Summary
Clinical characteristics.
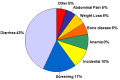
Celiac disease is a systemic autoimmune disease that can be associated with gastrointestinal findings (diarrhea, malabsorption, abdominal pain and distension, bloating, vomiting, and weight loss) and/or highly variable non-gastrointestinal findings (dermatitis herpetiformis, chronic fatigue, joint pain/inflammation, iron deficiency anemia, migraines, depression, attention-deficit disorder, epilepsy, osteoporosis/osteopenia, infertility and/or recurrent fetal loss, vitamin deficiencies, short stature, failure to thrive, delayed puberty, dental enamel defects, and autoimmune disorders). Classic celiac disease, characterized by mild to severe gastrointestinal symptoms, is less common than non-classic celiac disease, characterized by absence of gastrointestinal symptoms.
Diagnosis/testing.
The diagnosis of celiac disease is established in an individual with:
- Positive celiac serologic testing while on a gluten-containing diet (tissue transglutaminase IgA, anti-deamidated gliadin-related peptide IgA and IgG, endomysial antibody IgA),
- Characteristic histologic findings on small-bowel biopsy, and
- Human leukocyte antigen (HLA) haplotype DQ2 or DQ8 identified by molecular genetic testing of HLA-DQA1 and HLA-DQB1.
Management.
Treatment of manifestations: Lifelong adherence to a strict gluten-free diet (avoidance of wheat, rye, and barley); treatment of nutritional deficiencies (iron, zinc, calcium, fat-soluble vitamins, folic acid); standard treatment of osteoporosis. For individuals unresponsive to a gluten-free diet, evaluation for refractory celiac disease, ulcerative enteritis, T-cell lymphoma, and other gastrointestinal cancers.
Surveillance: For symptomatic individuals responsive to a gluten-free diet abnormal serologies should be followed to normalization, periodic physical examination and assessment of growth, nutritional status, and non-gastrointestinal disease manifestations.
Agents/circumstances to avoid: Dietary gluten.
Evaluation of relatives at risk: Molecular genetic testing of first-degree relatives of a proband (including young children) to monitor those with known celiac disease-susceptibility alleles for early evidence of celiac disease in order to institute gluten-free diet early in the disease course.
Genetic counseling.
Celiac disease is a multifactorial disorder resulting from the interaction of HLA-DQA1 and HLA-DQB1 allelic variants known to be associated with celiac disease susceptibility, less well-recognized variants in non-HLA genes, gliadin (a subcomponent of gluten), and other environmental factors. Some empiric risk data for at-risk relatives are available.
Diagnosis
Suggestive Findings
Celiac disease should be suspected in individuals with the following clinical and laboratory findings.
Clinical findings
- Gastrointestinal signs/symptoms (e.g., diarrhea, malabsorption, abdominal pain, distention, bloating, vomiting, weight loss)
- Dermatitis herpetiformis
- Chronic fatigue
- Joint pain/inflammation
- Neurologic symptoms (e.g., peripheral neuropathy, ataxia, seizures, migraines, attention-deficit disorder, poor school performance)
- Osteoporosis/osteopenia
- Infertility and/or recurrent fetal loss
- Short stature and/or failure to thrive
- Delayed puberty
- Dental enamel defects
- Autoimmune disorders
- Individuals with disorders associated with celiac disease (e.g., Down syndrome, Turner syndrome, Williams syndrome, selective IgA deficiency, insulin-dependent diabetes mellitus, Sjögren syndrome, thyroiditis)
- Absence of history of self-limited enteritis and/or tropical sprue
Laboratory findings
- Iron deficiency anemia
- Vitamin and/or mineral deficiencies (e.g., calcium, vitamin D, vitamin B12, folic acid)
- In individuals on a gluten-containing diet:
- Elevated serum tissue transglutaminase (tTG) IgA
- Elevated serum anti-deamidated gliadin-related peptide (a-DGP) IgA and IgG
- Elevated serum endomysial antibody (EMA) IgA; highest specificity (~99%) but sensitivity subject to observer variability
Note on measurement of serum total IgA to evaluate for IgA deficiency: Selective IgA deficiency is more prevalent in individuals with celiac disease (1:50). In individuals with IgA deficiency, measurement of tTG IgG and DGP-IgG should be performed instead of IgA levels.
Establishing the Diagnosis
The diagnosis of celiac disease is established in an individual with positive celiac serologic testing while on a gluten-containing diet (elevated serum tTG IgA, serum a-DGP IgA and IgG, and EMA IgA) by identification of:
- Characteristic histopathology of partial or complete villous atrophy, crypt hyperplasia, and increased intraepithelial lymphocytes identified on four to six duodenal biopsies, while the individual maintains a gluten-containing diet or has resumed a gluten-containing diet for at least two weeks;AND
- Human leukocyte antigen (HLA) haplotype DQ2 or DQ8, as determined by molecular genetic testing of HLA-DQA1 and HLA-DQB1, consistent with an increased risk of celiac disease (see Table 1).
Diagnosis without a duodenal biopsy. Pediatric guidelines from Europe present a pathway for the diagnosis of celiac disease in children that does not include a biopsy. Under these guidelines a diagnosis of celiac disease could be considered in a symptomatic child with a tTG IgA value of more than ten times the upper level of normal, the presence of HLA haplotype DQ2 or DQ8, positive celiac serologic testing on two occasions, and evaluation by a pediatric gastroenterologist [Husby et al 2012]. There is ongoing debate regarding this approach to diagnosis [Reilly et al 2018].
