NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010-.
We conducted a high-throughput screen for small molecule activators of the TRPML3 ion channel which, when mutated, causes deafness and pigmentation defects. Cheminformatics analyses of the 53 identified and confirmed compounds revealed nine different chemical scaffolds and 20 singletons. The two probes reported here [SID24801657 (ML123) and SID24787221 ML122)] exhibit potent EC50 values against TRPML3 (EC50 values of 0.873 μM and 1.43 μM), and activate TRPML2 (54.5%ACT and 23.5% ACT). Importantly, these probes induce a robust increase in intracellular calcium levels, assessed using fluorescence-based Fura-2 calcium assays and patch clamp studies performed by the assay provider, demonstrating their biological value. These probes exhibit no detectable activity against the TRPN1 channel or the YFP-HEK parental cell line, and are inactive against hTRPML1, hTRPM2, mTRPV2, hTRPC3, drTRPN1, and hTRPA1 ion channels. Additionally, these probes are inactive in other ion channel assays including TRPN1, (PubChem AID 1682), the X11L calcium channel (AID 2073), and the ROM K+ channel (AID 1918). We found that agonists strongly potentiated TRPML3 activation with low extracytosolic [Na+]. This synergism revealed existence of distinct and cooperative activation mechanisms, and a wide dynamic range of TRPML3 activity. Testing compounds on TRPML3-expressing sensory hair cells revealed absence of activator-responsive channels. Epidermal melanocytes showed only weak or no responses to the compounds. These studies validate the selectivity and biological relevance of these probes. Due to the lack of available compounds known to act as TRPML3/TRPML2 agonists (there is no prior art), these compounds are valuable tools for elucidating the functions of these ion channels. These results suggest that TRPML3 in native cells might be absent from the plasma membrane or that the protein is a subunit of heteromeric channels that are non-responsive to the activators identified in this screen.
Assigned Assay Grant #: 1 R03 MH083077-01
Screening Center Name & PI: Scripps Research Institute Molecular Screening Center (SRIMSC); Hugh Rosen
Chemistry Center Name & PI: SRIMSC; Hugh Rosen
Assay Submitter & Institution: Stefan Heller, Stanford University
PubChem Summary Bioassay Identifier (AID): 1809
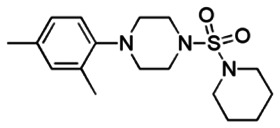
Probe 1 (ML123)
Secondary Arylsulfonamide Scaffold
(S, S)-trans isomer, CID1167615
racemate, SR-1984
original (Commercial), CID2911646
| CID/ML | Target Name | EC50 (nM) [SID, AID] | Anti-target | EC50 (μM) [SID, AID] | Fold Selective | Secondary Assays: EC50 (nM) [SID, AID] |
|---|---|---|---|---|---|---|
| Probe 1: CID2911646/ML123 | TRPML3 | 451 nM [powder SID 85786753, AID 1809] 873 nM [SID 24801657, AID 1562] | TRPN1 | 14.0 μM powder SID85786753, AID 1809] > 29.9 μM SID24801657, AID 1682 | >34-fold (TRPN1 Fluo8); 183-fold (YFP-HEK cell-patch clamp) | Inactive: hTRPML1, hTRPM2, mTRPV2, hTRPC3, drTRPN1, and hTRPA1 |
| TRPML2 | 54.5% ACT | |||||
| Probe 2: CID701237/ML122 | TRPML3 | 1430 nM [SID24787221, AID 1562] 2000 nM [powder SID85786752, AID 1809] | TRPN1 | > 29.9 μM [SID24787221, AID 1682] > 29.9 μM [powder SID85786752, AID 1809] | >20-fold (TRPN1 Fluo8); 60-fold (YFP-HEK cell-patch clamp) | Inactive: hTRPML1, hTRPM2, mTRPV2, hTRPC3, drTRPN1, and hTRPA1 |
| TRPML2 | 23.5% ACT |
Recommendations for scientific use of the probe (ML118)
Limitations in state of the art. There are currently no agonists for TRPML subfamily.
Probe Applications. These probes are useful for assays aiming to increase TRPML3 and TRPML2 channel activities, without activating hTRPML1, hTRPM2, mTRPV2, hTRPC3, drTRPN1, and hTRPA1 ion channels. The selectivity and potency of these compounds will enable further investigations into the biological and biochemical roles of TRPML3 and TRPML2. As suggested by the assay provider, compounds which modulate TRPML3 and TRPML2, such as the probes described here, represent potential starting points for the development of therapies for vertigo or tinnitus. Importantly, the probes have already been used to reveal that TRPML3 in native cells is differently configured than when overexpressed.
Expected end-users of the probe in the research community. The probes can be used by academic researchers studying ion channel biology, hair cell, vertigo, and hearing loss. Scientists in diverse fields will be able to apply these chemical probes to elucidate the roles of TRPML3 and TRPML2 in these cellular pathways.
Relevant biology of the probe. The transient receptor potential (TRP) ion channel subunit genes were first defined in the Drosophila visual system. The function of TRP channels is to depolarize cells and raise intracellular Na+ and Ca2+ levels. The goal of this probe development project was to identify small molecules that act as agonists of TRP ion channels.
1. Introduction
TRPML3 (mucolipin 3; MCOLN3) is a TRP channel expressed in inner ear hair cells and stereocilia [1–3], suggesting it may play a role in hearing and mechanotransduction. Reports that mice with mutations in TRPML3 (known as varitint-waddler mutants) exhibit early-onset hearing loss accompanied by head-bobbing and circling behaviors [4–6], provided further support for a role of TRPML3 in hearing and vestibular function.
TRPML2 is a human cation channel, previously known as Mucolipin 2, and is encoded by the MCOLN2 gene [7]. TRPML2 is associated with the Arf6-regulated trafficking pathway and is involved in the intracellular transport of membranes and membrane proteins [8], including TRPML3 [9]. As a result, the identification of probes for TRPML3 and TRPML2 would be useful to investigate the function of TRPML3 in inner ear mechanotransduction and hearing biology, as well as elucidate pathways of intracellular transport of membrane proteins. There are currently no agonists for TRPML2 or TRPML3 [10].
Selective ligands and activators are playing major roles in characterizing the physiological roles of TRP channels. In contrast to many TRP channels, there are no agonists known for members of the TRPML subfamily. This TRPML3 agonist HTS campaign has led to the identification of the first small molecule TRPML3 activators, providing an unparalleled set of novel tools to study the biophysical and physiological features of TRPML3. Maximal activation capabilities for the identified TRPML3 agonists was up to 2-fold higher than the activity observed for a constitutively active TRPML3 varitint-waddler mutant channel isoform. Recent research has identified the tight regulation of TRPML3 by dynamin-dependent endocytosis and altered trafficking through heteromerization with TRPML family members. TRPML2 and TRPML3 are known to co-localize in cells and homo- and hetero-multimerization of these channels may modulate channel function. The Heller Lab (Stanford University) is utilizing these newly discovered probes (SID 24801657 and SID 24787221) to study possible mechanisms of regulation for TRPML3. In particular the involvement of other TRPML family members such as TRPML2 can be better understood with small molecule modulators with differential selectivity. Towards this end TRPML3/TRPML2 targeted agonists will provide a unique tool for determining the role of these family members in functional hetero-multimerization. Tests with TRPML family-expressing sensory hair cells and epidermal melanocytes are already revealing that activator-responsive TRPML3 channels are tightly controlled in the plasma membrane of cells natively expressing the channel (publication in process).
