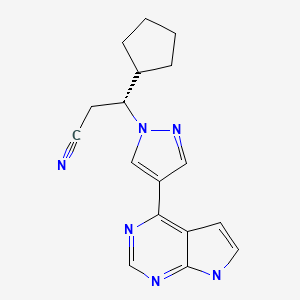
CASRN: 941678-49-5
Drug Levels and Effects
Summary of Use during Lactation
No information is available on the clinical use of ruxolitinib during breastfeeding. Because ruxolitinib is 97% bound to plasma proteins, the amount in milk is likely to be low. The manufacturer recommends that breastfeeding be discontinued during ruxolitinib therapy and for 2 weeks after the last dose for the oral tablets and for 4 weeks after the last dose for the topical cream.
Drug Levels
Maternal Levels. Relevant published information was not found as of the revision date.
Infant Levels. Relevant published information was not found as of the revision date.
Effects in Breastfed Infants
Relevant published information was not found as of the revision date.
Effects on Lactation and Breastmilk
Relevant published information was not found as of the revision date.
Substance Identification
Substance Name
Ruxolitinib
CAS Registry Number
941678-49-5
Drug Class
Breast Feeding
Lactation
Milk, Human
Antineoplastic Agents
Enzyme Inhibitors
Protein Kinase Inhibitors
Signal Transduction Inhibitors
Janus Kinase Inhibitors
JAK Inhibitors
Disclaimer: Information presented in this database is not meant as a substitute for professional judgment. You should consult your healthcare provider for breastfeeding advice related to your particular situation. The U.S. government does not warrant or assume any liability or responsibility for the accuracy or completeness of the information on this Site.
Publication Details
Publication History
Last Revision: December 15, 2023.
Copyright
Attribution Statement: LactMed is a registered trademark of the U.S. Department of Health and Human Services.
Publisher
National Institute of Child Health and Human Development, Bethesda (MD)
NLM Citation
Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006-. Ruxolitinib. [Updated 2023 Dec 15].