonable to expect that increased de novo production of increasingly heterogeneous ceramide species53 may affect domain formation/function and influence ceramide signaling. It will be interesting to determine if addition of more hydrophobic ceramides may affect channel formation by C16:0 ceramide.
Are There Any Relationships between Neurotrophins, Ceramide, Lipid Rafts and Neuronal Biology?
Neurotrophins comprise a family of growth factors that serve varied roles in the development, survival and differentiation of various neuronal populations.59 Neurotrophins mediate their affects via interaction with two receptors, the Trk family of receptor-linked tyrosine kinases and, p75NTR, the common neurotrophin receptor.60 p75NTR activation may contribute prominently to cell stress responses and apoptosis61 via increased ceramide production41,62–66, activation of stress-activated protein kinases,67–69 and modulation of NF-κB activity.70–72 Not surprisingly, p75NTR-dependent ceramide generation has been associated with the induction of apoptosis,73 neuroprotection64,74 and increasing axonal growth.41 Indeed, ceramide can directly increase74,75 or decrease76,77 axonal outgrowth depending, at least in part, upon the concentration and developmental stage of the neuron.74,75
The effect of ceramide on axonal growth may provide an interesting model to explore how lipid rafts and ceramide domain formation may contribute to or affect neurotrophin signaling. For example, increasing ceramide within distal axons of sympathetic neurons inhibits axonal growth.76,77 Interestingly, addition of ceramide to the cell bodies of sympathetic neurons had no effect on axonal elongation since the ceramide was not efficiently transported anterogradely.77 Further, increases in the ceramide content of distal axons inhibited the uptake of NGF but enhanced the activation of Trk A by NGF. This agrees with previous reports that ceramide can inhibit endocytosis78 and enhance both NGF-dependent and NGF-independent activation of Trk A.79 These results raise the possibility that ceramide domain formation may affect neurotrophin activity by inducing Trk A clustering in lipid rafts80 or enhancing clustering of Trk A pools localized outside of lipid rafts.34 Another twist may be that ceramide production via p75NTR may enhance clustering of this receptor (Dremina and Dobrowsky, unpublished observation). Although the role of p75NTR receptor clustering is unknown, this might be associated with the induction of apoptosis via p75NTR.73 It is intriguing to speculate that one role of ceramide production in p75NTR signaling may be to enhance clustering of the receptor and regulate coupling to other signal transduction pathways. It should also be noted that the effect of ceramide on neurotrophin activity may also be influenced by the presence of other protein components of lipid rafts. For example, caveolin-1 can bind to Trk A and inhibit its activation.81 Further, ceramide generated through activation of a lipid-raft-associated A-SMase may also inhibit downstream signaling through tyrosine kinase receptors.82 Interestingly, ceramide enrichment in caveolae was associated with inhibition of phosphatidylinositol 3-kinase (PI 3-kinase) via an interaction with caveolin-1;82 it is also relevant to note that Trk A activation may lead to inhibition of a lipid-raft associated A-SMase via activation of PI 3-kinase.83 Although the above results implicate a role for ceramide domain formation in these cell membranes, the data is not conclusive. The use of sterols which promote or inhibit formation of liquid-ordered domains may help to further define the role of ceramide interactions with these lipid domains and the contribution of this interaction to neurotrophin signaling.
