NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010-.
When mutated, the Transient Receptor Potential Channels 3 (TRPML3) ion channel causes deafness and pigmentation defects. Due to the lack of available compounds known to act as selective TRPML3 agonists, the identification of selective probes for TRPML3 are useful to investigate the function of TRPML3 in inner ear mechanotransduction and hearing biology. The two probes reported here, CID 776924 (ML268) and CID 53239838 (ML269), emerged from an HTS-based effort to identify small molecule activators of TRPML3. They exhibit submicromolar EC50 values against TRPML3 in intracellular calcium functional assays. Further, their selectivity & mechanism-of-action has been confirmed in various in patch clamp and functional assays. Interestingly, testing these probes and other TRPML3 activators on TRPML3-expressing sensory hair cells revealed the absence of activator-responsive channels. Similarly, epidermal melanocytes showed only weak or no responses when exposed to the compounds. These studies validate the biological relevance of these probes, as they have now been used to demonstrate that TRPML3 might be absent from the plasma membrane or that the protein is a subunit of heteromeric channels in native cells A comprehensive summary of their activity has been published [1].
ML268 and ML269 are first-in-class tools for elucidating the functions of the TRPML3 ion channel. The significance of their impact was the subject of a special review article [2].
Assigned Assay Grant #: 1 R03 MH083077-01
Screening Center Name & PI: Scripps Research Institute Molecular Screening Center (SRIMSC); Hugh Rosen
Chemistry Center Name & PI: SRIMSC, Hugh Rosen
Assay Submitter & Institution: Stefan Heller, Stanford University
PubChem Summary Bioassay Identifier (AID): 1809
Probe Structures & Characteristics

Figure 1Selective TRPML3 Agonist Probes
Table 1Selective TRPML3 Agonist Results Summary
| CID/ML# | Target Name | EC50 (nM) [SID, AID] | Anti-Target Name | EC50* (μM) [SID, AID] | Fold Selectivea | Secondary Assay EC50 (nM) [SID, AID] |
|---|---|---|---|---|---|---|
| Probe 1 CID 776924/ML268 | TRPML3 | LIQUID: 1030 nM [SID 14722627, AID 1562] POWDER: 950 nM [SID 87692368, AID 2510] | TRPN1 | LIQUID: >29.9 [SID 14722627, AID 1682] POWDER: >29.9 SID 87692368, AID 2583] >32-fold selectivity over TPRN1 in Fluo-8 screening assays. | LIQUID >29-fold POWDER >31-fold | Both probes were inactive against all TRP channels tested as determined using whole cell patch clamp assays. (AID 2116): hTRPML1 hTRPM2 mTRPV2 hTRPC3 drTRPN1 and hTRPA1 |
| TRPML2 | POWDER: Inactive [SID 87692368, AID 2770] | |||||
| Probe 2 CID 53239838/ ML269 | TRPML3 | LIQUID: 325nM [SID 26731169, AID 1562 DRUN] POWDER: 253 nM SID 87692372, AID 2510] 290 nM [SID 124349367, AID 602128] | TRPN1 | LIQUID: >29.9 [SID 26731169, AID 1682] POWDER: >29.9 [SID 124349367, AID 2583] | LIQUID >92-fold POWDER >118-fold | |
| TRPML2 | POWDER: Inactive [SID 87692372, AID 2770] | |||||
| TRPML2 | 23.5% ACT |
- a
Fold-selectivity was calculated as IC50 for anti-target/IC50 for target, using an approximate value for the anti-target due to its flat agonist curve (low maximal activation at the highest dose tested, 30μM).

Recommendations for the scientific use of this probe
These novel selective probes are useful for assays aiming to increase TRPML3 channel activities, without activating hTRPML1, hTRPML2, hTRPML1 NC (plasma membrane variant), hTRPM2, mTRPV2, hTRPC3, drTRPN1, and hTRPA1 ion channels. The selectivity and potency of these compounds will enable further investigations into the biological and biochemical roles of TRPML3. The Heller Lab at Stanford University is utilizing these probe molecules to study possible mechanisms of regulation for TRPML3. In particular, the involvement of other TRPML family members can be better understood with small molecule modulators with differential selectivity. Towards this end TRPML3-targeted agonists such as the probes reported here provide a unique tool for determining the role of these family members in functional hetero-multimerization. Tests with TRPML family-expressing sensory hair cells and epidermal melanocytes are already revealing that activator-responsive TRPML3 channels are tightly controlled in the plasma membrane of cells natively expressing the channel [1]. As suggested by the Assay Provider, selective TRPML3 modulators represent potential starting points for the development of therapies for vertigo or tinnitus. The HTS assays, chemistry effort, and biological experiments detailing the identification and characterization of these selective probes, related analogs, and our previously reported non-selective probes have been described together in [1].
1. Introduction
Cell signaling pathways that mediate osmosensation, photosensation, and thermosensation depend on a family of diverse transient receptor potential (TRP) cation channels, which are activated by agonist-receptor coupling [3–9]. A role for these channels in inner ear hair cell mechanotransduction was gleaned from TRP channel mutations identified in flies, worms, and lower vertebrates with defective balance and impaired sensitivity to touch [3–7]. TRPML3 (mucolipin 3; MCOLN3) is a TRP channel expressed in inner ear hair cells and stereocilia [7–9], suggesting it may play a role in hearing and mechanotransduction. Reports that mice with mutation in TRPML3 (known as varitint-waddler mutants) exhibit early-onset hearing loss accompanied by head-bobbing and circling behaviors [10–12], provided further support for a role of TRPML3 in hearing and vestibular function. The varitant-waddler defect is the result of a A419P substitution mutation in TRPML3, which causes the channel to be constitutively active, resulting in calcium overload and apoptosis in cells that express the channel, particularly melanocytes and sensory hair cells[13]. Unfortunately, although efforts into the roles of the wildtype and mutant TRPMLS channels have characterized the response of TRPML3 to luminal pH changes and extracellular ions such as H+ and Na2+, little is known about its physiological role, cellular localization, and heteromer formation under normal and diseased states. As a result, the identification of selective probes for TRPML3 would be useful to investigate the function of TRPML3 in inner ear mechanotransduction and hearing biology.
2. Materials and Methods
Chemistry: All chemical reagents and solvents were acquired from commercial vendors.
Biology: All protocols are reported in the relevant PubChem AIDs provided in Table 2 and in Grimm et al [1].
Table 2
PubChem BioAssay Table (Summary can be found at AID 1809).
Compound Properties: Solubility, stability, and glutathione reactivity analyses were conducted in accordance with NIH guidelines. CYP inhibition and microsome stability analyses were performed as previously described [14].
2.1. Assays
Table 2 lists all of the PubChem AIDs for the TRPML3 agonist project. Descriptions of the assay protocols follow the tables.
Assay Descriptions
TRPML3 Agonist Assays (AID 1448, AID 1526, AID 1562, AID 2510, AID 602128, and AID 602129)
The purpose of these assays is to identify test compounds that act as agonists of the TRPML3 cation channel. This assay employs a HEK293 cell line that stably expresses the human TRPML3-YFP cation channel. The cells are treated with test compounds followed by measurement of intracellular calcium as monitored by a fluorescent, cell permeable calcium indicator dye. As designed, compounds that act as TRPML3 agonists will increase calcium mobilization, resulting in increased relative fluorescence of the indicator dye, and thus increase well fluorescence. Compounds were tested in singlicate (AID 1448) or triplicate (AID 1526) at a nominal dose of 3 μM, and in a 10-point, 1:3 dilution series starting at a nominal concentration of 30 μM (AID 1562, AID 2510, AID 602128, and AID 602129).
The TRPML3 HEK293 cell line was routinely cultured in T-175 sq cm flasks at 37 degrees C and 95% relative humidity (RH). The growth media consisted of Minimum Essential Medium with GlutaMAX and supplemented with 10% v/v heat-inactivated qualified fetal bovine serum, 800 micrograms/mL Geneticin, and 1X antibiotic mix (penicillin, streptomycin, and neomycin). The day before the assay 1500 cells in 3 μl of growth media were seeded into each well of 1536 well microtiter plates and allowed to incubate at 37 degrees C, 5% CO2, and 95 % RH for 23 hours. Next, 2 μl of the fluorogenic Fluo-8 intracellular calcium indicator mixture with 1 mM trypan red plus (prepared according to the manufacturer’s protocol) was added to each well. After a 1 hour incubation at 37 degrees C, 5% CO2, and 95 % RH followed by a 30 minute incubation at room temperature, the assay was started by performing a basal read of plate fluorescence (470–495 nm excitation and 515–575 nm emission) for 5 seconds on the FLIPR Tetra (Molecular Devices). Next, 15 nL of test compound (3 μM final nominal concentration) in DMSO, DMSO alone (0.3% final concentration), or the cholinergic agonist carbachol (87 μM final concentration) in DMSO were dispensed to the appropriate wells. Then a real time fluorescence measurement was immediately performed for the remaining 120 seconds of the assay. A ratio for each well was calculated to normalize assay data, according to the following mathematical expression: Ratio = I_Max / I_Min. Where I_Max represents the maximum measured fluorescence emission intensity over the 125 second read and I_Min represents the minimum (basal) measured fluorescence emission intensity before compound was added. The percent activation was calculated from the median ratio as follows:
Median_Ratio_Low_Control)) *100
Where:
Test_Compound is defined as wells containing test compound.
High_Control is defined as wells with carbachol.
Low_Control is defined as wells with DMSO.
