OVERVIEW
Introduction
Ciprofloxacin is a second generation fluoroquinolone antibiotic that is widely used in the therapy of mild-to-moderate urinary and respiratory tract infections caused by susceptible organisms. Ciprofloxacin has been linked to rare but convincing instances of liver injury that can be severe and even fatal.
Background
Ciprofloxacin (sip" roe flox' a sin) is an oral fluoroquinolone that is used to treat mild-to-moderate urinary and respiratory tract infections. Ciprofloxacin is also used for infectious diarrhea, typhoid fever, uncomplicated gonorrhea, treatment of Neisseria meningitides nasal carriage and prophylaxis against anthrax. Like other fluoroquinolones, ciprofloxacin is active against a wide range of aerobic gram-positive and gram-negative organisms. The fluoroquinolones are believed to act by inhibition of bacterial DNA gyrase and topoisomerase IV that are required for synthesis of bacterial mRNAs (transcription) and DNA replication. In contrast, DNA gyrases are not present in human [and other eukarotic] cells and the equivalent topoisomerases are not sensitive to fluoroquinolone inhibition. Ciprofloxacin was approved for use in the United States in 1990 and, currently, more than 4 million prescriptions are filled yearly. Ciprofloxacin is available in multiple oral formulations of 100, 250, 500 and 750 mg tablets and extended release formulations of 500 and 1000 mg tablets. Ciprofloxacin is available generically and under several commercial names including Cipro and Proquin. The usual oral dose is 250 to 500 mg every 12 hours. Oral formulations are recommended for mild-to-moderate infections due to susceptible organisms, including urinary tract infections, sinusitis, bronchitis, skin infections, urethral and cervical infections. Intravenous formulations are available for moderate to severe infections, including pneumonia, sinusitis, septicemia, intraabdominal and bone and joint infections, the usual dosages being 200 to 400 mg intravenously every 8 hours. Oral therapy is typically continued for 7 to 10 days, but both shorter and longer courses are used. Common side effects include gastrointestinal upset, headaches, skin rash and allergic reactions. Less common, but more severe side effects include prolongation of the QT interval, seizures, hallucinations, tendon rupture, angioedema, Stevens Johnson syndrome and photosensitivity. Because of the frequency of severe adverse events, fluoroquinolones should not be used as first line therapy of mild and moderate infections.
Hepatotoxicity
Ciprofloxacin like other fluoroquinolones is associated with a low rate (1% to 3%) of serum enzyme elevations during therapy. These abnormalities are generally mild, asymptomatic and transient, resolving even with continuation of therapy. More importantly, ciprofloxacin has been linked to rare, but occasionally severe and even fatal cases of acute liver injury. The time to onset is typically short (2 days to 2 weeks) and the presentation is often abrupt with nausea, fatigue and abdominal pain, followed by dark urine and jaundice. The pattern of serum enzyme elevations can be either hepatocellular or cholestatic; cases with the shorter times to onset usually being more hepatocellular with markedly elevated ALT levels, and occasionally with rapid worsening of prothrombin time and early signs of hepatic failure. The onset of illness also may occur a few days after the medication is stopped. Cases with a cholestatic pattern of enzymes may run a prolonged course, but are usually self-limiting. Nevertheless, chronic cholestasis and vanishing bile duct syndrome have been reported with ciprofloxacin and other fluoroquinolones. Finally, the enzyme pattern can be initially hepatocellular and then evolve during the course of illness from a hepatocellular into a mixed or cholestatic pattern. Many (but not all) cases have had allergic manifestations with fever, rash and eosinophilia. Autoantibodies are usually not present.
Likelihood score: A (well established but uncommon cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism of ciprofloxacin hepatotoxicity is suspected to be hypersensitivity. Rechallenge leads to recurrence with a shorter time to onset and more severe course and should be avoided.
Outcome and Management
Severity ranges from mild and transient serum enzyme elevations to a self-limited hepatitis, to prolonged cholestatic hepatitis to a fulminant hepatic failure. If not fatal during the acute phase, complete recovery is expected after stopping the drug and is usually rapid (2 to 4 weeks) depending upon the severity and degree of cholestasis. Some instances of cholestatic liver injury from ciprofloxacin have resulted in vanishing bile duct syndrome. Corticosteroids have been used with variable degrees of success. Cross reactivity of the hepatic injury between different fluoroquinolones has been demonstrated in a small number of cases, but should be assumed based upon the similarity of clinical patterns of injury and latency. Thus, patients should be advised to avoid further exposure to the fluoroquinolones.