In contrast to the effect of ceramide on inhibiting axonal growth of sympathetic neurons, exogenous ceramide stimulates the early transition of hippocampal neurons from a rounded cell lacking processes (stage 1) to one bearing several short processes of similar length (stage 2). Brann et al41demonstrated that axonal elongation and maturation to stage 3 hippocampal neurons required nerve growth factor-induced ceramide production through p75NTR. Although the effect of ceramide on inducing axonal growth at stage 3 is attributed to its conversion to glucosylceramide,74,75,84 ceramide signaling was suggested to be critical to increasing the stage 1 to stage 2 transition since inhibitors of glucosylceramide production did not block this effect.75 Since stage 3, and presumably earlier stage neurons, lack lipid rafts due to their low SM content,84 these results raise the possibility that ceramide may form distinct and less ordered membrane phases in young neurons versus those possessing lipid rafts (stage 4–5).84 Indeed, low concentrations of ceramide do not promote survival but induce death of stage 4–5 neurons, a time when lipid raft domains are present.74,84 As discussed above, this would correlate with the potential to form more ordered ceramide domains in the membranes. However, if differences in the potential order/stability of a ceramide domain exist at different developmental stages, it remains unknown whether this is a critical factor in affecting how ceramide might differentially couple to differentiation or death signals. However, data does exist indicating that changes in the stability of liquid-ordered domains can influence protein-protein and lipid-protein interactions.18 Thus, it would seem that understanding how ceramide may interact with proteins would facilitate predicting if differences in the potential order/stability of a ceramide domain is relevant to directing the outcome of ceramide signaling.
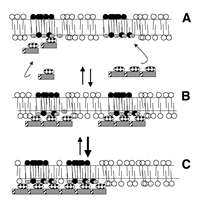
Several proteins, kinase suppressor of ras (KSR), raf-1 and PKC-ζ have been identified to bind ceramide with relatively high affinity.85,86 It is interesting that at least two of these proteins have been localized to lipid raft domains.18 Moreover, since ras also localizes to lipid rafts,18 it will not be surprising to find KSR in these domains. One hypothesis is that high affinity ceramide:protein interactions may occur via a cysteine-rich domain present in raf-1 and ζ. Molecular modeling predicts that this cysteine-rich region may form a cleft for interaction, via hydrogen bonding, with the amide and allylic hydroxyl of ceramide.88 Increased stability of a lipid raft-associated ceramide domain may provide a more competent scaffold for the stereospecific interaction of ceramide with KSR, raf-1 or ζ or possibly protein phosphatases.87 However, no evidence exists that the plethora of ceramide responses in various cells is regulated solely through the interaction of ceramide with high affinity binding proteins. Perhaps an example may also be taken from proteins that interact with other membrane lipids. It is well recognized that proteins containing pleckstrin homology (PH) domains can interact with high affinity to phosphatidylinositol 3,4-bisphosphate and phosphatidylinositol 3,4,5-trisphosphate.89 Some PH-domain containing proteins, however, bind phosphatidylinositol polyphosphates weakly or with low specificity.90 The regulated avidity model for recruitment of low affinity PH-domain containing proteins to membrane regions enriched in phosphatidylinositol polyphosphates implicates the formation of an oligomeric complex of individual proteins whose collective binding energies would be greater than any one individual component.91,92 Although phospholipids exist primarily in a fluid phase, in principle, this general concept should also be applicable to ceramide signaling. If cytosolic proteins that are activated/inhibited by increased ceramide levels interact with ceramide-enriched domains via hydrogen bonding, then it is not hard to envision that formation of an oligomeric protein complex, via phosphorylation or through interaction between protein-protein interaction modules, would have more potential sites for stable interaction. Thus, even a more fluid ceramide-enriched membrane region may be a sufficiently stable scaffold for (or stabilized by) oligomerized/self-associated low affinity binding proteins that collectively provide an increased avidity for the lipid domain (Fig. 1). Since ceramide present in liquid-ordered domains undergoes tighter packing distinct from that of a non-raft SM-ceramide domain,9,11 ceramide generation within lipid rafts may provide some spatial specificity by increasing the stringency of low affinity interactions. If ceramide generation is prolonged, changes in the size or number of ceramide-enriched domains may directly affect the avidity of the complex. Thus, it is conceivable that differences in the avidity of an oligomeric complex for a given ceramide-enriched domain may vary the amplitude and duration of the ceramide signal, even through a common signaling molecule, leading to qualitatively different biological responses.93 This is not unprecedented since transient activation of stress-activated protein kinases may promote cell growth whereas prolonged activation may induce apoptosis.94,95 Further identification of protein structures that may form a “sphingolipid-binding motif” would obviously facilitate exploration of this model.