TRPN1 Counterscreen Assays (AID 1424, AID 1525, AID 1682, and AID 2583)
The purpose of this assay is to identify test compounds that act as agonists of the TRPN1 cation channel. This assay serves as a counterscreen to determine whether compounds are non-selective TRP agonists. This assay employs a HEK293 cell line that stably expresses the zebrafish TRPN1-YFP cation channel. The cells are treated with test compounds followed by measurement of intracellular calcium as monitored by a fluorescent, cell permeable calcium indicator dye. As designed, compounds that act as TRPN1 agonists will increase calcium mobilization, resulting in increased relative fluorescence of the indicator dye, and thus increase well fluorescence. Compounds were tested in singlicate (AID 1424) or triplicate (AID 1525) at a nominal dose of 3 μM, and in a 10-point, 1:3 dilution series starting at a nominal concentration of 30 μM (AID 1682, and AID 2583).
The TRPN1 HEK293 cell line was routinely cultured in T-175 sq cm flasks at 37 degrees C and 95% relative humidity (RH). The growth media consisted of Minimum Essential Medium with GlutaMAX and supplemented with 10% v/v heat-inactivated qualified fetal bovine serum, 800 micrograms/mL Geneticin, and 1X antibiotic mix (penicillin, streptomycin, and neomycin). The day before the assay 1500 cells in 3 μl of growth media were seeded into each well of 1536 well microtiter plates and allowed to incubate at 37 degrees C, 5% CO2, and 95 % RH for 23 hours. Next, 2 μl of the fluorogenic Fluo-8 intracellular calcium indicator mixture with 1 mM trypan red plus (prepared according to the manufacturer’s protocol) was added to each well. After a 1 hour incubation at 37 degrees C, 5% CO2, and 95 % RH followed by a 30 minute incubation at room temperature, the assay was started by performing a basal read of plate fluorescence (470–495 nm excitation and 515–575 nm emission) for 5 seconds on the FLIPR Tetra (Molecular Devices). Next, 15 nL of test compound (3 μM final nominal concentration) in DMSO, DMSO alone (0.3% final concentration), or the cholinergic agonist carbachol (87 μM final concentration) in DMSO were dispensed to the appropriate wells. Then a real time fluorescence measurement was immediately performed for the remaining 120 seconds of the assay. A ratio for each well was calculated to normalize assay data, according to the following mathematical expression: Ratio = I_Max / I_Min. Where I_Max represents the maximum measured fluorescence emission intensity over the 125 second read and I_Min represents the minimum (basal) measured fluorescence emission intensity before compound was added. The percent activation was calculated from the median ratio as follows:
Median_Ratio_ Low_Control)) *100
Where:
Test_Compound is defined as wells containing test compound.
High_Control is defined as wells with carbachol.
Low_Control is defined as wells with DMSO.
Patch Clamp Assays (AID 2116, AID 2694, and AID 2692)
The purpose of these assays is to determine if test compounds can increase current recordings in TRPML3 ion channels (AID 2116 and AID 2694) or TRPN1 (AID 2692), along with controls employing the YFP-HEK parental background. Whole-cell currents were recorded with an Alembic Instruments VE-2 amplifier with 100% series resistance compensation, and acquired with JClamp software. The standard bath solution contained (in mM) 138 NaCl, 5.4 KCl, 2 MgCl2, 2 CaCl2, 10 HEPES, and 10 d-glucose, adjusted to pH 7.4 with NaOH. The standard pipette solution contained (in mM) 140 CsCl, 10 HEPES, 3 ATP-Na, 1 BAPTA, and 2 MgCl2, adjusted to pH 7.2. 100 μM 2-Aminoethyl-diphenyl borate was included in the bath solution to block gap junctions and had no effect on the expressed channels. Channel responses were plotted to 10 ms voltage steps (holding potential = +10 mV) between -200 mV and +100 mV in 20 mV incremental steps, normalized by cell capacitance (pF). Compounds were tested at 10 micromolar.
Fura-2 Calcium Influx Assays (AID 2116, AID 2719, and AID 2770)
The purpose of these assays is to determine whether compounds identified as probe candidates are able to increase whole cell Ca2+ influx in HEK293 cells transfected with human TRPML3, other human, or murine (m) TRP channels, or zebrafish TRPN1. In this assay cells transiently expressing channels or YFP control plasmid are perfused with test compound, followed by measurement of intracellular [Ca2+] for 2 minutes with the fluorescent indicator fura-2-AM. Compounds are added to cells 20–25 hours after transfection. Values are reported as mean values +/− SEM (n >= 3 independent experiments with 20–30 cells). The % activation values for TRPML2 in the SAR tables were calculated by normalizing the TRPML2 response ratios to TRPML3 response ratios. Compounds were tested at 10 micromolar.
2.2. Probe Chemical Characterization
Solubility
As shown in Table 3 the solubility of probes 1 and 2 in PBS was determined to be 0.28 μM and 0.02μM, respectively (solution used: 137 mM NaCl, 2.7 mM KCl, 10 mM sodium phosphate dibasic, 2 mM potassium phosphate monobasic, pH 7.4, room temperature). Owing to these poor solubilities, ML269 was tested in PBS supplemented with 6% fetal bovine serum (FBS), under conditions that are more relevant to the conditions used in the TRPML3 assay. Under these conditions, ML269 (specifically the re-assigned compound identified here as 6a) has a solubility of 12 μM. Therefore, probe solubilities are fully adequate to provide the high potency seen in multiple cell-based assays (< 1 μM) and are also adequate for broad use as a biological probe to be used in a variety of media.
Table 3
Probe Solubility and Stability.
Stability and Reactivity
The probes have a half-life of >48 hours in PBS at room temperature (Figures 2 and 3), when tested at 10 μM concentration. No erosion of LCMS peak intensity was seen in the 2 day duration of the study. The probes were found to be unreactive with excess glutathione, indicating that they are not a Michael acceptor under physiologically-relevant conditions. The compounds were tested at 10 μM concentration in the presence of 100 μM GSH (10-fold excess) and no erosion of LCMS peak intensity for the probe was seen for duration of the study.

Figure 2
Probe 1 Stability.

Figure 3
Probe 2 Stability.
2.3. Probe Preparation
(1) Synthesis of Probe 1 (CID 776924, compound 3)

Synthesis of 1
1-Naphthol (577 mg, 4 mmol, 1 equiv.) and potassium carbonate (1 g, 2 mmol) were ground together in a in a 10-mL round-bottomed flask until a fine powder was obtained. This mixture was then heated at 60 °C, and then diethyl sulfate (524 μL, 1 equiv.) was added under vigorous stirring. The mixture was heated at 60 °C until the reaction was complete as judged by TLC analysis. Water (10 mL) was added to the mixture and the aqueous layer was extracted three times with diethyl ether (10 mL). The combined organic layers were washed with 1N HCl, water and brine, and then dried over sodium sulfate. Solvent was removed under reduce pressure and compound 1 was obtained as a colorless oil (535 mg, 78 %) after purification by flash chromatography (silica gel, 100 % hexanes, Rf = 0.32).
1H NMR (400 MHz, CDCl3) δ 8.40-8.36 (1H, m), 7.87-7.84 (1H, m), 7.57-7.51 (2H, m), 7.49-7.40 (2H, m), 6.84 (1H, dd, J = 0.8 Hz, J = 7.6 Hz), 4.25 (2H, q, J = 7.1 Hz), 1.60 (3H, t, J = 7.0 Hz). 13C NMR (100 MHz, CDCl3) δ 154.7, 134.5, 127.4, 126.3, 125.9, 125.7, 125.0, 122.1, 120.0, 104.6, 63.6, 14.8.
Synthesis of 2
A solution of 1 (535 mg, 3.11 mmol, 1 equiv.) in chloroform (3.1 mL) in a 10-mL round-bottomed flask was cooled to −5 °C under an inert atmosphere. Chlorosulfonic acid (410 μL, 2 equiv.) was added dropwise to this solution. The stirred mixture was allowed to warm to room temperature and stirred for an additional 20 min. The mixture was then poured onto ice (13 g); chloroform (20 mL) was added along with a small amount of brine (5 mL). The heterogeneous solution was filtered through a short pad of Celite, the layers were separated and the organic layer was washed twice with water and once with brine. The organic phase was dried over sodium sulfate, filtered, and solvent was removed under reduce pressure to yield compound 2 as an off-white solid (511 mg, 61 %). This material was used in the next step without purification.
1H NMR (400 MHz, CDCl3) δ 8.71 (1H, ddd, J = 0.8 Hz, J = 0.8 Hz, J = 8.6 Hz), 8.41 (1H, ddd, J = 0.6 Hz, J = 1.2 Hz, J = 8.6 Hz), 8.28 (1H, d, J = 8.6 Hz), 7.78 (1H, ddd, J = 1.3 Hz, J = 6.9 Hz, J = 8.4 Hz), 7.62 (1H, ddd, J = 1.1 Hz, J = 6.9 Hz, J = 8.3 Hz), 6.77 (1H, d, J = 8.6 Hz), 4.28 (2H, q, J = 7.0 Hz), 1.59 (3H, t, J = 7.0 Hz). 13C NMR (100 MHz, CDCl3) δ 161.3, 132.0, 130.8, 129.7, 128.7, 126.7, 126.0, 123.7, 123.3, 102.2, 64.9, 14.4.
Synthesis of 3 (Probe 1, CID776924)
To a room temperature solution of sulfonyl chloride 2 (510 mg, 1.88 mmol, 1 equiv.) in dichloromethane (20 mL) were added triethylamine (525 μL, 2 equiv.) and azepane (318 μL, 1.5 equiv.). This mixture was stirred for 2 h. The mixture was poured into water (20 mL) and aqueous layer was extracted three times with dichloromethane. The combined organic layers were washed with 1N HCl, water and brine, and then dried over sodium sulfate. The mixture was filtered, solvent was removed under reduce pressure and compound 3 (493 mg, 79 %, white solid— mp = 103–104 °C) was obtained after purification by flash chromatography (silica gel, ethyl acetate-hexanes 20/80, Rf = 0.66).
