OVERVIEW
Introduction
Tenofovir is an acyclic nucleotide analogue of adenosine used in combination with other agents in the therapy of the human immunodeficiency virus (HIV) and as single agent in hepatitis B virus (HBV) infection. Tenofovir does not appear to be a significant cause of drug induced liver injury.
Background
Tenofovir (ten of' oh vir) is an acyclic nucleotide analogue of adenosine, but is poorly absorbed orally. For this reason, the prodrug is used, either tenofovir disoproxil fumarate or more recently tenofovir alafenamide, which are well absorbed from the intestines, rapidly hydrolyzed to tenofovir intracellularly and then phosphorylated to the active form, tenofovir diphosphate. Tenofovir diphosphate is a competitive inhibitor of the HIV reverse transcriptase (and the HBV polymerase) and is also incorporated into the nascent DNA strand causing chain termination. Tenofovir disoproxil fumarate (TDF) was approved for use in HIV infection in the United States in 2001 and for use in hepatitis B in 2008. Tenofovir alafenamide (TAF) was approved in 2015 for use both in HIV and HBV infection. Clinical indications include treatment and prevention of HIV infection, usually in combination with other reverse transcriptase, protease or integrase inhibitors. Tenofovir is also approved for use in chronic hepatitis B as a single agent. TDF is available generically and under the brand name Viread in 300 mg oral tablets. TAF is available under the brand name Vemlidy in 25 mg tablets. Both TDF and TAF are also available in various fixed combinations with other antiviral agents for use in treatment of HIV infection, usually in a single oral daily dose that is often referred to as a “single tablet regimen” (STR) which is extremely helpful in insuring compliance with HIV therapy. Examples of some of the single tablet regimen combinations and their brand names and doses are given in the Table below. The recommended daily dose of TDF in adults is 300 mg and of TAF is 25 mg, the lower dose of TAF being considered important in decreasing long term side effects, particularly those on phosphate metabolism and bone and kidney effects. Side effects of tenofovir are not common but can include asthenia, diarrhea, flatulence, nausea and vomiting, headache, renal dysfunction and rash. Rare but potentially severe adverse events associated with long term therapy include lactic acidosis and liver failure when given with other antiretroviral agents, severe withdrawal flares of hepatitis B upon discontinuation of tenofovir therapy, osteoporosis, and phosphate wasting proximal tubular dysfunction, renal tubular acidosis and renal failure. The bone and renal effects of tenofovir are believed to be less common with TAF than TDF.
Table: Tenofovir Combination Single Tablet Regimens Approved for HIV Therapy*†
Hepatotoxicity
Like all nucleoside analogues used as therapy of hepatitis B, tenofovir can cause transient increases in serum aminotransferases during or after therapy. These abnormalities appear to be due to an exacerbation or flare of the underlying hepatitis B. Three types of flares due to nucleoside analogue therapy have been described: transient flares during initiation of therapy (treatment flares), flares occurring in association with development of antiviral resistance (breakthrough flares) and flares occurring in the few months after stopping therapy (withdrawal flares). Treatment flares generally arise during the first few months of starting therapy, are usually mild, asymptomatic and self-limited and do not require dose modification or interruption of therapy. Breakthrough flares generally follow the development of antiviral resistance and subsequent rise in HBV DNA levels during nucleoside analogue therapy. Breakthrough flares can be symptomatic and severe. Because tenofovir is associated with a very low rate of antiviral resistance (<1% after 4 years), no convincing cases of breakthrough hepatitis have been linked to its use. Finally, sudden discontinuation of antiviral therapy is capable of causing a hepatitis B withdrawal flare. Withdrawal flares can be severe and several instances of acute liver failure resulting in death or the need for liver transplantation have been reported after stopping nucleoside analogue therapy. The rate of such flares after withdrawal of tenofovir therapy has not been clearly defined.
Tenofovir appears to have little or no direct hepatotoxicity. In patients without HBV and HIV infection, given tenofovir as a part of preexposure prevention, minor serum ALT and AST elevations are more frequent than with placebo, but are rarely above 5 times ULN (<1%). There have been no convincing reports of acute, clinically apparent liver injury attributable to tenofovir, although the combination of tenofovir and didanosine appears to lead to liver injury, with microvesicular fatty liver disease and lactic acidosis more commonly than didanosine with other antiretrovirals, perhaps because of drug-drug interactions. Tenofovir may also predispose to serum aminotransferase elevations during efavirenz therapy, again possibly because of drug-drug interactions.