Conclusions
At this point, our current knowledge of ceramide domain/pore formation can not help explain or predict the outcome of neurotrophin-induced ceramide signaling in the nervous system. However, it is apparent that formation of ceramide-enriched domains seems critical to some aspects of ceramide-mediated apoptosis.20,21 Recent development of a ceramide antibody (15B4 from Alexis) may help facilitate localization of ceramide-enriched domains in neuronal membranes. Future development of approaches to modify formation of these various regions will also facilitate identifying their potential role in signaling. Despite the lack of answers, there is little doubt that ceramide production is a component of neurotrophin signaling and can influence neuronal responses to cytokines and nerve growth factors. Whether domains or pores, determining if differences in phase partitioning in the membrane may direct ceramide signaling will stimulate a lot of neurons.
References
- 1.
- Goswami R, Dawson G. Does ceramide play a role in neural cell apoptosis? J Neurosci Res. 2000;60:141–149. [PubMed: 10740218]
- 2.
- Toman RE, Spiege lS, Faden AI. Role of ceramide in neuronal cell death and differentiation. J Neurotrauma. 2000;17:891–898. [PubMed: 11063055]
- 3.
- Hida H, Takeda M, Soliven B. Ceramide inhibits inwardly rectifying K+ currents via a ras- and raf-1-dependent pathway in cultured oligodendrocytes. J Neurosci. 1998;18:8712–8719. [PMC free article: PMC6793552] [PubMed: 9786978]
- 4.
- Yu SP, Yeh CH, Gottron F. et al. Role of the outward delayed rectifier K+ current in ceramide-induced caspase activation and apoptosis in cultured cortical neurons. J Neurochem. 1999;73:933–941. [PubMed: 10461882]
- 5.
- Guzman M, Galve-Roperh I, Sanchez C. Ceramide: A new second messenger of cannabinoid action. Trends Pharmacol Sci. 2001;22:19–22. [PubMed: 11165667]
- 6.
- Hannun YA. Functions of ceramide in coordinating cellular response to stress. Science. 1996;274:1855–1859. [PubMed: 8943189]
- 7.
- Kolesnick RN, Kronke M. Regulation of ceramide production and apoptosis. Annu Rev Physiol. 1998;60:643–665. [PubMed: 9558480]
- 8.
- Kronkë M. Biophysics of ceramide signaling Interaction with proteins and phase transition of membranes. Chem Phys Lipids. 1999;101:109–121. [PubMed: 10810929]
- 9.
- Xu X, Bittman R, Duportail G. et al. Effect of the structure of natural sterols and sphingolipids on the formation of ordered sphingolipid/sterol domains (rafts).Comparison of cholesterol to plant, fungal, and disease-associated sterols and comparison of sphingomyelin, cerebrosides, and ceramide. J Biol Chem. 2001;276:33540–33546. [PubMed: 11432870]
- 10.
- Siskind LJ, Colombini M. The lipids C2- and C16-ceramide form large stable channels. Implications for apoptosis. J Biol Chem. 2000;275:38640–38644. [PMC free article: PMC2094390] [PubMed: 11027675]
- 11.
- Massey JB. Interaction of ceramides with phosphatidylcholine, sphingomyelin and sphingomyelin/cholesterol bilayers. Biochim Biophys Acta. 2001;1510:167–184. [PubMed: 11342156]
- 12.
- Hannun YA, Luberto C, Argraves KM. Enzymes of sphingolipid metabolism: from modular to integrative signaling. Biochemistry. 2001;40:4893–4903. [PubMed: 11305904]
- 13.
- Spiegel S, Milstien S. Sphingosine-1-phosphate: signaling inside and out. FEBS Lett. 2000;476:55–57. [PubMed: 10878250]
- 14.
- Masserini M, Palestini P, Pitto M. Glycolipid-enriched caveolae and caveolae-like domains in the nervous system. J Neurochem. 1999;73:1–11. [PubMed: 10386949]
- 15.