Compound 3 (Probe 1, CID 776924): 1H NMR (400 MHz, CDCl3) δ 8.60 (1H, ddd, J = 0.9 Hz, J = 0.9 Hz, J = 8.5 Hz), 8.37 (1H, ddd, J = 0.8 Hz, J = 1.2 Hz, J = 8.4 Hz), 8.12 (1H, d, J = 8.3 Hz), 7.63 (1H, ddd, J = 1.4 Hz, J = 6.9 Hz, J = 8.4 Hz), 7.54 (1H, ddd, J = 1.1 Hz, J = 6.9 Hz, J = 8.3 Hz), 6.77 (1H, d, J = 8.4 Hz), 4.25 (2H, q, J = 7.0 Hz), 3.36 (4H, dd, J = 5.8 Hz, J = 6.0 Hz), 1.68-1.64 (4H, m), 1.59-1.55 (7H, m). 13C NMR (100 MHz, CDCl3) δ 158.7, 131.5, 129.9, 128.1, 126.2, 126.1, 125.8, 124.9, 122.8, 102.3, 64.2, 47.7, 29.1, 26.9, 14.6. HRMS ([M+Na]+) expected: 356.1291, observed: 356.1307. IR (cm−1): 2932, 2859, 1590, 1574, 1509, 1376, 1314, 1148, 1229, 1086, 1041, 961, 775, 709.
(2) Synthesis of Probe 2 (CID 532239838, compound 6a)

Synthesis of 4
3-(4-Chlorophenyl)-5-methylisoxazole-4-carboxaldehyde (1 g, 4.52 mmol, 1 equiv, purchased from Sigma-Aldrich, # 703281-1G) was dissolved in freshly distilled tetrahydrofuran (45 mL) under inert atmosphere in a flame-dried flask. The solution was cooled to −15 °C and then methylmagnesium bromide (3 M in diethyl ether, 2.26 mL, 1.5 equiv.) was slowly added. The mixture was allowed to warm up room temperature and was stirred for 1 h. The mixture was then poured into ice-water and 1N HCl was slowly added until the pH = 1. The aqueous layer was extracted with diethyl ether (3 × 100 mL) and the combined organic layers were washed with water (100 mL), brine (100 mL) and then dried over sodium sulfate. The solution was filtered, solvent was removed under reduced pressure to yield the corresponding secondary alcohol as a colorless oil (1.07 g, quant.) which was used in the following step without purification.
Chromic acid (904 mg, 9.04 mmol, 2 equiv.) and acetic anhydride (50 mL) were mixed with stirring in a 100-mL round-bottomed flask until homogeneous. A solution of secondary alcohol from the previous step (1.07 g, 4.52 mmol, 1 equiv.) in acetic anhydride (20 mL) was then added dropwise via syringe pump (10 min). The resulting mixture was stirred for 30 min, then was extracted with diethyl ether (4×50 mL). The combined organic layers were washed several times with saturated aqueous ammonium chloride solution. The organic layer was dried over sodium sulfate, filtered and then solvent was removed under reduced pressure. The crude methyl ketone was purified by flash chromatography over silica gel (ethyl acetate-hexanes 20/80, Rf = 0.28) to yield 4 (1.07 g, 99 % over two steps) as a colorless oil.
1H NMR (400 MHz, CDCl3) δ 7.45 (4H, s), 2.70 (3H, s), 2.13 (3H, s). 13C NMR (100 MHz, CDCl3) δ 192.7, 174.7, 161.0, 136.3, 130.4, 128.9, 127.3, 117.2, 30.6, 13.7.
Synthesis of 5
A solution of 4 (530 mg, 2.25 mmol, 1 equiv.) in dry toluene (7.5 mL) in a flame-dried sealed tube was treated with tert-butoxy bis(dimethylamino)methane (511 μL, 1.1 equiv.) under an inert atmosphere. The tube was sealed and the mixture was heated at 110 °C overnight. Toluene was then removed under reduce pressure to give the crude enamino-ketone intermediate as a pale yellow oil (641 mg, 98%) which was used in the next step without further purification.
The enamino-ketone from the preceding experiment (360 mg, 1.24 mmol, 1 equiv.) was dissolved in acetic acid (2 mL) in a 5-mL microwave-vial, and anhydrous hydrazine (43 μL, 1.1 equiv.) was added. The mixture was submitted to microwave irradiations for 1 h at 120 °C. Water (5 mL) and dichloromethane (5 mL) were added and then the resulting mixture was transferred to a separatory funnel. Saturated aqueous sodium hydrogenocarbonate solution was added until pH = 4–5, then the aqueous layer was extracted with dichloromethane (3 × 15 mL). The combined organic layers were washed with saturated aqueous ammonium chloride solution (30 mL), brine (30 mL) and dried over sodium sulfate. The solution was filtered and solvent was removed under reduce pressure. Pyrazole 5 was obtained as a pale yellow solid (300 mg, 91 %, 2 steps) after purification by flash chromatography (silica gel, ethyl acetate-hexanes 40/60, Rf = 0.65).
1H NMR (400 MHz, CDCl3) δ 10.31 (1H, br s), 7.51 (1H, d, J = 2.2 Hz), 7.46 (2H, d, J = 8.5 Hz), 7.30 (2H, d, J = 8.5 Hz), 6.16 (1H, d, J = 2.1 Hz), 2.50 (3H, s). 13C NMR (100 MHz, CDCl3) δ 168.4, 160.3, 139.7, 135.8, 131.5, 129.7, 128.8, 127.5, 108.1, 106.0, 12.0.
Synthesis of Probe 2 (6a, CID 53239838) and Its Regioisomer 6b
Method A: Pyrazole 5 (115 mg, 0.44 mmol, 1 equiv.) and p-tosyl chloride (84 mg, 1 equiv.) were dissolved in dry dichloromethane (4.4 mL) under inert atmosphere. Freshly distilled triethylamine (124 μL, 2 equiv.) was added and the mixture was stirred at room temperature for 24 h. At this time water (10 mL) and aqueous 1N HCl solution (10 mL) were added. The aqueous layer was extracted with dichloromethane (3 × 15 mL) The combined organic layers were washed with water (15 mL) and brine (15 mL) and dried over sodium sulfate. The solution was filtered, then solvent was removed under reduce pressure to give a 3:1 mixture of regioisomers 6a and 6b. The major regioisomer 6a (104 mg, white solid—mp = 133–134 °C) and the minor isomer 6b (34 mg, pale yellow solid) were separated by flash chromatography over silica gel (ethyl acetate/hexanes 20/80, Rf6a = 0.26, Rf6b= 0.14) with an overall yield of 76 %. The minor regioisomer6bhas the structure originally listed for Probe 2 the commercial supplier.
Method B: Pyrazole 5 (20 mg, 0.08 mmol, 1 equiv.), p-tosyl chloride (14.7 mg, 1 equiv.) and 97 % sodium hydride (3.8 mg, 2 equiv.) were weighed into a flame-dried 10-mL round-bottomed flask inside an inert atmosphere glovebox. The flask was sealed under inert atmosphere and the solids were dissolved in freshly distilled tetrahydrofuran (0.8 mL). The mixture was stirred at room temperature for 24 h. At this time water (5 mL) and aqueous 1N HCl solution (5 mL) were added with precaution. The aqueous layer was separated and extracted with dichloromethane (3 × 10 mL) The combined organic layers were washed with water (10 mL) and brine (10 mL) and dried over sodium sulfate. The solution was filtered, and solvent was removed under reduce pressure yielding regioisomers 6a (minor regioisomer 6b was not observed by 1H NMR analysis of the crude product). The major regioisomer 6a (26 mg, 82 %, white solid— mp = 133–134 °C) was purified by flash chromatography over silica gel (ethyl acetate/hexanes 20/80, Rf6a= 0.26).
Major regioisomer 6a (Probe 2, CID 53239838): 1H NMR (400 MHz, CDCl3) δ 8.08 (1H, d, J = 2.8 Hz), 7.87 (2H, d, J = 8.4 Hz), 7.37-7.33 (4H, m), 7.24 (2H, d, J = 8.7 Hz), 6.12 (1H, d, J = 2.7 Hz), 2.50 (3H, s), 2.46 (3H, s). 13C NMR (100 MHz, CDCl3) δ 169.3, 160.3, 147.7, 146.1, 135.6, 133.7, 131.8, 130.0, 129.9, 128.6, 128.2, 127.2, 108.8, 107.7, 21.7, 12.2. MS ([M+H]+): 414.1. IR (cm−1): 3142, 3126, 2921, 1641, 1475, 1370, 1178, 1157, 1093, 1048, 929, 778, 669.
Minor regioisomer 6b (with the structure listed for CID 2745583): 1H NMR (400 MHz, CDCl3) δ 7.83 (1H, d, J = 1.6 Hz), 7.48 (2H, d, J = 8.4 Hz), 7.15-7.14 (4H, m), 7.10 (2H, d, J = 8.1 Hz), 6. 04 (1H, d, J = 1.6 Hz), 2.34 (3H, s), 2.29 (3H, s). 13C NMR (100 MHz, CDCl3) δ 170.3, 159.6, 146.0, 143.5, 135.8, 134.8, 133.9, 129.7, 128.7, 128.4, 128.0, 127.1, 113.2, 103.9, 21.6, 11.7. MS ([M+H]+): 414.1. IR (cm−1): 3128, 2924, 2853, 1640, 1596, 1452, 1418, 1389, 1269, 1194, 1172, 1124, 1089, 914, 844, 809, 678, 661.