PubChem BioAssay Table
| AID | Assay Name | Assay Type | Target | Powder | Concentration |
|---|---|---|---|---|---|
| 1448 | Primary biochemical HTS assay for agonists of Transient Receptor Potential Channel ML3 (TRPML3) activity. | Primary Assay (1X%ACT) | TRPML3 | No | 3 μM |
| 1424 | Primary biochemical HTS assay for agonists of TRPN1 activity. | Counterscreen (1X%ACT) | TRPN1 | No | 3 μM |
| 1526 | Confirmation biochemical assay for agonists of TRPML3 activity | Confirmation Assay (3X %ACT) | TRPML3 | No | 3 μM |
| 1525 | Fluorescence counterscreen assay for TRPML3 agonists: cell-based HTS assay to identify agonists of TRPN1 | Counterscreen (3X%ACT) | TRPN1 | No | 3 μM |
| 1562 | Dose-response biochemical assay for agonists of TRPML3 activity. | Dose Response (3X EC50) | TRPML3 | No | 10-point, 1:3 dilution starting at 30 μM |
| 1682 | Fluorescence counterscreen assay for TRPML3 agonists: dose response cell-based HTS assay to identify agonists of TRPML3 activity. | Dose Response Counterscreen (3X EC50) | TRPN1 | No | 10-point, 1:3 dilution starting at 30 μM |
| 1809 | Summary of probe development efforts to identify agonists of TRPML3. | Summary | TRPML2,3 | No | N/A |
| 2116 | Late stage results from the probe development efforts to identify inhibitors of TRPML3 and TRPML2. | Late Stage | TRPML2,3 | Yes | 10 μM |
Table of Assay Rationale and Description
| AID | Assay Rationale | Assay Description | Z′ | S:B |
|---|---|---|---|---|
| 1448 | The purpose of this assay is to identify compounds that activate TRPML3. | This assay employs a HEK293 cell line that stably expresses human TRPML3-YFP cation channel. Cells are treated with compounds followed by measurement of intracellular calcium as monitored by a fluorescent, cell permeable calcium indicator dye. Compounds that act as TRPML3 agonists will increase calcium mobilization, resulting in increased relative fluorescence of the indicator dye, and increased well fluorescence. | 0.71 +/− 0.09 | 3.06 +/− 0.17 |
| 1424 | The purpose of this assay is to identify compounds that activate TRPN1. | Same as AID 1448, except that the assay employs HEK TRPN1-YFP cells. | 0.73 +/− 0.15 | 2.02 +/− 0.13 |
| 1526 | The purpose of this assay is to confirm activity of compounds active in AID 1448. | Same as AID 1448, except compounds are tested in triplicate. | 0.80 +/− 0.02 | 3.12 +/− 0.02 |
| 1525 | The purpose of this assay is to determine whether compounds active in AID 1448 were nonselective due to activation of TRPN1. | Same as AID 1424, except compounds are tested in triplicate. | 0.82 +/− 0.01 | 2.13 +/− 0.07 |
| 1562 | The purpose of this assay is to determine dose response curves for compounds active in AID 1448. | Same as AID 1448, except compounds are tested in triplicate using a dilution series. | 0.73 +/− 0.06 | 3.03 +/− 0.07 |
| 1682 | The purpose of this assay is to determine whether compounds active in AID 1448 were nonselective due to activation of TRPN1. | Same as AID 1424, except compounds are tested in triplicate using a dilution series. | 0.82 +/− 0.03 | 2.05 +/− 0.13 |
| 1809 | Summarize probe development efforts. | N/A | N/A | N/A |
| 2116 | Describe the assays performed during the probe development effort. | Assays include TRPML3, TRPML2, assays, TRPN1 counterscreens, patch clamp assays, and Fura-2 profiling against various TRP ion channels of different species. | N/A | N/A |
2.1. Assays
(Click on the hyperlinks to obtain itemized protocols directly from PubChem; also see Summary AID 1809 and reference [10])
TRPML3 Agonist Assays (PubChem AIDs 1448, 1526, 1562, and 2116)
The purpose of these assays is to identify compounds that act as agonists of the TRPML3 cation channel. These assays employ a HEK293 cell line that stably expresses the human TRPML3-YFP cation channel. The cells are treated with test compounds followed by measurement of intracellular calcium as monitored by the fluorescent, cell permeable calcium indicator dye Fluo-8. As designed, compounds that act as TRPML3 agonists will increase calcium mobilization, resulting in increased relative fluorescence of the indicator dye, and thus increase well fluorescence. Compounds were tested in singlicate (AID 1448) and in triplicate (AID 1526) at a nominal test concentration of 3 μM, and in triplicate using a 10-point, 1:3 dilution series starting at a nominal concentration of 29.9 micromolar (AID 1562 and 2116).
TRPN1 Agonist Assays (PubChem AIDs 1424, 1525, 1682, and 2116)
The purpose of these assays is to identify test compounds that act as agonists of the TRPN1 cation channel. These assays also serve as counterscreen to identify compounds that are nonselective TRP agonists due to activation of TRPN1. These assays employ a HEK293 cell line that stably expresses the zebrafish TRPN1-YFP cation channel. The cells are treated with test compounds followed by measurement of intracellular calcium as monitored by the fluorescent, cell permeable calcium indicator dye Fluo-8. As designed, compounds that act as TRPN1 agonists will increase calcium mobilization, resulting in increased relative fluorescence of the indicator dye, and thus increase well fluorescence. Compounds were tested in singlicate (AID 1424) and in triplicate (AID 1525) at a nominal test concentration of 3 μM, and in triplicate using a 10-point, 1:3 dilution series starting at a nominal concentration of 29.9 micromolar (AID 1682 and 2116).
Patch Clamp Assays (PubChem AID 2116)
The purpose of these assays is to determine if test compounds can increase current recordings in TRPML3 ion channels (Assay 3) or the YFP-HEK parental background (Assay 4). Whole-cell currents were recorded with an Alembic Instruments VE-2 amplifier with 100% series resistance compensation, and acquired with JClamp software. The standard bath solution contained (in mM) 138 NaCl, 5.4 KCl, 2 MgCl2, 2 CaCl2, 10 HEPES, and 10 d-glucose, adjusted to pH 7.4 with NaOH. The standard pipette solution contained (in mM) 140 CsCl, 10 HEPES, 3 ATP-Na, 1 BAPTA, and 2 MgCl2, adjusted to pH 7.2. 100 μM 2-Aminoethyl-diphenyl borate was included in the bath solution to block gap junctions and had no effect on the expressed channels. Channel responses were plotted to 10 ms voltage steps (holding potential = +10 mV) between −200 mV and +100 mV in 20 mV incremental steps, normalized by cell capacitance (pF). Compounds were tested at 10 micromolar. These assays were performed by the assay provider.
Fura-2 Assays (PubChem AID 2116)
The purpose of these assays is to determine whether compounds identified as probe candidates are able to increase whole cell Ca2+ influx in HEK293 cells transfected with human TRPML3, other human, or murine (m) TRP channels, or zebrafish TRPN1. In this assay cells transiently expressing channels or YFP control plasmid are perfused with test compound, followed by measurement of intracellular [Ca2+] for 2 minutes with the fluorescent indicator fura-2-AM. Compounds are added to cells 20–25 hours after transfection. Values are reported as mean values +/− SEM (n >= 3 independent experiments with 20–30 cells). The % activation values for TRPML2 in the SAR tables were calculated by normalizing the TRPML2 response ratios to TRPML3 response ratios. Compounds are tested at 10 micromolar. These assays were performed by the assay provider.
2.2. Probe Chemical Characterization
Probe chemical structure including stereochemistry. Separation of diastereomers (if necessary)
The structures of probes ML122 and ML123 are shown below. Because the stereochemistry of ML123 was not clearly defined in the information provided from commercial suppliers, we synthesized the stereoisomers of ML123 and determined that the most active isomer is the trans-S,S-isomer of ML123, as discussed in this report.

Probe 1 (ML123)
Secondary Arylsulfonamide Scaffold
(S, S)-trans isomer, CID1167615
racemate, SR-1984
original (Commercial), CID2911646
Structure verification with 1H NMR and LCMS results. See next section.
Solubility. The solubility of the probes was measured in phosphate buffered saline (PBS: 137 mM NaCl, 2.7 mM KCl, 10 mM sodium phosphate dibasic, 2 mM potassium phosphate monobasic and a pH of 7.4) at room temperature (23°C). The solubility of probe ML122 and ML123 and the associated stereomers are shown below.
