NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Fibroblasts are a heterogeneous population of structural cells whose primary function is the production of extracellular matrix for normal tissue maintenance and repair. However, fibroblasts provide much more than structural support as they synthesize and respond to many different cytokines and lipid mediators and are intimately involved in the processes of inflammation. It is now appreciated that fibroblasts exhibit phenotypic heterogeneity, differing not only between organ systems, but also within a given anatomical site. Subtypes of fibroblasts can be identified by the expression of markers such as Thy-1, a cell surface glycoprotein of unknown function. Initial characterization of fibroblasts as Thy-1+ or Thy-1- can be performed by immunofluorescence or flow cytometry. They can be sorted according to their expression of Thy-1 by fluorescence-activated cell sorting (FACS), cloning and/or magnetic beading, yielding greater than 99% purity. Fibroblasts that are separated into Thy-1+ and Thy-1- subsets exhibit differences in their morphological, immunological and proliferative responses and ability to differentiate into α-smooth muscle actin-expressing myofibroblasts and adipocyte-like lipofibroblasts, key cells for wound healing and fibrotic disorders. The identification of Thy-1 as a surface marker by which to separate fibroblast subtypes has yielded vital insight into diseases such as scarring and wound healing and highlights the concept of fibroblast heterogeneity. Future research into fibroblast subsets may lead to the tissue-specific treatment of disease such as idiopathic pulmonary fibrosis and Graves' ophthalmopathy.
Introduction
Fibroblasts are a heterogeneous population of structural cells whose primary function is the production of extracellular matrix (ECM) for tissue maintenance and repair.1 Thus, fibroblasts play a pivotal role not only in maintaining tissue integrity, but also in healing processes. They participate in fibrotic (scarring) disorders2 in lung, skin and other tissues.3 Wound healing is a reparative process that results in the restoration of tissue with minimal loss of function.3 Conversely, fibrosis is a response to tissue injury and the ensuing inflammatory response, which ultimately results in abnormal ECM production through the activation of fibroblasts.1 Fibroblasts participate in fibrosis by differentiating into cells called myofibroblasts,4,5 production of ECM and recruitment of lymphocytes to the site of inflammation.6,7 These effector functions are mediated through the production of proinflammatory cytokines and prostaglandins. It is now well-recognized that fibroblasts exhibit phenotypic heterogeneity, differing not only between organ systems but also within an anatomical site. Subtypes of fibroblasts can be identified by morphological and functional characteristics, proliferative potential, biosynthetic capacity and the expression of surface markers such as C1q receptors8 and Thy-1.2,9-12
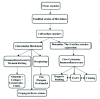
Thy-1 is a 25-kD cell surface glycoprotein belonging to the immunoglobulin-like superfamily. Originally described as a marker of thymocyte differentiation in mice13 it is expressed on subsets of neurons, epidermal cells, lymphocytes,9,14,15 endothelial cells13 and has been found on fibroblasts of all species studied thus far.16,17 Fibroblasts can be derived from tissues such as lung, skin, orbit of the eye, spleen and cornea and can be separated on the basis of Thy-1 surface expression. Following establishment of a primary culture, the fibroblastic phenotype is documented using morphological characteristics and immunohistochemistry. Further characterization of the cells as expressing (Thy-1+) or lacking Thy-1 (Thy-1-) can be performed by immunofluorescence and flow cytometry. They may be sorted according to their expression of Thy-1 by fluorescence-activated cell sorting (FACS) and/or magnetic beading, yielding greater than 99% purity. Once separated, pure Thy-1+ or Thy-1- fibroblast subsets can be propagated. Figure 1 provides an overview of fibroblast derivation and characterization (the reader is referred to ref. 18 for a detailed protocol).