Determination of the Structures of Regioisomers 6a and 6b
It is expected that sulfonylation of pyrazole 5 should lead selectively to regioisomer 6a. Indeed, isomer 6a was obtained exclusively when the sulfonylation was performed according to Method B. Fortunately, regioisomer 6b was obtained as the minor component of a 3 : 1 mixture from the sulfonylation performed as described in method A. Therefore, samples of both compounds were subjected to testing in the TRPML3 assay, from which it was determined that regioisomer 6a was the active probe compound.
Further evidence that regioisomer 6a is the correct structure of Probe 2 was provided as follows. TLC analysis demonstrated conclusively that the synthetic compound 6a is the same as a sample of Probe 2 obtained from a commercial supplier. However, compound 6b—which is inactive in the TRPML3 assay and which is different from commercial samples of Probe 2 by TLC analysis—has the structure as originally listed for Probe 2 in PubChem (CID 2745583) and in the commercial vendor’s catalogs. Therefore, we suspected that the structure of the compound is incorrect in the vendor catalogs, and that this error has been duplicated in the PubChem database.
We have performed NMR analysis of a commercial sample of the probe compound, obtained from Maybridge Ltd (the supplier ID is SPB04991), and have verified that synthetic probe 6a is IDENTICAL to the material—written with the wrong structure—in the vendor catalog.
Therefore, the following NMR experiments were performed in order to clarify the structures of regioisomers 6a and 6b. The 1H nOe summarized in the following figures are highly instructive. The nOe data in Figure 1, obtained by irradiation of the ortho hydrogen atoms of the toluenesulfonyl group, resulted in a strong enhancement of the indicated ortho pyrazoles ring hydrogen in structure 6a, but only a weak enhancement in 6b. Conversely, this nOe study (Figure 4) led to a stronger enhancement of the oxazoles methyl group in 6b than in 6a. These data are fully consistent with the assigned structures. This conclusion is enhanced by the 1H nOe study summarized in Figure 5, in which the ortho pyrazole hydrogen was irradiated in the two regioisomers. This resulted in a strong enhancement of the ortho hydrogen atoms of the toluenesulfonyl group in regioisomer 6a but only a weak enhancement in 6b.
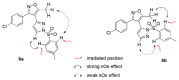
Figure 4
1H nOe enhancements from irradiation of 6a at 7.87 ppm and 6b at 7.48 ppm.

Figure 5
1H nOe enhancements from irradiation of 6a at 8.08 ppm and 6b at 7.83 ppm.
Therefore, based on these studies, the structure of Probe 2 must be reassigned from the original structure (listed in PubChem as CID 2745583, as in isomer 6b) to that of compound 6a (CID 53239838).

3. Results
Summary. Following primary HTS in singlicate to identify TRPML3 agonists (AID 1448), counterscreening against TRPN1 to identify nonselective agonists in singlicate (AID 1424) and triplicate (1525), confirmation of TRPML3 activity in triplicate (AID 1526), titration assays in triplicate to determine potency (AID 1562) and selectivity (AID 1682), certain compounds were identified as possible candidates for probe development. Compounds were ordered as powders samples for testing by the SRIMSC and assay provider labs. The results of powder dose response assays (Rounds 0 and 1: AID 2510; Round 2: 602128; Round 3: AID 602129), and selectivity dose response assays against TRPN1 (AID 2583), patch clamp assays against TRPML3 (AID 2694) and TRPN1 (AID 2692), and Fura-2 ion channel profiling (AID 2719 and AID 2770), resulted in the identification of two selective TRPML3 agonist probes ML268 and ML269.
3.1. Summary of Screening Results
The purpose of the HTS assays was to identify compounds that can increase calcium flux selectively through the TRPML3 channel, as measured using the Fluo-8 calcium reporter dye. These assays are cell-based, and employ HEK293 cells that stably express the human TRPML3-YFP cation or TRPN1 (anti-target) channel. The HTS assays were run in 1536-well plate format and were conducted at the SRISMC facility in Jupiter, Florida. Two HTS lead compounds belonging to the tertiary arylsulfonamides and Pyrazol-5-yl-isoxazole scaffolds (SID 14722627 and SID 26731169), exhibited EC50 values in HTS assays of 1.03 μM and 0.33 μM, respectively. These compounds and related analogs were ordered as powders (SID 87692368 and SID 87692372) for retesting by the SRIMSC in TRPML3 dose response assays. The powder samples exhibited EC50 values consistent with the liquid samples: 0.95 μM and 0.25 μM, respectively. Next, the assay provider tested these two probe candidates and select analogs in TRPML3 patch clamp, TRPN1 patch clamp counterscreening, and Fura-2 imaging assays. Results of the assay provider Fura-2 assays revealed that the two probes have selective activity against TRPML3, being inactive against all other ion channels tested, including the prior anti-targetsTRPN1 and TRPML2. See the SAR tables for additional data.
The flow chart (Figure 6) outlines the results of the ultra high throughput screening (uHTS) campaign. Briefly, 306 available hits were initially identified, 215 were confirmed as TRPML3-specific, and dose response potency and selectivity screens reduced the number of actives under consideration to 56.

Figure 6
TRPML3 Selective Agonist Screening Campaign Funnel.
3.2. Dose Response Curves for Probe
The dose response curves for the TRPML3 selective agonist probes as determined using Fluo-8 calcium dye cell-based fluorescence assays are shown in Figure 7. Please refer to the indicated PubChem AIDs for details.
3.3. Scaffold/Moiety Chemical Liabilities
There are no known chemical liabilities with the two probes described in this probe report.
3.4. SAR Tables
Please see next page for SAR tables.
SAR Table 1Tertiary arylsulfonamides Scaffold
| Compound Information | Screening Assays | Probe Development Data | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | SR-Number | Structure | CID | SID | MLS | Vendor | Vendor Catalog ID | AID 1448: TRPML3 Primary 1X%ACT | AID 1424: TRPN1 Primary 1X%ACT | AID 1526: TRPML3 CRUN 3X%ACT | AID 1525: TRPN1 3X%ACT | AID 1562: TRPML3 EC50, μM | TRPN1 CS DRUN (AID 1682) EC50, μM | AID 2510: TRPML3 EC50, μM | AID 2583: TRPN1 EC50, | AID 2694: TRPML3 Patch Clamp: nA/pF, −80 mV | AID 2692: TRPN1 Patch Clamp: nA/pF, −80 mV | AID 2719:TRPML3 Fura-2 Imaging: ΔF, 340 nm/ 380 nM | AID 2770: Fura-2 Assay: Other channels DF340/ F380 | AID 602128: TRPML3 EC50, μM | AID 602129: TRPML3 EC50, μM |
| Probe 1 (liquid SID) | SR-01000430563-2 |
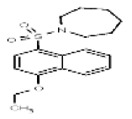
 | 776924 | 14722627 | MLS 000123160 | Asinex | BAS 00667918 | Active (101.27) | Inactive (−1.58) | Active (128.69) | Inactive (−0.72) | Active (1.03) | Inactive (>29.9) | Not tested: this SID is an MLSMR liquid and was not tested during MedChem | |||||||
| Probe 1 (ML268) (powder SID, purchased) | SR-01000430563-3 |
 | 776924 | 87692368 | None | ChemBridge | 5852491 | Not tested: this SID is a powder and was not tested during HTS | 0.95 (Active) CID776924 SID | >29.9 (Inactive) | −0.19 ±0.03 (Active) | − 0.003 ±0.001 (Inactive) | 1.0 ±0.1 (Active) | −0.011 ± 0.001 (Inactive) | SID Not Tested | SID Not Tested | |||||
| Probe 1 (powder SID, synthesized) | SR-03000002102-1 |
 | 776924 | 113584822 | MLS003875009 | TSRI | None | Not tested: this SID is a powder and was not tested during HTS | Compound was synthesized iin order to submit to MLSMR | ||||||||||||
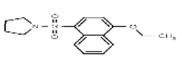
| Analog1a | SR-01000724392-1 |
 | 801435 | 24801969 | MLS000706525 | Asinex | BAS 02912185 | 67.73 (Active) | Inactive | 118.18 (Active) | Inactive | 1.33 (Active) | Inactive (>29.9) | Not tested: this SID is an MLSMR liquid and was not tested during MedChem | |||||||
| Analog 1b (purchased) | SR-01000724392-2 |
 | 801435 | 87692370 | MLS 000706525: | Asinex | BAS 02912185 | Not tested: this SID is a powder and was not tested during HTS | 1.124 (Active) | >29.9 (Inactive) | − 0.02 ±0.01 (Active) | − 0.003 ±0.001 (Inactive) | 0.52 ±0.05 (Active) | 0.008 ± 0.005 (Inactive) | Not Tested | Not Tested | |||||
| Analog 1c (synthesized) | SR-01000724392-3 |
 | 801435 | TBD | MLS003875013 | TSRI | None | Not tested: this SID is a powder and was not tested during HTS | Not tested: Compound was synthesized for submission to MLSMR | ||||||||||||
| Analog 2a (liquid) | SR-01000529615-2 |
 | 569100 | 14740076 | MLS 000530984 | Chem Bridge | 6930808 | Active (133.27) | Inactive (6.36) | Active (104) | Inactive (6.27) | Active (1.33) | Inactive (>29.9) | Not tested: this SID is an MLSMR liquid and was not tested during MedChem | |||||||
| Analog 2b (purchased) | SR-01000529615-3 |
