NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Varki A, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology [Internet]. 3rd edition. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2015-2017. doi: 10.1101/glycobiology.3e.007

Essentials of Glycobiology [Internet]. 3rd edition.
Show detailsThis chapter provides an overview of the biological functions of glycans in three broad categories: structural and modulatory roles, including nutrient storage and sequestration; specific recognition—most commonly by glycan-binding proteins of intrinsic or extrinsic origin; and molecular mimicry of host glycans. The chapter then presents some general principles for understanding and further exploring biological functions of glycans. For details, see the sources cited and the other chapters in this book.
GENERAL PRINCIPLES
As with other major macromolecules, the biological functions of glycans span the spectrum from relatively subtle to crucial for development, growth, maintenance, or survival of the organism that synthesizes them. For many glycans, a function is not yet evident. The same glycan may also have different functions depending on which aglycone it is attached. Over the years, many theories have been advanced regarding biological roles of glycans. Although there is evidence to support all the theories, exceptions to each can also be found. This should not be surprising, given the abundance and enormous diversity of glycans in nature. Complexities also arise because glycans are frequently bound by microbes and microbial toxins, making them a liability to the organism that synthesizes them.
Biological functions of glycans can be divided into three broad categories: (1) structural and modulatory properties, including nutrient storage and sequestration; (2) specific recognition by other molecules—most commonly, glycan-binding proteins (GBPs); and (3) molecular mimicry of host glycans (Figure 7.1). The GBPs can be subdivided into two groups: (1) intrinsic GBPs, which recognize glycans from the same organism and (2) extrinsic GBPs, which recognize glycans from another organism. Intrinsic GBPs typically mediate cell–cell interactions or recognize extracellular molecules, but they can also recognize glycans on the same cell. Extrinsic GBPs comprise pathogenic microbial adhesins, agglutinins, or toxins, but some also mediate symbiotic relationships or host defense directed at microbial glycans. Intrinsic and extrinsic glycan recognition likely act as opposing selective forces driving and constraining evolutionary change (Chapter 20), at least partly accounting for the enormous diversity of glycans in nature. Microbial pathogens engage in “molecular mimicry,” evading immune reactions by decorating themselves with glycans typical of their hosts, and also in “glycan gimmickry” for modulating host immunity toward increased tolerance. Finally, most microbes are themselves targets of pathogens (e.g., bacteriophages that invade bacteria), and glycan recognition is a common feature of these interactions as well.
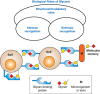
FIGURE 7.1.
General classification of the biological functions of glycans. A simplified and broad classification is presented, emphasizing the roles of organism-intrinsic and -extrinsic glycan-binding proteins in recognizing glycans. There is some overlap between (more...)
Other general principles emerge based on existing literature. The biological consequences of experimentally altering glycosylation in various systems seem to be highly variable and unpredictable. A given glycan can have different roles in different tissues, at different times in development (organism-intrinsic functions) or in different environmental contexts (organism-extrinsic functions). As a broad generalization, it can be stated that terminal sequences, unusual structures, and modifications of glycans are thought to mediate the more specific biological functions within the organism. However, such glycans or their modifications are also more likely to be targets for pathogens and toxins. Perhaps as a consequence, intra- and interspecies variations in glycosylation are relatively common, and at least some of the diversity of glycans in nature may represent signatures of past or current host–pathogen interactions (Chapter 20). Finally, genetic defects in glycosylation are easily obtained in cultured cells, but they often have limited biological consequences in vitro. In contrast, the same defects can have major and even catastrophic consequences in whole organisms. This indicates that many major functions of glycans are operative mainly within an intact, multicellular organism. Some of these principles are briefly discussed below.
BIOLOGICAL CONSEQUENCES OF EXPERIMENTALLY ALTERING GLYCOSYLATION ARE VARIABLE
Approaches to understand biological roles of glycans include prevention of initial glycosylation, prevention of glycan chain elongation, alteration of glycan processing, enzymatic or chemical deglycosylation of completed chains, genetic elimination of glycosylation sites, addition of unnatural monosaccharides, and studies of mutant and naturally occurring genetic variants in glycosylation enzymes. Consequences of such manipulations range from being essentially undetectable to the complete loss of particular functions or even loss of the entire glycoconjugate bearing the altered glycan. Even within a particular class of molecules (e.g., cell-surface receptors), the effects of altering glycosylation are variable and unpredictable. Moreover, the same glycosylation change can have markedly different effects in different cell types or when studied in vivo or in vitro. The effect may depend on the structure of the glycan, the biological context, and the specific biological function. Given all of the above considerations, it is difficult to predict the functions that a given glycan on a given glycoconjugate might mediate, and its relative importance to the organism.