Note: (1) HLA-DQA1 and HLA-DQB1 sequence variants that define haplotypes may be detected by sequencing or by a targeted genotyping assay (methodologies vary). Analysis of HLA genes is complicated by the presence of highly homologous gene family members. (2) Determination of HLA-DQA1 and HLA-DQB1 haplotypes is based on the combination of variants in cis across both genes. Interpretation of sequence data is complicated by detection of uncharacterized sequence variants and uninformative variant zygosity. (3) If haplotype-tag variants from only HLA-DQA1 or HLA-DQB1 are detected, the allele is referred to as a half-DQ2 haplotype (called half DQ-positive). (4) The combination of the haplotypes from homologous chromosomes is called a diplotype. An individual's diplotype at HLA-DQA1 and HLA-DQB1 determines the risk for celiac disease (Table 2).
Table 1.
HLA Haplotypes Associated with an Increased Risk for Celiac Disease
Clinical Characteristics
Clinical Description
Celiac disease is a systemic autoimmune disease with gastrointestinal symptoms and multiple, highly variable non-gastrointestinal symptoms (see Figure 2). It is induced by dietary gluten in genetically susceptible individuals. The onset of celiac disease may occur at any age after weaning; for adults, the peak age of diagnosis is between ages 30 and 50 years. The average time between the onset of symptoms and diagnosis is 11 years. The female-to-male ratio of diagnosed celiac disease is reported to be 3:1.
Gastrointestinal manifestations include chronic or recurrent diarrhea, malabsorption, abdominal pain and distention, bloating, vomiting, and weight loss. As many as 50% of individuals with celiac disease do not have daily diarrhea at the time of diagnosis [Rampertab et al 2006]. Additionally, many are overweight, even obese [Murray et al 2004].
Non-gastrointestinal manifestations include dermatitis herpetiformis, chronic fatigue, joint pain/inflammation, iron deficiency anemia, migraines, depression, attention-deficit disorder, epilepsy, osteoporosis/osteopenia, infertility and/or recurrent fetal loss, vitamin deficiencies, short stature, failure to thrive, delayed puberty, dental enamel defects, abnormal liver function tests, neurologic symptoms, and various secondary autoimmune disorders [Lebwohl et al 2018].
Types of Celiac Disease
Recently a consensus paper redefined the types of celiac disease [Ludvigsson et al 2013].
Classic celiac disease refers to the presence of symptoms of malabsorption such as diarrhea, failure to thrive, and weight loss and may occur in adults and children.
Non-classic celiac disease refers to celiac disease without prominent gastrointestinal symptoms or malabsorption (see Figure 2); however, individuals with atypical celiac disease can also have gastrointestinal symptoms such as reflux, abdominal pain, bloating, vomiting, constipation, and dyspepsia. Approximately 70% of individuals are diagnosed based on extraintestinal manifestations associated with celiac disease. This group includes monosymptomatic and subclinical forms.
Iron deficiency anemia is a common presentation of non-classic celiac disease and may be the only finding.
Dermatitis herpetiformis, an intensely pruritic rash found most commonly on the extensor surfaces of the extremities, is a common non-gastrointestinal manifestation.
Other extraintestinal presentations include osteoporosis/osteopenia, dental enamel hypoplasia, infertility and/or recurrent fetal loss, vitamin deficiencies, abnormal liver function tests (typically elevated transaminases), fatigue, psychiatric syndromes, and various neurologic conditions including peripheral neuropathy, ataxia, seizures, migraines, attention-deficit hyperactivity disorder, and poor school performance.
Non-classic celiac disease usually presents in later childhood or adulthood. Children with non-classic celiac disease can present with unexplained short stature, neurologic symptoms, and delayed puberty.
Non-classic celiac disease is more common than classic celiac disease [Ludvigsson at al 2013].
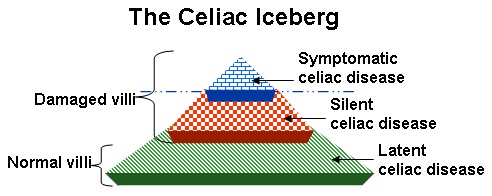
Potential or latent celiac disease. Latent celiac disease is defined as a normal small-bowel biopsy in an individual with a positive celiac disease serology. A subset of such individuals subsequently develop villous atrophy if they continue to ingest gluten; others may remain in this state or celiac serology testing may become negative. Only those with severe symptoms would be advised to start a gluten-free diet [Volta et al 2016] (see Figure 3).
Removal of gluten from the diet can result in:
- Reversal of (a) growth failure and (b) reduced bone mineralization in children with celiac disease;
- Decreased frequency of (a) spontaneous abortions and (b) low-birth-weight infants in women with celiac disease;
- Reduced risk for certain types of cancers including small-intestine adenocarcinoma, esophageal cancer, and non-Hodgkin's lymphoma;
- Reduced risk of mortality in symptomatic individuals.
Refractory sprue/celiac disease (RCD). Refractory sprue or RCD refers to persistence of symptoms of frank malabsorption with persistent intestinal inflammation and villous atrophy despite a strict gluten-free diet for at least six to 12 months. All individuals with refractory sprue are older than age 20 years.
- Primary refractory sprue describes the condition in which individuals have never responded to a gluten-free diet.