Drug Class: Antiinfective Agents
Other Drugs in the Subclass, Fluoroquinolones: Delafloxacin, Gemifloxacin, Levofloxacin, Moxifloxacin, Norfloxacin, Ofloxacin
CASE REPORTS
Case 1. Cholestatic hepatitis due to ciprofloxacin therapy.(1)
An 84 year old female resident of a long term care facility received a course of ciprofloxacin for urinary tract infection. Six days later she was found to have a rash and ciprofloxacin was stopped. Nevertheless, 3 days after stopping she was noted to be jaundiced. Serum bilirubin rose to as high as 8.2 mg/dL, but she recovered within 6 weeks.
Key Points
Laboratory Values
Comment
This patient received multiple medications, but the timing of onset and pattern of injury was typical of fluoroquinolone induced liver injury with short latency and rapid recovery upon withdrawal. The cholestatic pattern of injury is somewhat atypical, but occurs when the jaundice is severe and prolonged, particularly in the elderly. The lack of blood tests at earlier time points precludes a full assessment of the enzyme response which may have been more hepatocellular at onset of symptoms. Interestingly, this patient had received two previous courses of ciprofloxacin and was said to be allergic to sulfonamides.
Case 2. Severe acute hepatitis due to ciprofloxacin therapy.(2)
An 80 year old female developed nausea, anorexia and increasing forgetfulness followed by jaundice starting the week after completing a 10 day course of ciprofloxacin (500 mg twice daily) and while taking metronidazole (250 mg twice daily). She had a past medical history of hypertension, congestive heart failure, atrial fibrillation, colonic polyps and depression. Her other medications included furosemide, atenolol, valsartan, warfarin, vitamins and ginkgo biloba. She also took occasional acetaminophen with or without oxycodone for pain. She had no history of liver disease or risk factors for viral hepatitis and did not drink alcohol. Two months before onset of jaundice, she had developed diarrhea and abdominal pain which was presumed to be diverticulitis and for which she was given the two antibiotics. During the week before admission, she had increasing fatigue and forgetfulness and the day before presentation developed pruritus, dark urine and jaundice. Physical examination was unremarkable except for jaundice. She did not have fever, rash, hepatomegaly, splenomegaly, ascites or asterixis. Laboratory testing showed total bilirubin of 9.6 mg/dL (direct 6.8 mg/dL), ALT 705 U/L and INR 8.2 (Table). There was mild eosinophilia. Serum antinuclear antibody was negative, smooth muscle antibody was weakly positive (1:20). She was admitted and all medications including warfarin were stopped. Because of concern over acute liver failure, she was transferred to a tertiary care hospital for management. Tests for hepatitis A, B and C (including HCV RNA) were negative as were tests for Epstein Barr virus and cytomegalovirus infection. CT scans showed mild hepatosplenomegaly, mild ascites, absence of a gall bladder and no evidence of biliary obstruction. A liver biopsy showed severe acute hepatitis with giant cells and bridging necrosis. She developed worsening jaundice, ascites, and hepatic encephalopathy and died of multiorgan failure 5 weeks after onset of jaundice.
Key Points
Laboratory Values
Comment
An example of acute hepatocellular injury attributed to ciprofloxacin. Metronidazole can also cause acute hepatocellular injury and acute liver failure, but such cases are exceedingly rare. In contrast, the precipitous onset and relatively short latency are typical of fluoroquinolone induced acute liver injury. Also, ciprofloxacin induced liver injury tends to be more common and more severe in the elderly.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Ciprofloxacin – Generic, Cipro®, Proquin®
DRUG CLASS
Antiinfective Agents
Product labeling at DailyMed, National Library of Medicine, NIH
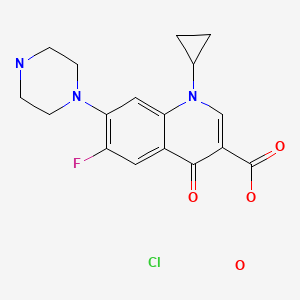
CHEMICAL FORMULA AND STRUCTURE
CITED REFERENCES
- 1.
- Sherman O, Beizer JL. Possible ciprofloxacin-induced acute cholestatic jaundice. Ann Pharmacother 1994; 28: 1162-4. [PubMed: 7841570]
- 2.
- Orman ES, Conjeevaram HS, Vuppalanchi R, Freston JW, Rochon J, Kleiner DE, Hayashi PH; DILIN Research Group. Clinical and histopathologic features of fluoroquinolone-induced liver injury. Clin Gastroenterol Hepatol 2011; 9: 517-23.e3. [PMC free article: PMC3718017] [PubMed: 21356330]
ANNOTATED BIBLIOGRAPHY
References updated: 09 May 2024
Abbreviations used: SJS, Stevens Johnson syndrome; TEN, toxic epidermal necrolysis.
