A new version of this title is available
See the updated version of this chapter
Several classes of successful commercial products are based on isolated or synthetic glycans. This chapter summarizes the use of glycans as vaccines and therapeutics. Applications of glycan mimics as drugs are also discussed.
GLYCANS AS COMPONENTS OF SMALL-MOLECULE DRUGS
Many well-known small-molecule drugs, such as antibiotics and anticancer therapeutic agents, are natural products that contain glycans as part of their core structure and/or as a sugar side chain (i.e., a glycoside). Some examples of natural products that bear glycan side chains are shown in Figure 57.1. The well-established area of natural product chemistry will not be reviewed in detail here. Modified glycans gave rise to synthetic drugs such as small-molecule inhibitors of influenza virus neuraminidase (see also Chapter 55). Recent advances in the functional understanding of carbohydrate–protein interactions have enabled the development of glycomimetics, a new class of small-molecule drugs that are briefly described.

FIGURE 57.1.
Examples of carbohydrate-based drugs. Zanamavir and oseltamivir phosphate are neuraminidase inhibitors and are used to prevent influenza infections, and miglustat is a treatment for type I Gaucher disease. Topiramate is an antiepilepsy drug that has been (more...)
Small-Molecule Inhibitors of Influenza Virus Neuraminidase
Influenza virus has two major surface proteins, hemagglutinin and neuraminidase (see Chapter 34). The hemagglutinin initiates infection by binding to cell-surface sialic acids. The neuraminidase assists virus release by cleaving sialic acids to prevent unwanted retention of newly synthesized virus on the cell surface. Neuraminidase may also function during the invasion phase by removing sialic acids on soluble mucins that would otherwise inhibit cell-surface binding. Because neuraminidase is essential to the viral life cycle, based on the crystal structure of the enzyme a rational drug design program yielded Zanamivir (Relenza). The addition of a bulky guanidino side chain at C-4 of a previously known neuraminidase inhibitor, 2-deoxy-2,3-dehydro-N-acetyl-neuraminic acid (DANA), markedly increased the affinity for influenza neuraminidase, without affecting host-cell neuraminidases (Figure 57.2; see also Chapter 55). Relenza blocks the influenza virus life cycle by preventing infection and by interrupting the spread of the virus during the early phase of an infection. Due to poor oral availability, Relenza has to be inhaled to work at the mucosal sites of infection in the upper airway. The orally available drug Oseltamivir (Tamiflu) achieves the same effects and has taken over most of the market due to the ease of use. The fear of avian influenza virus (“bird flu”) spreading into human populations has prompted stockpiling of Tamiflu. Fortunately, widespread use of the drug has not become necessary to date. The development of Tamiflu is a textbook example for rational drug design resulting in a powerful drug against a devastating disease.
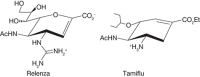
FIGURE 57.2.
The synthetic influenza neuraminidase inhibitors Relenza and Tamiflu.
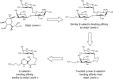
FIGURE 57.3.
Glycomimetic E-selectin inhibitors based on sialyl Lewis x.
TABLE 57.1.
Examples of glycan-based drugs, their target diseases, and modes of action
THERAPEUTIC GLYCOPROTEINS
Most biotherapeutic products are glycoproteins and include erythropoietin as well as various other cytokines, antibodies, glycosyltransferases, and glycosidases. This class of molecules sells in the tens of billions of U.S. dollars per year worldwide. Therapeutic glycoproteins are typically produced as recombinantly in cell culture systems or, less commonly, in the milk of transgenic animals. Control of glycosylation is of major importance during the development of these drugs, because their glycan chains have marked effects on stability, activity, antigenicity, and pharmacodynamics in intact organisms. In most cases, glycosylation must be optimized to ensure prolonged circulatory half-life in the blood. Manipulation of glycans to promote targeting to specific tissues and cell types has also been a useful element of drug design.