Stability. The stability of the probes was measured at room temperature (23°C) in PBS (no antioxidants or other protectants; DMSO concentration below 0.1%). The stability, represented by the half-life, was found to be > 48 hours. Below is a graph showing loss of compound with time over a 48 hour period with a minimum of 6 time points. The table indicates the percent of compound remaining at the end of the 48 hours.
| Probe | SR Number (Stereomer) | SID | CID | Solubility in PBS (μM) | Michael Acceptor 100 μM GSH trap | Stability in PBS t1/2 (hr) |
|---|---|---|---|---|---|---|
| ML123 | SR3-1984 (trans Racemate) | None | None | 49 | No | > 48 hr |
| ML123 | SR3-1985 (cis Racemate) | None | None | 121 | No | > 48 hr |
| ML123 | SR3-2020 (trans isomer R Enantiomer) | 104223082 | 1167614 | 22 | No | > 48 hr |
| ML123 | SR3-2021 (trans isomer S Enantiomer) | 104223083 | 1167615 | 28 | No | > 48 hr |
| ML122 | SR3-2103 (Resynthesis) | 113584823 | 701237 | 3.2 | No | > 48 hr |
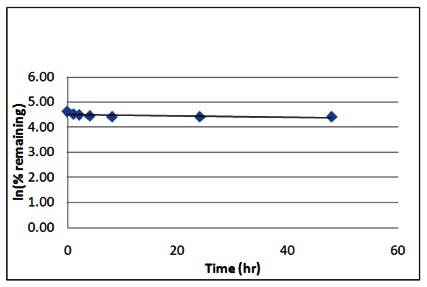
Stability of ML123 (trans racemate SR3-1984)
| SR3-1984 Stability in PBS Buffer (pH 7.4) | ||
|---|---|---|
| Sample concentration: 10 μM | ||
| Storage Microcentrifuge Tube on Lab Bench | ||
| Time (hr) | % remaining | In(%remaining) |
| 0 | 100 | 4.61 |
| 1 | 91 | 4.51 |
| 2 | 88 | 4.48 |
| 4 | 86 | 4.46 |
| 8 | 84 | 4.43 |
| 24 | 83 | 4.42 |
| 48 | 83 | 4.42 |

Stability of ML123 (cis racemate SR3-1985)
| SR3-1985 Stability in PBS Buffer (pH 7.4) | ||
|---|---|---|
| Sample concentration: 10 μM | ||
| Storage Microcentrifuge Tube on Lab Bench | ||
| Time (hr) | % remaining | In(%remaining) |
| 0 | 100 | 4.61 |
| 1 | 112 | 4.72 |
| 2 | 111 | 4.71 |
| 4 | 105 | 4.65 |
| 8 | 96 | 4.56 |
| 24 | 98 | 4.58 |
| 48 | 101 | 4.62 |

Stability of ML123 (R enantiomer of trans isomer SR3-2020)
| SR3-2020 Stability in PBS Buffer (pH 7.4) | ||
|---|---|---|
| Sample concentration: 6 μM | ||
| Storage Microcentrifuge Tube on Lab Bench | ||
| Time (hr) | % remaining | In(%remaining) |
| 0 | 100 | 4.61 |
| 1 | 98 | 4.58 |
| 2 | 101 | 4.62 |
| 4 | 97 | 4.57 |
| 8 | 101 | 4.62 |
| 24 | 95 | 4.55 |
| 48 | 105 | 4.65 |

Stability of ML123 (S enantiomer of trans isomer SR3-2021)
| SR3-2021 Stability in PBS Buffer (pH 7.4) | ||
|---|---|---|
| Sample concentration: 10 μM | ||
| Storage Microcentrifuge Tube on Lab Bench | ||
| Time (hr) | % remaining | In(%remaining) |
| 0 | 100 | 4.61 |
| 1 | 112 | 4.72 |
| 2 | 93 | 4.53 |
| 4 | 85 | 4.44 |
| 8 | 91 | 4.51 |
| 24 | 95 | 4.55 |
| 48 | 103 | 4.63 |

Stability of Probe ML122 (Resynthesis SR-03000002103)
| SR-03000002103 Stability in PBS Buffer (pH 7.4) | ||
|---|---|---|
| Sample concentration: 6 μM | ||
| Storage Microcentrifuge Tube on Lab Bench | ||
| Time (hr) | % remaining | In(%remaining) |
| 0 | 100.0 | 4.61 |
| 1 | 92.3 | 4.52 |
| 2 | 88.7 | 4.49 |
| 4 | 73.2 | 4.29 |
| 8 | 92.9 | 4.53 |
| 24 | 95.2 | 4.56 |
| 48 | 97.0 | 4.57 |
The probes were measured for their ability to form glutathione adducts. At concentrations of 100 μM reduced GSH, 10 μM of the probes do not appear to react with glutathione. These compounds do not have functionality that would render them to be Michael acceptors [11, 12].
2.3. Probe Preparation
Synthetic route for probes ML122 and ML123. Detailed experimental procedures for the synthesis of probes are below.
Synthesis and Characterization of Probe ML122. Probe ML122 was synthesized by acylation of N-(2,4-dimethylphenyl)-piperdine as shown in the following equation.
1-(2,4-Dimethylphenyl)-4-(piperidin-1-ylsulfonyl)piperazine (ML122, SID113584823, CID701237) To a solution of commercially available N-(2,4-dimethylphenyl)-piperdine (0.22 g, 1.15 mmol) and triethylamine (0.5 mL) in CH2Cl2 (5 mL) was added piperidine-1-sulfonyl chloride (ca. 2.0 mmol) at 0 °C. The mixture was stirred for 15 min, then was warmed to room temperature and stirred for an additional hour. The reaction mixture was diluted with dichloromethane (60 mL). The organic layer was separated and was washed with saturated aq. NaHCO3 (20 mL) and brine (20 mL). The combined organic extracts were dried over magnesium sulfate, filtered, and concentrated in vacuo. Purification of the crude product by flash chromatography eluting with a step gradient ranging from 6% to 50% EtOAc-hexane provided 0.33 g (0.990 mmol, 85%) of ML122 as a viscous oil; purity >98% by LCMS; IR (neat) 2939, 2853, 1713, 1500, 1445, 1361, 1336, 1262, 1220, 1161, 1143, 1115,1052, 926, 815, 735 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.00 (s, 1H), 6.97 (d, J = 8.0 Hz, 1H), 6.89 (d, J = 8.0 Hz, 1H), 3.36 (t, J = 4.8 Hz, 4H), 3.25 (t, J = 4.8 Hz, 4H), 2.90 (t, J = 4.8 Hz, 4H), 2.26 (s, 3H), 2.26 (s, 3H), 1.66-1.54 (m, 6H); 13C NMR (100 MHz, CDCl3) δ 139.11, 138.38, 129.26, 128.67, 67.01, 63.62, 49.71, 48.75, 28.48, 24.59, 24.17, 18.76; MS (ESI) 338 m/z [M+H]+; HRMS (ESI) 338.1910 m/z [M+H]+, 360.1713 m/z [M+Na]+, 697.3569 m/z [2M+Na]+; calc. 338.1897 [M+H]+, 360.1716 [M+Na]+, 697.3540 [2M+Na]+.
Synthesis of ML123 and Identification of the Stereochemistry of the Probe
Stereochemistry of the probe, ML123, was not specified by the original commercial supplier of samples identified from the HTS. Four possible stereoisomers exist: the racemic trans diastereomer (4) and the racemic cis diastereomer (10). Samples of the racemic 4 and 10 (both as 1:1 mixtures of enantiomers) were synthesized by the routes summarized below.
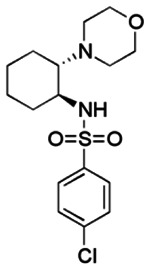
Synthesis of Racemic 4 (SR-03000001984)—Racemic Trans isomer. This synthesis involved ring-opening of cyclohexene oxide 1 with morpholine to give 2. Activation of the hydroxyl group of 2 as a mesylate and then displacement with ammonium hydroxide—via an azidinium ion—provided 3. Acylation of racemic 3 with p-chlorobenzenesulfonyl chloride then provided racemic 4 (SR-03000001984).
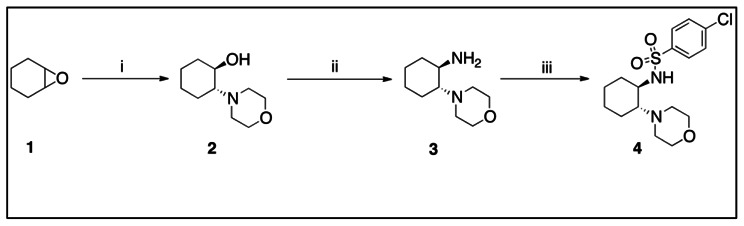
(i) morpholine, EtOH, reflux, 12h, 98%. (ii) mesyl chloride, Et3N, 2h, then NH4OH, 7h, 79%. (iii) 4-chlorophenylsulfonyl chloride, Et3N, DCM, 1h, 74%.
Synthesis of Racemic 10 (SR-03000001985)—Racemic Cis isomer. The synthesis of racemic 10 (SR-03000001985) was accomplished starting with the reaction of cyclohexene oxide 1 with benzylamine to give 5. Protection of the secondary amine as the Boc carbamate 6 and then oxidation of the hydroxyl group gave ketone 7. Reductive amination of 7 with morpholine gave the cis-diamine derivative 8. Deprotection of the Boc and N-benzyl groups gave the cis diamine derivative 9, which was then acylated with p-chlorobenzenesulfonyl chloride which provided racemic 10 (SR-03000001985).