- Secondary refractory sprue refers to the condition in which individuals have a full recovery, followed later by a relapse, despite adherence to the gluten-free diet.
An alternate classification for RCD involves the characterization of the intraepithelial lymphocytes (IELs) in persons with RCD:
- In active, uncomplicated celiac disease the IELs have surface expression of CD3 and CD8, a normal occurrence. In addition, these lymphocytes are not clonally restricted (i.e., polyclonal).
- In RCD1, the IELs are normal.
- In RCD2, the IELs are abnormal in the following ways:
- They have lost surface expression of CD3, CD8, and the T-cell receptor.
- CD3 is detectable within the cell.
- They have generally become clonal.
RCD1 is considered to be relatively common. Individuals usually respond to corticosteroids (e.g., budesonide, prednisone).
RCD2 is rare. An international series demonstrated a 30% five-year mortality rate and prognostic factors that determined survival [Rubio-Tapia et al 2016]. Poor survival is typically due to a high rate of progression to enteropathy-associated T-cell lymphoma [Chander et al 2018].
Genotype-Phenotype Correlations
Among affected individuals, no difference in clinical severity of celiac disease is observed between those who are homozygous for the DQ2 haplotype and those who are homozygous for the DQ8 haplotype.
Penetrance
The penetrance of celiac disease is low. The risk of developing celiac disease is affected by HLA diplotype (see Table 2).
Table 2.
Risk of Developing Celiac Disease Based on HLA Diplotype
Prevalence
Celiac disease affects approximately 1% of individuals in the US. Celiac disease is considered to be common in Europe, the US, Australia, Mexico, and some South American countries. The highest reported prevalence of celiac disease is 5.6%, found in a refugee population in North Africa.
The prevalence of celiac disease is increased in individuals with the following disorders [NIH Consensus Committee 2005]:
- Down syndrome (prevalence of celiac disease: 5%-12%)
- Turner syndrome (~3%)
- Williams syndrome (3%-10%)
- Selective IgA deficiency (~2%-10%)
- Insulin-dependent diabetes mellitus (~6%)
- Sjögren syndrome (~5%)
- Autoimmune thyroid disease (~2%-4%)
Genetically Related (Allelic) Disorders
No phenotypes other than those discussed in this GeneReview are known to be associated with these HLA haplotypes.
Differential Diagnosis
The clinical manifestations of celiac disease overlap with several other conditions, including:
- Helicobacter pylori gastritis
- Irritable bowel syndrome
- Inflammatory bowel disease
- Tropical sprue
- Various neurologic syndromes, including myasthesia gravis and peripheral neuropathy
- Drug-induced small-bowel disease (e.g., nonsteroidal anti-inflammatory drugs, olmesartan)
- Small-bowel bacterial overgrowth
Other (non-Celiac) Causes of Gluten Sensitivity
Allergic (wheat allergy). An adverse immunologic (allergic) reaction to proteins in wheat is characterized by production of anti-wheat IgE antibodies. Varieties of wheat allergy include: classic food allergy; wheat-dependent, exercise-induced anaphylaxis (WDEIA); occupational asthma (baker's asthma) and rhinitis; and contact urticaria. A review of wheat allergy is provided by Levy & Levy-Carrick [2014]. Skin prick tests, in vitro IgE assays, and oral food challenges are diagnostic approaches for wheat allergy. Wheat and/or gluten allergy has been reported to have a prevalence of 0.4% in the US. Wheat allergy is not commonly considered in the differential diagnosis of celiac disease though individuals with a wheat allergy do require a gluten-free diet.
Non-celiac gluten or wheat sensitivity. Non-celiac gluten or wheat sensitivity is considered to occur in individuals with symptoms that respond to withdrawal of gluten. Individuals who have non-celiac gluten sensitivity have intolerance to gluten but do not have histologic findings of celiac disease (e.g., characteristic findings on intestinal biopsy) or elevated levels of celiac-specific antibodies (e.g., tTG or EMA IgA) [Lebwohl et al 2015]. Although some may have elevated anti-native gliadin IgG antibodies (AGA), no diagnostic markers are specific for non-celiac, non-allergic gluten sensitivity. The condition is defined by improvement on a gluten-free diet and exclusion of celiac disease and wheat allergy. Non-celiac gluten sensitivity can present with intestinal symptoms (including IBS) and extraintestinal symptoms similar to those of celiac disease [Sapone et al 2011]. The etiology of non-celiac gluten or wheat sensitivity is unclear. One group has described a series of biomarkers that suggest that the condition is associated with altered intestinal permeability [Uhde et al 2019]. In other double-blind placebo-controlled studies, other dietary, non-absorbable sugars (fructans) were shown to be responsible for symptoms in self-identified gluten-sensitive individuals [Skodje et al 2018].
Management
Evaluations Following Initial Diagnosis
To establish the extent of disease in an individual diagnosed with celiac disease, the evaluations summarized in this section (if not performed as part of the evaluation that led to the diagnosis) are recommended:
- Baseline bone-density test in adults to evaluate for osteoporosis/osteopenia. In those with osteoporosis, vitamin D and parathyroid hormone concentrations should be evaluated.
- Screening tests for anemia, abnormal liver function, and nutrient deficiencies (iron, calcium, vitamin D, vitamin B12, folic acid)
- Evaluation for a coexisting autoimmune disease. Because of the frequent association of celiac disease with autoimmune thyroid disease, thyroid function assessment is often performed [Roy et al 2016].