Optimizing Glycans of Therapeutic Glycoproteins for Prolonged Serum Half-Life
Erythropoietin (EPO) is the most successful biotechnology product to date. It is a circulating cytokine that binds to the erythropoietin receptor, inducing proliferation and differentiation of erythroid progenitors in the bone marrow. EPO was developed to treat anemias caused by bone marrow suppression after chemotherapy or lack of erythropoietin (e.g., renal failure). Natural and recombinant forms of erythropoietin carry three sialylated complex N-glycans and one sialylated O-glycan. Although in vitro the activity of deglycosylated erythropoietin is comparable to that of the fully glycosylated molecule, its activity in vivo is reduced by ∼90%, because poorly glycosylated erythropoietin is rapidly cleared by filtration in the kidney. Undersialylated erythropoietin is also rapidly cleared by galactose receptors in hepatocytes and macrophages (see Chapter 31). Fully sialylated chains and increased tetra-antennary branching reduces these problems and increases EPO activity in vivo nearly 10-fold. Addition of an N-glycosylation site also increases half-life and activity in vivo. Covalently linking polyethylene glycol to the protein also reduces clearance by the kidney.
Erythropoietin is unusual because it is small enough to be cleared by the kidney if it is underglycosylated. For most glycoprotein therapeutics, a more important consideration is minimizing clearance by galactose-binding hepatic receptors by ensuring full sialylation of glycans. Because glycans greatly influence the efficacy of these drugs, control over glycosylation during production is very important in light of regulatory requirements for batch-to-batch product consistency. Changes in culture pH, the availability of precursors and nutrients, and the presence or absence of various growth factors and hormones can each affect the extent of glycosylation, the degree of branching, and the completeness of sialylation. Sialidases and other glycosidases that are either secreted or released by dead cells can also cause degradation of the previously intact product in the culture medium. These issues were hotly debated in recent years with the advent of “biosimilars” or generic versions of glycoproteins. The need to proof composition has fueled efforts devoted to glycan analysis and sequencing.
Impact of Glycosylation on Licensing, Patentability, and Prolonged Serum Half-Life of Therapeutic Glycoproteins
Patenting of new therapeutics is typically based on the composition of matter in the claimed molecule. Small molecules of defined structure and nonglycosylated proteins are easily captured in this manner. However, glycoproteins, especially those with multiple glycosylation sites, render it virtually impossible to obtain preparations that contain only a single glycoform. Thus, most biotherapeutic glycoproteins consist of a mixture of glycoforms. Licensing bodies allow for a certain range of variation in glycoforms and the complexity of the mixture. However, the manufacturer and the agency must agree on the extent such variation is acceptable for a given drug formulation. Biopharmaceutical companies therefore spend considerable effort in assuring that their products fall within these defined ranges, once these are approved by licensing bodies. The inherent difficulty in reproducing complex glycoform mixtures also complicates efforts to make generic forms of recombinant glycoprotein drugs. Given the complexities of producing glycotherapeutic agents in mammalian cells, even the smallest changes in growth conditions can have significant effects on the range of glycoforms found in any given product batch. The licensing agencies use consistency in glycoform composition as an indirect measure of the quality of process control in production. Differences in glycosylation can have implications for the patentability of agents in which the polypeptide remains constant. Marked differences in glycosylation have been used to define agents as being uniquely different. However, it is usually necessary to show that the differences in glycosylation being claimed also have a significant effect in changing the functionality of the drug in question. The associated pharmaceutical licensing and legal issues are rapidly evolving to keep pace with scientific advances in this area.
GLYCOSYLATION ENGINEERING
There are limits as to how much of a biotherapeutic glycoprotein an animal cell line can produce. Production becomes an issue in cases in which very large amounts of a particular glycoprotein is needed. Glycoprotein production in plants or yeast is attractive but makes it necessary to eliminate risks arising from the nonhuman glycans of plant and fungal cells that could cause excessively rapid clearance and/or antigenic reactions. Many plant and yeast glycans are immunogenic and elicit glycan-specific IgE and IgG antibodies in humans when delivered parenterally. A variety of mammalian genes have been added back into yeast and/or genes that are producing nonhuman glycosylation have been eliminated. Extensively engineered yeast strains are capable of producing biantennary N-linked glycans with the human sialic acid N-acetylneuraminic acid (Neu5Ac) but the productivity of such yeast strains are often low. Efforts to engineer yeast to make human-like O-glycans are under way, but glycosaminoglycans have not yet been addressed.