Likelihood score: C (has been associated with flares of hepatitis when it is withdrawn and rarely with a sudden antiviral effect early during therapy and finally linked to episodes of lactic acidosis due to its effects on drug levels of other nucleosides that can cause lactic acidosis).
Mechanism of Injury
The majority of cases of lactic acidosis and hepatic failure in patients receiving tenofovir appear to be due to didanosine, stavudine or zidovudine coadministration. Addition of tenofovir to an antiretroviral regimen including didanosine concurrently can increase didanosine concentrations by up to 60%, thus amplifying its potential to cause mitochondrial injury. Tenofovir by itself appears to have little hepatotoxic potential.
Outcome and Management
The minor ALT elevations associated with initiation of tenofovir therapy in chronic hepatitis B are usually asymptomatic and transient. Care should be taken in stopping tenofovir therapy in patients with chronic HBV infection. If administered concurrently with tenofovir, didanosine should be reduced in dosage and patients monitored carefully.
Agents used in therapy of HBV infection include adefovir, emtricitabine, entecavir, lamivudine, telbivudine, tenofovir, interferon alfa and peginterferon.
Drug Class: Antiviral Agents, Antiretroviral Agents, Hepatitis B Agents
Other Drugs in the Subclass, Nucleoside Analogues: Abacavir, Adefovir, Didanosine, Emtricitabine, Entecavir, Lamivudine, Stavudine, Telbivudine, Zidovudine
CASE REPORTS
Case 1. Lactic acidosis arising during therapy with didanosine after addition of tenofovir.(1)
A 45 year old woman with HIV infection and chronic hepatitis C developed vomiting, abdominal pain, and obtundation 8 weeks after the addition of tenofovir to her long term antiretroviral regimen of stavudine and didanosine. Tenofovir was used to replace nevirapine, which was discontinued because of minor serum enzymes elevations which then returned to initial values. On admission, she was jaundiced and disoriented and had tender hepatomegaly. Serum bilirubin was 12.6 mg/dL, ALT 157 U/L, and an international normalized ratio (INR) was 2.1. She had lactic acidosis with blood pH of 7.24 and lactate levels of 16.4 mmol/L. Imaging of the liver suggested fatty infiltration. Antiretrovirals were discontinued, but the lactic acidosis and hepatic failure worsened and she died two days after admission.
Key Points
Comment
Acute microvesicular hepatic steatosis with liver failure and lactic acidosis is a syndrome associated with several medications including the nucleoside analogues, particularly didanosine, stavudine and zidovudine. A similar syndrome occurs with intravenous tetracycline, aspirin (Reyes syndrome) and valproate, but the timing and course is different for those agents (shorter latency period), probably because they directly affect function of mitochondria rather than by causing functional failure by inhibition of mitochondrial replication and mitochondrial depletion. Mitochondria have a half-life of several weeks, so that inhibition of mitochondrial replication would be expected to lead to severe dysfunction (mitochondrial failure) after 2 to 3 months. Both didanosine and stavudine have been linked to many cases of hepatic steatosis and lactic acidosis and the addition of tenofovir appears to increase the risk of this complication. This syndrome has not been reported with the use of tenofovir alone. Other risk factors for hepatic steatosis with lactic acidosis include presence of underlying liver disease (such as hepatitis C), obesity and alcohol use.
Case 2. Transient flare of hepatitis B with initiation of tenofovir therapy.(2)
A 29 year old Asian-American woman was started on the combination of tenofovir and emtricitabine (Truvada) in a clinical trial of therapy of HBeAg-positive hepatitis B and developed a doubling of serum ALT levels to ~14 times the upper limit of normal within two weeks of starting therapy. At the same time, HBV DNA levels had fallen by four log10 IU (352 million to 36,160 IU/mL), but she remained HBsAg and HBeAg positive. Serum direct and total bilirubin levels increased slightly, but remained in the normal range. She had no symptoms of hepatitis and reported no other side effects. Tests for hepatitis A, C and D showed no evidence of de novo infection with these viruses. She was taking no other medications or herbal products. The dose of tenofovir and emtricitabine was not changed and, subsequently, her serum aminotransferase levels fell into the normal range and HBV DNA to undetectable. After 36 weeks of treatment, she became HBeAg-negative but did not develop anti-HBe. At one year, histologic evidence of inflammation and fibrosis had improved, but she remained HBsAg-positive and was continued on therapy.