STRUCTURAL AND MODULATORY FUNCTIONS OF GLYCANS
Glycans have many protective, stabilizing, organizational, and barrier functions. The glycocalyx that covers all eukaryotic cells and the polysaccharide coats of various prokaryotes represent a substantial physical barrier. Glycan constituents of matrix molecules, such as proteoglycans, are important for maintenance of tissue structure, porosity, and integrity. Such molecules can also contain binding sites for other specific glycans, which in turn, aid overall matrix organization. The external location of glycans on most glycoproteins can provide a general shield, protecting the underlying polypeptide from recognition by proteases, blocking antibody binding, and even (in the case of mucins) protecting entire tissue surfaces from microbial attachment. Glycans are also involved in folding of newly synthesized polypeptides in the endoplasmic reticulum (ER) and/or in the subsequent maintenance of protein solubility and conformation (Chapter 39). Indeed, if some proteins are incorrectly glycosylated, these fail to fold properly and/or to exit the ER and are subsequently consigned to degradation in proteasomes. Conversely, there are examples of glycoproteins whose synthesis, folding, trafficking, sensitivity to proteolysis, or immune recognition seem unaffected by altering their glycosylation. Moreover, inhibitors (Chapter 55) or genetic mutations (Chapter 45) that only affect later steps of glycan processing often do not interfere with basic structural functions. Although structural functions of glycans are obviously of great importance to the intact organism, they do not explain the evolution of such a diverse and complex range of molecules.
Glycosylation can also modulate interactions of proteins with one another. Some growth factor receptors acquire their binding abilities in a glycosylation-dependent manner while in transit through the Golgi apparatus. This may limit unwanted early interactions of a newly synthesized receptor with a growth factor that is synthesized in the same cell. Glycosylation of a polypeptide can also mediate an on-off switching effect. For example, when the hormone β-human chorionic gonadotrophin is deglycosylated, it still binds to its receptor with similar affinity, but it fails to stimulate adenylate cyclase. In most instances, the effects of glycosylation are incomplete; that is, glycosylation appears to be “tuning” a primary function of the protein rather than turning it on or off. For example, the activity of some glycosylated growth factors and hormones can be modulated over a wide range by the extent and type of their glycosylation. This becomes particularly evident when recombinant glycoprotein molecules are produced in biotechnology, bearing different glycosylation patterns based on the evolutionary history of the cell expression system in use.
The intricate modulation of Notch–ligand interactions by the Fringe glycosyltransferase, whereby cis and trans ligand interactions are tuned, is a prime example of such phenomena (Chapter 13). Another striking example is the role of polysialic acid (polySia) chains on the neural cell adhesion molecule (NCAM). This adhesion receptor normally mediates homophilic binding between neuronal cells. In the embryonic state, or in other states of neural “plasticity,” polySia chains tend to be long, thereby interfering with homophilic binding (Chapter 15). There are also instances wherein functions can be tuned by glycans attached to other neighboring structures. For example, polySias of embryonic NCAM can interfere with the interactions of other unrelated receptor–ligand pairs, simply by physically separating the cells. Also, tyrosine phosphorylation of the epidermal growth factor (EGF) and the insulin receptors can be modulated by endogenous cell-surface gangliosides, possibly by organizing them into membrane microdomains (Chapter 11). Although the precise mechanisms of the latter effects are uncertain, specificity is implied by the requirement for a defined glycan sequence on the ganglioside. Because most of such tuning effects of glycans are partial, their overall importance tends to be questioned. However, the sum total of several such partial effects can result in a dramatic effect on the final biological outcome. Thus, glycosylation appears to be a mechanism for generating important functional diversity from the limited set of basic receptor–ligand interactions that are possible, when using gene products from a typical genome. Of course, as with most other glycan functions, exceptions can be found. There are many receptors whose ligand binding is independent of glycosylation and many peptide ligands whose binding and action are not obviously affected by glycosylation.
Another structural/modulatory function of glycans is to act as a protective storage depot for biologically important molecules. For example, many heparin-binding growth factors (Chapters 17 and 38) bind glycosaminoglycan (GAG) chains of the extracellular matrix, adjacent to cells that need to be stimulated (e.g., in the basement membrane underlying epithelial and endothelial cells). This prevents diffusion of factors away from the site (sometimes generating morphogenic gradients), protects them from nonspecific proteolysis, prolongs their active lives, and allows them to be released under specific conditions. Likewise, GAG chains in secretory granules can bind and protect protein contents of the granule and modulate their functions. There are several other instances in which glycans act as sinks or depots for biologically important molecules, such as water, ions, and immune regulatory proteins. The asymmetry in localization of most glycans on the outer plasma membrane leaflet can contribute to a signal for the rupture of vesicles, in which exposed glycans are detected by cytoplasmic lectins (e.g., galectins) and trigger autophagy.