- Failure to regain weight or gastrointestinal bleeding should prompt evaluation for an intestinal malignancy with radiologic and endoscopic studies.
Treatment of Manifestations
Celiac disease. Ideally, the care of a newly diagnosed individual should be provided by a team including a gastroenterologist, primary care physician, and experienced nutritionist; see Figure 4 and Rubio-Tapia et al [2013].

Figure 4.
An approach to the management of newly diagnosed celiac disease [Pietzak 2005] With permission from MM Pietzak, MD
The only treatment for individuals with celiac disease is strict adherence to a gluten-free diet that requires lifelong avoidance of wheat, rye, and barley:
- Treatment with a gluten-free diet should be started only after the diagnosis has been established by intestinal biopsy.
- A dietitian experienced in treating celiac disease should be involved.
- Symptoms may improve rapidly while serologic tests may take up to 12 months to normalize on the gluten-free diet.
- For some individuals, even a small amount of gluten (i.e., 100 mg) can damage the small intestine. Note that a slice of bread contains approximately 2.5 grams of gluten.
- It can be difficult to adhere to the gluten-free diet, as gluten is found in many foods and other ingested products. Some hidden sources of gluten:
- Non-starchy foods such as soy sauce and beer
- Non-food items such as some medications and cosmetics (e.g., lipstick)
- Nutritional deficiencies and metabolic bone disease should be treated in the usual manner.
"Unresponsive celiac disease" refers to celiac disease in individuals who show no improvement on a gluten-free diet:
- The most common reason for unresponsive celiac disease is the presence of small amounts of gluten in the diet. This gluten ingestion may be intentional, such as "cheating" at social events or using communion wafers, or unintentional, including ingestion of gluten in medications and gluten-containing foods in restaurants. Advice from a nutritionist experienced in management of the gluten-free diet is recommended to achieve the best results.
- Assessment for lactose or fructose intolerance is important, as these conditions can be responsible for lack of response to the gluten-free diet [Lebwohl et al 2018].
- Assessment for alternative or additional diagnoses such as microscopic colitis, pancreatic exocrine insufficiency, IBS, small intestinal bacterial overgrowth, and eating disorders is necessary in those in whom gluten contamination is not the explanation.
Refractory sprue or celiac disease. Individuals with persistent symptoms and intestinal inflammation despite adherence to a gluten-free diet may need to be treated with corticosteroids (e.g., systemic steroids or locally active oral budesonide) and immunosuppressants [Green & Jabri 2003, NIH Consensus Committee 2005, Brar et al 2007, Lebwohl et al 2018].
Surveillance
Individuals with celiac disease
- Abnormal celiac disease serologies should be followed to normalization, which usually occurs within six to 12 months of starting a strict gluten-free diet.
- Periodic physical examination and assessment of growth, nutritional status, and non-gastrointestinal disease manifestations
- Follow-up biopsy can be considered to confirm healing of intestinal villi. Because some individuals on a strict gluten-free diet can heal gradually, this should typically be done at least two years after the initial diagnosis.
The most frequently used classification is the Marsh-Oberhuber classification (Table 3) [Oberhuber et al 1999].
Table 3.
Classification of Intestinal Lesions in Celiac Disease
For asymptomatic relatives who have the HLA-DQ2 or HLA-DQ8 celiac disease-susceptibility haplotype on molecular genetic testing and negative antibody results, tTG IgA testing should be performed at three- to five-year intervals to screen for the development of celiac disease-associated antibodies [Husby et al 2012].
Agents/Circumstances to Avoid
Avoid dietary gluten.
Evaluation of Relatives at Risk
Molecular genetic testing of first-degree relatives of a proband for the celiac disease-associated HLA-DQB1/HLA-DQA1 haplotypes DQ2 or DQ8 can identify those who are susceptible to developing celiac disease and who would benefit from serologic testing to screen for celiac disease or silent celiac disease.
Early diagnosis of celiac disease and treatment with a gluten-free diet can prevent secondary complications.
- Individuals who do not have the celiac disease-associated HLA-DQB1/HLA-DQA1 haplotypes DQ2 or DQ8 do not need to undergo serologic screening for celiac disease.
- Individuals who have celiac disease-associated HLA alleles (Table 2) are followed by celiac disease-associated antibody testing at three- to five-year intervals.Small-bowel biopsy is recommended when celiac disease-associated antibody testing is positive.A definitive diagnosis of celiac disease is made in a person with a positive small-bowel biopsy and clinical and/or histologic improvement on a gluten-free diet.
See Genetic Counseling for issues related to testing of at-risk relatives for genetic counseling purposes.
Therapies Under Investigation
Several novel therapeutic approaches that potentially could be used as alternatives for or additives to a gluten-free diet are being investigated (reviewed in Castillo et al [2015] and Rashtak & Murray [2012]):
- Larazotide, a tight junction regulatory peptide that is considered to prevent the passage of gliadin peptides into the mucosa. This drug is in clinical trials.
- Peptides that block the binding groove of DQ2 and DQ8 to prevent activation of gluten-sensitive T cells. The viability of this approach is currently uncertain.
- Transglutaminase (tTG) inhibitors
- Cytokine blockers, particularly for refractory celiac disease. Of particular interest is an anti-IL15 antibody.
- Drugs that selectively inhibit leukocyte adhesion and migration of lymphocytes into inflamed tissues
- Detoxifying gluten, using oral proteases. Latiglutenase, a glutenase, is currently in clinical trials.