Plants and algae have also been used to engineer recombinant glycoproteins, but, as in yeast, the glycans produced by plants differ from those found in vertebrates. The antigenic differences that arise in recombinant glycoproteins produced in plants become less problematic if used for topical or oral administration, because humans are normally exposed to plant glycans in the diet. The cost of production is much lower than in animal cell culture systems and animal sera are not needed. As in yeast, “humanizing plants” with respect to glycosylation may allow the production of nonimmunogenic glycoproteins. Chemical methods for synthesizing entire glycoproteins from scratch have been developed and single glycoforms of EPO have been prepared by total synthesis. Given the complexity of glycoprotein synthesis, scale up of these processes is challenging and an area of intense research activity (see Chapter 49).
GLYCAN THERAPEUTIC APPROACHES TO METABOLIC DISEASES
Salvage versus De Novo Synthesis
All monosaccharides needed for cellular glycan synthesis can be obtained from glucose through metabolic interconversions (see Chapter 4). Alternatively, monosaccharides can be derived from the diet or salvaged from degraded glycans. The relative contributions of different sources can vary with the cell type. For instance, even though all mammalian cells use sialic acid, only some contain high amounts of UDP-GlcNAc epimerase/N-acetylmannosamine kinase (GNE), which is required for the de novo synthesis of CMP-sialic acid. But sialic acid salvage from degraded glycans is quite efficient, decreasing the demand on the de novo pathway. Similarly, galactose, fucose, mannose, N-acetylglucosamine, and N-acetylgalactosamine can come from the diet or be salvaged for glycan synthesis, whereas glucuronic acid (GlcA), iduronic acid (IdoA), and xylose cannot. All monosaccharides derived from the diet or degraded glycans can be catabolized for energy, and again, cells vary in their reliance on the different pathways.
The variable contributions of these pathways are important for therapy of some diseases. For instance, patients with congenital disorder of glycosylation type Ib (CDG-Ib), who are deficient in phosphomannose isomerase, benefit greatly from oral mannose supplementation to bypass the insufficient supply of glucose-derived mannose-6-phosphate. A few CDG-IIc patients have been treated with fucose to restore synthesis of sialyl Lewis x on leukocytes (see Chapter 42). Some patients with Crohn's disease show clinical improvement with oral N-acetylglucosamine supplementation, but the mechanism is unknown. Mice deficient in GNE activity have kidney failure, but providing N-acetylmannosamine in the diet prevents this outcome. Clinical trials using N-acetylmannosamine to treat GNE-deficient patients with hereditary inclusion body myopathy type II (HIBM-II) have been conducted but have yielded inconclusive results.
Special Diets
Some monosaccharides and disaccharides can be toxic to humans who lack specific enzymes. For example, people who lack fructoaldolase (aldolase B) accumulate fructose-1-phosphate, which ultimately causes ATP depletion and disrupts glycogen metabolism. Prolonged fructose exposure in these people can be fatal, and fructose-limited diets are critical. Deficiencies in the ability to metabolize galactose (see Chapter 4) are mostly due to a severe reduction in galactose-1-phosphate uridyl transferase activity and cause galactosemia. Although these patients are asymptomatic at birth, ingesting milk leads to vomiting and diarrhea, cataracts, hepatomegaly, and even neonatal death. Low-galactose or galactose-free diets can prevent these life-threatening symptoms. However, even these diets do not prevent unexplained long-term complications, which include speech and learning disabilities and ovarian failure in females with galactosemia.
Infants hydrolyze lactose (Galβ1-4Glc) quite well, but the level of intestinal lactase can be much lower or absent in adults because of down-regulation of lactase gene expression. About two-thirds of the human population has lactase nonpersistence, making milk products a dietary annoyance. Unabsorbed lactose provides an osmotic load and is metabolized by colonic bacteria, causing diarrhea, abdominal bloating, flatulence, and nausea. Lactase persistence has evolved in certain pastoral populations from northwestern Europe, India, and Africa, allowing milk consumption in adult life. However, many adults either avoid lactose-containing foods or use lactase tablets to improve lactose digestion.