Key Points
Laboratory Values
Comment
A minor flare in hepatitis is not uncommon with initiation of antiviral therapy of hepatitis B and should not lead to dose modification, if HBV DNA levels are decreasing and no other cause for acute liver injury can be found. The flare of hepatitis probably represents an immunological reaction to the sudden decrease in HBV replication and may actually be a favorable sign, predictive of a serological and virological response (loss of HBeAg during treatment which occurred at 36 weeks).
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Tenofovir (Tenofovir disoproxil fumarate) – Generic, Viread®
Tenofovir (Tenofovir alafenamide) – Generic, Vemlidy®
DRUG CLASS
Antiviral Agents
COMPLETE LABELING (Tenofovir disoproxil fumarate)
COMPLETE LABELING (Tenofovir alafenamide)
Product labeling at DailyMed, National Library of Medicine, NIH
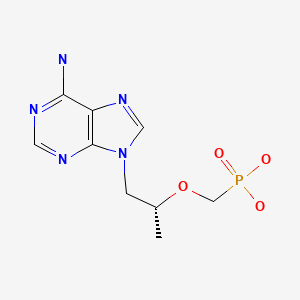
CHEMICAL FORMULA AND STRUCTURE
CITED REFERENCES
- 1.
- Rivas P, Polo J, de Górgolas M, Fernández-Guerrero ML. Drug Points: Fatal lactic acidosis associated with tenofovir. BMJ. 2003;327:711. [PMC free article: PMC200801] [PubMed: 14512477]
- 2.
- Case from the National Institutes of Health, Tenofovir Study Patient A2.
ANNOTATED BIBLIOGRAPHY
References updated: 20 October 2020
- Núñez M. Hepatic toxicity of antiviral agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 505-18.(Review of hepatotoxicity of antiviral agents).
- Flexner C. Antiretroviral agents and treatment of HIV infection. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1623-64.(Textbook of pharmacology and therapeutics).
- NIH. http://aidsinfo
.nih.gov/guidelines. (Clinical guidelines on the use of antiretroviral agents in HIV-1 infected adults, adolescents and children). - Brinkman K, ter Hofstede HJ, Burger DM, Smeitink JAM, Koopmans PP. Adverse effects of reverse transcriptase inhibitors: mitochondrial toxicity as a common pathway. AIDS. 1998;12:1735–44. [PubMed: 9792373](Review of mitochondrial function and role of mitochondrial toxicity or depletion in the adverse side effects of nucleoside analogues).
- Gallant JE, Deresinski S. Tenofovir disoproxil fumarate. Clin Infect Dis. 2003;37:944–50. [PubMed: 13130407](Review on the efficacy and safety of tenofovir mentions that it is well tolerated and has little evidence of mitochondrial toxicity).
- Cihlar T, Birkus G, Greenwalt DE, Hitchcock MJM. Tenofovir exhibits low cytotoxicity in various human cell types: comparison with other nucleotide reverse transcriptase inhibitors. Antiviral Research. 2002;54:37–45. [PubMed: 11888656](In vitro study of nucleoside analogues found that tenofovir and lamivudine were less cytotoxic to several human cell types than zidovudine, stavudine, didanosine or zalcitabine).
- Clark SJ, Creighton S, Portmann B, Taylor C, Wendon JA, Cramp ME. Acute liver failure associated with antiretroviral treatment for HIV: a report of six cases. J Hepatol. 2002;36:295–301. [PubMed: 11830344](6 HIV-positive patients were admitted with acute liver failure over a 25 month period, of whom five died; all had been treated with antiretrovirals and only two had had AIDS-defining illnesses).
- Pecora Fulco P, Kirian MA. Effect of tenofovir on didanosine absorption in patients with HIV. Ann Pharmacother. 2003;37:1325–8. [PubMed: 12921517](When given together, tenofovir increases plasma concentrations of didanosine).