Initial studies characterized fibroblast subsets obtained from the mouse lung9,19 and demonstrated that they possessed identifying morphological characteristics. Thy-1+ fibroblasts are generally spindle-shaped and synthesize large amounts of type I and III collagen. In contrast, Thy-1- fibroblasts are rounded, more spread and synthesize less collagen.19 Induction of collagen synthesis by IL-4 is also greater in Thy-1+ fibroblasts.20 Fibroblasts from several species and varying anatomic regions have been distinguished with respect to the expression of Thy-1.9,11,12,19,21-25 Although its precise function remains enigmatic, it is becoming increasingly clear that the level of Thy-1 expression correlates with distinct morphological and functional characteristics. These may play a role in the initiation and progression of inflammatory diseases such as pulmonary fibrosis, reproductive tract scarring and thyroid-associated ophthalmopathy.
Immunological and Inflammatory Characteristics of Thy-1 Fibroblast Subsets
The activation of fibroblasts by inflammatory stimuli results in their migration, proliferation and deposition of extracellular matrix components, important features involved in both wound healing and fibrosis. The differential expression of Thy-1 on fibroblast subsets may have important immunological consequences due, in part, to the inducible expression of MHC class II molecules, whose primary function is to present antigen to T cells to elicit an immune response. Phipps et al9 initially demonstrated that mouse lung Thy-1-, but not Thy-1+ fibroblasts, upregulated class II MHC molecules when treated with interferon-γ (IFN-γ). Further, coculture of IFN-γ-treated Thy-1- fibroblasts (but not untreated Thy-1- fibroblasts) with T helper cells (in the presence of antigen) results in the proliferation of these T cells. Therefore, the Thy-1- fibroblast subset can present antigen to T cells, strongly suggesting that Thy-1- fibroblasts are involved in chronic lung inflammation and the development of lung fibrosis.9 The ability of IFN-γ to upregulate MHC II molecules only on Thy-1- fibroblasts is not limited to mouse lung fibroblasts. Similar observations have been made in mouse splenic and human orbital fibroblasts where, only Thy-1- fibroblasts are able to upregulate class II MHC.22,26
In addition to the upregulation of MHC class II molecules on specific fibroblast subsets, the differential production of cytokines by Thy-1- versus Thy-1+ fibroblasts has been examined. IL-6 is a cytokine upregulated during inflammation that has been implicated in the pathogenesis of inflammatory conditions such as pulmonary fibrosis27 and Graves' disease. The latter is an autoimmune disease associated with localized manifestations, such as ophthalmopathy.28 Both Thy-1+ and Thy-1- fibroblasts subsets from the human orbit produced IL-6.29 However, Thy-1- fibroblasts isolated from the rat lung exhibited increased levels of IL-6 mRNA in response to IL-1β.24 This differential induction in IL-6 may reflect tissue and/or species-specific fibroblast heterogeneity.
In contrast to the uniform production of IL-6, IL-1β-activated Thy-1- fibroblasts from orbital tissue produce significantly more IL-8 than Thy-1+ cells. IL-8 can activate T cells and neutrophils and is found at sites of tissue injury and chronic inflammation.26,30 Collectively, these studies suggest that Thy-1- fibroblasts can act as antigen presenting cells and participate in the initial recruitment of inflammatory cells such as T cells and monocytes into tissue sites important in the initiation of wound healing and progression to fibrosis.
Another important regulator of inflammation is prostaglandin E2, (PGE2) one of the principal mediators of inflammatory conditions such as interstitial lung disease.31 PGE2, highly produced by fibroblasts, participates in leukocyte recruitment and synergizes with IL-8 for neutrophil recruitment. PGE2 is produced through the action of cyclooxygenase (COX) enzymes, of which there are two isoforms, COX-1 and COX-2. In most tissues, COX-1 is constitutively expressed and participates in tissue homeostasis. Conversely, COX-2 is an immediate-early gene that usually is expressed at very low levels, but is rapidly induced following proinflammatory stimulation with cytokines such as with IL-1β.12,32 In both human orbital26 and myometrial fibroblasts,12 Thy-1+ fibroblasts synthesize more PGE2 following IL-1β treatment than do Thy-1- fibroblasts. Similar results were obtained with fibroblast subsets isolated from the female reproductive tract. Koumas et al12 showed that PGE2 production in the Thy-1- subset was largely unaffected by IL-1β treatment (and therefore very low) whereas production of PGE2 increased two-fold in Thy-1+ cells. It was also found that COX-1 was highly expressed in untreated Thy-1+ fibroblasts and IL-1β upregulated COX-2.
