OVERVIEW
Introduction
Esketamine is unique antidepressant administered as an intranasal spray as therapy of treatment- resistant depression in adults. Esketamine has not been associated with serum enzyme elevations during treatment nor to instances of clinically apparent acute liver injury.
Background
Esketamine (es ket’ a meen) is an N-methyl-D-aspartate (NMDA) receptor antagonist that is used as an intranasal spray for treatment of adults with treatment-resistant depression. Esketamine is as isomer of ketamine, an anesthetic that was largely abandoned because of adverse events including dissociation syndrome. Ketamine later became a drug of abuse because of its psychotropic activities including dissociation and euphoria. Ketamine was used off label and experimentally to treat major depression and appeared to have rapid dramatic effects in anecdotal reports and in small controlled trials. Esketamine, the S-enantiomer of ketamine, was claimed to have more of an antidepressant and less sedative effect and was developed specifically as a therapy for treatment-resistant major depression. Multiple randomized controlled trials demonstrated that the addition of nasal spray esketamine to oral antidepressants significantly improved depression, as measured by standard scoring systems, in comparison to placebo in patients who had failed to respond to standard antidepressants. Esketamine was approved for this use in the United States in 2019. It is available under the commercial name Spravato as a nasal spray administered under medical supervision twice weekly for the first 4 weeks and then once weekly for 4 weeks of treatment, followed by once weekly or every other week thereafter. Esketamine is considered a Schedule III drug because of its potential for addiction and abuse. It has many adverse effects for which reason it is available only as a part of an FDA Risk Evaluation and Mitigation Strategy (REMS) program requiring training in its administration and direct monitoring of its effects. Common side effects include sedation and feelings of dissociation, dizziness, vertigo, nausea, anxiety, lethargy, hypoesthesia, nausea and vomiting, cystitis and lower urinary tract symptoms, and increases in blood pressure (within 1 to 2 hours of administration). Less common but potentially severe adverse events include suicidal ideation and behaviors, severe dissociation syndromes, interstitial cystitis, and embryo-fetal toxicity.
Hepatotoxicity
In patients on oral antidepressants, liver test abnormalities were no more frequent with the addition of nasal spray esketamine than with placebo. In the pivotal trials of esketamine as therapy of treatment- resistant depression, mean serum ALT, AST and alkaline phosphatase levels decreased during active therapy and there were no reports of serum enzyme elevations, jaundice, hepatitis, discontinuations for serum enzyme elevations or serious hepatic adverse events. Although long term ketamine use is known to be associated with bile duct injury and episodes of cholestatic jaundice, esketamine has not been linked to a similar pattern of biliary injury or cholestatic hepatitis when used under medical supervision to treat depression. There has been little clinical experience with long term use of esketamine, but no instances of clinically apparent liver injury have as yet been reported with its use.
Likelihood score: E* (unproven but suspected potential cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which esketamine might cause liver injury is not known. Esketamine is metabolized by the liver via the microsomal cytochrome P450 system (predominantly CYP 3A4 and 2B6), but plasma levels are minimally affected by agents that induce or inhibit the microsomal enzyme system. The liver injury reported with ketamine use appeared to be due to bile duct epithelial injury leading to bile duct strictures and cholestatic liver injury, in a manner similar to the urothelial cell injury and ureteral strictures that has been well documented to occur with ketamine use and abuse.
Drug Class: Antidepressant Agents
Other Drugs in the Subclass: Ketamine
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Esketamine – Spravato®
DRUG CLASS
Antidepressant Agents
Product labeling at DailyMed, National Library of Medicine, NIH
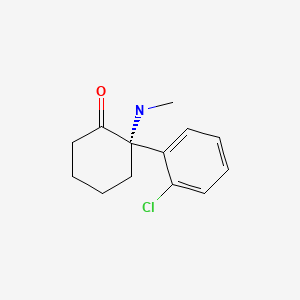
CHEMICAL FORMULA AND STRUCTURE
ANNOTATED BIBLIOGRAPHY
References updated: 18 October 2019
- Zimmerman HJ. Antidepressants. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 493-8.(Expert review of hepatotoxicity of antidepressants published in 1999; esketamine and ketamine are not discussed).