- Schroeder R, London E, Brown D. Interactions between saturated acyl chains confer detergent resistance on lipids and glycosylphosphatidylinositol (GPI)-anchored proteins:GPI-anchored proteins in liposomes and cells show similar behavior. Proc Natl Acad Sci USA. 1994;91:12130–12134. [PMC free article: PMC45390] [PubMed: 7991596]
- 16.
- Brown DA, London E. Structure and function of sphingolipid-and cholesterol-rich membrane rafts. J Biol Chem. 2000;275:17221–17224. [PubMed: 10770957]
- 17.
- Simons K, van Meer G. Lipid sorting in epithelial cells. Biochemistry. 1988;27:6197–6202. [PubMed: 3064805]
- 18.
- Smart EJ, Graf GA, McNiven MA. et al. Caveolins, liquid-ordered domains, and signal transduction. Mol Cell Biol. 1999;19:7289–7304. [PMC free article: PMC84723] [PubMed: 10523618]
- 19.
- Iwabuchi K, Handa K, Hakomori S. Separation of “glycosphingolipid signaling domain” from caveolin- containing membrane fraction in mouse melanoma B16 cells and its role in cell adhesion coupled with signaling. J Biol Chem. 1998;273:33766–33773. [PubMed: 9837965]
- 20.
- Grassme H, Jekle A, Riehle A. et al. CD95 signaling via ceramide-rich membrane rafts. J Biol Chem. 2001;276:20589–20596. [PubMed: 11279185]
- 21.
- Cremesti A, Paris F, Grassme H. et al. Ceramide enables fas to cap and kill. J Biol Chem. 2001;276:23954–23961. [PubMed: 11287428]
- 22.
- Kolesnick RN, Goni FM, Alonso A. Compartmentalization of ceramide signaling: physical foundations and biological effects. J Cell Physiol. 2000;184:285–300. [PubMed: 10911359]
- 23.
- Venkataraman K, Futerman AH. Ceramide as a second messenger: Sticky solutions to sticky problems. Trends Cell Biol. 2000;10:408–412. [PubMed: 10998592]
- 24.
- Brown DA, London E. Function of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–136. [PubMed: 9891780]
- 25.
- Holopainen JM, Subramanian M, Kinnunen P K J. Sphingomyelinase induces lipid microdomain formation in a fluid phosphatidylcholine/sphingomyelin membrane. Biochemistry. 1998;37:17562–17570. [PubMed: 9860872]
- 26.
- Prinetti A, Chigorno V, Prioni S. et al. Changes in the lipid turnover, composition, and organization, as sphingolipid-enriched membrane domains, in rat cerebellar granule cells developing in vitro. J Biol Chem. 2001;276:21136–21145. [PubMed: 11264283]
- 27.
- Schroeder RJ, Ahmed SN, Zhu Y. et al. Cholesterol and sphingolipid enhance the triton X-100 insolubility of glycosylphosphatidylinositol-anchored proteins by promoting the formation of detergent-insoluble ordered membrane domains. J Biol Chem. 1998;273:1150–1157. [PubMed: 9422781]
- 28.
- Ankaram MB, Thompson TE. Interaction of cholesterol with various glycerophospholipids and sphingomyelin. Biochemistry. 1990;29:10670–10675. [PubMed: 2176878]
- 29.
- Xu X, London E. The effect of sterol structure on membrane lipid domains reveals how cholesterol can induce lipid domain formation. Biochemistry. 2000;39:843–849. [PubMed: 10653627]
- 30.
- Sankaram MB, Thompson TE. Interaction of cholesterol with various glycerophospholipids and sphingomyelin. Biochemistry. 1990;29:10670–10675. [PubMed: 2176878]
- 31.
- Liu P, Wang P, Michaely P. et al. Presence of oxidized cholesterol in caveolae uncouples active platelet-derived growth factor receptors from tyrosine kinase substrates. J Biol Chem. 2000;275:31648–31654. [PubMed: 10918056]
- 32.