Thy-1+, but not Thy-1-, fibroblasts are also able to upregulate the expression of CD40 following IFN-γ treatment.11 CD40 was originally described as a receptor responsible for the activation and differentiation of B-lymphocytes.3,33,34 CD40 is an ˜50 kDa plasma membrane-spanning receptor and member of the tumour necrosis family (TNF)-α receptor superfamily. It is engaged by its ligand CD154 to promote cell survival, costimulatory protein (e.g., B7-1, B7-2) expression necessary for interaction with T-lymphocytes and B-cell immunoglobulin class switching. CD154 (also called CD40 ligand) up-regulates the synthesis of proinflammatory cytokines (IL-1, IL-6) and chemokines such as IL-8 and monocyte chemoattractant protein-1 (MCP-1) and PGE2.31,35 Thy-1+ myometrial fibroblasts treated with IFNγ and CD154 exhibit significantly increased IL-6, IL-8 and MCP-1 production.11 Interestingly, the expression of CD40 occurred in a bimodal pattern in fibroblast subsets isolated from the human orbit.26 IFN-γ treatment of isolated Thy-1+ and Thy-1- orbital fibroblasts increased CD40 expression in a portion of each subset. Functionally, CD40 ligation led to PGE2 production in both subsets, although Thy-1+ cells were able to produce significantly more PGE2. This observation suggests that the Thy-1+ subset plays an important role in scarring disorders in the orbit of the eye and lung through increased production of PGE2.
Although T cells provide an important source of CD154, it was recently demonstrated that human lung fibroblasts express intracellular CD154 and that fibroblasts strains from patients with idiopathic pulmonary fibrosis express higher levels of CD154 than fibroblasts isolated from normal tissue.3 Moreover, the expression of CD154 was heterogeneous in that there was a fraction of fibroblasts that did not stain positively.3 Although the level of Thy-1 expression was not examined in parallel with CD154 expression in this study, it is possible that the heterogeneous nature of CD154 expression is related to the presence, or absence, of Thy-1. Thus, fibroblasts play an important role in the propagation of inflammatory processes through the autocrine/paracrine actions of the CD40-CD154 system.3
Fibrogenic and Proliferative Characteristics of Thy-1+ and Thy-1- Subsets
Fibroblasts, given the appropriate microenvironment, can acquire some of the characteristics of smooth muscle cells, defined by the expression of α-smooth muscle actin (α-SMA).25 These differentiated fibroblasts, termed “myofibroblasts”, have features that are intermediate to both fibroblasts and smooth muscle cells.1 Although they occur in normal tissue, myofibroblasts are critically important in wound healing and fibrotic disorders4,25,36,37 and are active producers of cytokines and collagen.15 Alterations in fibroblast proliferation and increased density of myofibroblasts have important consequences in idiopathic pulmonary fibrosis, a poorly-treatable fibrotic lung disorder with a five-year mortality of more than 50%.38-41 The ability of fibroblasts to acquire the myofibroblastic phenotype and thus participate in the progression of fibrosis correlates well with Thy-1 expression. We have shown that only human myometrial (fig. 2) and orbital fibroblast subsets expressing Thy-1+ are capable of differentiating to myofibroblasts when treated with transforming growth factor-β (TGF-β),25 a key mediator in fibrosis and derivation of the myofibroblastic phenotype. Interestingly, human myometrial Thy-1+ fibroblasts express low levels of α-SMA constitutively, unlike Thy-1+ fibroblasts from the orbit. This difference in basal α-SMA expression further reflects the tissue-specific fibroblast heterogeneity and may indicate the requirement for myofibroblasts in maintaining the normal architecture and function of the human myometrium.