(i) Benzylamine 3% Zn(OTf)2, rt, 24h. (ii) (Boc)2O, DCM, rt, 11h, 85% (2 steps), (iii) PCC, DCM, rt, 24h, 96%. (iv) morpholine, 6% p-toluenesulfonic acid, benzene, 110 °C, 36h, then H2, Pd/C, THF, rt, 48 h, 53%. (v) TFA, 1h, then H2, Pd/C MeOH, 3h. (vi) 4-chlorophenylsulfonyl chloride, Et3N, DCM, 1h, 11% over 2 steps.
Because several of the TRPML3/2 agonist probe analogs—such as analogs 1–5—are ortho-substituted aniline sulfonamide derivatives, and because the racemic trans diastereomer 4 of ML123 (but not the cis diastereomer 10) maps best onto the structures of analogs 1–5, we surmised that the ML123 probe would have the trans stereochemistry. This assumption was verified by the results of testing of 4 and 10, which demonstrated that 4 is about 10-fold more active than 10 in the TRPML3/2 assay. Therefore, we proceeded to determine the structure of the most active enantiomer by the synthesis summarized below.
Synthesis of Single Trans Enantiomers 16 (SR-03000002020) and 18 (SR-03000002021). This synthesis was accomplished by treatment of cyclohexene oxide 1 with (R)-a-methyl-benzylamine. Conversion of 11 to the aziridine 12 set the stage for ring opening of the aziridine with morpholine. This reaction gave a ca. 3:2 mixture of the diastereomeric diamines 13 and 14 which were separated chromatographically. Debenzylation of 13 gave the (R,R)-15, whereas debenzylation of 14 gave the enantiomeric diamine, (S,S)-17. Acylation of 15 and 17 with p-chlorobenzenesulfonyl chloride then provided (R,R)-(−)-16 (SR-03000002020) and (S,S)-(+)-18 (SR-03000002021), respectively.

(i) (R)-alpha-methyl-benzylamine, 5% Zn(OTf)2, neat, rt, 24h.
(ii) DIAD, PPh3, DCM, 0 °C to rt, 8h, 73% (2 steps).
(iii) morpholine, 5% Zn(OTf)2, MeCN, 80 °C, 12h, 86% (ca. 3/2 ratio of 13/14).
(iv) H2, 20% Pd(OH)2/C, AcOH, rt, 1h.
(v) 4-chlorophenylsulfonyl chloride, Et3N, DCM, rt, 30 min. 59% (2 steps).
(vi) H2, 20% Pd(OH)2/C, MeOH/AcOH (9/1), rt, 24h.
(vii) 4-chlorophenylsulfonyl chloride, Et3N, DCM, rt, 30 min. 62% (2 steps).
Finally, the absolute stereochemistry of the primary amine intermediates 15 and 17 was verified by conversion of each to the corresponding Cbz derivatives (R,R)-(−)-19 and (S,S)-(+)-20, respectively. The optical rotation obtained for (S,S)-(+)-20 ([α]D28 = +37.9 (c = 1.0 in CHCl3)), but not for (R,R)-(−)-20 ([α]D28 = −35.7 (c = 1.0 in CHCl3)), was in close agreement with the value reported in the literature for this compound ([α]D28 = +45.7 (c = 1.0 in CHCl3); [13]

(i) CbzCI, Et3N, DCM, 0 °C to rt, 1h
Detailed experimental procedures for synthesis of Probe ML123 and Stereoisomers
trans-2-Morpholinocyclohexanol (2). To a solution of cyclohexene oxide (5.0 mL, 49.5 mmol) in anhydrous ethanol (25 mL) was added morpholine (7.0 mL, 79.3 mmol) at room temperature. The reaction mixture was stirred at 100 °C for 12 hours. After being cooled to ambient temperature, the solvent was removed under reduced pressure. Purification of the residue by flash chromatography eluting with a step gradient ranging from 0% to 20% MeOH-EtOAc provided 9.0 g (48.6 mmol, 98%) of 2 as a viscous oil. 1H NMR (400 MHz, CDCl3) δ 4.02 (d, J = 2.4 Hz, 1H), 3.60-3.52 (m, 4H), 3.38-3.31 (m, 1H), 2.63-2.40 (m, 4H), 2.13-2.07 (m, 1H), 1.91-1.87 (m, 1H), 1.74-1.66 (m, 2H), 1.60-1.58 (m, 1H), 1.19-1.07 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 69.29, 68.20, 66.77, 48.72, 33.91, 24.89, 23.74, 23.22; MS (ESI) 186 m/z [M+H]+.
trans-2-Morpholinocyclohexanamine (3). To a solution of 2 (2.3 g, 12.5 mmol) and triethylamine (2.6 mL, 18.6 mmol) in dry ether (50 mL) was added methanesulfonyl chloride (1.2 mL, 15.4 mmol) dropwise at 0 °C. A white precipitate formed, and the reaction mixture was stirred at room temperature for 2 hours. After completion of the mesylation step (monitored by LCMS for the absence of 186 m/z [M+H]+), NH4OH (50 mL) was added. The reaction mixture was vigorously stirred at ambient temperature for additional 7 hours. The reaction mixture was then diluted with ether (50 mL) and washed with saturated aq. NaHCO3 (30 mL) and brine (30 mL). The organic layer was dried over magnesium sulfate, filtered, and concentrated in vacuo. Purification of the crude product by flash chromatography using a SNAP KP-NH Cartridge by eluting with a step gradient ranging from 12% to 100% EtOAc-hexane provided 1.8 g (9.92 mmol, 79%) of 3 as a viscous oil. 1H NMR (400 MHz, CDCl3) δ 3.70-3.60 (m, 4H), 2.67-2.55 (m, 3H), 2.39-2.34 (m, 2H), 2.01-1.91 (m, 2H), 1.79-1.74 (m, 2H), 1.65-1.60 (m, 3H), 1.22-1.02 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 70.98, 67.90, 50.65, 49.01, 35.30, 25.98, 25.12, 22.85; MS (ESI) 185 m/z [M+H]+.
trans-4-Chloro-N-(trans-2-morpholinocyclohexyl)benzenesulfonamide (racemic-4, ML123, SR-0300001984-1). To a solution of 3 (0.11g, 0.60 mmol) and triethylamine (0.12 mL, 0.90 mmol) in dry CH2Cl2 (10 mL) was added 4-chlorophenylsulfonyl chloride (0.15 g, 0.72 mmol) at room temperature. The reaction mixture was stirred for 1 hour at ambient temperature. The solvent was removed under reduced pressure, and the crude product was diluted with ethyl acetate (50 mL). The organic layer was washed with saturated aq. NaHCO3 (10 mL) and brine (10 mL). The organic layer was dried over magnesium sulfate, filtered, and concentrated in vacuo. Purification of the crude product by flash chromatography eluting with a step gradient ranging from 12% to 100% EtOAc-hexane provided a viscous oil. The oil crystallized at 0°C to yield 0.16 g (0.449 mmol, 74%) of 4; purity >98% by LCMS; m. p. = 110 °C; IR (neat) 3236, 2942, 2861, 2817, 1588, 1479, 1451, 1333, 1166, 1070, 1013, 910, 827, 754 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.79 (d, J = 8.4 Hz, 2H), 7.44 (d, J = 8.4 Hz, 2H), 5.97 (s, 1H), 3.54-3.45 (m, 4H), 2.72 (td, J = 10.4, 4.0 Hz, 1H), 2.35-2.31 (m, 1H), 2.19-2.09 (m, 5H), 1.80-1.71 (m, 2H), 1.62-1.60 (m, 1H), 1.24-0.98 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 139.15, 138.80, 129.39, 128.72, 67.28, 66.92, 53.33, 48.13, 32.95, 25.24, 24.19, 22.93; MS (ESI) 359 m/z [M+H]+; HRMS (ESI) 359.1203 m/z [M+H]+, 381.1027 m/z [M+Na]+; calc. 359.1191 [M+H]+, 381.1010 [M+Na]+.
trans-2-(Benzylamino)cyclohexanol (5). To cyclohexene oxide (3 mL, 29.6 mmol) were added zinc trifluoromethanesulfonate (0.33 g, 3 mol%) and benzylamine (3 mL, 27.5 mmol) at room temperature. The reaction mixture was stirred neat at ambient temperature for 24 hour. The reaction mixture solidified, and the un-reacted reagents were removed under reduced pressure overnight. The crude solid product 5 (6.3 g, 30. 6 mmol) was directly used in the next step without further purification; MS (ESI) 206 m/z [M+H]+.