- Veldman RJ, Maestre N, Aduib OM. et al. A neutral sphingomyelinase resides in sphingolipid-enriched microdomains and is inhibited by the caveolin-scaffolding domain: Potential implications in tumour necrosis factor signalling. Biochem J. 2001;355:859–868. [PMC free article: PMC1221804] [PubMed: 11311151]
- 33.
- Prinetti A, Chigorno V, Tettamanti G. et al. Sphingolipid-enriched membrane domains from rat cerebellar granule cells differentiated in culture. A compositional study. J Biol Chem. 2000;275:11658–11665. [PubMed: 10766784]
- 34.
- Dobrowsky RT. Sphingolipid signaling domains: floating on rafts or buried in caves? Cell Signal. 2000;12:71–80. [PubMed: 10679576]
- 35.
- Liu P, Anderson R G W. Compartmentalized production of ceramide at the cell surface. J Biol Chem. 1995;270:27179–27185. [PubMed: 7592974]
- 36.
- Grigsby RJ, Dobrowsky RT. Inhibition of ceramide production reverses TNF-induced insulin resistance. Biochem Biophys Res Commun. 2001;287:1121–1124. [PubMed: 11587538]
- 37.
- Wang T -Y, Silvius JR. Different sphingolipids show differential partitioning into sphingolipid/cholesterol-rich domains in lipid bilayers. Biophys j. 79:1478–1489. [PMC free article: PMC1301041] [PubMed: 10969009]
- 38.
- Huang H -W, Goldberg EM, Zidovetzki R. Ceramide induces structural defects into phosphatidylcholine bilayers and activates phospholipase A2. Biochem Biophys Res Commun. 1996;220:834–838. [PubMed: 8607851]
- 39.
- Dbaibo GS, Perry DK, Gamard CJ. et al. Cytokine response modifier A (CrmA) inhibits ceramide formation in response to tumor necrosis factor (TNF)-a : CrmA and Bcl-2 target distinct components in the apoptotic pathway. J Exp Med. 1997;185:481–490. [PMC free article: PMC2196031] [PubMed: 9053448]
- 40.
- Jayadev S, Liu B, Bielawska AE. et al. Role for ceramide in cell cycle arrest. J Biol Chem. 1995;270:2047–2052. [PubMed: 7836432]
- 41.
- Brann AB, Scott R, Neuberger Y. et al. Ceramide signaling downstream of the p75 neurotrophin receptor mediates the effects of nerve growth factor on outgrowth of cultured hippocampal neurons. J Neurosci. 1999;19:8199–8206. [PMC free article: PMC6783007] [PubMed: 10493721]
- 42.
- Pike LJ, Casey L. Localization and turnover of phosphatidylinositol 4,5 bisphosphate in caveolin-enriched membrane domains. J Biol Chem. 1996;271:26453–26456. [PubMed: 8900109]
- 43.
- Pike LJ, Miller JM. Cholesterol depletion delocalizes phosphatidylinositol bisphosphate and inhibits hormone-stimulated phosphatidylinositol turnover. J Biol Chem. 1998;273:22298–22304. [PubMed: 9712847]
- 44.
- Bose R, Verheji M, Haimovitz-Friedman A. et al. Ceramide synthase mediates daunorubicin-induced apoptosis: an alternative mechanism for generating death signals. Cell. 1995;82:405–414. [PubMed: 7634330]
- 45.
- Shimeno H, Soeda S, Yasukouchi M. et al. Fatty acyl-Co A: sphingosine acyltransferase in bovine brain mitochondria: its solubilization and reconstitution onto the membrane lipid liposomes. Biol Pharm Bull. 1995;18:1335–1339. [PubMed: 8593432]
- 46.
- van Meer G. Transport and sorting of membrane lipids. Curr Opin Cell Biol. 1993;5:661–673. [PubMed: 8257608]
- 47.
- Brown DA, London E. Structure and origin of ordered lipid domains in biologic membranes. J Membr Biol. 1998;164:103–114. [PubMed: 9662555]
- 48.
- Rouser G, Kritchevsky G, Yamamoto H. Lipids in the nervous system of different species as a function of age: brain spinal cord, peripheral nerve, purified whole cell preparations, and subcellular particles: regulatory mechanisms and membrane structure. Adv Lipid Res. 1972;10:261–360.