Substrate Reduction Therapy
The failure to turn over glycans by lysosomal degradation causes serious problems for patients with lysosomal storage disorders. Deficiencies in individual lysosomal enzymes lead to pathological accumulation of their substrates in inclusion bodies inside the cells (see Chapter 41). One approach to treating these disorders is to inhibit initial glycan synthesis, a strategy termed substrate reduction therapy (SRT). Reduced synthesis of the initial compound decreases the load on the impaired enzyme, and some patients show significant clinical improvement. A small molecule drug used for SRT is N-butyldeoxynojirimycin (or N-butyl-DNJ) (Miglustat, Zavesca), that was approved in 2002 for treatment of Gaucher's disease (glucocerebrosidase deficiency).
Lysosomal Enzyme Replacement Therapy
Another approach for treating lysosomal storage disorders is enzyme replacement therapy (ERT). Unlike most therapeutic glycoproteins that interact with target receptors on the surface of cells, lysosomal enzymes developed for replacement therapy must be delivered intracellularly to lysosomes, their site of action. During the normal biosynthesis of lysosomal enzymes, their N-glycans become modified with mannose-6-phosphate (Man-6-P) residues, which target them to lysosomes using Man-6-P receptors (see Chapter 30). The challenge for ERT is to get the enzymes targeted properly to lysosomes, where they can degrade accumulated substrate. ERT for Gaucher's disease targets the lysosomes of macrophages via the cell-surface mannose receptor (see Chapter 31). The four recombinant enzyme products Imiglucerase (approved in 1995), Velaglucerase (approved in 2010), Taliglucerase alfa (Elelyso, approved in 2012), and Eliglustat (Cerdelga, approved in 2014) are marketed.
The success of glucocerebrosidase treatment stimulated the development of lysosomal enzymes for treatment of other lysosomal storage diseases such as Fabry's disease, mucopolysaccharidoses type I, II, and VI, and Pompe's disease. The replacement therapies clearly have beneficial effects and prolong life but are extremely expensive.
Chaperone Therapy
A third approach for treating lysosomal storage disorders takes advantage of the fact that some genetic defects lead to misfolding of the encoded enzyme in the endoplasmic reticulum (ER). Low-molecular-weight (LMW) competitive inhibitors of some of these enzymes can act as “chaperones” that stabilize the folded enzyme in the ER and effectively rescue the mutation and increase the steady-state concentration of active enzyme in the lysosome. The dose of the inhibitor must be carefully adjusted to ensure that the inhibitory effects on enzyme function do not overshadow beneficial effects on folding. Only a low level of enzyme restoration is needed to significantly reduce the accumulation of undigested glycan substrates, indicating that lysosomal hydrolases are normally present in large catalytic excess.
THERAPEUTIC APPLICATIONS OF GLYCOSAMINOGLYCANS
The use of purified glycans as therapeutics has received less attention than the development of glycoprotein-based treatments. Difficulties in establishing structure–activity relationships due to the large number of chiral centers and functional groups, undesirable pharmacokinetics of available formulations, poor oral absorption of the compounds, and low-affinity interactions with drug targets have limited their development. Some successful glycan drugs, such as the anticoagulant heparin, are given by injection, although efforts are under way to convert heparin into an orally absorbable form by complexing it with positively charged molecules. It may be possible to deliver other hydrophilic and/or negatively charged glycan drugs in this way to allow penetration of the intestinal barrier. Glycans are also sometimes attached to hydrophobic drugs to improve their solubility and alter their pharmacokinetics.