 | 569100 | 126723256 | MLS003875014 | Chemical Block Ltd | A2255/ 0095000 | Not tested: this SID is a powder and was not tested during HTS | Not tested: Compound was purchased for submission to MLSMR | ||||||||||||
| Analog 3 | SR-01000503984-2 |
 | 2920855 | 17412681 | MLS 000575809 | Chem Bridge | 6963034 | Active (68.49) | Inactive (−0.16) | Active (96.95) | Inactive (−1.04) | Active (1.37) | Inactive (>29.9) | Not tested: this SID is an MLSMR liquid and was not tested during MedChem | |||||||
| Analog 4 (MLSMR liquid) | SR-01000271732-2 |
 | 976573 | 24786634 | MLS 000693818 | Chem Bridge | 7726423 | Active (84.45) | Inactive (−1.46) | Active (77.37) | Inactive (−1.72) | Active (1.43) | Inactive (>29.9) | Not tested: this SID is an MLSMR liquid and was not tested during MedChem | |||||||
| Analog 4 (purchased) | SR-01000271732-3 |
 | 976573 | 87692367 | None | Enamine | T5579947 | Not tested: this SID is a powder and was not tested during HTS | 1.798 (Active) | >29.9 (Inactive) | Not tested due to lower TRPML3 activity, compared to probe. | ||||||||||
| Analog 5 (MLSMR liquid) | SR-01000683090-1 |
 | 1818631 | 14727674 | MLS 000548862 | Specs | AP-263/ 42611152 | Active (25.88) | Inactive (−2.06) | Active (68.51) | Inactive (−2.49) | Active (1.6) | Inactive (>29.9) | Not tested: this SID is an MLSMR liquid and was not tested during MedChem | |||||||
| Analog 5 (purchased) | SR-01000683090-2 |
 | 1818631 | 87692369 | None | Enamine | T5580266 | Not tested: this SID is a powder and was not tested during HTS | 4.724 (Active) | >29.9 (Inactive) | Not tested due to lower TRPML3 activity, compared to probe. | ||||||||||
| Analog 6 | SR-01000316809-2 |
 | 846974 | 125311233 | MLS003875010 | TSRI | None | Not tested: this SID is a powder and was not tested during HTS | Not tested: compound was synthesized after these assays were completed. | 0.476 (Active) | |||||||||||
| Analog 7 | SR-03000002558-1 |
 | 53384704 | 125311234 | MLS003875012 | TSRI | None | Not tested: this SID is a powder and was not tested during HTS | Not tested: compound was synthesized after these assays were completed. | 17.99 (Inactive) | |||||||||||
| Analog 8 | SR-01000407579-3 |
 | 772123 | 125311235 | MLS003875011 | TSRI | None | Not tested: this SID is a powder and was not tested during HTS | Was synthesized to complete SAR after Probe ML289 was fully characterized. While analog 1 is more active than probe ML289 in the primary assays, it has not been subjected to all final characterization assays (e.g., Patch Clamp assay, etc) | 3.457 (Active) | |||||||||||
| Analog 9 | SR-01000320627-3 |
 | 1085524 | 125311236 | None | TSRI | None | Not tested: this SID is a powder and was not tested during HTS | Not tested: compound was synthesized after these assays were completed. | >29.9 (Inactive) | |||||||||||
| Analog 10 | SR-03000002559-1 |
 | 53384709 | 125311237 | None | TSRI | None | Not tested: this SID is a powder and was not tested during HTS | Not tested: compound was synthesized after these assays were completed. | >29.9 (Inactive) | |||||||||||
| Analog 11 | SR-03000002560-1 |
 | 53384719 | 125311238 | None | TSRI | None | Not tested: this SID is a powder and was not tested during HTS | Not tested: compound was synthesized after these assays were completed. | >29.9 (Inactive) | |||||||||||
| Analog 12 | SR-01000803473-2 |
 | 818895 | 125311239 | None | TSRI | None | Not tested: this SID is a powder and was not tested during HTS | Not tested: compound was synthesized after these assays were completed. | >29.9 (Inactive) | |||||||||||
| Analog 13 | SR-03000002561-1 |
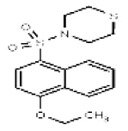 | 52418320 | 125311240 | None | TSRI | None | Not tested: this SID is a powder and was not tested during HTS | Not tested: compound was synthesized after these assays were completed. | >29.9 (Inactive) | |||||||||||
| Analog 14 | SR-01000321000-2 |
 | 801442 | 125311241 | None | TSRI | None | Not tested: this SID is a powder and was not tested during HTS | Not tested: compound was synthesized after these assays were completed. | >29.9 (Inactive) | |||||||||||
| Analog 15 (purchased) | SR-01000216251-1 |
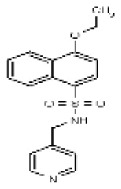 | 794388 | 131269030 | None | ChemBridge | 5661396 | Not tested: this SID is a powder and was not tested during HTS | Not tested: compound was purchased after these assays were completed. | >29.9 (Inactive) | |||||||||||
| Analog 15 (synthesized) | SR- 01000216251-2 |
 | 794388 | 125311242 | None | TSRI | None | Not tested: this SID is a powder and was not tested during HTS | Not tested: compound was synthesized after these assays were completed. | ||||||||||||
| Analog 16 | SR-03000002562-1 |
 | 53384693 | 125311243 | None | TSRI | None | Not tested: this SID is a powder and was not tested during HTS | Not tested: compound was synthesized after these assays were completed. | >29.9 (Inactive) | |||||||||||
| Analog 17 | SR-03000002563-1 |
 | 16645312 | 125311244 | None | TSRI | None | Not tested: this SID is a powder and was not tested during HTS | Not tested: compound was synthesized after these assays were completed. | >29.9 (Inactive) | |||||||||||
SAR Table 2Pyrazol-5-yl-isoxazole Scaffold
| Compound Information | HTS Assays | Probe Development Assays | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | SR-Number | Structure | Solubility in PBS (μM) | Solubility in PBS-6% FBS (μM) | CID | SID | MLS | Vendor | Vendor Catalog ID | AID 1448: TRPML3 Primary 1X%ACT | AID 1424: TRPN1 Primary 1X%ACT | AID 1526: TRPML3 CRUN 3X%ACT | AID 1525: TRPN1 3X%ACT | TRPN1 CS DRUN (AID 1682) EC50, μM | AID 2510: TRPML3 EC50, μM | AID 2583: TRPN1 EC50, | AID 2694: TRPML3 Patch Clamp: nA/pF, −80 mV | AID 2692: TRPN1 Patch Clamp: nA/pF, −80 mV | AID 2719: TRPML3 Fura-2 Imaging: ΔF, 340 nm/380 nM | AID 2770: Fura-2 Assay: Other channels DF340/F380 | AID 602128: TRPML3 EC50, μM | AID 602129: TRPML3 EC50, μM |
| Probe 2 Compound (6a) (this liquid SID is associated with an incorrect CID structure in PubChem). | SR-01000768170-1 |
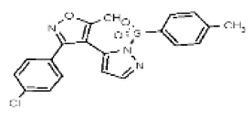 | 0.1 | 5.9 | 2745583 | 26731169 | MLS 001111122 | Maybridge | SPB 04991 | Active (81.8) | Inactive (1.01) | Active (106.23) | Inactive (0.49 | Inactive (>29.9) | Not tested: this SID is an MLSMR liquid and was not tested during MedChem | |||||||
| Probe 2 (this powder SID was purchased to confirm activity; SID is associated with an incorrect CID structure in PubChem) | SR-01000768170-2 |
 | 2745583 | 87692372 | None | Maybridge | SPB 04991 | Not tested: this SID is a powder and was not tested during HTS | 0.2526 (Active) | >29.9 (Inactive) | −0.14 ±0.02 (Active) | −0.004 ±0.001 (Inactive) | 0.72 ±0.05 (Active) | 0.005 ± 0.002 (Inactive) | SID Not Tested | SID Not Tested | ||||||
| Probe 2 (this powder SID was synthesized to prove that the CID structure in PubChem is incorrect; compound is inactive) | SR-01000768170-3 |
 | 0.02 | 12 | 2745583 | 124349366 | None | TSRI | None | Not tested: this SID is a powder and was not tested during HTS | Not tested: Compound was synthesized after these assays were completed. | >29.9 (Inactive) | SID Not Tested | |||||||||
| Probe 2 (ML269) Compound (6a) (this powder was synthesized to prove SAR; compound associated with this structure is active. Note new CID). | SR-03000002360-1 |
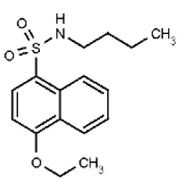 | 53239838 | 124349367 | MLS003675345 | TSRI | None | Not tested: this SID is a powder and was not tested during HTS | Not tested: Compound synthesized after these assays were completed. | 0.291 (Active) | SID Not Tested | |||||||||||
| Compound Information | HTS Assays | Probe Development Assays | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | SR-Number | Structure | CID | SID | MLS | Vendor | Vendor Catalog ID | AID 1448: TRPML3 Primary 1X%ACT | AID 1424: TRPN1 Primary 1X%ACT | AID 1526: TRPML3 CRUN 3X%ACT | AID 1525: TRPN1 3X%ACT | AID 1562: TRPML3 EC50, μM | TRPN1 CS DRUN (AID 1682) EC50, μM | AID 2510: TRPML3 EC50, μM | AID 2583: TRPN1 EC50, | AID 2694:TRPML3 Patch Clamp: nA/pF, −80 mV | AID 2692: TRPN1 Patch Clamp: nA/pF, −80 mV | AID 2719:TRPML3 Fura-2 Imaging: ΔF, 340 nm/380 nM | AID 2770: Fura-2 Assay: Other channels DF340/F380 | AID 602128: TRPML3 EC50, μM | AID 602129: TRPML3 EC50, μM |
| Analog 1a | SR-03000002573-1 |
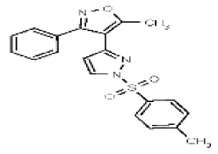 | 53384691 | 125311254 | MLS003875016 | TSRI | None | Not tested: this SID is a powder and was not tested during HTS | Not tested: Compound synthesized after these assays were completed. | 1.459 (Active) | |||||||||||
| Analog 1b (MLSMR liquid SID is associated with incorrect structure in PubChem) | SR-01000767070-1 |