- 49.
- Carrer DC, Maggio B. Phase behavior and molecular interactions in mixtures of ceramide with dipalmitoylphosphatidylcholine. J Lipid Res. 1999;40:1978–1989. [PubMed: 10553001]
- 50.
- Holopainen JM, Lehtonen JY, Kinnunen PK. Lipid microdomains in dimyristoylphosphatidylcholine-ceramide liposomes. Chem Phys Lipids. 1997;88:1–13. [PubMed: 9297850]
- 51.
- Holopainen JM, Brockman HL, Brown RE. et al. Interfacial interactions of ceramide with dimyristoylphosphatidylcholine: impact of the N-acyl chain. Biophys J. 2001;80:765–775. [PMC free article: PMC1301275] [PubMed: 11159444]
- 52.
- Weisner DA, Kilkus JP, Gottschalk AR. et al. Anti-immunoglobulin-induced apoptosis in WEHI 231 cells involves the slow formation of ceramide from sphingomyelin and is blocked by bcl-xL. J Biol Chem. 1997;272:9868–9846. [PubMed: 9092523]
- 53.
- Kroesen BJ, Pettus B, Luberto C. et al. Induction of apoptosis through B-cell receptor cross-linking occurs via de novo generated C16-ceramide and involves mitochondria. J Biol Chem. 2001;276:13606–13614. [PubMed: 11278517]
- 54.
- Gudz TI, Tserng K -Y, Hoppel CL. Direct inhibition of mitochondrial respiratory chain complex III by cell-permeable ceramide. J Biol Chem. 1997;272:24154–24158. [PubMed: 9305864]
- 55.
- Ghafourifar P, Klein SD, Schucht O. et al. Ceramide induces cytochrome c release from isolated mitochondria. Importance of mitochondrial redox state. J Biol Chem. 1999;274:6080–6084. [PubMed: 10037689]
- 56.
- Garcia-Ruiz C, Colell A, Maris M. et al. Direct effects of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species. J Biol Chem. 1997;272:11369–11377. [PubMed: 9111045]
- 57.
- Michel C, van Echten-Deckert G, Rother J. et al. Characterization of ceramide synthesis. A dihydroceramide desaturase introduces the 4,5-trans-double bond of sphingosine at the level of dihydroceramide. J Biol Chem. 1997;272:22432–22437. [PubMed: 9312549]
- 58.
- Simon C G J, Holloway PW, Gear AR. Exchange of C(16)-ceramide between phospholipid vesicles. Biochemistry. 1999;38:14676–14682. [PubMed: 10545193]
- 59.
- Huang EJ, Reichardt LF. Neurotrophins: Roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. [PMC free article: PMC2758233] [PubMed: 11520916]
- 60.
- Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci. 2001;24:1217–1281. [PubMed: 11520933]
- 61.
- Dobrowsky RT, Carter BD. p75 Neurotrophin receptor signaling: Mechanisms for neurotrophic modulation of cell stress? J Neurosci Res. 2000;61:237–243. [PubMed: 10900070]
- 62.
- Dobrowsky RT, Werner MH, Castellino AM. et al. Activation of the sphingomyelin cycle through the low-affinity neurotrophin receptor. Science. 1994;265:1596–1599. [PubMed: 8079174]
- 63.
- Frago LM, Leon Y, de la Rosa EJ. et al. Nerve growth factor and ceramides modulate cell death in the early developing inner ear. J Cell Sci. 1998;111:549–556. [PubMed: 9454729]
- 64.
- Kume T, Nishikawa H, Tomioka H. et al. p75-mediated neuroprotection by NGF against glutamate cytotoxicity in cortical cultures. Brain Res. 2000;852:279–289. [PubMed: 10678754]
- 65.
- Lievremont JP, Sciorati C, Morandi E. et al. The p75(NTR)-induced apoptotic program develops through a ceramide-caspase pathway negatively regulated by nitric oxide. J Biol Chem. 1999;274:15466–15472. [PubMed: 10336437]
- 66.