The anticoagulant heparin is, as discussed in Chapters 16 and 43, one of the most widely prescribed drugs today. Heparin binds and activates antithrombin, a protease inhibitor of the coagulation cascade. Antithrombin activation leads to rapid inhibition of thrombin and factor Xa, shutting down the production of fibrin clots. Billions of doses of heparin (several metric tons) is produced by autodigestion of pig intestines, followed by graded fractionation of the products. Unfractionated heparin produces a variable anticoagulant response as it also binds to several plasma, platelet, and endothelial proteins. LMW heparins are derived by chemical or enzymatic cleavage of heparin to form smaller fragments. The pharmacological properties and the relative efficacy of the various LMW heparins are superior to those of unfractionated heparin and fewer secondary complications are reported. LMW heparins have replaced unfractionated heparins as therapeutic of choice in virtually all developed countries. In price-sensitive markets the unfractionated products are still heavily used. The preparation of recombinant heparin based on heparin biosynthesis enzymes is still under development. Arixtra, a synthetic heparin pentasaccharide that binds antithrombin exactly as isolated heparin is used to prevent deep-vein thrombosis and pulmonary embolism and has gained market share in recent years. Still, the higher cost of the synthetic drug Arixtra has prevented an even larger success therapeutic.
To prevent excessive bleeding, rapid neutralization of heparin is desirable. Administration of the basic protein protamine, which binds to heparin, neutralizes its activity, and results in clearance of the complex by the kidney and liver. Heparin is also used to treat protein-losing enteropathy (PLE), likely working by competing for proinflammatory heparin-binding cytokines that trigger PLE in susceptible patients (see Chapter 43).
Hyaluronan (see Chapter 15) is a naturally occurring glycosaminoglycan that is extensively used in surgical applications. Because of its viscoelastic properties, hyaluronan has lubricating and cushioning properties that have made it useful for protecting the corneal endothelium during ocular surgery. Hyaluronan has antiadhesive properties and is useful in postsurgical wound healing. The mechanism of action is not well understood, but it may involve hyaluronan-binding proteins that mediate cell adhesion (see Chapter 15). Intra-articular injections of hyaluronan are used to treat knee and hip osteoarthritis. Modest improvement in patients treated with hyaluronan, may be the result of a mechanical (as a viscosupplement) and/or a biological (via signaling pathways) effect. Hyaluronan is used in very large quantities as a tissue filler in cosmetic medicine.
GLYCONUTRIENTS
“Glyconutrient” is a term used by the nutritional supplement industry to describe some of their products with wide-ranging claims concerning potential benefits. In most cases, these claims have not been substantiated through placebo-controlled, double-blind trials with defined, quantifiable outcomes. Much work is needed in this area to obtain insight into the potential role of dietary glycans on human health and to help consumers make wise decisions regarding their use. Mixtures of plant polysaccharides such as larchbark arabinogalactan and glucomannan are often termed “glyconutrients” that are claimed to contain “essential monosaccharides” needed for “cell communication.” Because all monosaccharides can be made from glucose (except in patients with rare genetic deficiencies; see Chapter 42), none of the other monosaccharides are actually known to be “essential.” Moreover, these polysaccharides are not degraded to available monosaccharides in the stomach or small intestine. Instead, anaerobic bacteria in the colon metabolize them and produce short-chain fatty acids. No peer-reviewed clinical studies support the efficacy of such “glyconutrients” for any disease or condition. Nevertheless, the following examples show how dietary glycans might have beneficial effects.
Glucosamine and Chondroitin Sulfate
Glucosamine (often mixed with chondroitin sulfate [CS]) has been promoted to relieve symptoms of osteoarthritis, which involves the age-dependent erosion of articular cartilage. Cartilage provides a cushion between the bones to minimize mechanical damage, and a net loss of cartilage occurs when the degradation rate exceeds the synthetic rate. A number of clinical trials report that glucosamine improves osteoarthritis symptoms, and some claim to restore partially the structure of the eroded cushion, in particular in the knees. Superficially, this would seem to make sense, because primary glycans of cartilage include hyaluronan (see Chapter 15) and CS, both of which contain hexosamines within their structure (see Chapter 16). Conflicting reports suggest that the outcome may depend on study design and the type and source of material. Nevertheless, veterinarians report positive results after treating animals with glucosamine for more than two decades. Double-blind, placebo-controlled studies in humans have shown a decreased rate of joint space narrowing. Glucosamine might also alter UDP-GlcNAc and potentially UDP-GalNAc levels, thus affecting cellular responses involving major classes of glycans.