The results presented in the study by Koumas et al25 are in contrast to a recent study where Zhou and colleagues15 separated rat lung fibroblasts on the basis of Thy-1 expression. They treated subsets with profibrotic cytokines such as IL-1β, IL-4 and platelet-derived growth factor (PDGF). Under basal conditions, Thy-1- fibroblasts expressed more α-SMA than did Thy-1+ cells and when stimulated with these cytokines, Thy-1- cells expressed significantly increased α-SMA levels. It was concluded that, at least in the rat lung, the presence of Thy-1 on the fibroblast surface confers resistance to the myofibroblastic phenotype.15 These findings fully support the concept of tissue and/or species-specific fibroblast heterogeneity where differences in function are linked to anatomical location.
PDGF is an important mitogen and chemoattractant for cells of mesenchymal origin.42 Moreover, its ability to activate fibroblasts appears to be related to the level of Thy-1 expression. For example, PDGF induces a myofibroblast phenotype in Thy-1- rat lung fibroblasts15 through differences in PDGF α-receptors (PDGFR-α).43 There are three isoforms of PDGF; PDGF-AA, PDGF-BB and PDGF-AB- and their biological activity is determined by the expression of PDGFR-α and β-receptors (PDGFR-β).42 Thy-1- cells isolated from the rat lung proliferate in response to PDGF-AA whereas Thy-1+ fibroblasts do not.43 This response correlated with expression levels of PDGFR-α, the receptor that confers the signal of PDGF-AA. In a study by Hagood and colleagues,43 PDGFR-α levels were higher in Thy-1- cells whereas PDGFR-β levels were similar in the two subsets. Further, PDGF-AA increased c-myc mRNA, an indicator of intracellular signaling, in Thy-1- fibroblasts.43
The response to PDGF-AA and PDGFR-α levels in the Thy-1- fibroblasts may result from differential responses to IL-1β, a regulator of PDGFR-α44 and a potent inducer of inflammation. Hagood and colleagues recently reported that IL-1β increases PDGFR-α levels and proliferation in Thy-1- cells and the IL-1 receptor antagonist (IL-1 RA) inhibits these processes.24 Interestingly, other signaling components such as IL-1β binding, activation of p38 MAP kinases and IL receptor subtypes were similar between Thy-1+ and Thy-1- fibroblasts, suggesting a prominent role for the Thy-1- phenotype in lung physiology.24 The discordant proliferative response between Thy-1+ and Thy-1- cells may be due to higher levels of IL-RA levels in Thy-1+ fibroblasts. Although we have found equivalent levels of secreted IL-1 RA from mouse lung fibroblasts,45 it remains possible that rat lung Thy-1- fibroblasts express different levels of IL-1 RA.
Human and rat Thy-1- lung fibroblasts proliferate in response to connective tissue growth factor (CTGF), an important fibroblast activator that works in concert with TGF-β to promote fibrogenesis.46 Not only do Thy-1- fibroblasts respond to CTGF with increased proliferation, but they secrete higher levels of CTGF compared to Thy-1+ cells.47 Collectively, these data suggest that Thy-1-null fibroblasts may be of importance in the development and progression of fibrotic lung disease through their enhanced proliferative and synthetic capacities. Thus, the increased mitogenic response of this fibroblast subset may condition Thy-1- cells to participate in pro-fibrotic responses by becoming activated in the presence of chronic inflammation.24
The cytoskeletal organization and migratory potential of fibroblasts, important in wound healing and fibroproliferation, may also be linked to the level of Thy-1 expression on the cell surface. Barker et al48 recently reported that rat lung fibroblasts exhibited a differential migratory potential. Thy-1- fibroblasts migrated more efficiently into wounds in vitro than Thy-1+ cells, which displayed elongated focal adhesions and actin stress fibers. Increasing the level of Thy-1 in these Thy-1-null fibroblasts, using a mouse Thy-1 expression vector, resulted in fibroblasts that failed to migrate in the wound region, similar to Thy-1+ fibroblasts.48 Thy-1 expression and migratory potential correlated positively with inhibition of Src family kinase, which has been linked to cytoskeletal organization and migration.48
The regulation of focal adhesion assembly in Thy-1+ fibroblasts is dependant on thrombospondin-1 (TSP-1), a protein that mediates the early phases of wound healing by inducing the disassembly of focal adhesions, thereby facilitating fibroblast migration.49 When treated with TSP-1 or the hep I peptide of TSP-1, only Thy-1+ fibroblasts underwent focal adhesion disassembly, an effect mediated through hep-1-induced activation of Src family kinases.49 These finding highlight the importance of Thy-1 in regulating fibroblasts in normal and pathological processes.