trans-tert-Butyl benzyl(trans-2-hydroxycyclohexyl)carbamate (6). To a solution of crude 5 (6.3 g, 30.6 mmol) in dry CH2Cl2 (50 mL) was added di-tert-butyl dicarbonate (8.8 g, 40.2 mmol) at room temperature. The reaction mixture was stirred for 11 hours at ambient temperature. The mixture was diluted with CH2Cl2 (100 mL) and washed with water (30 mL) and brine (30 mL). The organic layer was dried over magnesium sulfate, filtered, and concentrated in vacuo. Purification of the crude product by flash chromatography eluting with a step gradient ranging from 12% to 100% EtOAc-hexane provided 7.1 g (23.4 mmol, 85% over 2 steps) of 6 as a viscous oil; 1H NMR (400 MHz, DMSO-d6, Temp = 60 °C) δ 7.30-7.18 (m, 5H), 4.44-4.35 (m, 2H), 4.23 (d, J = 5.2 Hz, 1H), 3.60 (septet, J = 4.8 Hz, 1H), 3.48 (br, 1H), 1.94-1.91 (m, 1H), 1.57-1.53 (m, 3H), 1.36 (s, 10H), 1.24-1.09 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 157.09, 140.00, 128.41, 126.86, 80.17, 71.15, 62.07, 47.43, 35.14, 29.76, 28.36, 25.39, 24.38; MS (ESI) 206 (Boc decomposition) m/z [M+H]+.
tert-butyl Benzyl(2-oxocyclohexyl)carbamate (7). To a solution of 6 (7.1 g, 23.4 mmol) in dry CH2Cl2 (100 mL) was added pyridinium chlorochromate (PCC) (8.1 g, 37.6 mmol), and the reaction mixture was stirred at room temperature for 24 hours. After completion of the reaction (monitored by TLC), the mixture was diluted with ether and filtered through a pad of Celite. The solvent was removed under reduced pressure. Purification of the crude product by flash chromatography eluting with a step gradient ranging from 6% to 50% EtOAc-hexane provided 6.8 g (22.4 mmol, 96%) of 7 as a viscous oil; 1H NMR (400 MHz, DMSO-d6) δ 7.32-7.19 (m, 5H), 4.57-4.47 (m, 1H), 4.36 (q, J = 1.6 Hz, 1H), 4.12-4.02 (m, 1H), 2.41-2.29 (m, 2H), 1.99-1.53 (m, 6H), 1.31 (d, J = 41.2 Hz, 9H); 13C NMR (100 MHz, CDCl3) δ 207.37, 156.30, 140.29, 128.38, 126.75, 126.59, 80.49, 77.43, 64.73, 49.67, 41.69, 32.49, 31.09, 28.43, 26.58, 25.07; MS (ESI) 204 (Boc decomposition) m/z [M+H]+.
tert-butyl Benzyl(cis-2-morpholinocyclohexyl)carbamate (8). To a solution of 7 (0.41 g, 1.34 mmol) in dry benzene (10 mL) was added p-toluenesulfonic acid (17 mg, 0.089 mmol) at room temperature. The reaction mixture was stirred at 50 °C until all of acid had dissolved. Morpholine (0.15 mL, 1.73 mmol) was added to the reaction mixture, and the reaction mixture was refluxed at 100 °C for 3 hours. The solvent was removed by distillation. Additional morpholine (0.15 mL) and dry benzene (10 mL) was added to the reaction mixture, which was refluxed at 110 °C for 14 hours. The solvent was distilled at atmospheric pressure, and excess morpholine was removed under reduced pressure with a vacuum pump to give the crude enamine intermediate; MS (ESI) 373 m/z [M+H]+. The crude enamine was dissolved in dry THF (10 mL), and 10% Pd/C (78.5 mg) was added. After removing air in the flask using a vacuum pump, hydrogen gas was introduced using a balloon. The reaction mixture was stirred for 48 hours at ambient temperature. Palladium on carbon was removed by filtration. The filtrate was evaporated to give a crude viscous oil, which was purified by flash chromatography using SNAP KP-NH Cartridge by eluting with a step gradient ranging from 6% to 50% EtOAc-hexane to yield 8 (0.27 g, 0.712 mmol, 53%) as a viscous oil; MS (ESI) 375 m/z [M+H]+.
cis-2-Morpholinocyclohexanamine (9): To a solution of 8 (0.27 g, 0.712 mmol) in CH2Cl2 (4 mL) was added trifluoroacetic acid (4 mL) at room temperature. The reaction mixture was stirred at ambient temperature and monitored by mass spectrometer. The Boc-deprotection was complete after one hour, and the solvent and trifluoroacetic acid were removed under reduced pressure; MS (ESI) 275 m/z [M+H]+. To the crude intermediate were added MeOH (10 mL) and 10% Pd/C (ca. 0.1 g), and the reaction mixture was stirred at ambient temperature. Air in the flask was removed using a vacuum pump, and hydrogen gas was introduced using a balloon. The reaction was monitored by mass spectrometer and after 3 hours, benzyl deprotection was complete. Palladium on carbon was removed by filtration. The filtrate evaporated to give a crude viscous oil 9, which was directly used in the next step without further purification; MS (ESI) 185 m/z [M+H]+.
cis-4-Chloro-N-(cis-2-morpholinocyclohexyl)benzenesulfonamide (10, SR-0300001985-1). Racemic cis-isomer 10 (29 mg, 80.7 μmol, 11% over 2 steps) was obtained as a white solid by following the procedure described for the synthesis of 4; m. p. = 140 °C; IR (neat) 3171, 2928, 2867, 2806, 1584, 1449, 1392, 1370, 1329, 1170, 1113, 1093, 995, 754 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.80 (dt, J = 8.8, 2.0 Hz, 2H), 7.46 (dt, J = 8.8, 2.0 Hz, 2H), 5.34 (s, 1H), 3.49 (t, J = 4.8, 4H), 3.35 (d, J = 3.2, 1H), 2.27-2.23 (m, 3H), 2.09-2.02 (m, 3H), 1.74 (d, J = 10.8, 2H), 1.46 (qd, J = 13.2, 3.2 Hz, 1H), 1.34-1.30 (m, 1H), 1.24-1.09 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 148.56, 133.38, 132.76, 132.03, 127.26, 119.31, 51.80, 47.44, 47.01, 25.73, 24.00, 20.84, 17.75; MS (ESI) 359 m/z [M+H]+; HRMS (ESI) 359.1199 m/z [M+H]+, 381.1019 m/z [M+Na]+; calc. 359.1191 [M+H]+, 381.1010 [M+Na]+.
trans-2-(((R)-1-Phenylethyl)amino)cyclohexanol (11). Compound 11 (7.3 g, 33.5 mmol) was obtained by following the procedure described for the synthesis of 5 using 1 (3 mL, 29.7 mmol), (R)-alpha-methylbenzylamine (4 mL, 31.4 mmol), and zinc trifluoromethanesulfonate (0.32 g, 3 mol%); MS (ESI) 220 m/z [M+H]+.
7-((R)-1-Phenylethyl)-7-azabicyclo[4.1.0]heptanes (12). To a solution of 11 (ca. 30 mmol) in dry CH2Cl2 (30 mL) was added PPh3 (9.5 g, 36.0 mmol) at room temperature. The reaction mixture was stirred until PPh3 completely dissolved, then was cooled to 0 °C. Diisopropyl azodicarboxylate (DIAD, 7 mL, 36.0 mmol) was added dropwise to the reaction mixture. The mixture was warmed to ambient temperature and stirred for 8 hours. The solvent was removed under reduced pressure. The crude product was diluted with ethyl acetate (90 mL) and was washed with water (20 mL) and brine (20 mL). The organic layer was dried over magnesium sulfate, filtered, and concentrated in vacuo. Purification of the crude product by flash chromatography eluting with a step gradient ranging from 0% to 50% EtOAc-hexane provided 4.4 g (22.0 mmol, 73% over 2 steps) of 12 as a viscous oil; 1H NMR (400 MHz, CDCl3) δ 7.38 (d, J = 7.2 Hz, 2H), 7.30 (t, J = 7.2 Hz, 2H), 7.21 (t, J = 7.2 Hz, 1H), 2.43 (q, J = 6.8, 1H), 1.90-1.68 (m, 3H), 1.65-1.57 (m, 2H), 1.48-1.38 (m, 3H), 1.36 (d, J = 6.8, 3H), 1.22-1.10 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 145.93, 128.29, 126.88, 126.67, 70.04, 38.34, 38.05, 25.12, 24.82, 23.81, 20.94, 20.80; MS (ESI) 202 m/z [M+H]+.