Last but not least, polymerized glycans such as starch and cellulose (in plants) and glycogen (in animals) can serve a major role in nutrient storage and sequestration. The relative resistance to digestibility of the internal linkages of such polymers by enzymes of intrinsic or extrinsic origin determines their relative role as structural components or as a mechanism for nutrient sequestration.
GLYCANS AS SPECIFIC LIGANDS FOR CELL–CELL INTERACTIONS (INTRINSIC RECOGNITION)
The first intrinsic glycan receptors identified in vertebrates were those mediating clearance, turnover, and intracellular trafficking of soluble blood-plasma glycoproteins (Chapters 33–34), specifically recognizing certain terminal or subterminal glycans on the glycoprotein. However, even the most precise examples, such as the role of mannose-6-phosphate (Man-6-P) in the trafficking of lysosomal enzymes to lysosomes (Chapter 33), feature exceptions. For instance, Man-6-phosphorylation is not absolutely required for trafficking of lysosomal enzymes in certain cell types, nor is it operative in some “lower” eukaryotes. There are also endocytic receptors recognizing specific glycan sequences, whose functions have yet to be assigned. Several instances exist wherein free glycans can have hormonal actions that induce specific responses in a highly structure-specific manner. Examples include interaction of small glycans (lipochitooligosaccharides) from bacterial and fungal symbionts with plant roots (Chapter 40) and bioactive properties of hyaluronan fragments in mammalian systems (Chapter 16), both of which induce biological responses in a size- and structure-dependent manner. Likewise, free heparan or dermatan sulfate fragments released by certain cell types can have biological effects in situations such as wound healing. In many of these instances, the putative receptors for these molecules and their precise mechanisms are still being defined.
It is now clear that glycans have many specific biological roles in cell–cell recognition and cell–matrix interactions. A well characterized example is the selectin family of adhesion molecules, which recognize glycans on ligands and mediate critical interactions between blood cells and vascular cells in a variety of normal and pathological situations (Chapter 34). Glycans and GBPs on cell surfaces can interact specifically with molecules in the matrix or even with glycans on the same cell surface. Some critical recognition sites are actually combinations of glycans and protein. For example, P-selectin recognizes the generic selectin ligand sialyl Lewis x with high affinity only in the context of the amino-terminal 13 amino acids of P-selectin glycoprotein ligand-1 (PSGL-1), which include required sulfated tyrosine residues (Chapter 34). Glycan-binding sites of cell-surface receptors like Siglecs can be masked by cognate glycans on the same cell surface, making them unavailable for recognition of external ligands (Chapter 35). On the other hand, some glycans can act as “biological masks,” preventing recognition of underlying residues (e.g., sialic acids can mask recognition of underlying β-galactosides by galectins or other GBPs).
Carbohydrate–carbohydrate interactions can also have specific biological roles in cell–cell interactions. A dramatic example is the species-specific interaction between marine sponges, mediated via homotypic binding of glycans on a large cell-surface glycoprotein. Another example is the compaction of the mouse embryo at the morula stage, which seems to be facilitated by a Lewis x–Lewis x interaction. The single-site affinities of such interactions are not strong and sometimes difficult to measure. However, if the molecules are present in very high copy numbers on the cell surface, a large number of relatively low-affinity interactions can collaborate to produce a high-avidity “Velcro” effect that is sufficient to mediate biologically relevant interactions.
GLYCANS AS SPECIFIC LIGANDS FOR CELL–MICROBE INTERACTIONS (EXTRINSIC RECOGNITION)
Many glycans are specifically bound by various viruses, bacteria, and parasites, and also targeted by many toxins (Chapter 37). Given rapid evolution of pathogens and ongoing selection, there is typically excellent recognition specificity for the sequence of the glycans involved. For example, the hemagglutinins of many viruses specifically recognize the type of host sialic acid, its modifications, and its linkage to the underlying sugar chain. Likewise, various toxins bind with great specificity to certain gangliosides but not to related structures (Chapters 11 and 37). There is little doubt about the importance of structural specificity with respect to these functions of glycans. Indeed, many of the microbial binding proteins involved have been harnessed as molecular probes for studying the expression of their cognate glycans. However, providing signposts to aid the success of pathogenic microorganisms has little obvious value to the organism that synthesized such glycans. To counter such deleterious consequences, some organisms have also evolved the ability to mask or modify glycans recognized by microorganisms or toxins. Meanwhile, glycan sequences on soluble glycoconjugates, such as secreted mucins, act as decoys for microorganisms and parasites. Thus, a pathogenic organism or toxin evolving to bind to mucosal cell membranes may first encounter its cognate ligand attached to a soluble mucin, which can then be washed away, removing the danger to cells underneath. In contrast, instances occur in which symbiosis is mediated by specific glycan recognition, such as some commensal bacteria in the gut lumen of animals and the bacteria involved in forming plant root nodules in nitrogen fixing legumes or mycorrhizal symbioses between trees and fungi (Chapter 40). Last but not least, many pathogen-associated molecular patterns (PAMPs) often consist of foreign glycans and/or glycan patterns on invading microbes are recognized by innate immune cells, and are detected by specific receptors such as Toll-like receptors (TLRs) and C-type lectins (Chapter 34).