 | 2745600 | 26731235 | MLS 001111124 | Maybridge | SPB 05016 | 119.6 | Inactive (1.19) | Active (94.33) | Inactive (−1.09 | Active (0.819) | Inactive (>29.9) | Not tested: this SID is an MLSMR liquid and was not tested during MedChem | |||||||
| Analog 1b (this sample was purchased to confirm HTS activity) | SR-01000767070-2 |
 | 2745600 | 87692371 | None | Maybridge | SPB 05016 | Not tested: this SID is a powder and was not tested during HTS | 1.005 (Active) | >29.9 (Inactive) | This SID not tested in these assays | ||||||||||
| Analog 1b (this sample was synthesized to obtain correct structure shown) | SR-03000002582-1 |
 | 2745600 | 125311263 | None | TSRI | None | Not tested: this SID is a powder and was not tested during HTS | Compound was synthesized in order to confirm the structure was incorrect in PubChem. | >29.9 (Inactive) | |||||||||||
| Analog 2a | SR-03000002574-1 |
 | 53384705 | 125311255 | None | TSRI | None | Not tested: this SID is a powder and was not tested during HTS | Not tested: Compound synthesized after these assays were completed. | 3.336 (Active) | |||||||||||
| Analog 2b (MLSMR liquid SID is associated with an incorrect structure in PubChem) | SR-01000767049-1 |
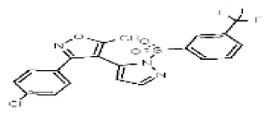 | 2745598 | 26731234 | MLS 001111123 | Maybridge | SPB 05013 | 33.0 | Inactive (1.49) | Active (51.03) | Inactive (−0.27 | Active (4.26) | Inactive (>29.9) | Not tested: this SID is an MLSMR liquid and was not tested during MedChem | |||||||
| Analog 2b (this sample was synthesized to obtain correct structure shown) | SR-03000002583-1 |
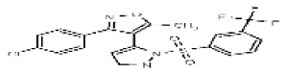 | 2745598 | 125311264 | None | TSRI | None | Not tested: this SID is a powder and was not tested during HTS | Not tested: Compound synthesized after these assays were completed. | >29.9 (Inactive) | |||||||||||
| Analog 3a | SR-03000002576-1 |
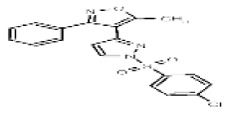 | 53384724 | 125311257 | None | TSRI | None | Not tested: this SID is a powder and was not tested during HTS | Not tested: Compound synthesized after these assays were completed. | >29.9 (Inactive) | |||||||||||
| Analog 3b (MLSMR SID is associated with an incorrect structure in PubChem) | SR-01000631911-2 |
 | 2745601 | 26731237 | MLS 001111125 | Maybridge | SPB 05017 | Inactive (8.45) | Inactive (2.08) | Not tested due to lack of TRPML3 activity in HTS Primary assay. | Not tested: this SID is an MLSMR liquid and was not tested during MedChem | ||||||||||
| Analog 3b (this sample was synthesized to obtain correct structure shown) | SR-03000002584-1 |
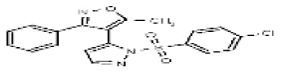 | 2745601 | 125311265 | None | TSRI | None | Not tested: this SID is a powder and was not tested during HTS | Not tested: Compound synthesized after these assays were completed. | >29.9 (Inactive) | |||||||||||
| Analog 4a | SR-03000002575-1 |
 | 53384697 | 125311256 | None | TSRI | None | Not tested: this SID is a powder and was not tested during HTS | Not tested: Compound synthesized after these assays were completed. | 15.82 (Inactive) | |||||||||||
| Analog 4b (the MLSMR liquid SID is associated with an incorrect structure in PubChem) | SR-01000773206-1 |
 | 2745582 | 26731233 | MLS 001111121 | Maybridge | SPB 04990 | Inactive (3.88) | Inactive (0.14) | Not tested due to lack of TRPML3 activity in HTS Primary assay. | Not tested: this SID is an MLSMR liquid and was not tested during MedChem | ||||||||||
| Analog 4b (this sample was synthesized to obtain correct structure shown) | SR-03000002585-1 |
 | 2745582 | 125311266 | None | TSRI | None | Not tested: this SID is a powder and was not tested during HTS | Not tested: Compound synthesized after these assays were completed. | >29.9 (Inactive) | |||||||||||
| Analog 5 (MLSMR Liquid) | SR-01000761039-1 |
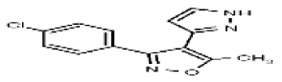 | 2745567 | 26729029 | MLS 000834032 | Maybridge | SPB 04969 | Inactive (−1.12) | Inactive (−1.49) | Not tested due to lack of TRPML3 activity in HTS Primary assay. | Not tested: this SID is an MLSMR liquid and was not tested during MedChem | ||||||||||
| Analog 5 (synthesized) | SR-03000002361-1 |
 | 2745567 | 124349368 | None | TSRI | None | Not tested: this SID is a powder and was not tested during HTS | Not tested: Compound synthesized after these assays were completed. | >29.9 (Inactive) | Not tested | ||||||||||
| Analog 6 | SR-03000002577-1 |
 | 53384721 | 125311258 | None | TSRI | None | Not tested: this SID is a powder and was not tested during HTS | Not tested: Compound synthesized after these assays were completed. | 2.988 (Active) | |||||||||||
| Analog 7 | SR-03000002578-1 |
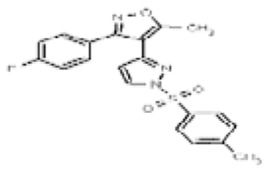 | 53384692 | 125311259 | MLS003875015 | TSRI | None | Not tested: this SID is a powder and was not tested during HTS | Not tested: Compound synthesized after these assays were completed. | 0.68 (Active) | |||||||||||
| Analog 8 | SR-03000002580-1 |
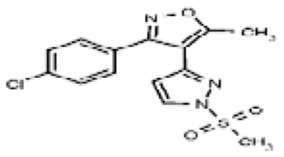 | 53384707 | 125311261 | None | TSRI | None | Not tested: this SID is a powder and was not tested during HTS | Not tested: Compound synthesized after these assays were completed. | >29.9 (Inactive) | |||||||||||
| Analog 9 | SR-03000002581-1 |
 | 53384683 | 125311262 | None | TSRI | None | Not tested: this SID is a powder and was not tested during HTS | Not tested: Compound synthesized after these assays were completed. | >29.9 (Inactive) | |||||||||||
| Analog 10 | SR-03000002579-1 |
 | 53384703 | 125311260 | None | TSRI | None | Not tested: this SID is a powder and was not tested during HTS | Not tested: Compound synthesized after these assays were completed. | >29.9 (Inactive) | |||||||||||
| Analog 11 | SR-03000002564-1 |
 | 53384708 | 125311245 | None | TSRI | None | Not tested: this SID is a powder and was not tested during HTS | Not tested: Compound synthesized after these assays were completed. | 4.89 (Active) | |||||||||||
| Analog 12 | SR-03000002565-1 |
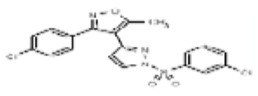 | 53384678 | 125311246 | MLS003875018 | TSRI | None | Not tested: this SID is a powder and was not tested during HTS | Not tested: Compound synthesized after these assays were completed. | 1.945 (Active) | |||||||||||
| Analog 13 | SR-03000002566-1 |
 | 53384730 | 125311247 | MLS003875019 | TSRI | None | Not tested: this SID is a powder and was not tested during HTS | Not tested: Compound synthesized after these assays were completed. | 2.386 (Active) | |||||||||||
| Analog 14 | SR-03000002567-1 |
 | 53384702 | 125311248 | None | TSRI | None | Not tested: this SID is a powder and was not tested during HTS | Not tested: Compound synthesized after these assays were completed. | 3.78 (Active) | |||||||||||
| Analog 15 | SR-03000002568-1 |
 | 53384723 | 125311249 | None | TSRI | None | Not tested: this SID is a powder and was not tested during HTS | Not tested: Compound synthesized after these assays were completed. | >29.9 (Inactive) | |||||||||||
| Analog 16 | SR-03000002569-1 |
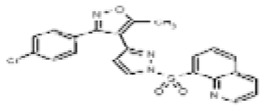 | 53384713 | 125311250 | None | TSRI | None | Not tested: this SID is a powder and was not tested during HTS | Not tested: Compound synthesized after these assays were completed. | >29.9 (Inactive) | |||||||||||
| Analog 17 | SR-03000002570-1 |
 | 53384729 | 125311251 | MLS003875017 | TSRI | None | Not tested: this SID is a powder and was not tested during HTS | Not tested: Compound synthesized after these assays were completed. | 1.533 (Active) | |||||||||||
| Analog 18 | SR-03000002571-1 |
 | 53384687 | 125311252 | None | TSRI | None | Not tested: this SID is a powder and was not tested during HTS | Not tested: Compound synthesized after these assays were completed. | 3.576 (Active) | |||||||||||
| Analog 19 | SR-03000002572-1 |
 | 53384682 | 125311253 | None | TSRI | None | Not tested: this SID is a powder and was not tested during HTS | Not tested: Compound synthesized after these assays were completed. | >29.9 (Inactive) | |||||||||||
3.5. Cellular Activity
Fura-2-AM Assays (AID 2719 and AID 2770) Reveal Cellular Activity of Probes. The SRIMSC next pursued cell-based assays to determine whether the powders samples of probes could increase whole cell Ca2+ influx in HEK293 cells transfected with human TRPML3. These assays were run in a manner similar to the HTS assays (Figure 8). Cells transiently expressing human TRPML3 channel or the YFP control plasmid were perfused with test compound (10 μM), followed by measurement of intracellular Ca2+ for 2 minutes with the fluorescent indicator fura-2-AM (the membrane-permeable derivative of Fura-2). Compounds were added to cells 20–25 hours after transfection. Values are reported as mean values +/− SEM (n ≥ 3 independent experiments with 20–30 cells). Note that these probes do not increase Ca2+ influx in cells expressing the YFP control plasmid (black bar).