Summary and Conclusions
It has become increasingly clear that fibroblasts are capable of providing more than structural support. They maintain a balance between proliferation, migration and the synthesis of immune mediators. Dysregulated inflammation and fibroproliferation underlies many disorders such as idiopathic pulmonary fibrosis. Thy-1-based separation of fibroblast subsets has demonstrated that fibroblast heterogeneity plays an important role in the progression of inflammation and appropriate resolution of wound healing. The production of cytokines by fibroblasts deficient in Thy-1 and upregulation of MHC II molecules and conversely, the generation of prostaglandins and upregulation of CD40 by Thy-1-expressing cells in many tissues highlights the contribution of both fibroblast subsets to the overall inflammatory cascade. However, tissue and species-specific differences in cytokine and prostaglandin production in fibroblast subsets highlight the concept of fibroblast heterogeneity.
Although an endogenous ligand for Thy-1 remains to be identified, recent reports suggest that Thy-1 levels directly correlate with fibroblast proliferative and migratory potential. By using a mouse Thy-1 expression vector, Barker and colleagues48,49 have shown that Thy-1 is essential for proper fibroblast migration. Further, the assembly of focal adhesions by thrombospondin-1 in Thy-1+ fibroblasts occurred through Src kinase activity.49 This represents one of the first reports characterizing an intracellular signaling pathway mediated by Thy-1 expression on fibroblasts. Identifying the function and signaling pathway utilized by Thy-1 should prove important in better understanding the role of fibroblasts in disease. These insights may eventually lead to the tissue-specific treatment of disease such as idiopathic pulmonary fibrosis and Graves' ophthalmopathy.
Grant Support
This work was supported by NIH grants EY011708, EY008976, EY014564, DK63121, HL04492, ES01247, HL75432, P. Harris U.S.A./International and an American Lung Association Fellowship (CJB).
References
- 1.
- White ES, Lazar MH, Thannickal VJ. Pathogenetic mechanisms in usual interstitial pneumonia/idiopathic pulmonary fibrosis. J Pathol. 2003;201(3):343–354. [PMC free article: PMC2810622] [PubMed: 14595745]
- 2.
- Korn JH, Thrall RS, Wilbur DC. et al. Fibroblast heterogeneity: Clonal selection of fibroblasts as a model for fibrotic disease. In: Phipps RP, ed. Pulmonary fibroblast heterogeneity. Boca Raton: CRC Press Inc. 1992:119–133.
- 3.
- Kaufman J, Sime PJ, Phipps RP. Expression of CD154 (CD40 ligand) by human lung fibroblasts: Differential regulation by IFN-gamma and IL-13, and implications for fibrosis. J Immunol. 2004;172(3):1862–1871. [PubMed: 14734771]
- 4.
- Gabbiani G. The role of contractile proteins in wound healing and fibrocontractive diseases. Methods Achiev Exp Pathol. 1979;9:187–206. [PubMed: 763158]
- 5.