MOLECULAR MIMICRY OF HOST GLYCANS AND GLYCOGIMMICKRY BY PATHOGENS AND HOSTS
Pathogens that invade multicellular animals sometimes decorate themselves with glycan structures that are identical or nearly identical to those on host cell surfaces (Chapters 42 and 43). Such glycans block recognition of underlying antigenic epitopes, restrict immune cell complement system activation, and can also mimic “self-associated molecular patterns” (SAMPs) of hosts, all of which are successful strategies for evading host immune responses. Perhaps not surprisingly, pathogens have evolved this state of molecular mimicry by making use of “every possible trick in the book,” including direct or indirect appropriation of host glycans, convergent evolution toward similar biosynthetic pathways, and even lateral gene transfer. In some instances, the impact of the pathogen is aggravated by autoimmune reactions, resulting from host reactions to these self-like antigens. Pathogens and parasites also engage in glycogimmickry—for example, gut symbionts induce tolerogenic states by the gut immunity using pentasaccharide called polysaccharide A, and helminthic parasites have evolved countless ways to modulate the immune response of their hosts using specific immunomodulatory glycans. Secreted and soluble milk oligosaccharides (all extensions of the disaccharide lactose) of mammals provide an example of glycan manipulation of microbial symbionts or pathogens in the infant by maternal glycans. These glycans have potent effects by favoring symbionts and blocking pathogenic microbes (bacteria and viruses) of the infant gut.
THE SAME GLYCAN CAN HAVE DIFFERENT FUNCTIONS WITHIN AN ORGANISM
Expression of certain glycans on different glycoconjugates in different tissues at different stages of development implies diverse roles for these glycan structures within the same organism. For example, Man-6-P-containing glycans were first found on lysosomal enzymes and are involved in lysosomal trafficking (Chapter 33). However, such glycans are now known to occur on a variety of apparently unrelated proteins, for different functional roles. Likewise, the sialylated fucosylated lactosamines critical for selectin recognition (Chapter 34) are found in a variety of unrelated cell types in mammals, and the polySia chains that play an important part in embryonic nervous system neural cell adhesion molecule (NCAM) function (Chapter 15) are also found on a G protein–coupled receptor (GPCR) (CCR7) expressed in dendritic cells where they appear to be important for targeting of these cells to the lymph nodes. Given that glycans are added posttranslationally, these observations should not be surprising. Once a new glycan or modification has been expressed in an organism, several distinct functions could evolve independently in different tissues and at different times in development. If any of these situations mediated a function valuable to survival and reproduction, the genetic mechanisms responsible for expression of the glycan and its expression pattern would remain conserved in evolution. A further example of dual roles is the use of fragments of structural glycans as danger or damage-associated molecular patterns (DAMPs) by the immune systems of multicellular organisms. Examples include cell wall fragments in plants and hyaluronan fragments in vertebrates.
INTRASPECIES AND INTERSPECIES VARIATIONS IN GLYCOSYLATION
The underlying core structures of major glycan classes tend to be conserved across many species; for example, the core structure of N-glycans is conserved across all eukaryotes and at least some Archaea (Chapter 9). However, there can be considerable diversity in outer-chain glycosylation, even among relatively similar species. Such interspecies variation in glycan structure indicates that some glycan sequences do not have fundamental and universal roles in all tissues and cell types in which they are expressed. Of course, such diversity could be involved in generating differences in morphology and function observed between species. Such variations could also reflect the outcome of differing selection pressures by exposure to variant pathogen regimes. Furthermore, significant intraspecies polymorphism in glycan structure can exist without obvious functional value, although they may be involved in the interplay between parasites and host populations (Chapter 20).
