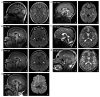
Clinical Description
To date, 40 individuals from 22 families have been identified with biallelic ENTPD1 pathogenic variants [Novarino et al 2014, Travaglini et al 2018, Mamelona et al 2019, Pashaei et al 2021, Calame et al 2022, Ölmez et al 2022]. The following description of the phenotypic features associated with this condition is based on these reports.
All individuals reported to date had onset younger than age five years. All individuals had developmental delay and intellectual disability, and progressive spastic paraplegia with gait impairment as well as abnormal deep tendon reflexes (hypereflexia, hyporeflexia, and/or areflexia). Additional neuromuscular findings can include weakness, neuropathy, and epilepsy. Other common findings are dysarthria, behavior abnormalities, and neurocognitive regression. Less commonly observed clinical findings include scoliosis and cataracts. At the time of this writing, ENTPD1-related neurodevelopmental disorder (ENTPD1-NDD) does not seem to affect life spa,n as affected individuals are known to have reached adulthood.
Onset. All affected individuals to date had onset before age five years. A common first manifestation is delayed walking or other motor and speech delays. First steps typically occur later than the average (i.e., age one year).
Intellectual disability ranges from borderline/mild to moderate/severe with impaired ability to engage independently in activities of daily living, which is not solely related to progressive spastic paraplegia.
Speech and language development are variable. Some individuals never achieve independent expressive language, whereas others communicate expressively with dysarthric speech.
Ability to engage in activities of daily living varies and depends on the level of intellectual disability, with some individuals requiring around-the-clock care.
Progressive spastic paraparesis manifests as weakness and spasticity of the lower extremities. Although individuals with this disorder learn to walk, albeit oftentimes delayed, progressive spastic paraplegia during childhood leads to progressive gait impairment, described as spastic, unsteady, toe walking, and ataxic. Issues with balance and frequent falling are common, potentially resulting in loss of independent ambulation and wheelchair dependence. Wheelchair dependence typically occurs in the teenage to young adult years.
Abnormal deep tendon reflexes include hyperreflexia, hyporeflexia, and areflexia. In the individuals reported to date, areflexia/hyporeflexia is more common than hyperreflexia. Although it is unclear why some individuals develop hyperreflexia and others develop hyporeflexia or areflexia, it could be a combination of uniform upper motor neuron disease with variable lower motor neuron disease (likely resulting from excitotoxicity due to impaired ATP metabolism). Even within the same individual, abnormal reflexes can vary between upper and lower extremities. Additionally, it is unclear if reflexes become progressively more hyperreflexic or hyporeflexic.
Muscle weakness varies. While the proximal lower extremities are most commonly involved, the upper extremities can also be involved. Amyotrophy was noted in some individuals.
Peripheral neuropathy, which can affect upper and lower limbs, manifests as hyporeflexia or areflexia, impaired sensation, and neuropathic pain. Neuropathic findings, including abnormal reflexes, tend to occur in advance of progressive spasticity.
Electromyography / nerve conduction studies revealed findings consistent with motor axonal neuropathy.
Epilepsy. Epilepsy, occurring in some affected individuals, typically starts in early childhood. Seizures are convulsive and respond well to standard anti-seizure medication such as valproic acid. EEG reveals generalized epileptiform activity.
Dysarthria ranges from nasal speech with mild dysarthria to anarthria (i.e., total inability to articulate speech). Individuals with dysarthria often also have dysphagia.
Dysphagia. Difficulty swallowing liquids and solid food with resultant coughing, choking, and aspiration can occur and tends to progress. Rarely, dysphagia requires gastrostomy tube placement.
Behavior abnormalities include attention-deficit/hyperactivity disorder, aggression, anger, impulsivity, and autism spectrum disorder. Behavior abnormalities range from mild/moderate to severe and can affect early childhood school performance if left untreated. Additionally, aggression, anger, impulsivity, and autistic behaviors affect interpersonal relationships and can be disabling.
Neurocognitive regression can manifest as progressively impaired ability to engage independently in activities of daily living, worsening academic performance, and speech and language impairment. Regression was observed in childhood with progressive difficulties with school performance, followed by slow progression into early adulthood. Longitudinal data are currently insufficient to determine if neurocognitive regression continues throughout the life span of affected individuals.
Other findings
Hand and foot deformities most commonly included camptodactyly of the hands and feet and spatulated digits. These deformities can affect day-to-day functioning, including fine and gross motor function.
Pes cavus can be evident early in the disease course and has been reported in young children.
Scoliosis. At this time it is unclear if scoliosis, present in five of 36 individuals, is progressive. Because scoliosis may be a late finding in neuromuscular disorders and because the individuals with ENTPD1-NDD reported to date are mostly children or adolescents, its frequency may be underestimated. There were no known vertebral anomalies. No individuals are known to have undergone surgical intervention.
Cataracts. While seen in approximately 10% of affected individuals, no information is available on cataract type or whether surgical intervention was necessary for any of these individuals.