NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010-.
Respiratory Syncytial Virus (RSV) is the most common cause of bronchiolitis and pneumonia among infants under one year of age. Most children will be infected with RSV prior to their second birthday, leading to 75,000-125,000 hospitalizations and medical costs exceeding $650 million annually. The virus is highly contagious and is associated with substantial morbidity and mortality. Nevertheless, severe lower respiratory tract disease may occur at any age, especially among the elderly or those with compromised cardiac, pulmonary, or immune systems. FDA-approved drugs for the acute infection are ribavirin and the prophylactic humanized monoclonal antibody, Synagis®, which is limited to use in high risk pediatric patients. Due to the lack of a vaccine and the presence of toxicological limitations in existing therapies, there is substantial need for effective treatments with an improved profile. Of the 313,816 Molecular Libraries Small Molecule Repository (MLSMR) compounds screened in a cell-based, RSV inhibition assay, 51 compounds were selected based on potency, selectivity and chemical tractability for further evaluation in dose response and secondary assays. Collaboration between the assay provider at the University of Louisville, the screening center at Southern Research Institute and the University of Kansas Specialized Chemistry Center narrowed the structure activity relationship (SAR) focus to three scaffolds. The probe, ML275, resulted from structural modification and optimization of a quinazolinedione chemical series, generating a compound with an antiviral EC50 value of 0.81 ± 0.75 μM and a 247-fold selectivity index (SI) for antiviral activity over HEp-2 cell cytotoxicity. Additionally, ML275 demonstrated a 6.7 log reduction of in vitro viral titer, or reduction by approximately 5,000,000-fold. ML275, determined to be a post-entry inhibitor of viral replication, has been broadly profiled for off-target liabilities and assessed for PAMPA permeability and hepatocyte toxicity.
Assigned Assay Grant #: 1 R03 MH082403-01A1
Screening Center & PI: Southern Research Institute, E. Lucile White
Chemistry Center & PI: University of Kansas Specialized Chemistry Center, Jeffrey Aubé
Assay Submitter & Institution: William Severson, University of Louisville
PubChem Summary Bioassay Identifier (AID): 2440
Probe Structure & Characteristics
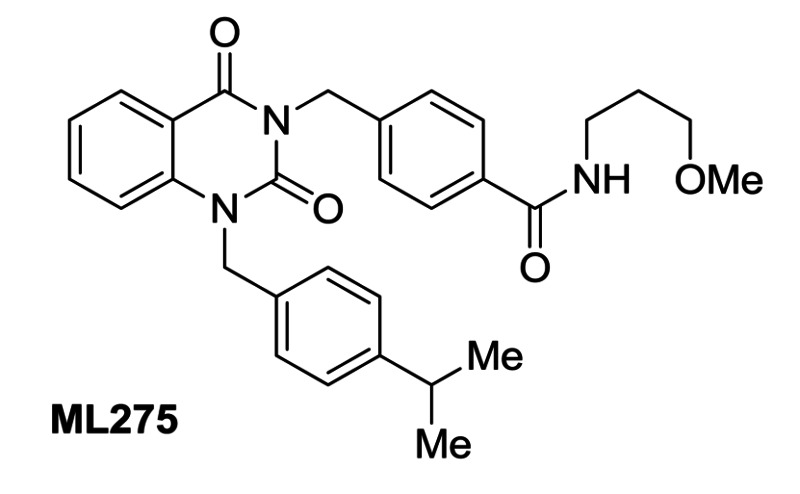
Figure 1Structure of probe ML275 and its associated data
| CID/ML # | Target Name | EC50 (nM) [SID, AID] | Anti-target Name | D=CC50 (μM) [SID, AID] | Fold Selectivea | Secondary Assay(s): IC50/EC50 (nM) [SID, AID] |
|---|---|---|---|---|---|---|
| CID 53301904/ML275 | Respiratory Syncytial Virus | 810± 750 Nm [SID 124756536, AID 504526, AID 504820, AID 504823] | Mammalian Cell toxicity, HEp-2 cells | CC50 ≥ 200 μM [SID 124756536, AID 504509, AID 504818, AID 504826] | >247 | 6.70 RSV Plaque Assay, Log Virus Titer reduction [SID 124756536, AID 504830] |
- a
Calculated as CC50/EC50.
1. Recommendations for Scientific Use of the Probe
Limitations in the current state of the art being addressed by the probe: There are currently two therapies approved for the treatment of RSV infection: ribavirin, a nucleoside analog used for therapeutic intervention that suffers from severe toxic liabilities that limits its use particularly in infants and children, and the prophylactic humanized monoclonal antibody, Synagis® (from MedImmune), that is limited to use in high risk pediatric patients. Moreover, Synagis is expensive, used only for prophylaxis, and requires monthly injections. Several small molecules have been developed; however, all to date have failed to yield positive clinical data. Most of these fall under the category of entry inhibitors which are associated with the quick emergence of resistance. Therefore, the identification of a novel chemotype with an improved profile of efficacy and safety as compared to ribavirin may provide a stage for further development and better treatment options.