Figure 8
Probes ML268 and ML269 increase TRPML3-mediated intracellular Ca2+ flux. Cell-based Fura-2-AM assays using HEK293 cells transfected with human TRPML3 or YFP control plasmid.
Lack of Effect of Probes on Native Cells provides insight into TRPML3 function. Because varitant-waddler epidermal melanocytes express mutant TRPML3 in the plasma membrane, leading to calcium loading, cell death, and variegated coat color [10], we next explored the effect of these TRPML3 probes in a relevant cell type: primary human epidermal skin melanocytes (HEMs). Melanocytes also express all three TRPML channels. Measurements of [Ca2+]i with the fluorescent indicators fura-2-AM (Invitrogen) were performed using a monochromator-based imaging system (iMIC platform and Polychrome V monochromator, TILL Photonics). HEK293 cells, plated onto glass coverslips, were loaded with 4 mM fura-2-AM (Invitrogen) in a standard bath solution (SBS) containing 138 mM NaCl, 6 mM KCl, 2 mM MgCl2, 2 mM CaCl2, 10 mM HEPES, and 5.5 mM D-glucose (adjusted to pH 7.4 with NaOH).
Confirmation of the ability of native HEM TRPML3 to respond was provided by studies using compound SN-2, a cell impermeable compound thought to act at an unknown extracellular site (Figure 9, far right bars). However, in contrast to the results with TRPML3-transfected HEK cells, no significant increase in intracellular Ca2+ was observed in untransfected HEMs treated with 100 μM of the probe compounds (Figure 9). HEMs transfected with TRPML3 do show a calcium response, similar to that of transfected HEK cells. Together these results showing a lack of effect of our probes in non-recombinant HEMs provide three novel insights into native TRPML3 physiology and structure. First, that TRPML3 might be absent from the plasma membrane in native cells (in contrast to its plasma membrane localization in the varitant waddler mutant). Second, that plasma membrane localization of TRPML3 channels is tightly regulated. Third, that the TRPML3 protein is a subunit of heteromeric channels [15, 16] that are nonresponsive to the probes. Future studies using mouse models alone and in combination with these new probes will allow us and other TRP biologists to explore these questions further.
3.6. Profiling Assays
Fura-2 Assays (AID 2719 and AID 2770) Reveal Cellular Activity and TRPML3 Selectivity. We next pursued additional cell-based TRP channel profiling studies to determine whether compounds identified as selective TRPML3 agonist probe candidates also increase whole cell Ca2+ influx in cells transfected with human (h) TRPML3, other human, or murine (m) TRP channels, or zebrafish (dr) TRPN1. In these assays HEK293 cells transiently expressing channels or YFP control plasmid are perfused with test compound, followed by measurement of intracellular [Ca2+] for 2 minutes with the fluorescent indicator fura-2-AM. Compounds were added to cells 20–25 hours after transfection (Figure 10). The bar diagrams represent average [Ca2+]i levels 2 min after 10 μM compound application relative to the respective calcium level before application (mean values ± SEM, n ≥3 independent experiments with 20–30 cells). In contrast to our two previously reported TRPML3/2 dual agonist probes, the current probes exhibit no activity on TRPML2. As a result these new probes are a significant improvement over the prior art. Furthermore, because these probes do not increase Ca2+ flux in non-transfected (NT) HEK293 cells nor in HEK293 cells expressing the YFP control plasmid, it is highly unlikely that these probes activate non-TRPML3-dependent calcium pathways.
These probes exhibit no detectable activity against the TRPN1 channel or the YFP-HEK parental cell line, and are inactive against hTRPML1, hTRPM2, mTRPV2, hTRPC3, drTRPN1, and hTRPA1 ion channels. The MLSMR liquid sample of probe 1 (SID 14722627) was tested in 521 PubChem BioAssays and active in only 4 (3 TRPML3 agonist assays, and one ER stress assay). The liquid sample of probe 2 (SID 26731169) obtained from a commercial supplier was tested in 371 PubChem Bioassays and active only in 8 assays (3 of these are TRPML3 agonist assays). These TRPML3 agonist probes were inactive in other ion channel assays including TRPN1 (AID 1682), TRPML2 (AID 2770 the X11L calcium channel (AID 2073), and the ROM K+ channel (AID 1918).
4. Discussion
4.1. Comparison to existing art and how the new probe is an improvement
As shown in Table 4, the new probes ML268 and ML269 demonstrate selective activity against TRPML3. Our prior probes ML122 and ML123 were active against both TRPML2 and TRPML3. In addition, our prior art probe ML123 exhibited activity against TRPN1. As a result, these new selective probes will allow investigations into the specific biology of the TRPML3 channel. This is significant as selective ligands such as ML268 and ML269 can be used to characterize the specific function and interactions of TRPML3. Further, there are currently no known agonists for this channel and the physiologic roles of this TRP ion channel are unknown.
Table 4
Comparison of probes to prior art.
4.2. Mechanism of Action Studies
Probes ML268 and ML269 increase TRPML3 ion channel currents. We next pursued whole cell patch clamp studies to determine whether probe compounds could increase current recordings in TRPML3 ion channels. Whole-cell currents were recorded with an Alembic Instruments VE-2 amplifier with 100% series resistance compensation, and acquired with JClamp software. The standard bath solution contained (in mM) 138 NaCl, 5.4 KCl, 2 MgCl2, 2 CaCl2, 10 HEPES, and 10 d-glucose, adjusted to pH 7.4 with NaOH. The standard pipette solution contained (in mM) 140 CsCl, 10 HEPES, 3 ATP-Na, 1 BAPTA, and 2 MgCl2, adjusted to pH 7.2. 100 μM 2-Aminoethyl-diphenyl borate was included in the bath solution to block gap junctions and had no effect on the expressed channels. Channel responses were plotted to 10 ms voltage steps (holding potential = +10 mV) between −200 mV and +100 mV in 20 mV incremental steps, normalized by cell capacitance (pF). Compounds were tested at 10 micromolar (Figure 11). These assays showed that the probes increase TRPML3 channel activity as measured by the current density. No effect was seen in YFP-transfected cells. See PubChem AIDs 2694 (TRPML3), and AID 2692 (TRPN1) for details.
RNA Interference Assays (RNAi) to Investigate TRPML3 Heteromers
Heteromerization of TRPML channels has been postulated [15–17]. Previous reports by other groups have suggested that TRPML3 may interact with TRPML1, and that TRPML1 may regulate the plasma membrane concentrations of TRPML3 [16]. Figure 8 showed that our probes increased calcium flux in TRPML3-transfected HEK cells, with no effect on other TRP channels nor the YFP control plasmid. We next repeated these studies in the relevant native HEM cell line (Figure 12, black bars) and found that these same probes had no effect on calcium flux (see Figure 9). We hypothesized that the proposed heteromeric channels may have very distinct features from the homomeric channels, thereby altering the calcium response in native cell types. To determine whether the effect in our HEK cells was dependent upon formation of a heteromer between TRPML3 and TRPML1 (since HEK cells express all three TRPML3 channels) we tested the effect of compounds in the presence of shRNA 1208 to block TRPML1 (Figure 12, red bars). Specific and efficient RNAi-mediated knock down of endogenous human TRPML1 expression has been recently reported [18, 19]. Oligonucleotide 50-GCTACCTGACCTTCTTCCACA-30 [19] was cloned into the shRNA expression vector U6 RNA Pol III (Invitrogen). HEK293 cells were cotransfected with 3 mg of shRNA to human TRPML1 and 1 mg human TRPML1-YFP plasmid using Genejammer reagent. Human epidermal melanocytes were cotransfected with 5 mg TRPML1 shRNA (1208) using Amaxa Nucleofector technology, followed by treatment with probe compounds and measurement of Fura-2-AM fluorescence ratios. These assays revealed no change in calcium flux upon loss of TRPML1, suggesting that the compounds activate TRPML3 via a mechanism independent of TRPML1. We also tested several other compounds in these assays, in particular the cell-impermeable SN-2, which has no activity on other TRP channels. SN-2 was found to dramatically increase Ca2+ flux in native HEMs (Figure 12) an effect that was also independent of TRPML1, confirming that this compound may act on an extracellular channel activation site and that heteromer formation between TRPML3 and TRPML1 is not likely in this in vitro system.
4.3. Planned Future Studies
Due to the lack of available compounds known to act as selective TRPML3 agonists (there is no prior art), the identification of these selective probes for TRPML3 will be useful to investigate the function of TRPML3 in inner ear mechanotransduction and hearing biology. Likewise, because the other two related mucolipins TRPML1 and TRPML2 are involved in regulation of lysosomal function as well as intracellular trafficking of membrane proteins, there is a potential role for TRPML3 in these processes as well. Nevertheless, two laboratories recently reported targeted knock outs of the murine Trpml3 gene and have not detected strong inner ear or other overt phenotypes [20, 21], which suggests that TRPML3’s role in cellular physiology and disease remains obscure. It is obvious that these mice have not been characterized thoroughly because functional tests have focused on the inner ear. More experiments are certainly needed to elucidate TRPML3’s function. The identified selective probes reported herein will be very useful in this process because the probes enable researchers to characterize the gating of this elusive ion channel as well as structure-function relationships with respect to interactions between probe activators and extracytosolic sodium ions.