POTENTIAL IMPORTANCE OF TERMINAL SEQUENCES, MODIFICATIONS, AND UNUSUAL STRUCTURES
Given all of the above, it is challenging to predict which glycan structures are likely to mediate the more specific or crucial biological roles within an organism. As mentioned above, terminal sugar sequences, unusual structures, or modifications of glycans are more likely to be involved in such specific roles. The predictive value of this observation is reduced by the fact that such entities are also more likely to be involved in interactions with microorganisms and other potentially noxious agents. Further complexity arises from “microheterogeneity” in glycan structure (Chapter 20), wherein the same glycosylation site on the same protein in the same species can carry a variety of related glycan structures or none at all. The challenge then is to predict and sort out which distinct roles can be assigned to a given glycan structure in a given cell type and organism. In the coevolutionary arms race between pathogens and hosts, pathogens will tend to exploit glycans that are difficult for the host to change (those with host intrinsic function), and because hosts are unable to alter their glycans easily owing to their slower evolutionary rates and functional constraints of existing glycans, the extrinsic and intrinsic functions of these glycans are bound to overlap. Hosts can, however, to the degree possible, be expected to evolve glycans that are difficult for microbes to mimic, such as the vertebrate-specific sialic acid Neu5Gc (Chapter 15) and sulfated GAGs, which prokaryotes do seem to have reinvented (Chapter 17).
ARE THERE “JUNK” GLYCANS?
Because microorganisms and parasites that bind glycans evolve in parallel with their multicellular hosts, they must adapt their glycan-binding “repertoire” to any change in glycan structure presented by the host. In response, the host population may select for new modifications of the target structure, especially if the latter had meanwhile evolved a vital function elsewhere within the organism. Thus, there would be no choice but to preserve the underlying scaffolding on which the latest modification was placed, while adding yet another layer of complexity to its glycans. Such cycles of evolutionary interaction between microbes and hosts might explain some of the complex and extended sugar chains found in multicellular organisms, especially in areas of frequent microbial contact, such as in secreted mucins on mucosal surfaces. In this manner, “junk” glycans could accumulate, akin to “junk” DNA. An important distinction is that much of the junk DNA consists of selfish DNA elements capable of replicating, whereas glycans cannot encode additional copies of themselves, given that their synthesis relies on the activity of a coordinated network of gene products. Although such structures may still function as structural scaffolding, they may have no other specific role in a particular cell type at that particular time in evolution. They would, of course, provide fodder for future evolutionary selection, either for new organism-intrinsic functions or selective responses to a new pathogen. Additionally, neutral drift (which is now acknowledged as a major process in evolution) may explain some of the apparent “junk” glycans. The microheterogeneity inherent in glycan synthesis process may be producing “neutral noise,” but this noise may also contribute to evolutionary flexibility.
APPROACHES TO ELUCIDATING SPECIFIC BIOLOGICAL FUNCTIONS OF GLYCANS
Some functions of glycans are discovered serendipitously. In other instances, the investigator who has elucidated complete details of the structure and biosynthesis of a specific glycan is left without knowing its functions. It is necessary to design experiments that can differentiate between trivial and crucial functions mediated by each glycan. Various approaches are discussed below, emphasizing the advantages and disadvantages of each approach (also presented in schematic form in Figure 7.2).
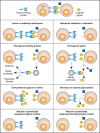
FIGURE 7.2.
Approaches for elucidating the biological functions of glycans. The figure assumes that a specific biological role is being mediated by recognition of a certain glycan structure by a specific glycan-binding protein. Clues to this biological role could (more...)
Localization or Interference with Specific Glycans Using Glycan-Recognizing Probes (GRPs)
Many current approaches to understanding glycan diversity (Chapters 50 and 51) involve extraction and identification of the entire complement of glycans found in a given organ or tissue, without regard to the fact that individual cell types and even basal versus apical sides of the same cell can have widely varying glycan expression patterns. However, cell type–specific localization of glycans can be explored using highly specific GRPs (GBPs or antibodies, see Chapter 48). Once a specific glycan has been localized in an interesting biological context, it is natural to consider introducing the cognate GRP into the intact system, hoping to interfere with a specific function and generate an interpretable phenotype. Such an approach is likely to give confusing results with regard to glycan function. Some GRPs (e.g., antibodies against glycans) tend to have weak affinity and show cross-reactivity. Although some plant lectins seem very specific for animal glycans, they originate from organisms that typically do not contain the same ligand. Thus, their apparent specificity may not be as reliable when introducing them into complex animal biological systems where they might bind unknown cross-reacting glycans. Finally, most GRPs are multivalent, and their cognate ligands (the glycans) tend to be present in multiple copies on multiple glycoconjugates. Thus, introduction of a GRP into a complex biological system may cause nonspecific aggregation of various molecules and cell types, and the effects seen may extend well beyond the biological functions of the glycan in question. It would seem worthwhile to develop recombinant monovalent GRP modules that are derived from the same system being investigated, provided they are of high enough affinity. Effects of introducing such monovalent GRPs as competitors of the native function may yield more interpretable clues.