- Blanchard JN, Wohlfeiler M, Canas A, King K, Lonergan JT. Pancreatitis with didanosine and tenofovir disoproxil fumarate. Clin Infect Dis. 2003;37:e57–62. [PubMed: 12942419](4 patients developed pancreatitis and lactic acidosis arising 2-6 months after adding tenofovir to HIV regimen including didanosine; 1 died, 3 who survived were able to restart tenofovir without didanosine; liver tests mentioned only in fatal case [bilirubin 5.3 mg/dL, ALT 89 U/L]).
- Callens S, De Schacht C, Huyst V, Colebunders R. Pancreatitis in an HIV-infected person on a tenofovir, didanosine and stavudine containing highly active antiretroviral treatment. J Infect. 2003;47:188–9. [PubMed: 12860159](33 year old woman developed pancreatitis 5 months after starting didanosine and 1 month after addition of tenofovir to HIV regimen [ALT 386 U/L, Alk P 208 UL, amylase 11395 U/L], resolving rapidly when antivirals were stopped).
- Rivas P, Polo J, de Górgolas M, Fernández-Guerrero ML. Drug Points: Fatal lactic acidosis associated with tenofovir. BMJ. 2003;327:711. [PMC free article: PMC200801] [PubMed: 14512477](45 year old woman with HIV infection treated with didanosine and stavudine developed jaundice and hepatomegaly 8 weeks after switching from nevirapine to tenofovir [bilirubin 12.6 mg/dL, ALT 157 U/L, CT showed fatty liver], despite stopping antivirals and medical support lactic acidosis worsened and she died 36 hours later: Case 1).
- Murphy MD, O’Hearn M, Chou S. Fatal lactic acidosis and acute renal failure after addition of tenofovir to an antiretroviral regimen containing didanosine. Clin Infect Dis. 2003;36:1082–5. [PubMed: 12684925](49 year old man with HIV infection and renal insufficiency on long term didanosine developed progressive, fatal lactic acidosis 6 weeks after starting tenofovir [lactate 5.5 rising to 16.7 mmol]; no mention of liver injury).
- Gallant JE, Staszewski S, Pozniak AL, DeJesus E, Suleiman JM, Miller MD, Coakley DF, et al. 903 Study Group. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naïve patients: a 3-year randomized trial. JAMA. 2004;292:191–201. [PubMed: 15249568](Controlled trial of 3 years of tenofovir vs stavudine added to lamivudine and efavirenz in 600 treatment-naïve patients with HIV; ALT rises above 5 times normal occurred in 4% of tenofovir- vs 5% of stavudine-treated; lactic acidosis arose in no tenofovir- vs 3 [1%] stavudine-treated subjects).
- Guo Y, Fung HB. Fatal lactic acidosis associated with coadministration of didanosine and tenofovir disoproxil fumarate. Pharmacotherapy. 2004;24:1089–94. [PubMed: 15338857](63 year old man with HIV-HCV coinfection developed fatal lactic acidosis 1.5 years after starting didanosine-tenofovir-lopinavir-ritonavir regimen with pancreatitis, multiorgan failure and death; liver injury not mentioned).
- Giola M, Basilico C, Grossi P. Fatal lactic acidosis associated with tenofovir and abacavir. Int J Infect Dis. 2005;9:228–9. [PubMed: 15916912](60 year old man with HIV infection developed fatal lactic acidosis and aminotransferase elevations 5 months after being switched from stavudine to abacavir while continuing tenofovir and nevirapine [ALT 70 U/L, lactate 9.7 mmol], and progressive acidosis).
- Abrescia N, D’Abbraccio M, Figoni M, Busto A, Maddaloni A, De Marco M. Hepatotoxicity of antiretroviral drugs. Curr Pharm Des. 2005;11:3697–710. [PubMed: 16305505](Review of risk factors, epidemiology and pathogenic mechanisms of hepatotoxicity caused by antiretroviral drugs).
- Masiá M, Gutiérrez F, Padilla S, Ramos JM, Pascual J. Severe toxicity associated with the combination of tenofovir and didanosine: case report and review. Int J STD AIDS. 2005;16:646–8. [PubMed: 16176639](45 year old man with HIV-HCV coinfection developed lactic acidosis and “mild cholestasis” 3 months after adding tenofovir to didanosine, resolving slowly after stopping therapy).