Positive effects of CS on osteoarthritis are less well-documented. It remains unclear how the acidic CS polymer can be absorbed and delivered to its proposed site of action. Further studies are needed to determine whether CSs are absorbed by the target tissue, and if they actually lead to changes in cartilage metabolism.
Xylitol and Sorbitol in Chewing Gum
Many studies suggest that chewing gum containing sugar alditols, such as xylitol and sorbitol, can help control the development of dental caries. Mothers who chew xylitol-sweetened gum may even block transmission of caries-causing bacteria to their children. The benefit of these reduced sugars seems to be based on stimulation of salivary flow, but an antimicrobial effect is also possible. Xylitol also inhibits the expression and secretion of proinflammatory cytokines from macrophages and inhibits the growth of Porphyromonas gingivalis, one of the suspected causes of periodontal disease. Children who drank xylitol solutions also had a lower occurrence of otitis media.
Milk Oligosaccharides
Human milk contains about 70 g/L of lactose and 5–10 g/L of free oligosaccharides. More than 130 different glycan species have been identified with lactose at the reducing end, including poly-N-acetyllactosamine units. Some glycans are α2-3- and/or α2-6-sialylated and/or fucosylated in α1-2, α1-3, and/or α1-4 linkages. In contrast, bovine milk, the typical mainstay in human infant formulas, contains much smaller amounts of these glycans. These differences may account for some of the physiological advantages seen for breast-fed versus formula-fed infants. The glycans may also favor growth of a nonpathogenic bifidogenic microflora and/or block pathogen adhesion that causes infections and diarrhea. Surprisingly, a substantial number of human milk oligosaccharides remain almost undigested in the infant's intestine and are excreted intact into the urine. Whether supplementing infant formula with specific, biologically active free glycans enhances infant health is unknown.
GLYCANS AS VACCINE COMPONENTS
Microbial Vaccines
Polysaccharide vaccines consisting solely of glycan components typically elicit poor immunity, especially in infants. Because glycans are T-cell-independent antigens they do not effectively stimulate T-helper-dependent activation and class switching of B-cell-mediated immunity. Conjugate vaccines consisting of glycans coupled to carrier proteins have proven to be highly effective. Three major conjugate vaccines are marketed today: Haemophilus influenzae type b (Hib) causes an acute lower respiratory infection among young children. Children up to this age constitute a high risk group for H. influenzae type B infections. Consequently, the Hib PSV introduced in 1985 was withdrawn from the market in 1988 and replaced by CPS-protein conjugate vaccine formulations. A conjugated form of an Hib-derived oligosaccharide coupled to a protein carrier is part of routine vaccination schedules and has been so successful that infectious diseases caused by this bacterium are nearly eradicated in vaccinated populations. A potent semisynthetic Hib glycoconjugate vaccine marketed in Cuba contains glycan chains with an average length of 16 monosaccharides. Pneumococcal conjugate vaccines have been developed to cover an increasing number of serotypes, and current formulations are 10- (Synflorix, GSK) and 13-valent (Prevnar13, Pfizer). Prevnar 13 provides protection against serotypes that account for >70% of cases of invasive pneumococcal disease worldwide and is the best-selling vaccine exceeding revenues of five billion U.S. dollars in 2015. Conjugate vaccines to protect from Neisseria meningitidis are also very successful on the market. Due to the fast onset and rapid progression of meningococcal infections, vaccination is required to protect against this disease. Several conjugated CPS vaccines are licensed in different parts of the world: the tetravalent serogroup A, C, W, and Y (Menactra, Menveo, and Nimenrix) and a few monovalent vaccines based on serogroup C CPS (Meningitec, Menjugate, NeisVac-C). Two combination vaccines for N. meningitidis and H. influenzae type b (Hib) are available against meningococcal serogroups C/Y (MenHibrix) and against meningococcal serogroup C (Menitorix). A monovalent serogroup A vaccine (MenAfriVac) is widely used in the sub-Saharan meningitis belt of Africa.