Metabolic Inhibition or Alteration of Glycosylation
Many pharmacological agents can metabolically inhibit or alter glycosylation in intact cells and animals (Chapter 49). Although such agents are powerful tools to elucidate biosynthetic pathways, they can yield confusing results in complex systems. One concern is that an inhibitor may have effects on other unrelated pathways. For example, the inhibitor tunicamycin that blocks N-linked glycosylation can also cause ER stress and inhibit UDP-Gal uptake into the Golgi. The second concern is that the inhibitor may cause such global changes in glycan synthesis that the physical properties of the glycoconjugates and/or membranes are altered, making it difficult to interpret the results. Somewhat more useful results can be obtained by introducing low-molecular-weight primers of terminal glycosylation (Chapter 55), which can act as alternate substrates for Golgi enzymes, diverting synthesis away from the endogenous glycoproteins. However, this approach can simultaneously generate incomplete glycans on endogenous glycoconjugates, as well as produce secreted glycan chains, each of which could have its own biological effects.
Finding Natural Glycan Ligands for Specific Receptors
Because specific “carbohydrate-recognition domains” can be identified within a primary amino acid sequence (Chapters 28 and 29), it is now possible to predict whether a newly cloned protein can bind glycans. If a potential GBP can be produced in sufficient quantities, techniques such as hemagglutination, flow cytometry, surface plasmon resonance, and affinity chromatography (Chapters 29 and 28) can then be used to search for specific ligands. However, the monovalent affinity of the putative GBP for its ligand may not be high. Thus, high densities and/or multivalent arrays may be needed to avoid missing a biologically relevant interaction. The question also arises as to where exactly to look for the biologically relevant ligands in a complex multicellular system. Furthermore, because many glycan structures can be expressed in different tissues at different times in development and growth, a recombinant GBP may detect a cognate structure in a location and at a time that it is not of major biological relevance. Careful consideration of the natural occurrence and expression profile of the GBPs should lead to a rational decision as to where to look for its biologically relevant glycan ligands.
Finding Receptors That Recognize Specific Glycans
The converse situation arises when an unusual glycan is found to be expressed in an interesting context and is hypothesized to be a ligand for a specific receptor. It is possible to search for such a receptor by techniques similar to those above, such as hemagglutination, flow cytometry, and affinity chromatography (Chapters 28 and 29). To facilitate the search, it is necessary to have reasonable quantities of the pure defined glycan in question, as well as a variety of closely related structures as negative controls. Because many biologically relevant lectin-like interactions are of low affinity, it is probably advisable to use a multivalent form of the glycan as the probe (bait). Finally, it may not be obvious where to look for the glycan-binding protein. For example, the receptor that recognizes the unusual sulfated N-glycans of pituitary glycoprotein hormones was eventually found not in the pituitary nor in any of the target tissues for these hormones, but in liver endothelial cells, wherein it regulates the circulating half-life of the hormones (Chapter 31). Indeed, the most biologically relevant receptor for a particular glycan might even be found in another organism (a pathogen, a symbiont, or a mate).
Interference with Soluble Glycans or Structural Mimics
The addition of soluble glycans or structural mimics into a system can block the interaction between an endogenous GBP and a specific glycan (Chapters 28 and 29). If a sufficient concentration of the specific inhibitor can be achieved, the resulting phenotypic changes can be instructive. When studying in vitro systems, even monosaccharides can be used in such experiments, as exemplified by the Man-6-P receptor pathway (Chapter 33). However, it is often necessary to use competing glycans at high concentrations to block the relatively low-affinity site interactions between a GBP and its ligand. Intrinsically low site affinity can sometimes be overcome using a multivalent form of the cognate glycan. Finally, especially when studying complex multicellular systems, introduced glycans could be cross-recognized by other known or unknown binding proteins, giving a confusing phenotypic readout.
Eliminating Specific Glycan Structures by Glycosidases
A powerful approach is to use degradative enzymes known to be highly specific for a particular glycan sequence. Many such specific enzymes can be obtained from microbial pathogens. The advantage of this approach is that one is eliminating certain structures selectively after normal synthesis has been completed rather than interfering with the biosynthetic cellular machinery. Thus, for example, sialidase treatment abolished lymphocyte binding to the high endothelial venules of lymph nodes and provided the first indication of endogenous ligands for L-selectin (Chapter 32); injection of endoneuraminidase into the developing retina suggested specific roles for polySias (Chapter 15); and injection of heparanase into developing embryos gave randomization of left–right axis formation (Chapter 17). In all such studies, the purity of the enzyme used is critical and appropriate controls are necessary (preferably including a specific inhibitor of the enzyme or a catalytically inactive version of the enzyme). If the enzyme is of bacterial origin, trace amounts of contaminants such as endotoxin are also of concern. A genetic approach can be used to avoid problems of contamination by expressing a cDNA for the glycan-modifying enzyme in the intact cell or animal. For example, transgenic expression of an influenza sialic acid–specific 9-O-acetylesterase in mice resulted in either early or late abnormalities in development, depending on the promoter used. Unfortunately, many such glycosidases may not function well or at all in the context of an intact animal, which can limit the spectrum of glycan structures that may be probed for function with this approach.