- Gluten-sequestering polymers. An oral polymeric resin, P(HEMA-co-SS), binds to gluten and is under study.
- Gluten tolerization. A peptide-based vaccine could desensitize or induce tolerance in individuals with celiac disease. A prototype vaccine, Nexvax2, involving a set of gluten peptides recognized by HLA-DQ2, is in clinical trials.
- Rho/Rho kinase inhibition to theoretically reverse gluten-dependent increase in intestinal permeability [Sollid & Khosla 2011]
- Antibodies to proteins involved in autoimmune pathologies, including anti-IFN-γ, anti-CD3, anti-CD20 therapy, and anti-IL-15. [Sollid & Khosla 2011]
- Another approach: interfering with the homing of gluten-specific T cells to the gut mucosa by using CCR9 antagonists. A Phase II clinical trial is listed in Sollid & Khosla [2011].
Search ClinicalTrials.gov in the US and EU Clinical Trials Register in Europe for information on clinical studies for a wide range of diseases and conditions.
Genetic Counseling
Genetic counseling is the process of providing individuals and families with information on the nature, mode(s) of inheritance, and implications of genetic disorders to help them make informed medical and personal decisions. The following section deals with genetic risk assessment and the use of family history and genetic testing to clarify genetic status for family members; it is not meant to address all personal, cultural, or ethical issues that may arise or to substitute for consultation with a genetics professional. —ED.
Risk to Family Members
In infant feeding studies in children at risk for celiac disease due to the existence of an affected family member, the major risk for the development of celiac disease was the dosage of the genetic risk (see Table 4). This risk is compounded by sex (females are at greater risk) and country of residence, with Sweden having the greatest risk [Liu et al 2014].
- Children who were homozygous for HLA-DQ2 were regarded as high risk; 25.8% who had a double dose of DQ2 developed celiac disease [Lionetti et al 2014]. This high risk for those children who are homozygous for HLA-DQ2 was confirmed in another international study of children at risk for celiac disease due to the presence of these genes.
- Other at-risk HLA-DQ2 or -DQ8 combinations were regarded as low risk / average risk with 15.8% developing celiac disease while those without either HLA-DQ2 or -DQ8 were regarded as having no risk [Lionetti et al 2014].
Note: Risks in Table 4 are increased over those in Table 2 due to the existence of a close affected relative.
Table 4.
Risk of Developing Celiac Disease to Family Members
Related Genetic Counseling Issues
See Management, Evaluation of Relatives at Risk for information on evaluating at-risk relatives for the purpose of early diagnosis and treatment.
DNA banking. Because it is likely that testing methodology and our understanding of genes, allelic variants, and diseases will improve in the future, consideration should be given to banking DNA from probands in whom a molecular diagnosis has not been confirmed (i.e., the causative genetic alteration/s are unknown).
Prenatal Testing
While technically possible, prenatal testing of celiac disease-susceptibility HLA variants is not relevant in this complex disorder because:
- The genetic change is common in the general population;
- The genetic change is predisposing to but not predictive of celiac disease;
- A highly effective treatment is available.
Resources
GeneReviews staff has selected the following disease-specific and/or umbrella support organizations and/or registries for the benefit of individuals with this disorder and their families. GeneReviews is not responsible for the information provided by other organizations. For information on selection criteria, click here.
- Celiac Disease FoundationPhone: 844-593-8169
- MedlinePlus
Molecular Genetics
Information in the Molecular Genetics and OMIM tables may differ from that elsewhere in the GeneReview: tables may contain more recent information. —ED.
Table A.
Celiac Disease: Genes and Databases
Table B.
OMIM Entries for Celiac Disease (View All in OMIM)
Molecular Pathogenesis
Celiac disease is a multifactorial disorder caused by an immune-mediated response to gliadin (a subcomponent of gluten) in genetically susceptible individuals leading to inflammation of the small bowel, villous damage, and resultant malabsorption. Haplotypes across adjacent HLA-DQA1 and HLA-DQB1 known to be associated with celiac disease susceptibility are necessary but not sufficient to cause the disease. The etiology of many of the extraintestinal manifestations has not been fully elucidated. Other genes (many involved in the immune system or in intestinal permeability) and environmental factors are involved.
The Immunologic Mechanisms of Celiac Disease
When working properly, inflammatory mechanisms and immunologic responses in the digestive system provide protection from bacteria, toxins, and other foreign elements in the food and water supply. IgA, made in abundance by the intestinal immune system, is important in local (mucosal) immunity. In celiac disease, inappropriate immune responses lead to chronic inflammation and damage. The two main categories of immune response involved in celiac disease are: the adaptive immune response (HLA-specific) and the innate immune response (independent of HLA type).
Gene structure. HLA-DQA1 and HLA-DQB1 are adjacent MHC class II genes that lie in the HLA gene complex.
Pathogenic variants. The combination of sequence variants across HLA-DQA1 and HLA-DQB1 define the haplotype for the HLA-DQ protein. Due to the proximity of HLA-DQA1 and HLA-DQB1 to other HLA class II genes (e.g., HLA-DR), haplotype blocks may extend beyond these genes.
Normal gene product. The HLA-DQ protein is a heterodimer, encoded by adjacent HLA-DQA1 (encodes the alpha-chain) and HLA-DQB1 (encodes with beta-chain), found on the surface of antigen-presenting cells (APCs).