- Pozniak AL, Gallant JE, DeJesus E, Arribas JR, Gazzard B, Campo RE, Chen SS, et al. Tenofovir disoproxil fumarate, emtricitabine, and efavirenz versus fixed-dose zidovudine/lamivudine and efavirenz in antiretroviral-naive patients: virologic, immunologic, and morphologic changes—a 96-week analysis. J Acquir Immune Defic Syndr. 2006;43:535–40. [PubMed: 17057609](Controlled trial of tenofovir and emtricitabine vs zidovudine and lamivudine combined with efavirenz in 517 patients with HIV infection; elevations in ALT >3 times normal occurred in 8% vs 9% in first 2 years of study).
- Jain MK. Drug-induced liver injury associated with HIV medications. Clin Liver Dis. 2007;11:615–39. vii-viii. [PubMed: 17723923](Review of hepatotoxicity of antiretroviral medications; ALT elevations occur in 2-18% of patients, but often resolve spontaneously even without dose modification; classes of injury include hypersensitivity [nevirapine, efavirenz, abacavir], mitochondrial injury [stavudine, didanosine, zidovudine], flares of hepatitis B [lamivudine, emtricitabine, tenofovir], flares of hepatitis C [any potent regimen], idiosyncratic injury [ritonavir, nevirapine, efavirenz], and cholestatic hepatitis [many agents]).
- Lapadula G, Izzo I, Costarelli S, Cologni G, Bercich L, Casari S, Gambarotti M, et al. Dideoxynucleoside HIV reverse transcriptase inhibitors and drug-related hepatotoxicity: a case report. J Med Case Rep. 2007;1:19. [PMC free article: PMC1868747] [PubMed: 17488516](43 year old with HIV infection on stavudine for 3 years and tenofovir for 6 months developed rise in ALT levels (222 to 392 U/L), which persisted despite stopping indinavir and resolved slowly after stopping stavudine; liver biopsy showed inflammation, fat and Mallory bodies).
- Lattuada E, Lanzafame M, Carolo G, Gottardi M, Concia E, Vento S. Does tenofovir increase efavirenz hepatotoxicity? AIDS. 2008;22:995. [PubMed: 18453862](Three patients with HIV infection without hepatitis B or C on efavirenz therapy developed ALT elevations 4-6 weeks after starting tenofovir [ALT 144, 186 and 392 U/L] [previously normal], resolving with stopping tenofovir).
- Marcellin P, Heathcote EJ, Buti M, Gane E, de Man RA, Krastev Z, Germanidis G, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359:2442–55. [PubMed: 19052126](Two controlled trials of tenofovir vs adefovir in 641 patients with chronic hepatitis B; ALT levels above 5 times the ULN occurred in 6% of tenofovir vs 3% of adefovir recipients, usually within 8 weeks of starting; all self-limited even with continuing drug).
- Hammer SM, Eron JJ Jr, Reiss P, Schooley RT, Thompson MA, Walmsley S, Cahn P, et al. International AIDS Society-USA. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA. 2008;300:555–70. [PubMed: 18677028](Recent recommendations on use of antiviral therapy in adults with HIV infection including use of recently approved agents raltegravir, maraviroc and etravirine).
- Inductivo-Yu I, Bonacini M. Highly active antiretroviral therapy-induced liver injury. Current Drug Safety. 2008;3:4–13. [PubMed: 18690975](Review of drug induced liver injury due to antiretroviral agents).
- Soriano V, Puoti M, Garcia-Gascó P, Rockstroh JK, Benhamou Y, Barreiro P, McGovern B. Antiretroviral drugs and liver injury. AIDS. 2008;22:1–13. [PubMed: 18090386](Review of hepatotoxicity of antiretroviral drugs with recommendations on management, stopping therapy if symptoms arise, with overt jaundice [direct bilirubin], evidence of mitochondrial toxicity, ALT >10 times ULN, ALT at lower levels if newly marketed agent; important to rule out other causes: problematic agents include didanosine, stavudine and zidovudine, nevirapine and efavirenz, full dose ritonavir and tipranavir).