(1R,2R)-2-Morpholino-N-((R)-1-phenylethyl)cyclohexanamine (13) and (1S,2S)-2-Morpholino-N-((R)-1-phenylethyl)cyclohexanamine (14). To a solution of 12 (1.5 g, 7.64 mmol) in dry CH3CN were added zinc trifluoromethanesulfonate (0.13g, 5 mol%) and morpholine (0.8 mL, 9.25 mmol) at room temperature. The reaction mixture was stirred at 80 °C for 12 hours. The mixture was cooled to ambient temperature, then the solvent was removed by evaporation. The reaction mixture was diluted with ethyl acetate (80 mL) and washed with water (20 mL) and brine (20 mL). The organic layer was dried over magnesium sulfate, filtered, and concentrated in vacuo. Purification of the crude product by flash chromatography using a SNAP KP-NH Cartridge by eluting with a step gradient ranging from 0% to 50% EtOAc-hexane provided 1.2 g (4.29 mmol, 56%) of 13 as a white solid (Rf = 0.51 at 25% EtOAc/Hexane on KP-NH TLC plate) and 0.66 g (2.29 mmol, 30%) of 14 as a viscous oil (Rf = 0.39 at 25% EtOAc/Hexane on KP-NH TLC plate)
Data for 13: 1H NMR (400 MHz, CDCl3) δ 7.36 (d, J = 7.2 Hz, 2H), 7.29 (t, J = 7.2 Hz, 2H), 7.22-7.17 (m, 1H), 3.77-3.65 (m, 5H), 2.90 (s, 1H), 2.69-2.64 (m, 2H), 2.52-2.40 (m, 3H), 2.27-2.20 (m, 1H), 1.86-1.82 (m, 1H), 1.74-1.66 (m, 2H), 1.53-1.49 (m, 1H), 1.36 (d, J = 6.8 Hz, 3H), 1.23-1.00 (m, 3H), 0.93-0.87 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 147.73, 128.23, 126.54, 126.50, 67.82, 67.77, 57.62, 57.49, 48.60, 33.58, 25.60, 24.64, 24.41, 22.96; MS (ESI) 289 m/z [M+H]+.
Data for 14: 1H NMR (400 MHz, CDCl3) δ 7.32-7.28 (m, 2H), 7.24-7.20 (m, 3H), 3.77 (q, J = 6.8 Hz, 1H), 3.70-3.58 (m, 4H), 3.09 (br, 1H), 2.24-2.13 (m, 6H), 2.03 (td, J = 10.4, 4.4 Hz, 1H), 1.77-1.70 (m, 2H), 1.66-1.62 (m,1H), 1.43 (d, J = 6.8 Hz, 3H), 1.19-0.93 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 146.33, 128.44, 126.96, 126.47, 67.87, 67.81, 53.95, 53.26, 48.67, 31.88, 25.73, 24.56, 24.53, 22.68; MS (ESI) 289 m/z [M+H]+.
(1R,2R)-2-Morpholinocyclohexanamine (15). To a solution of 13 (16 mg, 56.5 μmol) in AcOH (10 mL) was added 20% Pd(OH)2/C (9.6 mg) at room temperature. After removing air in the flask using a vacuum pump, hydrogen gas was introduced using a balloon. The mixture was stirred for 5 minutes, the balloon was removed, and the reaction mixture was stirred for 1 hour at ambient temperature. Palladium on carbon was removed by filtration through a Celite pad. The filtrate was evaporated to give a crude viscous oil 15, which was directly used in the next step without further purification; MS (ESI) 185 m/z [M+H]+.
4-chloro-N-((1R,2R)-2-Morpholinocyclohexyl)benzenesulfonamide (16, CID1167614, SID104223082, SR-0300002020). Compound 16 (12 mg, 33.4 μmol, 59% over 2 steps) was obtained as a white solid by following the procedure described for synthesis of 4. The spectroscopic data (1H, 13C, and MS) of 16 are same to those of 4; m.p. = 168 °C; [α]D28 = −72.2 (c = 1.0 in CHCl3). The purity of 16 was >98% based on LCMS.
(1S,2S)-2-Morpholinocyclohexanamine (17). To a solution of 14 (0.20 g, 0.695 mmol) in MeOH/AcOH (50 uL/5 mL) was added 20% Pd(OH)2/C (38 mg) at room temperature. After removing air in the flask using a vacuum pump, hydrogen gas was introduced using a balloon. The reaction mixture was stirred for 24 hour at ambient temperature. Palladium on carbon was removed by filtration through a Celite pad. The filtrate was collected and evaporated to give a crude viscous oil, which was directly used for the next step without further purification. The spectra data of 17 (1H and MS) are same to those of 3.
4-Chloro-N-((1S,2S)-2-morpholinocyclohexyl)benzenesulfonamide (18, CID1167615, SID104223083, SR-0300002021). Compound 18 (0.15 g, 0.428 mmol, 62% over 2 steps) was obtained as a white solid by following the procedure described for synthesis of 4. The spectroscopic data (1H, 13C, and MS) for 18 are same to those of 4; m.p. = 173 °C; [α]D28 = +79.9 (c = 1.0 in CHCl3). The purity of 18 was >98% based on LCMS.
Benzyl ((1R, 2R)-2-Morpholinocyclohexyl)carbamate (19). To a solution of 15 (20 mg, 0.107 mmol) and triethylamine (0.1 mL) in dry CH2Cl2 (5 ml) was added benzyl chloroformate (0.02 mL) dropwise at 0 °C. After being stirred 15 minutes, the reaction mixture was warmed to room temperature and stirred for additional 1 hour. The reaction mixture was diluted with dichloromethane (40 mL) and washed with saturated aq. NaHCO3 (10 mL) and brine (10 mL). The organic layer was dried over magnesium sulfate, filtered, and concentrated in vacuo. Purification of the crude product by flash chromatography eluting with a step gradient ranging from 12% to 100% EtOAc-hexane provided 24 mg (77 μmol, 72%) of 19 as a viscous oil: [α]D28 = −35.7 (c = 1.0 in CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.35-7.28 (m, 5H), 5.36 (br, 1H), 5.08 (s, 2H), 3.66-3.55 (m, 4H), 3.31 (septet, J = 4.4 Hz, 1H), 2.65-2.60 (m, 2H), 2.43-2.41 (m, 1H), 2.35-2.30 (m, 2H), 2.15 (td, J = 10.8, 3.2 Hz, 1H), 1.88-1.77 (m, 2H), 1.68-1.62 (m, 1H), 1.28-1.03 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 156.77, 137.08, 128.71, 128.23, 128.22, 67.88, 67.69, 66.56, 51.47, 48.58, 33.28, 25.61, 24.79, 23.34; MS (ESI) 319 m/z [M+H]+.
Benzyl ((1S, 2S)-2-Morpholinocyclohexyl)carbamate (20). Compound 20 (23 mg, 73 μmol, 75%) was obtained as a viscous oil by following the procedure described for synthesis of 19: [α]D28 = +37.9 (c = 1.0 in CHCl3), (ref. [α]D28 = +45.7 (c = 1.0 in CHCl3) [13]; MS (ESI) 319 m/z [M+H]+.
3. Results
3.1. Summary of Screening Results
Following primary HTS in singlicate to identify TRPML3 agonists (AID 1448), counterscreening against TRPN1 to identify nonselective agonists in singlicate (AID 1424) and triplicate (1525), confirmation of TRPML3 activity in triplicate (AID 1526), titration assays in triplicate to determine potency (AID 1562) and selectivity (AID 1682), certain compounds were identified as possible candidates for probe development. Two HTS lead compounds belonging to the secondary arylsulfonamide and sulfonarylpiperazine scaffolds (SID 24801657 and SID 24787221), exhibited EC50 values of 0.873 μM and 1.43 μM, respectively. These compounds and related analogs were ordered as powders (SID 85786753 and SID 85786752) for retesting by the SRIMSC in TRPML3 dose response assays. The powder samples exhibited EC50 values consistent with the liquid samples: 0.451 μM and 2.00 μM, respectively. Next, the assay provider tested these two probe candidates and select analogs in TRPML3 patch clamp, TRPN1 patch clamp counterscreening, and Fura-2 imaging assays. Results of the assay provider Fura-2 assays revealed that the two probes have activity against both TRPML3 and TRPML2, and are inactive against hTRPML1, hTRPM2, mTRPV2, hTRPC3, drTRPN1, and hTRPA1 ion channels. As a result, two probes were identified. Probe compounds SID 24801657 and SID 24787221 were tested in more than 190 PubChem Bioassays and active only in 3 TRPML3 assays.

TRPML3 Agonist Campaign Summary
3.2. Dose Response Curves for Probes
The dose response curves shown below demonstrate that the racemic trans-isomer, SR-1984, is about 10-fold more potent than the racemic cis-isomer, SR-1985, and that the (S,S)-trans isomer of ML123 (e.g., SR-2021) is >10-fold more potent than the (R,R)-trans isomer SR-2020. Therefore, the most potent isomer of ML123 is SR-2021 (442 nM EC50).