- Zimmerman HJ. Quinolones. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999. p 603.(Expert review of hepatotoxicity published in 1999 mentions that cinoxacin, nalidixic acid, ciprofloxacin, norfloxacin, enoxacin, and ofloxacin are associated with minor serum enzyme elevations during therapy and with rare instances of clinically apparent liver injury).
- Moseley RH. Fluoroquinolones. Hepatotoxicity of antimicrobial and antifungal agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd Edition. Amsterdam: Elsevier, 2013. p. 468-9.(Review of hepatotoxicity of antibiotics mentions that hepatocellular and cholestatic forms of injury have been reported due to the quinolones, including cases of ductopenia, acute liver failure and death).
- MacDougall C. The quinolones. Sulfonamides, trimethoprim-sulfamethoxazole, quinolones, and agents for urinary tract infections. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1015-8.(Textbook of pharmacology and therapeutics).
- Halkin H. Adverse effects of the fluoroquinolones. Rev Infect Dis 1988; 10 (Suppl 1): S258-61. [PubMed: 3279499](Combined analysis of databases provided by manufacturers on adverse events of fluoroquinolones in approximately 30,000 persons receiving ciprofloxacin, ofloxacin, pefloxacin, norfloxacin and enoxacin found similar types and rates of adverse events among the agents, overall in 4-8%, elevated liver enzymes in 1.8-2.5%, but eosinophilia in 2.4% with ciprofloxacin and 5-19% with ofloxacin).
- Arcieri GM, Becker N, Esposito B, et al. Safety of intravenous ciprofloxacin. A review. Am J Med 1989; 87 (Suppl 5A): 92S-97S. [PubMed: 2686431](Summary on safety of intravenous ciprofloxacin based upon 1869 patients treated in 59 clinical trials; ALT elevations occurred in 2.0% and one patient had "hepatic necrosis").
- Kljucar S, Heimesaat M, von Pritzbuer E, Timm J, Scholl H, Beermann D. Efficacy and safety of higher-dose intravenous ciprofloxacin in severe hospital-acquired infections. Am J Med 1989; 87: 52S-56S. [PubMed: 2589385](Elevated ALT levels occurred in 7 of 54 patients given high dose intravenous ciprofloxacin; no symptomatic hepatotoxicity).
- Schacht P, Arcieri G, Hullmann R. Safety of oral ciprofloxacin. An update based on clinical trial results. Am J Med 1989; 87 (Suppl 5A): 98S-102S. [PubMed: 2686432](Industry report on 9473 courses of oral ciprofloxacin, usually given for 7-14 days: 9.3% had an adverse event, mostly mild, only 0.5% were serious. ALT elevations occurred in 1.3%, but jaundice in only 1 patient).
- Slama TG. Serum sickness-like illness associated with ciprofloxacin. Antimicrob Agents Chemother 1990; 34: 904-5. [PMC free article: PMC171716] [PubMed: 2360826](56 year old man developed rash, fever, arthralgias and eosinophilia after 6 days of ciprofloxacin accompanied by mild enzyme elevations [bilirubin normal, ALT 102-307 U/L, Alk P 128-178 U/L], resolving rapidly with corticosteroid therapy).
- Wolfson JS, Hooper DC. Overview of fluoroquinolone safety. Am J Med 1991; 91 (Suppl 6A): 153S-61S. [PubMed: 1767803](Review of side effects reported in 22 clinical trials of fluoroquinolones; elevations in ALT and/or Alk P levels occurred in 1.8-2.7% of patients on cipro-, nor-, or ofloxacin).
- Grassmick BK, Lehr VT, Sundareson AS. Fulminant hepatic failure possibly related to ciprofloxacin. Ann Pharmacother 1992; 26: 636-9. [PubMed: 1591420](66 year old man developed symptoms and jaundice after 1 day of starting ciprofloxacin [bilirubin 10.9 mg/dL, ALT 2608 U/L, Alk P 269 U/L] with shock, lactic acidosis and death 7 days later).
- Jick SS, Jick H, Dean AD. A follow-up safety study of ciprofloxacin users. Pharmacotherapy 1993; 13: 461-4. [PubMed: 8247912](Follow up of ~37,000 patients for 45 days after receiving oral ciprofloxacin, none developed clinically apparent liver disease).