Currently, several new vaccines based on synthetic oligosaccharide antigens are being developed to protect children and the elderly from a variety of bacterial infections. Vaccines to protect from hospital acquired infections that are increasingly antibiotic resistant are in preclinical evaluation.
Cancer Vaccines
Several carbohydrate-based cancer vaccines are at different stages of development to treat cancer. Ganglioside immunogens present on certain types of cancer cells, such as gangliosides GM2 and GD2 in melanomas and globo H in breast cancer, are being explored. The shorter glycan sequences such as sialyl-Tn (sialylα2-6GalNAcα-) found on cancer mucins (see Chapter 44) has seen little progress in twenty years. The synthetic GloboH hexasaccharide (see Figure 57.1) resembling the breast and prostate cancer antigen has failed Phase 3 clinical trials in early 2017.
BLOCKING GLYCAN RECOGNITION IN DISEASES
Blocking Infection
As discussed in Chapter 34, many microbes and toxins bind to mammalian tissues by recognizing specific glycan ligands. Thus, small soluble glycans or glycan mimetics can be used to block the initial attachment of microbes and toxins to cell surfaces (or block their release), and thus prevent or suppress infection. Because many of these organisms naturally gain access through the airways or gut, the glycan-based drugs can be delivered directly without being distributed systemically. Milk oligosaccharides are believed to be natural antagonists of intestinal infection in infants (see above) and polymers that will block the binding of viruses such as influenza. Although backed by a strong scientific rationale and robust in vitro studies, such “antiadhesive” therapies have not yet found much practical application.
Inhibition of Selectin-Mediated Leukocyte Trafficking
When specific glycan–protein interactions are responsible for selective cell–cell interactions and a resulting pathology, then administration of small-molecule glycomimetics of the natural ligand is a useful means of intervention. Selectin-mediated recruitment of neutrophils and other leukocytes into sites of inflammation or ischemia/reperfusion injury involves specific selectin–glycan interactions in the vascular system (see Chapter 31). The use of sialyl Lewis x tetrasaccharide derivatives failed due to poor oral availability and a short serum half-life. Glycomimetics that preserve the essential functionality of the parent tetrasaccharide but eliminate unwanted polar functional groups and synthetically cumbersome glycan components have been successful. The design of a monosaccharide glycomimetic starting from sialyl Lewis x is shown in Figure 51.3. First, the sialic acid residue was replaced with a charged glycolic acid group, the N-acetylglucosamine residue was then replaced with an ethylene glycol linker, and finally the galactose residue was replaced with a linker moiety. The resulting glycomimetic had E-selectin binding affinity comparable to sialyl Lewis x.
TRANSFUSION AND TRANSPLANTATION REJECTION BY ANTIGLYCAN ANTIBODIES
As discussed in Chapter 13, a variety of glycans, including the classical A and B blood group determinants, can act as barriers to blood transfusion and transplantation of organs. Rejection of mismatched blood or organs occurs because hosts have a high-titer of preexisting antibodies against the glycan epitopes, presumably as a prior reaction to related structures found on bacteria or other microbes. In the case of the ABO blood groups, incompatibility is routinely managed by blood and tissue typing and finding an appropriate donor for the recipient. Bacterial enzymes can be used in vitro to remove the A and B blood group determinants from A and B red cells, converting them into “universal donor” O red cells.
A related problem is found in xenotransplantation (i.e., the transplantation of organs between species) which is actively being pursued as a solution for the shortage of human organs for patients. The animal donors of preference are pigs, because many porcine organs resemble those of humans in size, physiology, and structure. However, unlike humans and certain other primates, pigs and most other mammals produce the terminal “α-Gal” epitope on glycoproteins and glycolipids. Because humans have naturally occurring high-titer antibodies in blood directed toward this epitope, this results in hyperacute rejection of porcine organ transplants, via reaction of the antibodies with endothelial cells of blood vessels. Attempts to prevent this reaction include blood filtration over glycan affinity columns to remove xenoreactive antibodies and blockade of the interaction by infusing soluble competing oligosaccharides. Transgenic pigs lacking the reactive epitope have also been produced, as have animals with an excess of complement-controlling proteins on their cell surfaces. Pig organs also have high levels of the nonhuman sialic acid (Neu5Gc), against which most humans have antibodies. Even if this problem is solved, there are other glycan and protein structural differences between humans and pigs that cause later stages of graft rejection, thus necessitating immunosuppression.