A useful probe from this project was defined as a small molecule with novel structure as compared to ribavirin and known compounds in development for RSV. Furthermore, the point of intervention in viral replication would need to be defined as an entry or post-entry inhibitor. Once the point of intervention for the probe was determined as either entry or post-entry inhibition, the required probe criteria were set as CPE EC50 < 1 μM for entry inhibitors; CPE EC50 < 5 μM for post-entry inhibitors; a selective index > 30× the EC50 for the primary assay versus the observed cytotoxicity; titer reduction ≥ 1 log in virus reduction; and a differential response over time in the time of addition assay. A probe that met these expectations would represent an improvement over the use of ribavirin (comparison between probe ML275 and ribavirin is provided in Section 4.1). Probe ML275 is a post-entry inhibitor with a CPE assay EC50 = 0.81 ± 0.75 μM, a selective index of >247, and a 6.7 log reduction in viral titer at 10 μM. Given that the probe has an IC50 in the sub-micromolar range, a selective index >200, and > one million-fold reduction in viral titer, it ranks very highly when compared to the compounds described in the prior art section below. This, combined with the novel, putative post-entry mechanism, places the probe as a best-in-class compound.
Use of the probe: The probe will be used as a novel tool for studying the replication cycle of RSV in human cells. Additional mechanisms of action studies that are outside the scope of the current project are in progress by the assay provider, Dr. Severson, and will define the specific host or viral component that is the target of the probe. This will provide preliminary data to support continued investigations, including an extended characterization proposal, and subsequent NIH/NIAID grant proposals to be submitted by Dr. Severson. Specifically, these studies will attempt to elucidate the antiviral mechanism and investigate the probe as a potential antiviral therapeutic using additional in vitro and in vivo models. The combination of mechanism of action studies and in vivo therapeutic data will allow chemistry optimization to enhance the pharmacological properties of the probe as the basis of identifying a novel treatment for RSV.
Use of the probe in the research community: The limitations associated with currently-approved RSV treatments, such as host toxicity and off-target effects, emphasize a special need for new probes that specifically target viral gene products and multiple stages of the viral propagation cycle. The probe will be used by the research community to isolate and study the functions of the viral targets in the context of host infection, and will provide new insights into the development of pathogen-specific antivirals. Alternatively, it is possible that the probe, whose mechanism has not yet been defined, modulates a host target that is essential for efficient viral replication. Similarly, this would allow the research community to study the host interactions with virus gene products and might form the basis for identification of host-specific, broadly effective antiviral therapeutics.
Relevant biology to which the probe can be applied: All stages of the RSV life cycle (attachment, entry, genomic replication, assembly, and budding) are valid targets for the actions of the probe. In addition, cellular functions that are preempted by the virus and participate in viral replication, trafficking, and release are also possible targets. This includes, but is not limited to, kinase-regulated host cell signaling, metabolic regulation, cell cycle regulation, microtubule and actin cytoskeleton modeling, and innate immunity.
We seek to identify small molecule compounds that inhibit the virus-induced cytopathic effect (CPE) by reducing respiratory syncytial virus replication. To do so, a well-characterized in vitro human cell/virus infection model has been combined with a simple, phenotypic, cytoprotection high-throughput screening (HTS) assay.
RSV was discovered ~40 years ago, and was initially isolated from chimpanzees during an epizootic upper respiratory tract disease outbreak. RSV, which belongs to the family Paramyxoviridae, was subsequently found to be the most important cause of infectious pulmonary disease in human infants, and is a major causative agent of respiratory tract infections among children worldwide. Infants, immune-compromised children, or those with underlying respiratory disorders are at a particularly high risk of developing severe and lethal RSV respiratory tract infections that can be complicated by the resultant viral pneumonia and respiratory distress. Elderly and immune-compromised individuals are also susceptible to severe respiratory infections, thereby highlighting the importance of medical intervention in the form of early diagnosis and implementation of supportive or antiviral therapies [1].
RSV is a negative sense, single-stranded, non-segmented RNA virus of approximately 15 kb. Ten viral genes encode 11 viral proteins. These are NS1 and NS2 (which inhibit type I interferon activity); N, which is the genome-coating nucleocapsid protein; M, which encodes the matrix protein; and SH, G and F (fusion protein) which are incorporated into the viral coat. The G protein is a post-translationally glycosylated surface protein and also recognizes cell surface components during viral attachment. The F protein mediates entry of the virus into the cell cytoplasm and also promotes the formation of syncytia. Typically, antibodies directed at the F protein are neutralizing because this protein is conserved in both subtypes of RSV, while the G protein varies in sequence considerably between the two subtypes. The M2 gene encodes the elongation factor M2-1 and transcription factor M2-2. The L gene encodes the viral RNA polymerase, and the phosphoprotein P, which is a cofactor for L function [2].
Existing therapies for acute RSV infections in infants are ribavirin and the prophylactic humanized monoclonal antibody (Synagis® from MedImmune) that is limited to use in high risk pediatric patients. Although the side effects of ribavirin use are an increased risk for hemolytic anemia and teratogenicity, it remains an approved antiviral therapy. Multiple potential antivirals (discussed below as prior art) are under clinical investigation, but none of these have been governmentally approved as therapies or are available to the research community as tools to study the life cycle of the virus or host/virus interactions. Therefore, there are significant research opportunities for the discovery of additional probes and potential therapeutics for RSV [2, 3].