- Levinson JR, Kumar A. Ciprofloxacin induced cholestatic jaundice: a case report. Am J Gastroenterol 1993; 88: 1619.(Abstract: 46 year old woman developed pruritus and jaundice within a few days of starting ciprofloxacin [bilirubin 10.6 mg/dL, ALT 519 U/L, Alk P 1459 U/L], resolving several weeks after stopping).
- Fuchs S, Simon Z, Brezin M. Fatal hepatic failure associated with ciprofloxacin. Lancet 1994; 343: 738-9. [PubMed: 7907714](92 year old man developed "progressive hepatic failure" after 2 days of IV ciprofloxacin; no details given).
- Sherman O, Beizer JL. Possible ciprofloxacin-induced acute cholestatic jaundice. Ann Pharmacother 1994; 28: 1162-4. [PubMed: 7841570](84 year old woman developed jaundice and rash after 7 days of ciprofloxacin therapy [bilirubin 8.2 mg/dL, ALT 126 U/L, Alk P 1119 U/L, resolving over ensuing 6 weeks: Case 1).
- Ball P, Tillotson G. Tolerability of fluoroquinolone antibiotics. Past, present and future. Drug Saf 1995; 13: 343-58. [PubMed: 8652079](Review of the nature and frequency of adverse reactions to the fluoroquinolones mentions that clinically apparent liver injury is rare, but that severe and occasional fatal cases have been reported with ciprofloxacin, levofloxacin, norfloxacin and ofloxacin and from postmarketing surveillance has estimated the frequency of clinically apparent liver injury to be 0.8 per 100,000 recipients).
- Aggarwal A, Gurka J. Probable ciprofloxacin induced cholestasis. Aust N Z J Med 1995; 25: 541-2. [PubMed: 8588783](36 year old man developed marked Alk P elevations [peak 792 U/L] with minimal increases in ALT [61 U/L], without symptoms or jaundice within a week of starting ciprofloxacin, falling to baseline within 6 weeks of stopping).
- Alcalde M, Donoso MS, Carcfa-Diaz M, Pascasio JM, Narvaez I. Liver dysfunction due to ciprofloxacin. Acta Gastroenterol Belg 1995; 58: 475-6. [PubMed: 8776005](27 year old man developed fatigue after 10 days of ciprofloxacin therapy [bilirubin 2.5 mg/dL, ALT 248 U/L, Alk P not available], resolving within 2 months of stopping).
- Hautekeete ML, Kockx MM, Naegels S, Holvoet JK, Hubens H, Kloppel G. Cholestatic hepatitis related to quinolones: a report of two cases. J Hepatol 1995; 23: 759-60. [PubMed: 8750178](50 year old man developed fatigue after 5 days of oral ciprofloxacin [bilirubin 4.8 rising to 9.3 mg/dL, ALT 202 U/L, Alk P 314 U/L], resolving within 9 weeks of stopping; second case due to ofloxacin).
- Villeneuve JP, Davies C, Côté J. Suspected ciprofloxacin-induced hepatotoxicity. Ann Pharmacother 1995; 29: 257-9. [PubMed: 7606070](44 year old woman developed jaundice 2 weeks after finishing a 7 day course of ciprofloxacin [bilirubin 9.6 mg/dL, ALT 745 U/L, Alk P 284 U/L], with full recovery requiring 5 months).
- Labowitz JK, Silverman WB. Cholestatic jaundice induced by ciprofloxacin. Dig Dis Sci 1997; 42: 192-4. [PubMed: 9009137](47 year old man developed jaundice and pruritus within 2 days of starting ciprofloxacin [bilirubin 10 rising to 34 mg/dL, ALT 308 U/L, Alk P 163 U/L], resolving slowly to normal over next 12 weeks).
- Jones SE, Smith RH. Quinolones may induce hepatitis. BMJ 1997; 314(7084): 869. [PMC free article: PMC2126221] [PubMed: 9093098](21 year old man developed jaundice following a 5 day course of ofloxacin and two doses of ciprofloxacin [bilirubin 5.7 rising to 8.2 mg/dL, AST 348 U/L, Alk P 321 U/L], worsening for 7 days and then resolving within 5 weeks of stopping).
- Contreras MA, Luna R, Mulero J, Andreu JL. Severe ciprofloxacin-induced acute hepatitis. Eur J Clin Microbiol Infect Dis 2001; 20: 434-5. [PubMed: 11476450](32 year old man developed abdominal pain, fever and rash within 2 days of starting ciprofloxacin [bilirubin not given, ALT 147 rising to 2144 U/L, GGT 98 U/L], with subsequent signs of liver failure, but ultimate recovery after methylprednisolone therapy).