Abnormal gene product. Variant proteins encoded by haplotypes HLA-DQ2 and HLA-DQ8, referred to as DQ2 and DQ8, are the single most important genetic risk factor in celiac disease susceptibility, with the remainder of disease susceptibility attributed to unknown sequence variants in non-HLA genes. Haplotypes with DQ2-associated variants in either HLA-DQA1 or HLA-DQB1 are referred to as half-DQ2.
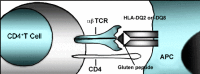
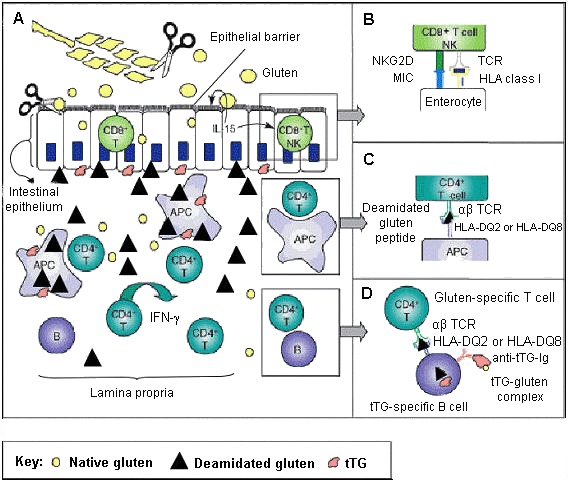
The great majority (>90%) of individuals with celiac disease express at least one copy of DQ2, referred to as being DQ2-positive, and most of the remainder express at least one copy of DQ8, referred to as DQ8-positive (see Table 1 and Table 2). A small percentage express a half-DQ2 variant. DQ2 and DQ8 confer susceptibility to celiac disease by presenting the gliadin subcomponent of gluten to specific CD4+ T-helper cells of the immune system in the intestinal mucosa (see Figure 5 and Figure 6) [Sollid 2002, Sollid & Lie 2005].
The DQ2 at-risk haplotypes include several allelic variants of HLA-DQA1 and HLA-DQB1 that extend into HLA-DR. At-risk individuals:
- Have the DR17-DQ2 celiac disease-susceptibility haplotype [HLA-DRB1*0301;HLA-DQA1*0501;HLA-DQB1*0201];OR
- Are heterozygous for the celiac disease-susceptibility haplotypes DR11 or DR12-DQ7 [HLA-DRB1*11/12;HLA-DQA1*0505;DQB1*0301] or DR7-DQ2 [HLA-DRB1*07;HLA-DQA1*0201;HLA-DQB1*0202].
Individuals with celiac disease who have DQ8 have the DR4-DQ8 celiac disease-susceptibility haplotype (HLA-DRB1*04;HLA-DQA1*03;HLA-DQB1*0302) [Sollid & Lie 2005] (see Figure 1).
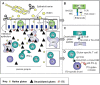
Tissue transglutaminase (tTG), an enzyme found in every tissue of the body, protects the body through wound healing and bone growth. In the intestine, tTG deamidates gliadin, introducing negative charges to the gluten peptides. Both DQ2 and DQ8 preferentially bind these negatively charged deamidated gluten peptides (see Figure 5 and Figure 6). Gluten-reactive T-helper cells (positive for the surface marker CD4) become activated upon recognition of deamidated gluten peptides bound to DQ2 or DQ8 and produce cytokines including interferon gamma (IFN-γ). The ensuing inflammatory response results in the release of additional cytokines and chemicals leading to villous damage and atrophy. In response to inflammation, plasma cells in the inflamed intestinal tissue release antibodies including anti-gliadin and anti-endomysial antibodies and the autoimmune antibody against tTG (see Figure 6) [Treem 2004, Alaedini & Green 2005, Sollid & Lie 2005].
Innate Immune Response
In addition to the adaptive immune response (see Figure 5), an innate response involving intraepithelial CD8+ cytotoxic T lymphocytes (IELs) plays a role in the pathogenesis of celiac disease. In individuals with celiac disease, gluten independently induces epithelial stress through overproduction of interleukin-15 cytokine (IL-15) from IELs. Other stress-response proteins are also induced and confer NK-like properties to CD8+ T cells, which then attack intestinal epithelial cells indiscriminately, leading to intestinal damage [Hüe et al 2004, Jabri et al 2005] (see Figure 6).
References
Literature Cited
- Alaedini A, Green PH. Narrative review: celiac disease: understanding a complex autoimmune disorder. Ann Intern Med. 2005;142:289–98. [PubMed: 15710962]
- Bardella MT, Elli L, Velio P, Fredella C, Prampolini L, Cesana B. Silent celiac disease is frequent in the siblings of newly diagnosed celiac patients. Digestion. 2007;75:182–7. [PubMed: 17848794]
- Brar P, Lee S, Lewis S, Egbuna I, Bhagat G, Green PH. Budesonide in the treatment of refractory celiac disease. Am J Gastroenterol. 2007;102:2265–9. [PubMed: 17581265]
- Castillo NE, Theethira TG, Leffler DA. The present and the future in the diagnosis and management of celiac disease. Gastroenterol Rep (Oxf). 2015;3:3–11. [PMC free article: PMC4324867] [PubMed: 25326000]
- Chander U, Leeman-Neill RJ, Bhagat G. Pathogenesis of enteropathy-associated T cell lymphoma. Curr Hematol Malig Rep. 2018;13:308–17. [PubMed: 29943210]
- Fasano A, Berti I, Gerarduzzi T, Not T, Colletti RB, Drago S, Elitsur Y, Green PH, Guandalini S, Hill ID, Pietzak M, Ventura A, Thorpe M, Kryszak D, Fornaroli F, Wasserman SS, Murray JA, Horvath K. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States. Arch Intern Med. 2003;163:286–92. [PubMed: 12578508]
- Freeman HJ. Risk factors in familial forms of celiac disease. World J Gastroenterol. 2010;16:1828–31. [PMC free article: PMC2856821] [PubMed: 20397258]
- Green PH, Jabri B. Coeliac disease. Lancet. 2003;362:383–91. [PubMed: 12907013]
- Guandalini S. Celiac disease. In: Guandalini S, ed. Textbook of Pediatric Gastroenterology and Nutrition. London, UK: Taylor & Francis Books, Ltd; 2004:435-50.