- Larrey D, Ripault MP. Hepatotoxicity of psychotropic drugs and drugs of abuse. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 443-62.(Review of antidepressant hepatotoxicity; ketamine and esketamine are not discussed).
- O'Donnell JM, Shelton RC. Pharmacotherapy of depression and anxiety disorders. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 267-78.(Textbook of pharmacology and therapeutics).
- https://www
.accessdata .fda.gov/drugsatfda_docs /nda/2019/211243Orig1s000MedR.pdf . (FDA website with medical review of efficacy and safety of esketamine in support of its approval; laboratory test results discussed on pages 215-6). - Voican CS, Corruble E, Naveau S, Perlemuter G. Antidepressant-induced liver injury: a review for clinicians. Am J Psychiatry. 2014;171:404–15. [PubMed: 24362450](Review of the frequency and clinical features of drug induced liver injury due to antidepressants; SSRIs such as venlafaxine, fluoxetine, sertraline and paroxetine are discussed, but not esketamine).
- Singh JB, Fedgchin M, Daly E, Xi L, Melman C, De Bruecker G, Tadic A, et al. Intravenous esketamine in adult treatment-resistant depression: a double-blind, double-randomization, placebo-controlled study. Biol Psychiatry. 2016;80:424–31. [PubMed: 26707087](Among 30 patients with treatment resistant depression treated with single intravenous doses of esketamine [0.2 or 0.4 mg/kg] or placebo, depression scales improved more with esketamine [-16.8 and -16.9] than placebo [-3.8] and side effects were somewhat dose related, particularly dissociation, but “no clinically significant changes in laboratory tests … were observed”).
- Canuso CM, Singh JB, Fedgchin M, Alphs L, Lane R, Lim P, Pinter C, et al. Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am J Psychiatry. 2018;175:620–30. [PubMed: 29656663](Among 68 patients with major depression on oral antidepressants treated with addition of esketamine [84 mg] or placebo intranasally twice weekly for 4 weeks, depression scores were significantly improved compared to placebo at 4 and 24 hours but not at 25 days, and side effects were greater with esketamine including nausea [37% vs 3%], dizziness [31% vs 13%], headache [31% vs 26%] and dissociation [31% vs 13%]; no mention of ALT elevations or laboratory abnormalities).
- Morrison RL, Fedgchin M, Singh J, Van Gerven J, Zuiker R, Lim KS, van der Ark P, et al. Effect of intranasal esketamine on cognitive functioning in healthy participants: a randomized, double-blind, placebo-controlled study. Psychopharmacology (Berl). 2018;235:1107–19. [PMC free article: PMC5869899] [PubMed: 29392371](Among 24 healthy adult volunteers treated with a single dose of intranasal esketamine [84 mg] or placebo in a crossover design, cognitive performance was impaired at 40 minutes but not 2, 4 or 6 hours after esketamine dosing and “overall, no clinically significant effect on the laboratory parameters … was observed”).
- Daly EJ, Singh JB, Fedgchin M, Cooper K, Lim P, Shelton RC, Thase ME, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2018;75:139–48. [PMC free article: PMC5838571] [PubMed: 29282469](Among 67 patients with treatment resistant depression treated with esketamine [28, 56 or 84 mg] intranasally twice weekly for 2 weeks followed by open label therapy, changes in depression rating scores were greater with esketamine in a dose related manner while common side effects were dizziness, nausea, headache, hypertension and dissociation, 3 subjects [5%] stopping therapy early; no mention of laboratory test abnormalities).
- Molero P, Ramos-Quiroga JA, Martin-Santos R, Calvo-Sánchez E, Gutiérrez-Rojas L, Meana JJ. Antidepressant efficacy and tolerability of ketamine and esketamine: a critical review. CNS Drugs. 2018;32:411–20. [PubMed: 29736744](Review of the mechanism of action and history of clinical evaluation of ketamine and esketamine as therapy of depression, and discussion of controversy of their safety and long term efficacy in comparison to conventional treatments of depression).