Probe ML275 is a quinazolinedione that demonstrates submicromolar potency against RSV, with an excellent margin of selectivity against mammalian cell cytotoxicity and ability to effectively reduce in vitro viral titer, thus providing a basis for evaluation in small animal toxicity and efficacy studies.
Prior Art
As discussed above, the only FDA approved small molecule therapeutic for the treatment of severe RSV infection is ribavirin, a nucleoside anti-metabolite prodrug with notable primary clinical toxicity (Figure 2). Ribavirin may also resemble GMP and decrease cellular GTP pools due to the inhibition of the enzyme inosine monophosphate dehydrogenase (IMPDH) [4]. Patients treated with ribavirin have suffered hemolytic anemia and worsening of cardiac disease that has led to myocardial infarctions. While the significant teratogenic and/or embryocidal effects that have been demonstrated in animals exposed to ribavirin may be less pertinent for the indication of RSV in young children [3], the drug’s long half-life is of concern. Ribavirin accumulates in erythrocytes and cannot be eliminated, thus requiring regeneration of the affected red blood cell population to remove the drug from circulation – a process estimated to take as long as 6 months [5]. These effects underscore the importance of finding safer and more effective treatments [6–10]. In our assay system, ribavirin had marginal potency, CPE EC50 = 28.37 ± 3.75 μM and a CC50 of 113.90 ± 38.52 μM, providing a SI of 3.6. In the plaque reduction assay, ribavirin demonstrated a log reduction of viral titer by 2.47, or ~ 300-fold, at 10 μM.

Figure 2
Ribavarin.
A search of the literature was performed prior to the start of the project to accurately gauge what properties the probe should have in order to out-perform existing lead compounds that have been reported in journals and in patents related to RSV. A SciFinder search and Prous Integrity search was performed using the terms: respiratory syncytial virus inhibtors, RSV, RSV inhibitors. Patents and journal articles were evaluated for relevant content. Additionally, a substructure SciFinder search was done once chemistry resources were assigned to this chemical class to evaluate the chemical space related to the probe. Many compounds have been reported in patent and journal forms; however, only those most relevant to and having bearing on the probe criteria are discussed. Several compounds have been the subject of development programs; however, all to date have failed to yield positive clinical data. Most of these fall under the category of entry inhibitors which are associated with the quick emergence of resistance. Some of these are discussed in more detail below. Wyeth has described a lead compound that is effective against RSV with an EC50 of ~ 0.020 μM. The compound, RFI-641 (WAY-15641), is reportedly effective against both RSV A and RSV B (Figure 3). WAY-15641, characterized as an anionic sodium salt, has also been described in its protonated form (SID 28730297, CID 16130904) [11–13].

Figure 3
RFI-641 (WAY-15641) as a disodium salt.
Viropharma has described a bis-tetrazole, VP-14637, as an entry inhibitor for RSV with an EC50 = 0.0014 μM (Figure 4A). The tautomeric, carbon analog of this structure has also been reported (SID 815578 CID 6479288). While VP-14637 is potent, both it and its analogs bear several reactive, electrophilic sites that may have toxicological implications. VP-14637 has been reportedly dropped from development [14–16].

Figure 4
A. VP-14637; B. Arrow Therapeutics/Novartis inhibitor; C. JNJ-2408068 (R-170591); D. BMS-433771.
Novartis and Arrow Therapeutics partnered to investigate a series of benzodiazepines as entry RSV inhibitors (Figure 4B). The potency of their lead compound, SID 8615639, in a whole cell RSV assay was reported with an EC50 = 3.3 μM (17). Johnson and Johnson pursued benzimidazole-derived compounds as potential RSV inhibitors, describing JNJ-2408068 (previously known as R-170591, Figure 4C) as an entry inhibitor with an EC50 = 0.16 μM and CC50 > 100 μM [18–20]. Bristol-Myers-Squibb also reported entry inhibitors (Fig. 4D.) effective against RSV in the benzimidazole series with a potency of EC50 = 0.010 μM; CC50 = 218 μM [21–23].
Novartis recently identified a series of host-targeted, broad-spectrum antivirals, and potent, in vitro EC50 values in the range of 0.002 to 0.3 μM were reported for RSV, influenza virus, hepatitis C virus (HCV), dengue virus, yellow fever virus, and HIV (Figure 5). The isoxazolylpyrazole and the proline series are identified. The observed efficacy was lost when exogenous UDP was added to the system. This suggests that dihydroorotate dehydrogenase (involved in the pyrimidine de novo biosynthesis pathway) is the target. In vivo studies showed that the compounds did not reduce mortality or viral load, and actually increased viral load in the late stage of infection [24].
Figure 5
Novartis broad-spectrum antivirals.
Most recently, this team (SRI, KU SCC and the assay provider) developed an entry-inhibitor probe derived from a sulfonylpyrrolidine scaffold, ML232, which attenuated a CPE effect with an EC50 = 2.25 μM with a selectivity index of 13.7, and showed ability to reduce viral plaques by a log reduction of 2 (Figure 6) [25a-b]. Comparatively, the probe described in this report, ML275, represents a significant improvement in potency, cytotoxicity, plaque reduction and novelty in mechanism of action.