- Breen E, Cutroneo KR. Biochemical and molecular aspects of pulmonary fibroblast heterogeneity. In: Phipps RP, ed. Pulmonary fibroblast heterogeneity. Boca Raton: CRC Press Inc. 1992:27–53.
- 6.
- Smith TJ, Sempowski GD, Berenson CS. et al. Human thyroid fibroblasts exhibit a distinctive phenotype in culture: Characteristic ganglioside profile and functional CD40 expression. Endocrinology. 1997;138(12):5576–5588. [PubMed: 9389546]
- 7.
- Smith TJ, Koumas L, Gagnon A. et al. Orbital fibroblast heterogeneity may determine the clinical presentation of thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2002;87(1):385–392. [PubMed: 11788681]
- 8.
- Bordin S, Page RC, Narayanan AS. Heterogeneity of normal human diploid fibroblasts: Isolation and characterization of one phenotype. Science. 1984;223(4632):171–173. [PubMed: 6691142]
- 9.
- Phipps RP, Penney DP, Keng P. et al. Characterization of two major populations of lung fibroblasts: Distinguishing morphology and discordant display of Thy 1 and class II MHC. Am J Respir Cell Mol Biol. 1989;1(1):65–74. [PubMed: 2576218]
- 10.
- McIntosh JC, Hagood JS, Richardson TL. et al. Thy1 (+) and (-) lung fibrosis subpopulations in LEW and F344 rats. Eur Respir J. 1994;7(12):2131–2138. [PubMed: 7536165]
- 11.
- Koumas L, King AE, Critchley HO. et al. Fibroblast heterogeneity: Existence of functionally distinct Thy 1(+) and Thy 1(-) human female reproductive tract fibroblasts. Am J Pathol. 2001;159(3):925–935. [PMC free article: PMC1850439] [PubMed: 11549585]
- 12.
- Koumas L, Phipps RP. Differential COX localization and PG release in Thy-1(+) and Thy-1(-) human female reproductive tract fibroblasts. Am J Physiol Cell Physiol. 2002;283(2):C599–608. [PubMed: 12107070]
- 13.
- Lee WS, Jain MK, Arkonac BM. et al. Thy-1, a novel marker for angiogenesis upregulated by inflammatory cytokines. Circ Res. 1998;82(8):845–851. [PubMed: 9576104]
- 14.
- Froncek MJ, Derdak S, Felch ME. et al. Cellular and molecular characterization of Thy-1+ and Thy-1- murine lung fibroblasts. In: Phipps RP, ed. Pulmonary Fibroblast Heterogeneity. Boca Raton: CRC Press Inc. 1992:135–198.
- 15.
- Zhou Y, Hagood JS, Murphy-Ullrich JE. Thy-1 expression regulates the ability of rat lung fibroblasts to activate transforming growth factor-beta in response to fibrogenic stimuli. Am J Pathol. 2004;165(2):659–669. [PMC free article: PMC1618578] [PubMed: 15277239]
- 16.
- Haeryfar SM, Hoskin DW. Thy-1: More than a mouse pan-T cell marker. J Immunol. 2004;173(6):3581–3588. [PubMed: 15356100]
- 17.
- Pont S. Thy-1: A lymphoid cell subset marker capable of delivering an activation signal to mouse T lymphocytes. Biochimie. 1987;69(4):315–320. [PubMed: 2888493]
- 18.
- Baglole CJ, Reddy SY, Pollock SJ. et al. Phenotypic characterization and isolation of primary fibroblast strains and their derivative subsets. Methods in Molecular Medicine, Fibrosis: Experimental Approaches and Protocols. Totowa: Humana; In Press.
- 19.
- Penney DP, Keng PC, Derdak S. et al. Morphologic and functional characteristics of subpopulations of murine lung fibroblasts grown in vitro. Anat Rec. 1992;232(3):432–443. [PubMed: 1543267]
- 20.