- Morelli MS, O'Brien FX. Stevens-Johnson Syndrome and cholestatic hepatitis. Dig Dis Sci 2001; 46: 2385-8. [PubMed: 11713940](19 year old woman developed fever followed by rash and jaundice 4 days after starting ibuprofen and 2 days after starting metoclopramide and ketorolac [bilirubin 3.3 rising to 9.2 mg/dL, ALT 300 U/L, Alk P 409 U/L], with worsening fever and rash diagnosed as Stevens Johnson syndrome [SJS], resolving without corticosteroid therapy).
- Bataille L, Rahier J, Geubel A. Delayed and prolonged cholestatic hepatitis with ductopenia after long-term ciprofloxacin therapy for Crohn's disease. J Hepatol 2002; 37: 696-9. [PubMed: 12399240](63 year old woman with Crohn disease developed jaundice while being treated with ciprofloxacin and ornidazole [a nitroimidazole derivative also linked to cases of cholestatic jaundice] for 6 months [bilirubin 3.4 rising to 8.1 mg/dL, ALT 740 U/L, Alk P 662 U/L], liver biopsy showing paucity of bile ducts, slowly resolving over the 8 months after discontinuation of both drugs).
- Hällgren J, Tengvall-Linder M, Persson M, Wahlgren CF. Stevens-Johnson syndrome associated with ciprofloxacin: a review of adverse cutaneous events reported in Sweden as associated with this drug. J Am Acad Dermatol 2003; 49 (5 Suppl): S267-9. [PubMed: 14576649](31 and 33 year old women developed rash and oral ulcers diagnosed as SJS 2 and 8 days after starting ciprofloxacin [one with serum enzyme elevations without jaundice], and review of Swedish Drug Information System found 8 other cases of serious cutaneous reactions due to ciprofloxacin).
- Goetz M, Galle PR, Schwarting A. Non-fatal acute liver injury possibly related to high-dose ciprofloxacin. Eur J Clin Microbiol Infect Dis 2003; 22: 294-6. [PubMed: 12739107](79 year old woman developed confusion and lactic acidosis within 2 days of starting iv ciprofloxacin [peak bilirubin 1.6 mg/dL, ALT 4878 U/L, Alk P 581 U/L, LDH 6111 U/L], resolving within 2 weeks).
- Zaidi SA. Hepatitis associated with amoxicillin/clavulanic acid and/or ciprofloxacin. Am J Med Sci 2003; 325: 31-3. [PubMed: 12544082](80 year old man received both ciprofloxacin and amoxicillin/clavulanate, developing rash and eosinophilia within 1 week followed by self-limiting serum enzyme elevations without jaundice [bilirubin 1.9 mg/dL, ALT 972 U/L, Alk P 358 U/L]).
- Zimpfer A, Propst A, Mikuz G, Vogel W, Terracciano L, Stadlmann S. Ciprofloxacin-induced acute liver injury: case report and review of literature. Virchows Arch 2004; 444: 87-9. [PubMed: 14994731](22 year old man developed jaundice 1 week after finishing a 7 day course of ciprofloxacin [bilirubin 16.9 mg/dL, ALT 890 U/L, Alk P 180 U/L], symptoms and liver test abnormalities resolving within a week of starting methylprednisolone).
- Thakur BS, Jain AK, Sirkar S, Joshi G, Joshi R. Ciprofloxacin-induced cholestatic jaundice. Indian J Gastroenterol 2007; 26 (1): 51-2. [PubMed: 17401251](26 year old man developed rash and then jaundice within 5 days of starting ciprofloxacin [ALT 1700 U/L], with slow recovery by 6 months after onset).
- Bhagirath KM. A case report of highly suspected ciprofloxacin-induced hepatotoxicity. Turk J Gastroenterol 2008; 19: 204-6. [PubMed: 19115163](39 year old woman developed jaundice 1 month after a 14 day course of ciprofloxacin and metronidazole [bilirubin 16.3 mg/dL, ALT 1406 U/L, Alk P 160 U/L, IRN 1.6], resolving within the next 3 months with prednisone therapy).
- Dichiara AJ, Atkinson M, Goodman Z, Sherman KE. Ciprofloxacin-induced acute cholestatic liver injury and associated renal failure. Case report and review. Minerva Gastroenterol Dietol 2008; 54: 307-15. [PubMed: 18614979](65 year old man developed jaundice 6 days into a course of ciprofloxacin for cellulitis [bilirubin 8.5 rising to 25 mg/dL, ALT 157 U/L, Alk P 711 U/L], with concurrent renal failure, and slow but eventual recovery 5 months later).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, the fluoroquinolones accounting for 10 cases [3%], including 5 attributed to ciprofloxacin [ranking 8th], 4 to levofloxacin, and 1 to moxifloxacin).