Studying Natural or Genetically Engineered Glycan Mutants
This is intuitively a powerful approach for understanding glycan function. It is easiest to study glycosylation mutants in cultured cell lines (Chapter 49). Although genetic or acquired defects in glycosylation are obtained relatively easily in cells, these defects may have limited or not easily discernible biological consequences. This may be because of lack of other factors or cell types that would be present in the intact organism. For example, the cognate receptor for the glycan may not be present in the same cell type. Of course, such mutants can still be used to analyze basic structural functions of the glycans and their relevance to the physiology of a single cell. Furthermore, one can reintroduce external factors or other cell types thought to interact with the modified glycan. Some mutants can also be reintroduced into intact organisms, for example, to study tumorigenicity or metastatic behavior of malignant cells.
Although much useful information can be gained by such approaches, many of the more specific roles of glycans need to be uncovered by studying mutations in the intact multicellular organism. Looking at the various glycosylation mutants that have been recently discovered in flies, worms, mice, and humans, it is clear that glycan changes often affect multiple systems (are pleiotropic) and that the phenotypes are unpredictable and highly variable. This has become apparent, in part, by comparing the genotype-phenotype relationships in naturally occurring human disorders of glycosylation and in experimentally induced glycosylation disorders in mice. In humans, naturally occurring disease-associated mutations in glycosylation pathways often leave some residual enzymatic function intact (Chapters 44 and 45), whereas deletion of the corresponding enzymatic locus in mice often leads to a lethal phenotype during embryogenesis. Regardless, the value of engineering glycosylation mutants in intact animals is evident. Indeed, complete elimination of most major glycan classes of vertebrates has been accomplished in mice, and in every instance has lead to embryonic lethality. Given the complex phenotypes and the potential for early developmental lethality, the ability to disrupt glycosylation-related genes in a temporally controlled and cell type–specific manner can be particularly valuable.
Studying Natural or Genetically Engineered Glycan Receptor Mutants
Eliminating a specific glycan receptor can yield a phenotype that may be very instructive with regard to the functions of the glycan. As with genetic modification of the glycan, the results are more likely to be useful if studied in the intact organism. However, the receptor protein may have other functions unrelated to glycan recognition. Conversely, the glycan in question may have other functions not mediated by the receptor. For example, genetic elimination of the CD22/Siglec-2 receptor and the ST6Gal-I enzyme that generates its ligand gave complementary, but not identical, phenotypes (Chapter 35). However, breeding the two mutations into the same mouse indicated that there were indeed epistatic interactions. Similar results were obtained by mating mice deficient in making polysialic acid and in synthesizing the protein carrier of polysialic acids, NCAM.
ACKNOWLEDGMENTS
The authors appreciate helpful comments and suggestions from Merrina Anugraham, Donald Bernsteel, Martina Delbianco, and Steve M. Fernandes.