Figure 6
Structure of ML232, an entry inhibitor for hRSV.
As discussed above, ribavirin has a narrow therapeutic index in humans, and while several potential therapeutics appear to be in development (in preclinical or clinical trials), none have been approved for use by regulatory agencies. Therefore, the identification of a novel chemotype with an improved profile of efficacy and safety as compared to ribavirin may provide a stage for further development and better treatment options.
2. Materials and Methods
Overall Assay Strategy
The inhibition of the CPE caused by RSV infection in HEp-2 cells was used as the primary assay to identify the antiviral effects of compounds screened. The phenotypic end-point assay measured the luminescence generated by cellular ATP as a marker of cell viability. A total of 313,816 compounds have been screened and Z values of the screen ranged between 0.6 and 0.9 with the median of 0.83. Seven thousand five hundred eighty-three (7583) compounds were evaluated as active using the criteria of the average of the negative control + 3 times SD (22.3%) of the entire screen. Two thousand four hundred sixty-five compounds (2465) were subjected to the dose response assays (using the primary assay methodology) to verify their activity and cytotoxicity. Confirmed, non-toxic compounds were further investigated and subjected to chemical optimization, followed by secondary assay evaluation. Secondary assays more closely characterized the ability of the compounds to reduce RSV replication and can be used to examine the mechanism of action of the compounds by determining their point of intervention in the viral life cycle. The combination of primary assay (to measure cytoprotection), counter assay (for general eukaryotic cell toxicity) and secondary assay (to measure reduction in viral replication rates and point of intervention) combine to allow a determination of probe efficacy, selectivity, and specificity.
A detailed description of all assays is located in the Appendix.
2.1. Assays
Primary Assay: A Cell Based HTS Approach for the Discovery of New Inhibitors of Respiratory Syncytial Virus (RSV)
Purpose: The primary inhibition assay was conducted to screen the MLSMR 300K compound library, to confirm 2456 hits from the primary screen, and to verify purchased/synthesized compounds.
Summary AID: AID 2440
Assigned AID: AID 2391, AID 488972, AID 492966, AID 493016, AID 493088, AID 504526, AID 504655, AID 504820, AID 504823, AID 507827
Counterscreen: An HTS Cytotoxicity Screen to Evaluate New Inhibitors of Respiratory Syncytial Virus (RSV)
Purpose: This cell-based assay measures the cytotoxicity of compounds in parallel with inhibition of RSV-induced CPE in cells using luminescent cell viability assay readout.
Summary AID: AID 2440
Assigned AID: AID 2410, AID 488976, AID 492968, AID 493015, AID 493090, AID 504509, AID 504674, AID 504818, AID 504826, AID 504825
Secondary Assay: Secondary Screen for RSV Inhibitors by a Titer Reduction Assay
Purpose: This cell-based assay provides an alternative measurement of inhibitory activity on virus replication. It measures reduction in total progeny virus titer after treating the infected cells with test compounds, measured by quantitative real time PCR. The assay was used to confirm antiviral activities of the selected compounds.
Summary AID: AID 2440
Assigned AID: AID 449732
Secondary Assay: Secondary Screen for RSV Inhibitors by Time of Addition CPE Assay
Purpose: Time of addition assays were performed to determine the window in the RSV lifecycle that the lead compounds inhibit.
Summary AID: AID 2440
Assigned AID: AID 504829
Secondary Assay: Inhibition of Progeny Virus Production - RSV Plaque Assay
Purpose: A plaque assay is used to confirm antiviral compound effect and determine the potency of compounds. This method specifically determines the degree of reduction in the number of functional virus particles produced by viral replication in the presence of the compound, and acts as a complement to the primary assay (which determines the degree of cytoprotection).
Summary AID: AID 2440
Assigned AID: AID 504830.
2.2. Probe Chemical Characterization
Probe Chemical Structure, Physical Parameters and Probe Properties
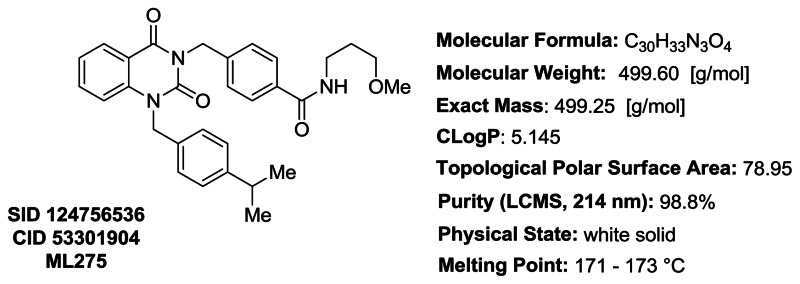
Figure 7Probe characteristics for ML275
Structure Verification and Purity: 1H NMR, 13C NMR, LCMS, and HRMS Data
Proton and carbon NMR data for ML275/SID 124756536/CID 53301904: Detailed analytical methods and instrumentation are described in section 2.3, entitled “Probe Preparation” under general experimental and analytical details. The numerical experimental proton and carbon data are represented below. The experimental proton and carbon spectra are included for reference (Appendix, Figures A1A and A1B, respectively).