ACKNOWLEDGMENTS
The authors appreciate helpful comments and suggestions from Wu Di, Benjamin Schulz, Jonathan Viola, and Paeton L. Wantuch.
FURTHER READING
- Kunz C, Rudloff S, Baier W, Klein N, Strobel S. 2000. Oligosaccharides in human milk: Structural, functional, and metabolic aspects. Annu Rev Nutr 20: 699–722. [PubMed: 10940350]
- Gomord V, Chamberlain P, Jefferis R, Faye L. 2005. Biopharmaceutical production in plants: Problems, solutions and opportunities. Trends Biotechnol 23: 559–565. [PubMed: 16168504]
- Joshi L, Lopez LC. 2005. Bioprospecting in plants for engineered proteins. Curr Opin Plant Biol 8: 223–226. [PubMed: 15753005]
- Mhurchu CN, Dunshea-Mooij C, Bennett D, Rodgers A. 2005. Effect of chitosan on weight loss in overweight and obese individuals: A systematic review of randomized controlled trials. Obes Rev 6: 35–42. [PubMed: 15655037]
- Pastores GM, Barnett NL. 2005. Current and emerging therapies for the lysosomal storage disorders. Expert Opin Emerg Drugs 10: 891–902. [PubMed: 16262569]
- Beck M. 2007. New therapeutic options for lysosomal storage disorders: Enzyme replacement, small molecules and gene therapy. Hum Genet 121: 1–22. [PubMed: 17089160]
- Brown JR, Crawford BE, Esko JD. 2007. Glycan antagonists and inhibitors: A fount for drug discovery. Crit Rev Biochem Mol Biol 42: 481–515. [PubMed: 18066955]
- Butters TD. 2007. Pharmacotherapeutic strategies using small molecules for the treatment of glycolipid lysosomal storage disorders. Expert Opin Pharmacother 8: 427–435. [PubMed: 17309337]
- Eklund EA, Bode L, Freeze HH. 2007. Diseases associated with carbohydrates/glycoconjugates. In Comprehensive glycoscience (ed. Kamerling JP, Boone GJ, Lee YC, editors. ), Vol. 4, pp. 339–372. Elsevier, New York.
- Hamilton SR, Gerngross TU. 2007. Glycosylation engineering in yeast: The advent of fully humanized yeast. Curr Opin Biotechnol 18: 387–392. [PubMed: 17951046]
- Schultz BL, Laroy W, Callewaert N. 2007. Clinical laboratory testing in human medicine based on the detection of glycoconjugates. Curr Mol Med 7: 397–416. [PubMed: 17584080]
- von Itzstein M. 2007. The war against influenza: Discovery and development of sialidase inhibitors. Nat Rev Drug Discov 6: 967–974. [PubMed: 18049471]
- Schnaar RL, Freeze HH. 2008. A “glyconutrient sham”. Glycobiology 18: 652–657. [PubMed: 17855741]
- Ernst B, Magnani JL. 2009. From carbohydrate leads to glycomimetic drugs. Nat Rev Drug Discov 8: 661–677. [PMC free article: PMC7097102] [PubMed: 19629075]
- Anish C, Schumann B, Pereira CL, Seeberger PH. 2014. Chemical biology approaches to designing defined carbohydrate vaccines. Chem Bio 21: 38–50. [PubMed: 24439205]
Publication Details
Author Information and Affiliations
Authors
Peter H. Seeberger and Richard D. Cummings.Publication History
Published online: 2017.
Copyright
PDF files are not available for download.
Publisher
Cold Spring Harbor Laboratory Press, Cold Spring Harbor (NY)
NLM Citation
Seeberger PH, Cummings RD. Glycans in Biotechnology and the Pharmaceutical Industry. 2017. In: Varki A, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology [Internet]. 3rd edition. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2015-2017. Chapter 57. doi: 10.1101/glycobiology.3e.057