- Hüe S, Mention JJ, Monteiro RC, Zhang S, Cellier C, Schmitz J, Verkarre V, Fodil N, Bahram S, Cerf-Bensussan N, Caillat-Zucman S. A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity. 2004;21:367–77. [PubMed: 15357948]
- Husby S, Koletzko S, Korponay-Szabó IR, Mearin ML, Phillips A, Shamir R, Troncone R, Giersiepen K, Branski D, Catassi C, Lelgeman M, Maki M, Ribes-Koninckx C, Ventura A, Zimmer KP, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136–60. [PubMed: 22197856]
- Jabri B, Kasarda DD, Green PH. Innate and adaptive immunity: the yin and yang of celiac disease. Immunol Rev. 2005;206:219–31. [PubMed: 16048552]
- Lebwohl B, Ludvigsson JF, Green PH. Celiac disease and non-celiac gluten sensitivity. BMJ. 2015;351:h4347. [PMC free article: PMC4596973] [PubMed: 26438584]
- Lebwohl B, Sanders DS, Green PHR. Coeliac disease. Lancet. 2018;391:70–81. [PubMed: 28760445]
- Levy J, Levy-Carrick N. Wheat allergy. In: Fassano A, ed. Clinical Guide to Gluten-Related Disorders. NASPGHAN Foundation. Philadelphia, PA: Lippincott Williams & Wilkins; 2014.
- Lionetti E, Castellaneta S, Francavilla R, Pulvirenti A, Tonutti E, Amarri S, Barbato M, Barbera C, Barera G, Bellantoni A, Castellano E, Guariso G, Limongelli MG, Pellegrino S, Polloni C, Ughi C, Zuin G, Fasano A, Catassi C, et al. Introduction of gluten, HLA status, and the risk of celiac disease in children. N Engl J Med. 2014;371:1295–303. [PubMed: 25271602]
- Liu E, Lee HS, Aronsson CA, Hagopian WA, Koletzko S, Rewers MJ, Eisenbarth GS, Bingley PJ, Bonifacio E, Simell V, Agardh D, et al. Risk of pediatric celiac disease according to HLA haplotype and country. N Engl J Med. 2014;371:42–9. [PMC free article: PMC4163840] [PubMed: 24988556]
- Lo W, Sano K, Lebwohl B, Diamond B, Green PH. Changing presentation of adult celiac disease. Dig Dis Sci. 2003;48:395–8. [PubMed: 12643621]
- Ludvigsson JF, Leffler DA, Bai JC, Biagi F, Fasano A, Green PH, Hadjivassiliou M, Kaukinen K, Kelly CP, Leonard JN, Lundin KE, Murray JA, Sanders DS, Walker MM, Zingone F, Ciacci C. The Oslo definitions for coeliac disease and related terms. Gut. 2013;62:43–52. [PMC free article: PMC3440559] [PubMed: 22345659]
- Megiorni F, Mora B, Bonamico M, Barbato M, Nenna R, Maiella G, Lulli P, Mazzilli MC. HLA-DQ and risk gradient for celiac disease. Hum Immunol. 2009;70:55–9. [PubMed: 19027045]
- Murray JA, Watson T, Clearman B, Mitros F. Effect of a gluten-free diet on gastrointestinal symptoms in celiac disease. Am J Clin Nutr. 2004;79:669–73. [PubMed: 15051613]
- NIH Consensus Committee. National Institutes of Health consensus development conference statement on celiac disease, June 28-30, 2004. Gastroenterology. 2005;128:S1–9. [PubMed: 15825115]
- Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol 1999;11:1185e94. [PubMed: 10524652]
- Pietzak MM. Follow-up of patients with celiac disease: achieving compliance with treatment. Gastroenterology. 2005;128:S135–41. [PubMed: 15825121]
- Pietzak MM, Schofield TC, McGinnis FM, Nakamura RM. Stratifying risk for celiac disease in a large at-risk United States population by using HLA alleles. Clin Gastroenterol Hepatol. 2009;7:966–71. [PubMed: 19500688]
- Rampertab SD, Pooran N, Brar P, Singh P, Green PH. Trends in the presentation of celiac disease. Am J Med. 2006;119:355.e9–14. [PubMed: 16564784]
- Rashtak S, Murray JA. Review article: Coeliac disease, new approaches to therapy. Aliment Pharmacol Ther. 2012;35:768–81. [PMC free article: PMC3912561] [PubMed: 22324389]
- Reilly NR, Husby S, Sanders DS, Green PHR. Coeliac disease: to biopsy or not? Nat Rev Gastroenterol Hepatol. 2018;15:60–6. [PubMed: 29018278]
- Roy A, Laszkowska M, Sundström J, Lebwohl B, Green PH, Kämpe O, Ludvigsson JF. Prevalence of celiac disease in patients with autoimmune thyroid disease: a meta-analysis. Thyroid. 2016;26:880–90. [PubMed: 27256300]
- Rubio-Tapia A, Hill ID, Kelly CP, Calderwood MD, Murray JA. ACG clinical guidelines Diagnosis and management of celiac disease. Am J Gastroenterol. 2013;108:656–76. [PMC free article: PMC3706994] [PubMed: 23609613]