- Short B, Fong J, Galvez V, Shelker W, Loo CK. Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry. 2018;5:65–78. [PubMed: 28757132](Review of the reported side effects of ketamine use in depression based upon 60 studies in 899 patients, usually short term [80%] using iv therapy [82%], the most common effects being psychiatric including anxiety, sedation and dissociation; liver injury has been reported anecdotally and with prolonged infusions or ketamine abuse).
- Popova V, Daly EJ, Trivedi M, Cooper K, Lane R, Lim P, Mazzucco C, et al. Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: a randomized double-blind active-controlled study. Am J Psychiatry. 2019;176:428–38. [PubMed: 31109201](Among 67 adults with treatment resistant depression started on a new antidepressant combined with esketamine [54 or 86 mg] or placebo nasal spray twice weekly for 4 weeks, depression scores improved more with esketamine [-21 vs -17], and side effects were greater including dissociation [26% vs 4%], nausea [26% vs 6%] and vertigo [26% vs 3%], leading 7% of patients to stop therapy; no mention of ALT or laboratory test abnormalities).
- Kim J, Farchione T, Potter A, Chen Q, Temple R. Esketamine for treatment-resistant depression - first FDA-approved antidepressant in a new class. N Engl J Med. 2019;381:1–4. [PubMed: 31116916](Review of the FDA assessment of efficacy and safety of esketamine that led to the approval as therapy of treatment resistant depression and requirement for a REMS program for monitoring based upon its common side effects and potential for abuse, but also because of lack of data on its long term efficacy and safety).
- Esketamine nasal spray (Spravato) for treatment-resistant depression. Med Lett Drugs Ther. 2019;61(1569):54–7. [PubMed: 31169797](Concise review of the mechanism of action, clinical efficacy, safety and costs of esketamine nasal spray shortly after its approval as therapy of treatment resistant depression, does not mention hepatotoxicity or serum enzyme elevations as a potential side effect of its use).
- Daly EJ, Trivedi MH, Janik A, Li H, Zhang Y, Li X, Lane R, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatr 2019 Jun 5. [Epub ahead of print] [PMC free article: PMC6551577] [PubMed: 31166571](Among 297 patients who achieved remission or a stable response on a 16 week course of an oral antidepressant and nasal spray esketamine and were then either continued on esketamine or switched to placebo, relapses occurred more frequently with placebo [45% and 58%] than with continuing esketamine [27% and 26%], but side effects were less; no mention of ALT or other laboratory test abnormalities).
- Fedgchin M, Trivedi M, Daly EJ, Melkote R, Lane R, Lim P, Vitagliano D, et al. Efficacy and safety of fixed-dose esketamine nasal spray combined with a new oral antidepressant in treatment-resistant depression: results of a randomized, double-blind, active-controlled study (TRANSFORM-1). Int J Neuropsychopharmacol. 2019 Jul 10; [Epub ahead of print] [PMC free article: PMC6822141] [PubMed: 31290965](Among 346 patients with treatment resistant depression treated with an oral antidepressant and nasal spray esketamine [56 or 84 mg] or placebo twice weekly for 4 weeks, depression scores decreased more on esketamine than placebo, but the differences were not statistically significant at week 4, and side effects were greater with esketamine; no mention of ALT or laboratory abnormalities).
- Ritter P, Findeis H, Bauer M. Ketamine in the treatment of depressive episodes. Pharmacopsychiatry. 2019 Aug 21; [Epub ahead of print] [PubMed: 31434140](Review of the chemistry, mechanism of action and clinical efficacy of ketamine as a therapy of major depression, in which it demonstrates dramatic rapid clinical effects, but usually in studies that could not be “blinded” because of its side effects; most studies using intravenous therapy, careful monitoring and thrice weekly infusions for optimal effects).
Publication Details
Publication History
Last Update: October 18, 2019.
Copyright
Publisher
National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda (MD)
NLM Citation
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Esketamine. [Updated 2019 Oct 18].