- Nüesch R, Ananworanich J, Srasuebkul P, Chetchotisakd P, Prasithsirikul W, Klinbuayam W, Mahanontharit A, et al. Interruptions of tenofovir/emtricitabine-based antiretroviral therapy in patients with HIV/hepatitis B virus co-infection. AIDS. 2008;22:152–4. [PubMed: 18090405](Among 10 patients with HIV-HBV coinfection on tenofovir therapy, 6 had a rise in HBV DNA levels and one had a clinically significant flare of hepatitis within 6 months of tenofovir withdrawal; all responded to restarting).
- Sulkowski MS. Management of hepatic complications in HIV-infected persons. J Infect Dis. 2008;197 Suppl 3:S279–93. [PubMed: 18447614](Review of the complications, natural history and management of hepatitis B in patients with HIV infection).
- Fontana RJ. Side effects of long-term oral antiviral therapy for hepatitis B. Hepatology. 2009;49(5) Suppl:S185–95. [PubMed: 19399802](Review of side effects of nucleoside analogues used to treat chronic hepatitis B).
- Ortu F, Weimer LE, Floridia M, Manconi PE. Raltegravir, tenofovir, and emtricitabine in an HIV-infected patient with HCV chronic hepatitis, NNRTI intolerance and protease inhibitors-induced severe liver toxicity. Eur J Med Res. 2010;15:81–3. [PMC free article: PMC3352050] [PubMed: 20452889](43 year old woman with HIV/HCV coinfection developed symptomatic elevations of serum enzymes on saquinavir, fosamprenavir and again on darunavir, was adequately maintained on tenofovir/emtricitabine and raltegravir).
- Garg H, Sarin SK, Kumar M, Garg V, Sharma BC, Kumar A. Tenofovir improves the outcome in patients with spontaneous reactivation of hepatitis B presenting as acute-on-chronic liver failure. Hepatology. 2011;53:774–80. [PubMed: 21294143](Randomized trial of tenofovir versus placebo in 90 patients with acute liver failure due to spontaneous reactivation of hepatitis B found improved survival at 3 months with tenofovir therapy and no evidence of renal or liver toxicity from the antiviral).
- Dore GJ, Soriano V, Rockstroh J, Kupfer B, Tedaldi E, Peters L, Neuhaus J, et al. SMART INSIGHT study group. Frequent hepatitis B virus rebound among HIV-hepatitis B virus-coinfected patients following antiretroviral therapy interruption. AIDS. 2010;24:857–65. [PMC free article: PMC2881334] [PubMed: 20216301](Among 54 patients with HIV-HBV coinfection who were withdrawn from chronic antiretroviral therapy, 12 [22%] had a >3 log rebound in HBV DNA levels including 7 of 17 [41%] who had been on tenofovir vs 3 of 27 [11%] on lamivudine; ALT flares were uncommon and no patient developed hepatic decompensation).
- DeJesus E, Rockstroh JK, Henry K, Molina JM, Gathe J, Ramanathan S, Wei X, et al. GS-236-0103 Study Team. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate versus ritonavir-boosted atazanavir plus co-formulated emtricitabine and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet. 2012;379(9835):2429–38. [PubMed: 22748590](Controlled trial of two combination regimens of once daily antiviral agents in 708 patients with HIV infection for at least 48 weeks; ALT elevations occurred in 18% vs 22% and bilirubin elevations in 11% vs 96% of patients in the tenofovir/emtricitabine/elvitegravir/cobicistat vs the atazanavir/ritonavir/tenofovir/ emtricitabine combination, the ALT elevations being attributable to underlying liver disease in most cases and the bilirubin elevations largely due to atazanavir).
- Vermund SH. Safety and tolerability of tenofovir for preexposure prophylaxis among men who have sex with men. J Acquir Immune Defic Syndr. 2013;64:3–6. [PMC free article: PMC3819194] [PubMed: 23881239](Editorial in response to Grohskopf et al [2013]).
- Grohskopf LA, Chillag KL, Gvetadze R, Liu AY, Thompson M, Mayer KH, Collins BM, et al. Randomized trial of clinical safety of daily oral tenofovir disoproxil fumarate among HIV-uninfected men who have sex with men in the United States. J Acquir Immune Defic Syndr. 2013;64:79–86. [PubMed: 23466649](Controlled trial of daily oral tenofovir vs placebo for 24 months in 373 HIV negative men who have sex with men found no excess of adverse events in tenofovir- vs placebo-treated subjects and no hepatic severe adverse events; changes in ALT levels were not discussed).
- Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, Washington MK, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381(9865):468–75. [PubMed: 23234725](Among 348 patients with chronic hepatitis B who underwent liver biopsy before and after a 4 year course of tenofovir, liver histology improved in 304 [87%] and fibrosis regressed in 176 [51%], including 74% of those who had cirrhosis on initial biopsy; no antiviral resistance breakthroughs occurred).
- Köklü S, Tuna Y, Gülşen MT, Demir M, Köksal AŞ, Koçkar MC, Aygün C, et al. Long-term efficacy and safety of lamivudine, entecavir, and tenofovir for treatment of hepatitis B virus-related cirrhosis. Clin Gastroenterol Hepatol. 2013;11:88–94. [PubMed: 23063679](Retrospective analysis of 227 patients with cirrhosis due to hepatitis B who were treated with lamivudine [n=74], entecavir [n=77] or tenofovir [n=72]; viral breakthrough occurred in 32% on lamivudine, 2.5% on entecavir, but none on tenofovir; no discussion of ALT flares).
- Mandala J, Nanda K, Wang M, De Baetselier I, Deese J, Lombaard J, Owino F, et al. Liver and renal safety of tenofovir disoproxil fumarate in combination with emtricitabine among African women in a pre-exposure prophylaxis trial. BMC Pharmacol Toxicol. 2014;15:77. [PMC free article: PMC4297367] [PubMed: 25539648](Among 2058 HIV-positive African women enrolled in a controlled trial of preexposure prophylaxis against HIV who were treated with tenofovir/emtricitabine vs placebo, minor elevations in creatinine and ALT were more frequent in those on antivirals but marked ALT elevations [above 5 times ULN] were rare and occurred in a similar proportion [8 women in both groups]).
- Patil R, Ona MA, Papafragkakis H, Carey J, Moshenyat Y, Alhaddad A, Anand S. Acute liver toxicity due to efavirenz/ emtricitabine/tenofovir. Case Reports Hepatol. 2015;2015:280353. [PMC free article: PMC4487274] [PubMed: 26161275](24 year old man with HIV infection developed hepatitis 2 months after starting a regimen of efavirenz with tenofovir and emtricitabine [bilirubin 0.6 mg/dL, ALT 1793 U/L], which resolved after switching from efavirenz to rilpivirine).
- Habib G, Nashashibi M. Tenofovir-induced severe hepatitis in occult hepatitis B reactivation. Dig Liver Dis. 2015;47:898–9. [PubMed: 26346266](56 year old man with B-cell lymphoma treated with rituximab-CHOP developed reactivation of hepatitis B but did not develop abnormal liver tests until treated with tenofovir, serum ALT rising from normal to 1418 U/L and bilirubin from normal to 18.6 mg/dL, then resolving when he was switched to entecavir).
- Gowda C, Newcomb CW, Liu Q, Carbonari DM, Lewis JD, Forde KA, Goldberg DS, et al. Risk of acute liver injury with antiretroviral therapy by viral hepatitis status. Open Forum Infect Dis. 2017;4:ofx012. [PMC free article: PMC5407218] [PubMed: 28470014](Among 10,083 patients with HIV followed after initiation of antiretroviral therapy, 206 [2%] developed de novo ALT elevations above 200 U/L but no instances of acute liver failure, elevations being more common among those with hepatitis B or C co-infection).
- Kang MK, Park JG. Tenofovir disoproxil fumarate-induced severe liver injury in a patient with chronic hepatitis B virus infection. Dig Liver Dis. 2018;50:628–30. [PubMed: 29625906](68 year old woman with cirrhosis due to hepatitis B developed a flare on starting therapy with tenofovir [ALT rising from 62 to 357 U/L, bilirubin 1.69 to 3.63 mg/dL], resolving on switching to entecavir and adefovir).
Publication Details
Publication History
Last Update: October 20, 2020.
Copyright
Publisher
National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda (MD)
NLM Citation
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Tenofovir. [Updated 2020 Oct 20].