Probe ML123 (Resynthesis of Stereomers)
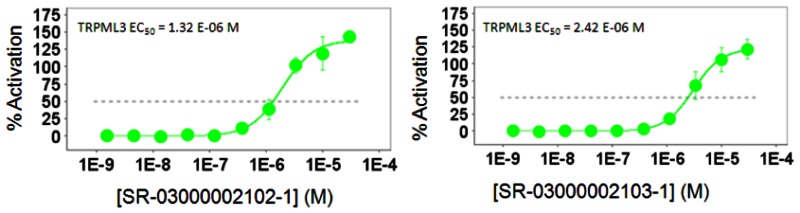
Probe ML122 (Resynthesis)
TRPML3 % Activation vs Agonist Concentration. TRPML3 Activation is shown in percentage, normalized to the maximum Carbachol response. EC50 values are reported as mean values (n = 3).
3.3. Scaffold/Moiety Chemical Liabilities
There are no known instability or chemical liabilities associated with probes ML122 or ML123.
3.4. SAR Tables
TRPML3/2 Agonist SAR Table 1Secondary Arylsulfonamides Scaffold
| Compound Information | HTS Information | Probe Development Information | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | Structure | CID | SID | MLS | Vendor | Vendor Catalog ID | TRPML3 Dose Response (AID 1562) EC50, μM | TRPN1 Dose Response Counterscreen (AID 1682) EC50, μM | SID | TRPML3 Dose Response EC50, μM (AID 2116) | TRPN1 Dose Response EC50, μM (AID 2116) | TRPML3 Patch Clamp Assay (AID 2116) | TRPN1 Patch Clamp Assay (AID 2116) | TRPML3, TRPML2 Fura-2 Imaging (AID 2116) | Fura-2 Profiling Assays (AID 2116) |
| Probe |
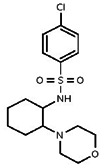 | 2911646 | 24801657 | MLS 000713637 | Asinex | BAS 03787124 | Active (0.873) | Inactive (>29.9) | 85786753 | Active (0.451) | Inactive (14.0) | Active | Inactive | Active | Inactive in hTRPML1, hTRPM2, mTRPV2, hTRPC3, drTRPN1, and hTRPA1. |
| Analog 1 |
 | 2111037 | 14746905 | MLS 000570381 MLS | Enamine | T5241132 | Active (0.872) | Inactive (>29.9) | 85786755 | Active (0.624) | Inactive (>29.9) | Active | Inactive | Inactive | Inactive in hTRPML1, hTRPM2, mTRPV2, hTRPC3, drTRPN1, and hTRPA1. Inactive in hTRPML1, |
| Analog 2 |
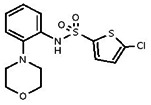 | 2124908 | 3716245 | 000054019 MLS | Enamine | T5252809 | Active (1.36) | Inactive (>29.9) | 85786754 | Active (1.09) | Inactive (>29.9) | Active | Inactive | Inactive | hTRPM2, mTRPV2, hTRPC3, drTRPN1, and hTRPA1. Inactive in hTRPML1, |
| Analog 3 |
 | 818647 | 17414375 | 000575428 | Chem Bridge | 5182458 | Active (1.59) | Inactive (>29.9) | 85786756 | Active (2.14) | Inactive (>29.9) | Active | Inactive | Inactive | hTRPM2, mTRPV2, hTRPC3, drTRPN1, and hTRPA1. |
| Analog 4 |
 | 2814040 | 26731263 | MLS 000850372 | Maybridge | HTS 08158 | Active (2.3) | Inactive (>29.9) | Not tested due to lower target potency in AID 1562, compared to the probe. | ||||||
| Analog 5 |
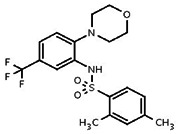 | 2078255 | 22405272 | MLS 000394056 | Enamine | T5268454 | Active (2.48) | Inactive (>29.9) | |||||||
TRPML3/2 Agonist SAR Table 2Sulfonylaryl piperazines Scaffold
| Compound Information | HTS Information | Probe Development Information | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | Structure | CID | SID | MLS | Vendor | Vendor Catalog ID | TRPML3 Dose Response (AID 1562) EC50, μM | TRPN1 Dose Response Counterscreen (AID 1682) EC50, μM | SID | TRPML3 Dose Response EC50, μM (AID 2116) | TRPN1 Dose Response EC50, μM (AID 2116) | TRPML3 Patch Clamp Assay (AID 2116) | TRPN1 Patch Clamp Assay (AID 2116) | TRPML3, TRPML2 Fura-2 Imaging (AID 2116) | Fura-2 Profiling Assays (AID 2116) |
| Probe |
 | 701237 | 24787221 | MLS 000715887 | Asinex | BAS 08196462 | Active (1.43) | Inactive (>29.9) | 85786752 | Active (2.00) | Inactive (>29.9) | Active | Inactive | Active | Inactive in hTRPML1, hTRPM2, mTRPV2, hTRPC3, drTRPN1, and hTRPA1. |
| Analog 1 |
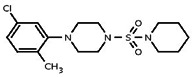 | 991660 | 24792359 | MLS 000690533 | Chem Bridge | 7797638 | Active (1.84) | Inactive (>29.9) | 85786751 | Active (3.46) | Inactive (>29.9) | Not tested due to lower target potency in AID 1562, compared to the probe. | |||
| Analog 2 |
 | 991661 | 24787220 | MLS 000715888 | Asinex | BAS 08196464 | Active (2.07) | Inactive (>29.9) | Not tested due to lower target potency in AID 1562, compared to the probe. | ||||||
| Analog 3 |
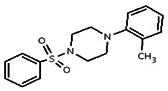 | 740952 | 14720556 | MLS 000035471 | Asinex | BAS 00898171 | Active (5.11) | Inactive (>29.9) | |||||||
| Analog 4 |
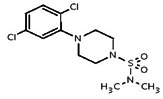 | 849896 | 4255423 | MLS 000063673 | Chem Bridge | 7727036 | Inactive (26.5) | Inactive (>29.9) | |||||||
| Analog 5 |
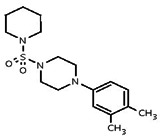 | 849900 | 24784768 | MLS 000715886 | Asinex | BAS 08196460 | Not tested because it was inactive in the TRPML3 Primary Assay (AID 1448). | ||||||||
3.5. Cellular Activity
With regard to the cytotoxicity of the probes, ML123 (SID 24801657CID 2911646) was found inactive in several cytotoxicity assays in PubChem, including AIDs 1486, 1554, 1825, 449728. Similarly, because this compound was inactive in numerous assays examining inhibition of diverse cellular pathways such as STAT3, STAT1, luciferase reporters, PERK, P97 ATPase, Rml C and D reductase, TNAP, alkaline phosphatase, Mcl/Bid, aldehyde dehydrogenase, and bacterial streptokinase, it is likely not a general inhibitor of cell-based transcription or metabolism at the doses and timepoints tested.
Probe ML122 (SID 24787221/CID 701237) was found inactive in several cytotoxicity/viability assays in PubChem, including AIDs 1554, and 504466. ML122 was also found inactive in assays examining cell-based pathways such as DNA repair, beta arrestin, NFkB, ERK Map Kinase, aldehyde dehydrogenase, histone methyltransferase, cysteine protease, and alpha glucosidase.
Probes were also tested in a variety of cell-based assays performed by the SRIMSC and assay provider, as shown below. These assays were performed to determine the probes’ selectivity, cellular activity, and mechanism of action.
Fura-2 Assays
These assays employ the calcium indicator dye fura-2-AM to detect increases in intracellular calcium in TRPML3-transfected HEK cells. The results of these assays for the two probes are shown below.
Patch Clamp Assays
The purpose of these assays is to determine if test compounds can increase current recordings in TRPML3 and TRPN1 ion channels. Whole-cell currents were recorded with an Alembic Instruments VE-2 amplifier with 100% series resistance compensation, and acquired with JClamp software. The standard bath solution contained (in mM) 138 NaCl, 5.4 KCl, 2 MgCl2, 2 CaCl2, 10 HEPES, and 10 d-glucose, adjusted to pH 7.4 with NaOH. The standard pipette solution contained (in mM) 140 CsCl, 10 HEPES, 3 ATP-Na, 1 BAPTA, and 2 MgCl2, adjusted to pH 7.2. 100 μM 2-Aminoethyl-diphenyl borate was included in the bath solution to block gap junctions and had no effect on the expressed channels. Channel responses were plotted to 10 ms voltage steps (holding potential = +10 mV) between −200 mV and +100 mV in 20 mV incremental steps, normalized by cell capacitance (pF). Compounds were tested at 10 micromolar.