Proton NMR Data for ML275/SID 124756536/CID 53301904:1H NMR (500 MHz; CDCl3): δ (ppm) 8.24 (dd, J = 7.9 and 1.6 Hz, 1H), 7.71 (d, J = 8.3 Hz, 2H), 7.61–7.53 (m, 3H), 7.22 (apparent t, J = 7.5 Hz, 1H), 7.20–7.13 (m, 5H), 6.89 (t, J = 4.5 Hz, 1H), 5.36 (s, 2H), 5.33 (broad s, 2H), 3.60–3.52 (m, 4H), 3.37 (s, 3H), 2.92–2.81 (m, 1H), 1.87 (quintet, J = 5.9 Hz, 2H), 1.21 (d, J = 6.9 Hz, 6H).
Carbon NMR Data for ML275/SID 124756536/CID 53301904:13C NMR (126 MHz; CDCl3): δ (ppm) 166.98, 161.89, 151.45, 148.51, 140.38, 140.13, 135.36, 134.12, 132.86, 129.22, 129.07, 127.15, 127.14, 126.53, 123.26, 115.71, 114.65, 72.55, 59.06, 47.31, 44.90, 39.27, 33.84, 28.91, 24.01.
LCMS and HRMS Data for ML275/SID 124756536/CID 53301904: Detailed analytical methods and instrumentation are described in section 2.3, entitled “Probe Preparation” under general experimental and analytical details. The numerical experimental LCMS and HRMS data are represented below. LCMS retention time: 3.422 min. LCMS purity at 214 nm: 98.8%. HRMS: m/z calcd for C30H33N3O4 (M + H+) 500.2544, found 500.2540. The experimental LCMS and HRMS spectra are included for reference (Appendix, Figure A1C and A1D, respectively).
Solubility
Solubility was measured in phosphate buffered saline (PBS) at room temperature (23 °C). PBS by definition is 137 mM NaCl, 2.7 mM KCl, 10 mM sodium phosphate dibasic, 2 mM potassium phosphate monobasic and a pH of 7.4 [26, 27]. Probe ML275 (SID 124756536) was found to have a solubility measurement of 0.18 μg/mL, or 0.36 μM, under these conditions. Solubility was also assessed in RSV CPE assay media (DMEM/F12(r) (Sigma, Cat # D6434)/1× Pen/Strep/Glutamine (Gibco, Cat # 10378)/2% Heat Inactivated FBS (Gibco Cat # 10082)). Probe ML275 (SID 124756536) was determined to have an assay media solubility of 5.1 μg/mL, or 10.21 μM. The solubility in PBS buffer was limited; however, in assay media, the compound concentration was determined to be > 12-fold higher than that of the measured CPE assay EC50. The presence of fetal bovine serum in the media likely accounts for the observed solubility, though the degree of protein binding was not assessed independently.
Stability
Stability was measured under two distinct conditions with ML275 (SID 124756536, Figure 8). Stability, depicted as closed circles in the graph, was assessed at room temperature (23 °C) in PBS (no antioxidants or other protectants and DMSO concentration below 0.1%). Stability, illustrated with closed squares in the graph, was also assessed with 50% acetonitrile added as the solubility of the compound in PBS was 0.18 μg/mL. Stability data in each case is depicted as a graph showing the loss of compound with time over a 48 hr period with a minimum of 6 time points and providing the percent remaining compound at the end of the 48 hr [26, 28]. With no additives (closed circles), 27% of ML275 remains after 48 hours; however, this data is dependent on and misleading due to the solubility limitations in PBS buffer. With the addition of 50% acetonitrile to account for solubility (closed squares), 100% of ML275 remained after 48 hours.

Figure 8
Graph depicting stability of ML275 after 48 h under two separate conditions.
2.3. Probe Preparation
The probe and analogs were generally synthesized by the method shown (Figure 9A and 9B). Commercially available 2-aminobenzoic acid 1, coupled to the requisite benzylamine, afforded aminoamide 2 which was cyclized with CDI to provide quinazolinedione intermediate 3. Intermediate 3 was used to generate the probe and corresponding analogs in this report through two related methods, each offering selective, orthogonal, late stage diversification of key functionality. Most analogs were afforded by the same protocol (9A) leading to the probe which involved hydrolysis of the ester, subsequent coupling with the desired alkylamine (e.g.,5), and installation of the core N-alkyl appendage to generate final compounds (e.g.,6). For analogs bearing the oxetane moiety, it was advantageous to incorporate that structural entity late-stage (9B). The core N-alkyl appendage was introduced (e.g.,7) from intermediate 3, followed by routine coupling manipulation to deliver final compounds (e.g.,9). Specific experimental details for the probe are detailed in section 2.3, entitled, “Probe Preparation,” and experimental protocols for analogs are described in the Appendix.