- Sempowski GD, Derdak S, Phipps RP. Interleukin-4 and interferon-gamma discordantly regulate collagen biosynthesis by functionally distinct lung fibroblast subsets. J Cell Physiol. 1996;167(2):290–296. [PubMed: 8613470]
- 21.
- Smith TJ, Sempowski GD, Wang HS. et al. Evidence for cellular heterogeneity in primary cultures of human orbital fibroblasts. J Clin Endocrinol Metab. 1995;80(9):2620–2625. [PubMed: 7673404]
- 22.
- Borrello MA, Phipps RP. Differential Thy-1 expression by splenic fibroblasts defines functionally distinct subsets. Cell Immunol. 1996;173(2):198–206. [PubMed: 8912877]
- 23.
- Phipps RP, Borrello MA, Blieden TM. Fibroblast heterogeneity in the periodontium and other tissues. J Periodontal Res. 1997;32(1 Pt 2):159–165. [PubMed: 9085227]
- 24.
- Hagood JS, Mangalwadi A, Guo B. et al. Concordant and discordant interleukin-1-mediated signaling in lung fibroblast thy-1 subpopulations. Am J Respir Cell Mol Biol. 2002;26(6):702–708. [PubMed: 12034569]
- 25.
- Koumas L, Smith TJ, Feldon S. et al. Thy-1 expression in human fibroblast subsets defines myofibroblastic or lipofibroblastic phenotypes. Am J Pathol. 2003;163(4):1291–1300. [PMC free article: PMC1868289] [PubMed: 14507638]
- 26.
- Koumas L, Smith TJ, Phipps RP. Fibroblast subsets in the human orbit: Thy-1+ and Thy-1- subpopulations exhibit distinct phenotypes. Eur J Immunol. 2002;32(2):477–485. [PubMed: 11813166]
- 27.
- Fries KM, Felch ME, Phipps RP. Interleukin-6 is an autocrine growth factor for murine lung fibroblast subsets. Am J Respir Cell Mol Biol. 1994;11(5):552–560. [PubMed: 7946384]
- 28.
- Prabhakar BS, Bahn RS, Smith TJ. Current perspective on the pathogenesis of Graves' disease and ophthalmopathy. Endocr Rev. 2003;24(6):802–835. [PubMed: 14671007]
- 29.
- Sempowski GD, Rozenblit J, Smith TJ. et al. Human orbital fibroblasts are activated through CD40 to induce proinflammatory cytokine production. Am J Physiol. 1998;274(3 Pt 1):C707–714. [PubMed: 9530102]
- 30.
- Mukaida N. Interleukin-8: An expanding universe beyond neutrophil chemotaxis and activation. Int J Hematol. 2000;72(4):391–398. [PubMed: 11197203]
- 31.
- Zhang Y, Cao HJ, Graf B. et al. CD40 engagement up-regulates cyclooxygenase-2 expression and prostaglandin E2 production in human lung fibroblasts. J Immunol. 1998;160(3):1053–1057. [PubMed: 9570516]
- 32.
- Wang HS, Cao HJ, Winn VD. et al. Leukoregulin induction of prostaglandin-endoperoxide H synthase-2 in human orbital fibroblasts. An in vitro model for connective tissue inflammation. J Biol Chem. 1996;271(37):22718–22728. [PubMed: 8798446]
- 33.
- Kehry MR. CD40-mediated signaling in B cells. Balancing cell survival, growth, and death. J Immunol. 1996;156(7):2345–2348. [PubMed: 8786287]
- 34.
- Noelle RJ. CD40 and its ligand in host defense. Immunity. 1996;4(5):415–419. [PubMed: 8630727]
- 35.
- Cao HJ, Wang HS, Zhang Y. et al. Activation of human orbital fibroblasts through CD40 engagement results in a dramatic induction of hyaluronan synthesis and prostaglandin endoperoxide H synthase-2 expression. Insights into potential pathogenic mechanisms of thyroid-associated ophthalmopathy. J Biol Chem. 1998;273(45):29615–29625. [PubMed: 9792671]
- 36.