- Okan G, Yaylaci S, Peker O, Kaymakoglu S, Saruc M. Vanishing bile duct and Stevens-Johnson syndrome associated with ciprofloxacin treated with tacrolimus. World J Gastroenterol 2008; 14: 4697-700. [PMC free article: PMC2738797] [PubMed: 18698687](26 year old woman developed fever and rash 2 weeks after starting ciprofloxacin [bilirubin 4.1 rising to 34 mg/dL, ALT 326 U/L, Alk P 229 U/L], biopsies showing SJS and bile duct loss, and ultimately improving and recovery with ursodiol, prednisone and tacrolimus therapy).
- Miftode E, Leca D, Luca V. [A severe case of infectious mononucleosis associated with ciprofloxacin and salazopyrin administration]. Rev Med Chir Soc Med Nat Iasi 2008; 112: 652-5. Romanian. [PubMed: 20201247](22 year old woman developed rash and severe hepatitis 32 days after starting sulfasalazine and 2 days after ciprofloxacin [bilirubin 36 mg/dL, ALT 488 U/L]).
- Cholongitas E, Georgousaki C, Spyrou S, Dasenaki M. Ciprofloxacin-induced acute cholestatic hepatitis. Ann Hepatol 2009; 8: 400-1. [PubMed: 20009146](66 year old man developed liver enzyme elevations 3 days after starting intravenous ciprofloxacin [direct bilirubin 1.9, ALT 582 U/L, Alk P 1234 U/L], with worsening for 7 days and then resolving within 3 months of stopping).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, of which 1 was attributed to ciprofloxacin, but none to other fluoroquinolones).
- Alan C, Koçoğlu H, Ersay AR, Ertung Y, Kurt HA. Unexpected severe hepatotoxicity of ciprofloxacine: two case reports. Drug Chem Toxicol 2011; 34(2): 189-91. [PubMed: 21314468](Two cases; 56 and 62 year old men developed liver injury 2 and 5 days after starting ciprofloxacin [bilirubin ~5.0 mg/dL, ALT 500 and 320 U/L, Alk P 152 and 132 U/L, prothrombin time 57 and 34 secs], both recovering rapidly upon stopping).
- Orman ES, Conjeevaram HS, Vuppalanchi R, Freston JW, Rochon J, Kleiner DE, Hayashi PH; DILIN Research Group. Clinical and histopathologic features of fluoroquinolone-induced liver injury. Clin Gastroenterol Hepatol 2011; 9: 517-23.e3. [PMC free article: PMC3718017] [PubMed: 21356330](Among 679 cases of drug induced liver injury presenting between 2004 and 2010 at 8 US medical centers, 12 [1.8%] were attributed to fluoroquinolones including 6 cipro-, 4 moxi-, 1 levo-, and 1 gatifloxacin; average time to onset was 4 days [range 1-39], with both hepatocellular and cholestatic enzyme patterns, 7 with rash or fever, mortality limited to those with hepatocellular injury and jaundice; the pattern of hepatic injury appeared to be shared among the fluoroquinolones: Case 2).
- Paterson JM, Mamdani MM, Manno M, Juurlink DN; Canadian Drug Safety and Effectiveness Research Network. Fluoroquinolone therapy and idiosyncratic acute liver injury: a population-based study. CMAJ 2012; 184: 1565-70. [PMC free article: PMC3470619] [PubMed: 22891208](In a population based study using Canadian health care databases, the risk of admission to hospital for acute liver injury was increased for persons who received a prescription for moxifloxacin or levofloxacin relative to clarithromycin, but not for ciprofloxacin).
- Hayashi PH, Chalasani NP. Liver injury in the elderly due to fluoroquinolones: should these drugs be avoided? CMAJ 2012; 184: 1555-6. [PMC free article: PMC3470615] [PubMed: 22891207](Editorial in response to Paterson [2013] stressing the low absolute risk of liver injury from the fluoroquinolones [4-9 per 100,000 exposures]).
- Kwon H, Lee SH, Kim SE, Lee JH, Jee YK, Kang HR, Park BJ, et al. Spontaneously reported hepatic adverse drug events in Korea: multicenter study. J Korean Med Sci 2012; 27: 268-73. [PMC free article: PMC3286773] [PubMed: 22379337](Summary of 2 years of adverse event reporting in Korea; of 9360 reports, 567 were liver related, including 29 [5.1%] attributed to quinolones).