General experimental and analytical details:1H and 13C NMR spectra were recorded on a Bruker AM 400 spectrometer (operating at 400 and 101 MHz respectively) or a Bruker AVIII spectrometer (operating at 500 and 126 MHz respectively) in CDCl3 with 0.03% TMS as an internal standard or DMSO-d6. The chemical shifts (δ) reported are given in parts per million (ppm) and the coupling constants (J) are in Hertz (Hz). The spin multiplicities are reported as s = singlet, br. s = broad singlet, d = doublet, t = triplet, q = quartet, dd = doublet of doublet and m = multiplet. The LCMS analysis was performed on an Agilent 1200 RRL chromatograph with photodiode array UV detection and an Agilent 6224 TOF mass spectrometer. The chromatographic method utilized the following parameters: a Waters Acquity BEH C-18 2.1 × 50mm, 1.7 um column; UV detection wavelength = 214 nm; flow rate = 0.4ml/min; gradient = 5 - 100% acetonitrile over 3 minutes with a hold of 0.8 minutes at 100% acetonitrile; the aqueous mobile phase contained 0.15% ammonium hydroxide (v/v). The mass spectrometer utilized the following parameters: an Agilent multimode source which simultaneously acquires ESI+/APCI+; a reference mass solution consisting of purine and hexakis(1H, 1H, 3H-tetrafluoropropoxy) phosphazine; and a make-up solvent of 90:10:0.1 MeOH:Water:Formic Acid which was introduced to the LC flow prior to the source to assist ionization. Melting points were determined on a Stanford Research Systems OptiMelt apparatus.
The probe was prepared using the following protocols:
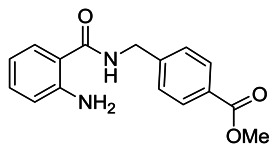
Methyl 4-((2-aminobenzamido)methyl)benzoate: To a solution of 2-aminobenzoic acid (1.50 g, 10.94 mmol) in N,N-dimethylformamide (12 mL) was added methyl 4-(aminomethyl)benzoate hydrochloride (2.21 g, 10.94 mmol), HATU (4.57 g, 12.03 mmol) and N,N-diisopropylethylamine (5.42 mL, 32.80 mmol). The reaction mixture was stirred for 16 h at room temperature, then diluted with dichloromethane (50 mL) and washed sequentially with 1M HCl (40 mL), sat. aqueous NaHCO3 (40 mL) and water (2 × 200 mL). The separated organic extract was dried (MgSO4), filtered, and concentrated under reduced pressure to afford a crude product which was purified by silica gel flash column chromatography (0 – 60% v/v EtOAc/Hexane), yielding the product as a white solid (1.88 g, 6.61 mmol, 61% yield). 1H NMR (400 MHz; CDCl3): δ (ppm) 8.01 (d, J = 8.4 Hz, 2H), 7.41 (d, J = 8.6 Hz, 2H), 7.35 (dd, J = 7.9 and 1.4 Hz, 1H), 7.25–7.19 (m, 1H), 6.70 (dd, J = 8.3 and 0.9 Hz, 1H), 6.67–6.61 (m, 1H), 6.43 (broad s, 1H), 5.56 (broad s, 2H), 4.66 (d, J = 5.9 Hz, 2H), 3.91 (s, 3H).

Methyl 4-((2,4-dioxo-1,2-dihydroquinazolin-3(4H)-yl)methyl)benzoate: After stirring a solution of methyl 4-((2-aminobenzamido)methyl)benzoate (3.05 g, 10.73 mmol) and N,N-diisopropylethylamine (8.87 ml, 53.60 mmol) in CH2Cl2 (125 mL) for 10 minutes at room temperature under nitrogen, 1,1′-carbonyldiimidizaole (5.22 g, 32.20 mmol) was added, and the reaction mixture was heated 16 h at reflux. The formed precipitate was filtered, dried under vacuum and the desired product was furnished as a white solid without further purification (3.16 g, 10.18 mmol, 95% yield). 1H NMR (400 MHz; DMSO): δ (ppm) 11.58 (s, 1H), 7.94 (dd, J = 8.3 and 1.4 Hz, 1H), 7.90 (d, J = 8.4 Hz, 2H), 7.72–7.64 (m, 1H), 7.43 (d, J = 8.4 Hz, 2H), 7.26–7.18 (m, 2H), 5.15 (s, 2H), 3.83 (s, 3H).

4-((2,4-dioxo-1,2-dihydroquinazolin-3(4H)-yl)methyl)benzoic acid: To a solution of methyl 4-((2,4-dioxo-1,2-dihydroquinazolin-3(4H)-yl)methyl)benzoate (1.00 g, 3.23 mmol) in THF (20 mL) was added 1 M lithium hydroxide in water (19.35 mL, 19.35 mmol). The reaction mixture was stirred at 40 °C for 1 hr, at which point TLC confirmed reaction completion. Then 1 M HCl was cautiously added until the reaction mixture reached pH 2, at which point the product precipitated out of solution. The precipitate was collected by filtration, washed with water (2 × 25 mL), dried under high vacuum to afford the desired product as a white solid (0.84 g, 2.84 mmol, 88% yield). 1H NMR (400 MHz; DMSO): δ (ppm) 12.90 (broad s, 1H), 11.58 (s, 1H), 7.95 (d, J = 7.3 Hz, 1H), 7.88 (d, J = 8.3 Hz, 2H), 7.74–7.65 (m, 1H), 7.40 (d, J = 8.3 Hz, 2H), 7.28–7.18 (m, 2H), 5.15 (s, 2H).