- Roy SG, Nozaki Y, Phan SH. Regulation of alpha-smooth muscle actin gene expression in myofibroblast differentiation from rat lung fibroblasts. Int J Biochem Cell Biol. 2001;33(7):723–734. [PubMed: 11390280]
- 37.
- Liu T, Dhanasekaran SM, Jin H. et al. FIZZ1 stimulation of myofibroblast differentiation. Am J Pathol. 2004;164(4):1315–1326. [PMC free article: PMC1615359] [PubMed: 15039219]
- 38.
- Sime PJ, O'Reilly KM. Fibrosis of the lung and other tissues: New concepts in pathogenesis and treatment. Clin Immunol. 2001;99(3):308–319. [PubMed: 11358425]
- 39.
- Moodley YP, Caterina P, Scaffidi AK. et al. Comparison of the morphological and biochemical changes in normal human lung fibroblasts and fibroblasts derived from lungs of patients with idiopathic pulmonary fibrosis during FasL-induced apoptosis. J Pathol. 2004;202(4):486–495. [PubMed: 15095276]
- 40.
- Bjoraker JA, Ryu JH, Edwin MK. et al. Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1998;157(1):199–203. [PubMed: 9445300]
- 41.
- Ramos C, Montano M, Garcia-Alvarez J. et al. Fibroblasts from idiopathic pulmonary fibrosis and normal lungs differ in growth rate, apoptosis, and tissue inhibitor of metalloproteinases expression. Am J Respir Cell Mol Biol. 2001;24(5):591–598. [PubMed: 11350829]
- 42.
- Bonner JC. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev. 2004;15(4):255–273. [PubMed: 15207816]
- 43.
- Hagood JS, Miller PJ, Lasky JA. et al. Differential expression of platelet-derived growth factor-alpha receptor by Thy-1(-) and Thy-1(+) lung fibroblasts. Am J Physiol. 1999;277(1 Pt 1):L218–224. [PubMed: 10409250]
- 44.
- Bonner JC, Lindroos PM, Rice AB. et al. Induction of PDGF receptor-alpha in rat myofibroblasts during pulmonary fibrogenesis in vivo. Am J Physiol. 1998;274(1 Pt 1):L72–80. [PubMed: 9458803]
- 45.
- Silvera MR, Phipps RP. Synthesis of interleukin-1 receptor antagonist by Thy-1+ and Thy-1- murine lung fibroblast subsets. J Interferon Cytokine Res. 1995;15(1):63–70. [PubMed: 7648435]
- 46.
- Watts KL, Spiteri MA. Connective tissue growth factor expression and induction by transforming growth factor-beta is abrogated by simvastatin via a Rho signaling mechanism. Am J Physiol Lung Cell Mol Physiol. 2004;287(6):L1323–L1332. [PubMed: 15298857]
- 47.
- Hagood JS, Lasky JA, Nesbitt JE. et al. Differential expression, surface binding, and response to connective tissue growth factor in lung fibroblast subpopulations. Chest. 2001;120(1 Suppl):64S–66S. [PubMed: 11451929]
- 48.
- Barker TH, Grenett HE, MacEwen MW. et al. Thy-1 regulates fibroblast focal adhesions, cytoskeletal organization and migration through modulation of p190 RhoGAP and Rho GTPase activity. Exp Cell Res. 2004;295(2):488–496. [PubMed: 15093746]
- 49.
- Barker TH, Pallero MA, MacEwen MW. et al. Thrombospondin-1-induced focal adhesion disassembly in fibroblasts requires Thy-1 surface expression, lipid raft integrity, and Src activation. J Biol Chem. 2004;279(22):23510–23516. [PubMed: 15033989]
- Functional Assessment of Fibroblast Heterogeneity by the Cell-Surface Glycoprote...Functional Assessment of Fibroblast Heterogeneity by the Cell-Surface Glycoprotein Thy-1 - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...