- Blochl A, Sirrenberg C. Neurotrophins stimulate the release of dopamine from rat mesencephalic neurons via Trk and p75Lntr receptors. J Biol Chem. 1996;271:21100–21107. [PubMed: 8702878]
- 67.
- Cassacia-Bonnefil P, Carter BD, Dobrowsky RT. et al. Nerve growth factor-mediated death of oligodendrocytes by the p75 neurotrophin receptor. Nature. 1996;383:716–719. [PubMed: 8878481]
- 68.
- Bamji SX, Majdan M, Pozniak CD. et al. The p75 neurotrophin receptor mediates neuronal apoptosis and is essential for naturally occurring sympathetic neuron death. J Cell Biol. 1998;140:911–923. [PMC free article: PMC2141754] [PubMed: 9472042]
- 69.
- Wang JJ, Tasinato A, Ethell DW. et al. Phosphorylation of the common neurotrophin receptor p75 by p38beta2 kinase affects NF-kappaB and AP-1 activities. J Mol Neurosci. 2000;15:19–29. [PubMed: 11211234]
- 70.
- Carter BD, Kaltschmidt C, Kaltschmidt B. et al. Selective activation of NF-kB by nerve growth factor through the neurotrophin receptor p75. Science. 1996;272:542–545. [PubMed: 8614802]
- 71.
- Gentry JJ, Casaccia-Bonnefil P, Carter BD. Nerve growth factor activation of NF-kB through its p75 receptor is an anti-apoptotic signal in RN22 schwannoma cells. J Biol Chem. 2000;275:7558–7565. [PubMed: 10713062]
- 72.
- Bhakar AL, Roux PP, Lachance C. et al. The p75 neurotrophin receptor (p75NTR) alters tumor necrosis factor- mediated NF-kappaB activity under physiological conditions, but direct p75NTR-mediated NF-kappaB activation requires cell stress. J Biol Chem. 1999;274:21443–21449. [PubMed: 10409708]
- 73.
- Barker PA. p75NTR: A study in contrasts. Cell Death Differ. 1998;5:346–356. [PubMed: 10200483]
- 74.
- Mitoma J, Ito M, Furuya S. et al. Bipotential roles of ceramide in the growth of hippocampal neurons: promotion of cell survival and dendritic outgrowth in dose- and developmental stage-dependent manners. J Neurosci Res. 1998;51:712–722. [PubMed: 9545085]
- 75.
- Schwarz A, Futerman AH. Distinct roles for ceramide and glucosylceramide at different stages of neuronal growth. J Neurosci. 1997;17:2929–2938. [PMC free article: PMC6573634] [PubMed: 9096129]
- 76.
- Posse de Chaves E, Bussiere M, Vance DE. et al. Elevation of ceramide within distal neurites inhibits neurite growth in cultured rat sympathetic neurons. J Biol Chem. 1997;272:3028–3035. [PubMed: 9006952]
- 77.
- Posse de Chaves E, Bussiere M, MacInnis B. et al. Ceramide inhibits axonal growth and nerve growth factor uptake without compromising the viability of sympathetic neurons. J Biol Chem. 2001;276:36207–36214. [PubMed: 11454862]
- 78.
- Chen CS, Rosenwald AG, Pagano RE. Ceramide as a modulator of endocytosis. J Biol Chem. 1995;270:13291–13297. [PubMed: 7768929]
- 79.
- MacPhee I, Barker PA. Extended ceramide exposure activates the trkA receptor by increasing receptor homodimer formation. J Neurochem. 1999;72:1423–1430. [PubMed: 10098845]
- 80.
- Huang C, Zhou J, Feng AK. et al. Nerve growth factor signaling in caveolae-like domains at the plasma membrane. J Biol Chem. 1999;274:36707–36714. [PubMed: 10593976]
- 81.