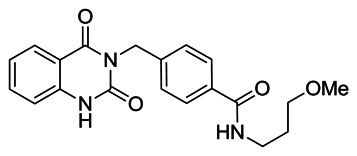
4-((2,4-dioxo-1,2-dihydroquinazolin-3(4H)-yl)methyl)-N-(3-methoxypropyl)benzamide: To a solution of 4-((2,4-dioxo-1,2-dihydroquinazolin-3(4H)-yl)methyl)benzoic acid (1.00 g, 3.38 mmol) in N,N-dimethylformamide (15 mL) was added 3-methoxypropylamine (0.35 mL, 3.38 mmol), HATU (1.41 g, 3.71 mmol) and N,N-diisopropylethylamine (1.67 mL, 10.13 mmol). The reaction mixture was stirred for 16 h at room temperature, then diluted with CH2Cl2 (90 ml) and washed sequentially with 1 M HCl (60 mL), sat. aqueous NaHCO3 (60 mL) and water (2 × 180 mL). The organic extract was separated, dried (MgSO4), filtered, and concentrated under reduced pressure to afford a crude product which was purified by silica gel flash column chromatography (0 – 5% v/v MeOH/CH2Cl2) yielding the desired product as a white solid (0.91 g, 2.48 mmol, 73% yield). 1H NMR (400 MHz; DMSO): δ (ppm) 11.56 (s, 1H), 8.40 (t, J = 5.6 Hz, 1H), 7.95 (d, J = 7.3 Hz, 1H), 7.76 (d, J = 8.4 Hz, 2H), 7.71–7.63 (m, 1H), 7.36 (d, J = 8.4 Hz, 2H), 7.26–7.17 (m, 2H), 5.13 (s, 2H), 3.35 (t, J = 6.3 Hz, 2H), 3.31–3.24 (m, 2H), 3.22 (s, 3H), 1.78–1.67 (m, 2H).
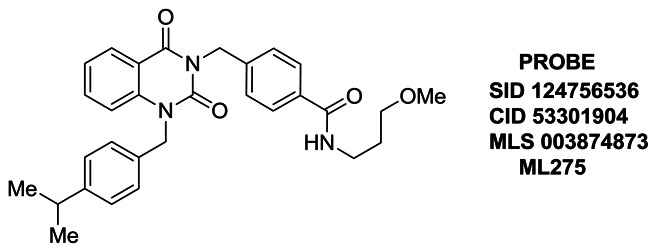
PROBE ML275: 4-((1-(4-isopropylbenzyl)-2,4-dioxo-1,2-dihydroquinazolin-3(4H)-yl)methyl)-N-(3-methoxypropyl)benzamide (SID 124756536, CID 53301904). To a solution of 4-((2,4-dioxo-1,2-dihydroquinazolin-3(4H)-yl)methyl)-N-(3-methoxypropyl)benzamide (0.050 g, 0.14 mmol) in N,N-dimethylformamide (2 mL) was added 4-isopropylbenzyl bromide (0.028 mL, 0.16 mmol) and potassium carbonate (0.056 g, 0.41 mmol). The resulting reaction mixture was stirred at 40 °C for 16 h. The formed residue was dissolved in CH2Cl2 (6 mL) and sequentially washed with 1 M HCl (4 mL), water (3 × 20 mL) and brine (8 mL). The organic layer was separated, dried (MgSO4), filtered, and concentrated under reduced pressure to give a crude product which was purified by silica gel flash column chromatography (0 – 5% v/v MeOH/DCM) yielding 4-((1-(4-isopropylbenzyl)-2,4-dioxo-1,2-dihydroquinazolin-3(4H)-yl)methyl)-N-(3-methoxypropyl)benzamide as a white solid (0.030 g, 0.060 mmol, 44% yield). 1H NMR (500 MHz; CDCl3): δ (ppm) 8.24 (dd, J = 7.9 and 1.6 Hz, 1H), 7.71 (d, J = 8.3 Hz, 2H), 7.61–7.53 (m, 3H), 7.22 (apparent t, J = 7.5 Hz, 1H), 7.20–7.13 (m, 5H), 6.89 (t, J = 4.5 Hz, 1H), 5.36 (s, 2H), 5.33 (broad s, 2H), 3.60–3.52 (m, 4H), 3.37 (s, 3H), 2.92–2.81 (m, 1H), 1.87 (quintet, J = 5.9 Hz, 2H), 1.21 (d, J = 6.9 Hz, 6H). 13C NMR (126 MHz; CDCl3): δ (ppm) 166.98, 161.89, 151.45, 148.51, 140.38, 140.13, 135.36, 134.12, 132.86, 129.22, 129.07, 127.15, 127.14, 126.53, 123.26, 115.71, 114.65, 72.55, 59.06, 47.31, 44.90, 39.27, 33.84, 28.91, 24.01. LCMS retention time: 3.422 min. LCMS purity at 214 nm: 98.8%. HRMS: m/z calcd for C30H33N3O4 (M + H+) 500.2544, found 500.2540. Melting point: 171–173 °C.