- Rubio-Tapia A, Malamut G, Verbeek WH, van Wanrooij RL, Leffler DA, Niveloni SI, Arguelles-Grande C, Lahr BD, Zinsmeister AR, Murray JA, Kelly CP, Bai JC, Green PH, Daum S, Mulder CJ, Cellier C. Creation of a model to predict survival in patients with refractory coeliac disease using a multinational registry. Aliment Pharmacol Ther. 2016;44:704–14. [PMC free article: PMC5018234] [PubMed: 27485029]
- Sapone A, Lammers KM, Casolaro V, Cammarota M, Giuliano MT, De Rosa M, Stefanile R, Mazzarella G, Tolone C, Russo MI, Esposito P, Ferraraccio F, Carteni M, Giegler G, de Magistris L, Fasano A. Divergence of gut permeability and mucosal immune gene expression in two gluten-associated conditions: celiac disease and gluten sensitivity. BMC Med. 2011;9:23. [PMC free article: PMC3065425] [PubMed: 21392369]
- Skodje GI, Sarna VK, Minelle IH, Rolfsen KL, Muir JG, Gibson PR, Veierød MB, Henriksen C, Lundin KEA. Fructan, rather than gluten, induces symptoms in patients with self-reported non-celiac gluten sensitivity. Gastroenterology. 2018;154:529–39.e2. [PubMed: 29102613]
- Sollid LM. Coeliac disease: dissecting a complex inflammatory disorder. Nat Rev Immunol. 2002;2:647–55. [PubMed: 12209133]
- Sollid LM, Jabri B. Is celiac disease an autoimmune disorder? Curr Opin Immunol. 2005;17:595–600. [PubMed: 16214317]
- Sollid LM, Khosla C. Novel therapies for coeliac disease. J Intern Med. 2011;269:604–13. [PMC free article: PMC3101315] [PubMed: 21401739]
- Sollid LM, Lie BA. Celiac disease genetics: current concepts and practical applications. Clin Gastroenterol Hepatol. 2005;3:843–51. [PubMed: 16234020]
- Treem WR. Emerging concepts in celiac disease. Curr Opin Pediatr. 2004;16:552–9. [PubMed: 15367850]
- Uhde M, Indart AC, Yu XB, Jang SS, De Giorgio R, Green PHR, Volta U, Vernon SD, Alaedini A. Markers of non-coeliac wheat sensitivity in patients with myalgic encephalomyelitis/chronic fatigue syndrome. Gut. 2019;68:377–8. [PMC free article: PMC6352651] [PubMed: 29550784]
- Volta U, Caio G, Giancola F, Rhoden KJ, Ruggeri E, Boschetti E, Stanghellini V, De Giorgio R. Clin Gastroenterol Hepatol. 2016;14:686–93.e1. [PubMed: 26538207]
Chapter Notes
Acknowledgments
We would like to acknowledge genetic counselor Katie Storm, MS for her contribution to revisions.
We would also like to thank Dr Ludvig Sollid for sharing his knowledge and insight.
Author History
Peter HR Green, MD (2008-present)
Benjamin Lebwohl, MD, MS (2015-present)
Cara L Snyder, MS, CGC (2008-present)
Annette K Taylor, MS, PhD, CGC (2008-present)
Danielle O Young, MS, CGC; Kimball Genetics, Inc (2008-2015)
Revision History
- 31 January 2018 (sw) Comprehensive update posted live
- 17 September 2015 (me) Comprehensive update posted live
- 3 July 2008 (me) Review posted live
- 28 September 2006 (cs) Original submission
Publication Details
Author Information and Affiliations
Publication History
Initial Posting: July 3, 2008; Last Update: January 31, 2019.
Copyright
GeneReviews® chapters are owned by the University of Washington. Permission is hereby granted to reproduce, distribute, and translate copies of content materials for noncommercial research purposes only, provided that (i) credit for source (http://www.genereviews.org/) and copyright (© 1993-2024 University of Washington) are included with each copy; (ii) a link to the original material is provided whenever the material is published elsewhere on the Web; and (iii) reproducers, distributors, and/or translators comply with the GeneReviews® Copyright Notice and Usage Disclaimer. No further modifications are allowed. For clarity, excerpts of GeneReviews chapters for use in lab reports and clinic notes are a permitted use.
For more information, see the GeneReviews® Copyright Notice and Usage Disclaimer.
For questions regarding permissions or whether a specified use is allowed, contact: ude.wu@tssamda.
Publisher
University of Washington, Seattle, Seattle (WA)
NLM Citation
Taylor AK, Lebwohl B, Snyder CL, et al. Celiac Disease. 2008 Jul 3 [Updated 2019 Jan 31]. In: Adam MP, Feldman J, Mirzaa GM, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2024.