- Harr T, French LE. Stevens-Johnson syndrome and toxic epidermal necrolysis. Chem Immunol Allergy 2012; 97: 149-66. [PubMed: 22613860](Review of the clinical features, epidemiology, genetics and pathogenesis of SJS and TEN).
- Patel TK, Barvaliya MJ, Sharma D, Tripathi C. A systematic review of the drug-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Indian population. Indian J Dermatol Venereol Leprol 2013; 79: 389-98. [PubMed: 23619444](Systematic review of 10 case series of SJS/TEN from India identified 352 cases, among which 342 implicated a medication with most common being antimicrobials [37%], anticonvulsants [16%] and NSAIDs [16%]; fluoroquinolones accounted for 33 cases [10%], 4 of which were due to ciprofloxacin, 1 ofloxacin and 1 levofloxacin).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013; 144: 1419-25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, none of which were attributed to ciprofloxacin or other fluoroquinolones).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol 2014; 13: 231-9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, one due to trovafloxacin [acute liver failure], but none attributed to ciprofloxacin or other fluoroquinolones).
- Alshammari TM, Larrat EP, Morrill HJ, Caffrey AR, Quilliam BJ, LaPlante KL. Risk of hepatotoxicity associated with fluoroquinolones: a national case-control safety study. Am J Health Syst Pharm 2014; 71: 37-43. [PubMed: 24352180](Retrospective analysis of Veterans Affairs patients receiving a fluoroquinolone [n=7862] found a higher relative risk of developing acute liver injury after receipt of ciprofloxacin compared to matched controls [adjusted odds ratio: OR=1.29], but not after receipt of levofloxacin [OR=1.16) or moxifloxacin [OR=0.98]).
- Goldberg DS, Forde KA, Carbonari DM, Lewis JD, Leidl KB, Reddy KR, Haynes K, et al. Population-representative incidence of drug-induced acute liver failure based on an analysis of an integrated health care system. Gastroenterology 2015; 148: 1353-61.e3. [PMC free article: PMC4446162] [PubMed: 25733099](Analysis of Kaiser Permanente health care database from 2004 to 2011 identified 62 patients with suspected acute liver failure, 32 [52%] of whom had a presumed drug etiology, the most common being acetaminophen [18: 56%] and various herbal products [5: 16%], with single instances attributed to imatinib, simvastatin, leflunomide, isoniazid and valproate, but none to ciprofloxacin or other fluoroquinolones).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 38 cases [4%] were attributed to fluoroquinolones, including 16 due to ciprofloxacin [the 8th most common prescription drug cause], 13 due to levofloxacin and 8 to moxifloxacin).
- Moreno L, Sánchez-Delgado J, Vergara M, Casas M, Miquel M, Dalmau B. Recurrent drug-induced liver injury (DILI) with ciprofloxacin and amoxicillin/clavulanic. Rev Esp Enferm Dig 2015; 107: 767-8. [PubMed: 26671593](56 year old woman developed pruritus and dark urine 4 and jaundice 10 days after starting ciprofloxacin [bilirubin 9.5 mg, ALT 506 U/L, Alk P 455 U/L, eosinophils 10%, INR 0.98] and, after recovery, developed jaundice again 9 days after starting amoxicillin/clavulanate [bilirubin 8.5 mg/dL, ALT 692 U/L, Alk P 348 U/L, eosinophils 3%], no information on recovery).
- Kaddu-Mulindwa D, Lammert F, Maßmann A, Link A. [Acute liver failure after ingestion of ciprofloxacin]. Dtsch Med Wochenschr 2016; 141: 1173-6. [PubMed: 27509349](23 year old woman developed nausea 1 day and jaundice 5 days after starting ciprofloxacin [bilirubin 10.8 mg/dL, ALT 3431 U/L, Alk P 152 U/L, INR 2.5], with severe but self-limiting course, resolving completely over the next 6 months).
- Goldie FC, Brogan A, Boyle JG. Ciprofloxacin and statin interaction: a cautionary tale of rhabdomyolysis. BMJ Case Rep 2016; 2016. pii: bcr2016216048. [PMC free article: PMC4986139] [PubMed: 27469384](62 year old woman on long term simvastatin developed rhabdomyolysis 4 days after starting ciprofloxacin [bilirubin and Alk P not given, ALT 240 U/L, CPK 24524 U/L], resolving with stopping both drugs, ALT elevations likely due to muscle rather than liver injury).