The probes generated in this report are first in class, and thus best in class, selective agonists for TRPML3. The leads have excellent selectivity against all other TRP receptors and ion channels tested. SAR studies suggest that additional analogs may exhibit greater potency and may be superior probes than the leads selected. Analog 6 of probe #1 (which was generated after the Fura-2 assays described in Section 3.5 were performed with Probe 1 ML268) is particularly interesting in this regard, as it is the most potent of the TRPML3 agonists identified in this work. Such analogs are likely to be increasingly useful compounds for elucidating the biology of TRPML3.
5. References
- 1.
- Grimm C, Jors S, Saldanha SA, Obukhov AG, Pan B, Oshima K, Cuajungco MP, Chase P, Hodder P, Heller S. Small molecule activators of TRPML3. Chem Biol. 2010;17(2):135–48. [PMC free article: PMC2834294] [PubMed: 20189104]
- 2.
- Yamaguchi S, Muallem S. Opening the TRPML gates. Chem Biol. 2010;17(3):209–10. [PMC free article: PMC2857704] [PubMed: 20338511]
- 3.
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426(6966):517–24. [PubMed: 14654832]
- 4.
- Cuajungco MP, Grimm C, Heller S. TRP channels as candidates for hearing and balance abnormalities in vertebrates. Biochim Biophys Acta. 2007;1772(8):1022–7. [PMC free article: PMC1961624] [PubMed: 17300924]
- 5.
- Gillespie PG, Walker RG. Molecular basis of mechanosensory transduction. Nature. 2001;413(6852):194–202. [PubMed: 11557988]
- 6.
- Eberl DF, Hardy RW, Kernan MJ. Genetically similar transduction mechanisms for touch and hearing in Drosophila. J Neurosci. 2000;20(16):5981–8. [PMC free article: PMC6772586] [PubMed: 10934246]
- 7.
- Qian F, Noben-Trauth K. Cellular and molecular function of mucolipins (TRPML) and polycystin 2 (TRPP2). Pflugers Arch. 2005;451(1):277–85. [PubMed: 15971078]
- 8.
- Atiba-Davies M, Noben-Trauth K. TRPML3 and hearing loss in the varitint-waddler mouse. Biochim Biophys Acta. 2007;1772(8):1028–31. [PubMed: 17329082]
- 9.
- Gong Z, Son W, Chung YD, Kim J, Shin DW, McClung CA, Lee Y, Lee HW, Chang DJ, Kaang BK, Cho H, Oh U, Hirsh J, Kernan MJ, Kim C. Two interdependent TRPV channel subunits, inactive and Nanchung, mediate hearing in Drosophila. J Neurosci. 2004;24(41):9059–66. [PMC free article: PMC6730075] [PubMed: 15483124]
- 10.
- Di Palma F, Belyantseva IA, Kim HJ, Vogt TF, Kachar B, Noben-Trauth K. Mutations in Mcoln3 associated with deafness and pigmentation defects in varitint-waddler (Va) mice. Proc Natl Acad Sci U S A. 2002;99(23):14994–9. [PMC free article: PMC137533] [PubMed: 12403827]
- 11.
- Nagata K, Zheng L, Madathany T, Castiglioni AJ, Bartles JR, Garcia-Anoveros J. The varitint-waddler (Va) deafness mutation in TRPML3 generates constitutive, inward rectifying currents and causes cell degeneration. Proc Natl Acad Sci U S A. 2008;105(1):353–8. [PMC free article: PMC2224216] [PubMed: 18162548]
- 12.
- van Aken AF, Atiba-Davies M, Marcotti W, Goodyear RJ, Bryant JE, Richardson GP, Noben-Trauth K, Kros CJ. TRPML3 mutations cause impaired mechano-electrical transduction and depolarization by an inward-rectifier cation current in auditory hair cells of varitint-waddler mice. J Physiol. 2008;586(Pt 22):5403–18. [PMC free article: PMC2655368] [PubMed: 18801844]
- 13.
- Grimm C, Cuajungco MP, van Aken AF, Schnee M, Jors S, Kros CJ, Ricci AJ, Heller S. A helix-breaking mutation in TRPML3 leads to constitutive activity underlying deafness in the varitint-waddler mouse. Proc Natl Acad Sci U S A. 2007;104(49):19583–8. [PMC free article: PMC2148332] [PubMed: 18048323]
- 14.
- Brothers SP, Saldanha SA, Spicer TP, Cameron M, Mercer BA, Chase P, McDonald P, Wahlestedt C, Hodder PS. Selective and brain penetrant neuropeptide y y2 receptor antagonists discovered by whole-cell high-throughput screening. Mol Pharmacol. 2010;77(1):46–57. [PMC free article: PMC2802430] [PubMed: 19837904]
- 15.
- Zeevi DA, Frumkin A, Bach G. TRPML and lysosomal function. Biochim Biophys Acta. 2007;1772(8):851–8. [PubMed: 17306511]
- 16.
- Venkatachalam K, Hofmann T, Montell C. Lysosomal localization of TRPML3 depends on TRPML2 and the mucolipidosis-associated protein TRPML1. J Biol Chem. 2006;281(25):17517–27. [PMC free article: PMC4196876] [PubMed: 16606612]
- 17.
- Kim J, Chung YD, Park DY, Choi S, Shin DW, Soh H, Lee HW, Son W, Yim J, Park CS, Kernan MJ, Kim C. A TRPV family ion channel required for hearing in Drosophila. Nature. 2003;424(6944):81–4. [PubMed: 12819662]
- 18.
- Miedel MT, Rbaibi Y, Guerriero CJ, Colletti G, Weixel KM, Weisz OA, Kiselyov K. Membrane traffic and turnover in TRP-ML1-deficient cells: a revised model for mucolipidosis type IV pathogenesis. J Exp Med. 2008;205(6):1477–90. [PMC free article: PMC2413042] [PubMed: 18504305]
- 19.
- Samie MA, Grimm C, Evans JA, Curcio-Morelli C, Heller S, Slaugenhaupt SA, Cuajungco MP. The tissue-specific expression of TRPML2 (MCOLN-2) gene is influenced by the presence of TRPML1. Pflugers Arch. 2009;459(1):79–91. [PMC free article: PMC2913554] [PubMed: 19763610]
- 20.
- Castiglioni AJ, Remis NN, Flores EN, Garcia-Anoveros J. Expression and vesicular localization of mouse Trpml3 in stria vascularis, hair cells, and vomeronasal and olfactory receptor neurons. J Comp Neurol. 2011;519(6):1095–114. [PMC free article: PMC4105223] [PubMed: 21344404]
- 21.
- Jors S, Grimm C, Becker L, Heller S. Genetic inactivation of Trpml3 does not lead to hearing and vestibular impairment in mice. PLoS One. 2010;5(12):e14317. [PMC free article: PMC3001452] [PubMed: 21179200]
- PMCPubMed Central citations
- PubChem BioAssay for Chemical ProbePubChem BioAssay records reporting screening data for the development of the chemical probe(s) described in this book chapter
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Campaign to Identify Agonists of Transient Receptor Potential Channels 3 and 2 (TRPML3 & TRPML2).[Probe Reports from the NIH Mol...]Review Campaign to Identify Agonists of Transient Receptor Potential Channels 3 and 2 (TRPML3 & TRPML2).Saldanha SA, Grimm C, Mercer BA, Choi JY, Allais C, Roush WR, Heller S, Hodder P. Probe Reports from the NIH Molecular Libraries Program. 2010
- Small molecule activators of TRPML3.[Chem Biol. 2010]Small molecule activators of TRPML3.Grimm C, Jörs S, Saldanha SA, Obukhov AG, Pan B, Oshima K, Cuajungco MP, Chase P, Hodder P, Heller S. Chem Biol. 2010 Feb 26; 17(2):135-48.
- Review TRPML3 and hearing loss in the varitint-waddler mouse.[Biochim Biophys Acta. 2007]Review TRPML3 and hearing loss in the varitint-waddler mouse.Atiba-Davies M, Noben-Trauth K. Biochim Biophys Acta. 2007 Aug; 1772(8):1028-31. Epub 2007 Jan 23.
- Constitutive activity of TRPML2 and TRPML3 channels versus activation by low extracellular sodium and small molecules.[J Biol Chem. 2012]Constitutive activity of TRPML2 and TRPML3 channels versus activation by low extracellular sodium and small molecules.Grimm C, Jörs S, Guo Z, Obukhov AG, Heller S. J Biol Chem. 2012 Jun 29; 287(27):22701-8.
- The varitint-waddler (Va) deafness mutation in TRPML3 generates constitutive, inward rectifying currents and causes cell degeneration.[Proc Natl Acad Sci U S A. 2008]The varitint-waddler (Va) deafness mutation in TRPML3 generates constitutive, inward rectifying currents and causes cell degeneration.Nagata K, Zheng L, Madathany T, Castiglioni AJ, Bartles JR, García-Añoveros J. Proc Natl Acad Sci U S A. 2008 Jan 8; 105(1):353-8. Epub 2007 Dec 27.
- Identification of Selective Agonists of the Transient Receptor Potential Channel...Identification of Selective Agonists of the Transient Receptor Potential Channels 3 (TRPML3) - Probe Reports from the NIH Molecular Libraries Program
Your browsing activity is empty.
Activity recording is turned off.
See more...





![Figure 12. Ca2+-imaging results showing [Ca2+]i increases in primary human epidermal melanocytes (HEM) Black bars, non-transfected cells.](/books/NBK169454/bin/ml269f12.gif)