FURTHER READING
- Berger EG, Buddecke E, Kamerling JP, Kobata A, Paulson JC, Vliegenthart JF. 1982. Structure, biosynthesis and functions of glycoprotein glycans. Experientia 38: 1129–1162. [PubMed: 6754417]
- Kobata A. 1992. Structures and functions of the sugar chains of glycoproteins. Eur J Biochem 209: 483–501. [PubMed: 1358608]
- Lis H, Sharon N. 1993. Protein glycosylation. Structural and functional aspects. Eur J Biochem 218: 1–27. [PubMed: 8243456]
- Varki A. 1993. Biological roles of oligosaccharides: All of the theories are correct. Glycobiology 3: 97–130. [PMC free article: PMC7108619] [PubMed: 8490246]
- Drickamer K, Taylor ME. 1998. Evolving views of protein glycosylation. Trends Biochem Sci 23: 321–324. [PubMed: 9787635]
- Ferguson MA. 1999. The structure, biosynthesis and functions of glycosylphosphatidylinositol anchors, and the contributions of trypanosome research. J Cell Sci 112: 2799–2809. [PubMed: 10444375]
- Gagneux P, Varki A. 1999. Evolutionary considerations in relating oligosaccharide diversity to biological function. Glycobiology 9: 747–755. [PubMed: 10406840]
- Spiro RG. 2002. Protein glycosylation: Nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology 12: 43R–56R. [PubMed: 12042244]
- Haltiwanger RS, Lowe JB. 2004. Role of glycosylation in development. Annu Rev Biochem 73: 491–537. [PubMed: 15189151]
- Ohtsubo K, Marth JD. 2006. Glycosylation in cellular mechanisms of health and disease. Cell 126: 855–867. [PubMed: 16959566]
- Bishop JR, Schuksz M, Esko JD. 2007. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature 446: 1030–1037. [PubMed: 17460664]
- Moremen KW, Tiemeyer M, Nairn AV. 2012. Vertebrate protein glycosylation: Diversity, synthesis and function. Nat Rev Mol Cell Biol 13: 448–462. [PMC free article: PMC3934011] [PubMed: 22722607]
- Hart GW. 2013. Thematic minireview series on glycobiology and extracellular matrices: Glycan functions pervade biology at all levels. J Biol Chem 288: 6903. [PMC free article: PMC3591599] [PubMed: 23329844]
- Hardivillé S, Hart GW. 2014. Nutrient regulation of signaling, transcription, and cell physiology by O-GlcNAcylation. Cell Metab 20: 208–213. [PMC free article: PMC4159757] [PubMed: 25100062]
- Van Breedam W, Pöhlmann S, Favoreel HW, de Groot RJ, Nauwynck HJ. 2014. Bitter-sweet symphony: Glycan–lectin interactions in virus biology. FEMS Microbiol Rev 38: 598–632. [PMC free article: PMC7190080] [PubMed: 24188132]
- Schnaar RL. 2017. Glycobiology simplified: Diverse roles of glycan recognition in inflammation. J Leukoc Biol 99: 825–838. [PMC free article: PMC4952015] [PubMed: 27004978]
- Stanley P. 2017. What have we learned from glycosyltransferase knockouts in mice? J Mol Biol 428: 3166–3182. [PMC free article: PMC5532804] [PubMed: 27040397]
- Varki A. 2017. Biological rules of glycans. Glycobiology 27: 3–49. [PMC free article: PMC5884436] [PubMed: 27558841]
- GENERAL PRINCIPLES
- BIOLOGICAL CONSEQUENCES OF EXPERIMENTALLY ALTERING GLYCOSYLATION ARE VARIABLE
- STRUCTURAL AND MODULATORY FUNCTIONS OF GLYCANS
- GLYCANS AS SPECIFIC LIGANDS FOR CELL–CELL INTERACTIONS (INTRINSIC RECOGNITION)
- GLYCANS AS SPECIFIC LIGANDS FOR CELL–MICROBE INTERACTIONS (EXTRINSIC RECOGNITION)
- MOLECULAR MIMICRY OF HOST GLYCANS AND GLYCOGIMMICKRY BY PATHOGENS AND HOSTS
- THE SAME GLYCAN CAN HAVE DIFFERENT FUNCTIONS WITHIN AN ORGANISM
- INTRASPECIES AND INTERSPECIES VARIATIONS IN GLYCOSYLATION
- POTENTIAL IMPORTANCE OF TERMINAL SEQUENCES, MODIFICATIONS, AND UNUSUAL STRUCTURES
- ARE THERE “JUNK” GLYCANS?
- APPROACHES TO ELUCIDATING SPECIFIC BIOLOGICAL FUNCTIONS OF GLYCANS
- ACKNOWLEDGMENTS
- FURTHER READING
- Review Biological Functions of Glycans.[Essentials of Glycobiology. 2022]Review Biological Functions of Glycans.Gagneux P, Hennet T, Varki A. Essentials of Glycobiology. 2022
- Review Biological Roles of Glycans.[Essentials of Glycobiology. 2009]Review Biological Roles of Glycans.Varki A, Lowe JB. Essentials of Glycobiology. 2009
- Review Evolution of Glycan Diversity.[Essentials of Glycobiology. 2015]Review Evolution of Glycan Diversity.Gagneux P, Aebi M, Varki A. Essentials of Glycobiology. 2015
- Review Evolution of Glycan Diversity.[Essentials of Glycobiology. 2009]Review Evolution of Glycan Diversity.Varki A, Freeze HH, Gagneux P. Essentials of Glycobiology. 2009
- Review Evolution of Glycan Diversity.[Essentials of Glycobiology. 2022]Review Evolution of Glycan Diversity.Gagneux P, Panin V, Hennet T, Aebi M, Varki A. Essentials of Glycobiology. 2022
- Biological Functions of Glycans - Essentials of GlycobiologyBiological Functions of Glycans - Essentials of Glycobiology
Your browsing activity is empty.
Activity recording is turned off.
See more...