- Bonkovsky HL, Kleiner DE, Gu J, Odin JA, Russo MW, Navarro VM, Fontana RJ, Ghabril MS, et al.; U.S. Drug Induced Liver Injury Network Investigators. Clinical presentations and outcomes of bile duct loss caused by drugs and herbal and dietary supplements. Hepatology 2017; 65: 1267-77. [PMC free article: PMC5360519] [PubMed: 27981596](Among 363 patients with drug induced liver injury who underwent liver biopsy, 26 [7%] had bile duct loss of whom 94% developed evidence of chronic liver injury suggestive of vanishing bile duct syndrome, 2 of which were due to fluoroquinolones, 1 moxifloxacin and 1 levofloxacin).
- Comparison table: some systemic fluoroquinolones. Med Lett Drugs Ther 2018; 60: e57-e58. [PubMed: 29635268](Table comparing 4 fluoroquinolones [cipro-, levo-, dela- and moxifloxacin] mentions that ALT and AST elevations are a class adverse event).
- Mavros MN, Theochari NA, Kyriakidou M, Economopoulos KP, Sava JA, Falagas ME. Fluoroquinolone-based versus β-lactam-based regimens for complicated intra-abdominal infections: a meta-analysis of randomised controlled trials. Int J Antimicrob Agents 2019; 53: 746-54. [PubMed: 30639629](Systematic review of controlled trials of fluoroquinolones versus β-lactam-based antibiotic regimens found similar rates of efficacy and adverse events, no discussion of ALT elevations or liver related toxicities).
- Kuula LSM, Viljemaa KM, Backman JT, Blom M. Fluoroquinolone-related adverse events resulting in health service use and costs: A systematic review. PLoS One 2019; 14: e0216029. [PMC free article: PMC6485715] [PubMed: 31026286](Systematic review of observational studies on safety of fluoroquinolones concluded that due to lack of published literature, health service and costs could not be evaluated).
- Napier DJ, Bevan AV, di Mambro A. A fatal case of ciprofloxacin-induced fulminant hepatitis. Eur J Case Rep Intern Med. 2020;7:001612. [PMC free article: PMC7473698] [PubMed: 32908821](72 year old man with Waldenstrom’s macroglobulinemia developed dark urine and jaundice 3 days after starting ciprofloxacin for suspected pneumonia [bilirubin 9.2 mg/dL, ALT 1779 U/L, Alk P 234 U/L, protime 15 sec], with rapid progression and death from hepatic failure 46 days after presentation).
- Ahmad W, Waqar M, Hadi MH, Muhammad AS, Iqbal N. Acute cholestatic liver injury due to ciprofloxacin in a young healthy adult. Cureus. 2021;13:e13340. [PMC free article: PMC7967918] [PubMed: 33747648](32 year old woman developed pruritus and jaundice 2 weeks after starting a 10-day course of ciprofloxacin for a urinary tract infection [bilirubin 16.7 mg/dL, ALT 172 U/L, Alk P 866 U/L], biopsy showing cholestatic hepatitis and improvement after stopping ciprofloxacin with normal liver tests 6 weeks later).
- Gu S, Rajendiran G, Forest K, Tran TC, Denny JC, Larson EA, Wilke RA. Drug-induced liver injury with commonly used antibiotics in the All of Us Research Program. Clin Pharmacol Ther. 2023;114:404-412. [PMC free article: PMC10484299] [PubMed: 37150941](Analysis of electronic health records from a large cohort of US subjects [n=318,598] identified 44,202 who received a course of ciprofloxacin, of whom 24 developed liver injury attributable to the drug, yielding a rate of 54 per 100,000 or 1:1,852 persons exposed).
- Mąsior MN, Rostkowska OM, Furmańczyk-Zawiska A, Wieczorek-Godlewska R, Wyzgał M, Durlik M. DRESS syndrome: renal involvement in two cases - a comprehensive analysis and literature review of improved diagnosis and treatment. Am J Case Rep. 2024;25:e942315. [PMC free article: PMC10792591] [PubMed: 38204155](Description of 2 patients with DRESS syndrome, the first an 85 year old woman who developed diarrhea, fever, and rash with edema 4 weeks after stating ciprofloxacin and 3 weeks after starting piperacillin/tazobactam for a surgical site infection [eosinophils 5430/uL, liver tests normal, creatinine 4.2 mg/dL], which resolved slowly after stopping both antibiotics).
Publication Details
Publication History
Last Update: May 9, 2024.
Copyright
Publisher
National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda (MD)
NLM Citation
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Ciprofloxacin. [Updated 2024 May 9].