- Bilderback TR, Lisanti MP, Dobrowsky RT. Caveolin interacts with Trk A and p75NTR and regulates neurotrophin signaling pathways. J Biol Chem. 1999;274:257–263. [PubMed: 9867838]
- 82.
- Zundel W, Swiersz LM, Giaccia A. Caveolin 1-mediated regulation of receptor tyrosine kinase-associated phosphatidylinositol 3-kinase activity by ceramide. Mol Cell Biol. 2000;20:1507–1514. [PMC free article: PMC85322] [PubMed: 10669728]
- 83.
- Bilderback TR, Gazula V -R, Dobrowsky RT. Phosphoinositide 3-kinase regulates crosstalk between Trk A tyrosine kinase and p75NTR-dependent sphingolipid signaling pathways. J Neurochem. 2001;76:1540–1551. [PubMed: 11238738]
- 84.
- Ledesma MD, Brugger B, Bunning C. et al. Maturation of the axonal plasma membrane requires upregulation of sphingomyelin synthesis and formation of protein-lipid complexes. EMBO J. 1999;18:1761–1771. [PMC free article: PMC1171262] [PubMed: 10202140]
- 85.
- Zhang Y, Yao B, Delikat S. et al. Kinase suppressor of ras is ceramide-activated protein kinase. Cell. 1997;89:63–72. [PubMed: 9094715]
- 86.
- Muller G, Ayoub M, Storz P. et al. PKC z is a molecular switch in signal tranduction of TNF-a, bifunctionally regulated by ceramide and arachidonic acid. EMBO J. 1995;14:1961–1969. [PMC free article: PMC398295] [PubMed: 7744003]
- 87.
- Chalfant CE, Kishikawa K, Mumby MC. et al. Long chain ceramides activate protein phosphatase-1 and protein phosphatase-2A. Activation is stereospecific and regulated by phosphatidic acid. J Biol Chem. 1999;274:20313–20317. [PubMed: 10400653]
- 88.
- van Blitterswijk WJ. Hypothesis: ceramide conditionally activates atypical protein kinases C, raf-1 and KSR through binding to their cysteine-rich domains. Biochem J. 1998;331:679–680. [PMC free article: PMC1219404] [PubMed: 9531568]
- 89.
- Franke TF, Kaplan DK, Cantley LC. et al. Direct regulation of the Akt proto-oncogene product by phosphatidyl-3,4-bisphosphate. Science. 1997;275:665–668. [PubMed: 9005852]
- 90.
- Kavran JM, Klein DE, Lee A. et al. Specificity and promiscuity in phosphoinositide binding by pleckstrin homology domains. J Biol Chem. 1998;273:30497–30508. [PubMed: 9804818]
- 91.
- Lemmon MA, Ferguson KM. Signal-dependent membrane targeting by pleckstrin homology (PH) domains. Biochem J. 2000;350:1–18. [PMC free article: PMC1221219] [PubMed: 10926821]
- 92.
- Mao Y, Nickitenko A, Duan X. et al. Crystal structure of the VHS and FYVE tandem domains of Hrs, a protein involved in membrane trafficking and signal transduction. Cell. 2000;100:447–456. [PubMed: 10693761]
- 93.
- Pawson T, Nash P. Protein-protein interactions define specificity in signal transduction. Genes Dev. 2000;14:1027–1047. [PubMed: 10809663]
- 94.
- Roulston A, Reinhard C, Amiri P. et al. Early activation of c-Jun N-terminal kinase and p38 kinase regulate cell survival in response to tumor necrosis factor alpha. J Biol Chem. 1998;273:10232–10239. [PubMed: 9553074]
- 95.
- Guo YL, Baysal K, Kang B. et al. Correlation between sustained c-Jun N-terminal protein kinase activation and apoptosis induced by tumor necrosis factor-alpha in rat mesangial cells. J Biol Chem. 1998;273:4027–4034. [PubMed: 9461593]
- Neurons, Neurotrophins and Ceramide Signaling: Do Domains and Pores Contribute t...Neurons, Neurotrophins and Ceramide Signaling: Do Domains and Pores Contribute to the Dichotomy? - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...