3.6. Profiling Assays
The MLSMR sample of probe ML123 (SID 24801657) has been tested in a total of 371 PubChem Bioassays and was identified as active in only the 3 TRPML3 assays (AIDs 1562, 1526, and 1448). This gives a 0.8% promiscuity ratio. These findings demonstrate the high level of selectivity of this novel probe.
In addition, the MLSMR sample of probe ML122 (SID24787221) has been tested in a total of 363 PubChem Bioassays and was identified as active in only 4 assays. This result gives a 1.1% promiscuity ratio. Three of these assays are the SRIMSC’s TRPML3 agonist assays (AIDs 1562, 1526, and 1448). The fourth assay (AID 504444) is a fluorescence-based cell-based reporter assay which attempted to identify inhibitors of the Nrf2 transcription factor out of the entire MLP collection. The compound did not confirm activity in the subsequent validation study, thereby confirming the selectivity of this probe.
4. Discussion
4.1. Comparison to existing art and how the new probe is an improvement
There is no prior art for agonists of TRPML3 and TRPML2.
4.2. Mechanism of Action Studies
These novel probes activate the TRPML3 channel to increase intracellular Ca2+ influx (see Section 3.5 for Fura-2 based Calcium assay results). In addition, these probes act synergistically in the presence of endolymph-like extracellular solution (ELS) containing high K+ (150 mM) and low Na+ (2 mM) concentrations, leading to a 10-fold increase in current response [10]. These findings indicate that these probes act cooperatively with extracellular Na+ on TRPML3.
4.3. Planned Future Studies
The probes generated in this report are first in class, and thus best in class, agonists for TRPML3. The leads have excellent selectivity against other TRP receptors and ion channels, with moderate activity against TRPML2. The SAR studies suggest that additionally synthesized compounds may exhibit greater potency and may be superior probes than the leads selected. We encourage members of the community to use the (S,S)-trans isomer of ML123 as the starting point for further optimization, as it is the most potent of the TRPML3 agonists identified in this work, and are chemically amenable to further structural optimization. Such analogs are likely to be increasingly useful compounds for elucidating the biology of TRPML3. The SRIMSC is pursuing the development of additional TRPML-3–selective probes, based on other chemical scaffolds. These studies will be presented in a future probe report.
5. References
- 1.
- Qian F, Noben-Trauth K. Cellular and molecular function of mucolipins (TRPML) and polycystin 2 (TRPP2). Pflugers Arch. 2005;451(1):277–85. [PubMed: 15971078]
- 2.
- Atiba-Davies M, Noben-Trauth K. TRPML3 and hearing loss in the varitint-waddler mouse. Biochim Biophys Acta. 2007;1772(8):1028–31. [PubMed: 17329082]
- 3.
- Gong Z, Son W, Chung YD, Kim J, Shin DW, McClung CA, Lee Y, Lee HW, Chang DJ, Kaang BK, Cho H, Oh U, Hirsh J, Kernan MJ, Kim C. Two interdependent TRPV channel subunits, inactive and Nanchung, mediate hearing in Drosophila. J Neurosci. 2004;24(41):9059–66. [PMC free article: PMC6730075] [PubMed: 15483124]
- 4.
- Di Palma FIA, Belyantseva HJ, Kim TF, Vogt B, Kachar, Noben-Trauth K. Mutations in Mcoln3 associated with deafness and pigmentation defects in varitint-waddler (Va) mice. Proc Natl Acad Sci U S A. 2002;99(23):14994–9. [PMC free article: PMC137533] [PubMed: 12403827]
- 5.
- Nagata K, Zheng L, Madathany T, Castiglioni AJ, Bartles JR, Garcia-Anoveros J. The varitint-waddler (Va) deafness mutation in TRPML3 generates constitutive, inward rectifying currents and causes cell degeneration. Proc Natl Acad Sci U S A. 2008;105(1):353–8. [PMC free article: PMC2224216] [PubMed: 18162548]
- 6.
- van Aken AF, Atiba-Davies M, Marcotti W, Goodyear RJ, Bryant JE, Richardson GP, Noben-Trauth K, Kros CJ. TRPML3 mutations cause impaired mechano-electrical transduction and depolarization by an inward-rectifier cation current in auditory hair cells of varitint-waddler mice. J Physiol. 2008;586(Pt 22):5403–18. [PMC free article: PMC2655368] [PubMed: 18801844]
- 7.
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426(6966):517–24. [PubMed: 14654832]
- 8.
- Karacsonyi C, Miguel AS, Puertollano R. Mucolipin-2 localizes to the Arf6-associated pathway and regulates recycling of GPI-APs. Traffic. 2007;8(10):1404–14. [PubMed: 17662026]
- 9.
- Venkatachalam K, Hofmann T, Montell C. Lysosomal localization of TRPML3 depends on TRPML2 and the mucolipidosis-associated protein TRPML1. J Biol Chem. 2006;281(25):17517–27. [PMC free article: PMC4196876] [PubMed: 16606612]
- 10.
- Grimm C, Jors S, Saldanha SA, Obukhov AG, Pan B, Oshima K, Cuajungco MP, Chase P, Hodder P, Heller S. Small molecule activators of TRPML3. Chem Biol. 2010;17(2):135–48. [PMC free article: PMC2834294] [PubMed: 20189104]
- 11.
- Li X, He Y, Ruiz CH, Koenig M, Cameron MD, Vojkovsky T. Characterization of dasatinib and its structural analogs as CYP3A4 mechanism-based inactivators and the proposed bioactivation pathways. Drug Metab Dispos. 2009;37(6):1242–50. [PMC free article: PMC3202349] [PubMed: 19282395]
- 12.
- Li X, Kamenecka TM, Cameron MD. Bioactivation of the epidermal growth factor receptor inhibitor gefitinib: implications for pulmonary and hepatic toxicities. Chem Res Toxicol. 2009;22(10):1736–42. [PubMed: 19803472]
- 13.
- Gonzalez-Sabin J, Gotor V, Rebolledo F. Chemoenzymatic preparation of optically active trans-cyclohexane-1,2-diamine derivatives: an efficient synthesis of the analgesic U-(−)-50,488. Chem. Eur. J. 2004;10(22):5788–94. [PubMed: 15472932]
- PMCPubMed Central citations
- PubChem BioAssay for Chemical ProbePubChem BioAssay records reporting screening data for the development of the chemical probe(s) described in this book chapter
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Identification of Selective Agonists of the Transient Receptor Potential Channels 3 (TRPML3).[Probe Reports from the NIH Mol...]Review Identification of Selective Agonists of the Transient Receptor Potential Channels 3 (TRPML3).Saldanha SA, Grimm C, Allais C, Smith E, Ouizem S, Mercer BA, Roush WR, Heller S, Hodder P. Probe Reports from the NIH Molecular Libraries Program. 2010
- Small molecule activators of TRPML3.[Chem Biol. 2010]Small molecule activators of TRPML3.Grimm C, Jörs S, Saldanha SA, Obukhov AG, Pan B, Oshima K, Cuajungco MP, Chase P, Hodder P, Heller S. Chem Biol. 2010 Feb 26; 17(2):135-48.
- Constitutive activity of TRPML2 and TRPML3 channels versus activation by low extracellular sodium and small molecules.[J Biol Chem. 2012]Constitutive activity of TRPML2 and TRPML3 channels versus activation by low extracellular sodium and small molecules.Grimm C, Jörs S, Guo Z, Obukhov AG, Heller S. J Biol Chem. 2012 Jun 29; 287(27):22701-8.
- Review Mechanisms of cellular synchronization in the vascular wall. Mechanisms of vasomotion.[Dan Med Bull. 2010]Review Mechanisms of cellular synchronization in the vascular wall. Mechanisms of vasomotion.Matchkov VV. Dan Med Bull. 2010 Oct; 57(10):B4191.
- Review TRPML2 and the evolution of mucolipins.[Adv Exp Med Biol. 2011]Review TRPML2 and the evolution of mucolipins.Flores EN, García-Añoveros J. Adv Exp Med Biol. 2011; 704:221-8.
- Campaign to Identify Agonists of Transient Receptor Potential Channels 3 and 2 (...Campaign to Identify Agonists of Transient Receptor Potential Channels 3 and 2 (TRPML3 & TRPML2) - Probe Reports from the NIH Molecular Libraries Program
Your browsing activity is empty.
Activity recording is turned off.
See more...