3. Results
3.1. Dose Response Curves for Probe
The primary assay methodology (Summary AID: 2440; Assigned AID: 504526, 504820, 504823) was used to measure both probe efficacy and cytotoxicity. The probe ML275 potency in the RSV CPE assay was determined: EC50 = 0.81 ± 0.75 μM, and the CC50 > 200 μM. The calculated selectivity was determined as (CC50/EC50) > 247. The ML275 dose response profiles for efficacy and cytotoxicity curves are graphed in Figure 10.

Figure 10
Dose response efficacy (green circles) and cytotoxicity (red squares) for ML275.
3.2. Cellular Activity
A cell-based assay was employed to measure the cytotoxicity of the probes in cells using a luminescent cell viability assay readout. This assay is detailed in Summary AID: AID 2440 and additionally assigned AIDs: AID 2410, AID 488976, AID 492968, AID 493015, AID 493090, AID 504509, AID 504674, AID 504818, AID 504826, and AID 504825. This assay directly measures phenotypic cytotoxicity, but indirectly measures the solubility of the probe and its ability to pass through the cellular membrane. Insoluble or impermeable probes will not result in cell death.
3.3. Profiling Assays
Antiviral selectivity and tested cell types: The ML275 probe class was tested in two different human cell types. The primary assay was performed in HEp2 cells and the secondary assays were performed in HEp2 and A549 cells. The probe class was effective against RSV in both cell lines. The probe itself was also tested for activity against vaccinia, influenza A, Venezuelan equine encephalitis, and dengue viruses. The probe showed no efficacy in inhibiting these viruses, thus showing selectivity for RSV.
Broad spectrum target profiling: ML275 was submitted for assessing off-target pharmacology using a Ricerca LeadProfiling® screen made up of 67 assays. The probe was assayed in duplicate at a concentration of 10 μM for all targets, and the following responses (Table 1) were noted as ≥ 50% inhibition or stimulation for biochemical assays:
Table 1
Targets against which ML275 demonstrated > 50% inhibition.
Determination of actual IC50 information for any of these will be handled as part of a MLPCN extended characterization proposal. Inhibition for the human adenosine A3 receptor and human platelet activating factor was noted at 44% and 43%, respectively; however, all other inhibition levels were reported below 34 percent. Full results, including target profile list, percent inhibition for each assay, methods and experimental details are provided in the Appendix [29].
4. Discussion
4.1. Comparison to Existing Art and How the New Probe is an Improvement
Currently, two FDA approved drugs are available for RSV infections. The first is Virozole, the brand name for ribavirin, and is delivered in aerosolized form directly to the lungs [3,4]. Ribavirin causes hemolytic anemia by the accumulation of ribavirin triphosphate and subsequent depletion of intracellular ATP in red blood cells. It has a narrow therapeutic index in humans. The second therapeutic (Synagis, MedImmune) is a humanized monoclonal antibody used prophylactically to protect at risk infants through their first two RSV seasons. The prophylactic opportunity is limited due to high treatment cost, and the therapeutic efficacy of this application has not been defined. While several potential therapeutics appear to be in development (in preclinical or clinical trials), none have been approved for use by regulatory agencies.
The discovery of novel, small molecular antiviral therapeutics would potentially fill this therapeutic gap. Understanding the probe’s mechanism of action may deconvolute the nuances of virus-host interaction, thus aiding in finding scaffold modifications that effectively target these interactions and increase efficacy and therapeutic potential. A side-by-side comparison of ML275 and ribavirin is provided in Table 2.
Table 2
Comparison of ribavirin to ML275.
Compared to ribavirin, probe ML275 has an improved CPE EC50 of 0.81 μM and selective index of >247 (ribavirin: 28.37 μM and 3.6, respectively). ML275 was shown to reduce viral replication in a cell-based assay by 6.7 log, intervenes at a different stage in the viral life cycle than ribavirin, and has strong potential for therapeutic use instead of as a prophylactic agent. Therefore, probe ML275 represents an improvement both in its therapeutic index and potential.
Mechanism-of-action studies have classified this probe as a post-entry inhibitor of late stage infection processes. Ribavirin is a post-entry inhibitor of viral replication, but its toxicological profile (small therapeutic index) prohibits its use except for the most severe cases of lower respiratory infections of RSV in infants. Most of the other known compounds that have been described in the literature are entry inhibitors, prone to induce rapid emergence of viral resistance and none have progressed to reach FDA approval. Therefore, a probe which inhibits post-entry processes and has a low cytotoxicity (large therapeutic index) will address an unmet need.
5. References
- 1.
- Byrd, Prince Animal Models of Respiratory Syncytial Virus Infection. Clinical Infectious Diseases. 1997;25:1363–8. [PubMed: 9431379]
- 2.
- Olszewska W, Openshaw P. Emerging drugs for respiratory syncytial virus infection. Expert Opin Emerg Drugs. 2009;14(2):207–17. [PMC free article: PMC2705842] [PubMed: 19453286]
- 3.
- Ribavirin is formulated and administered as an aerosol. Pregnant women including female parents and hospital staff are advised not to be exposed to children receiving ribavirin due to the risk of birth defects - Taken from Virazole drug information.
- 4.
- Leyssen P, et al. The predominant mechanism by which ribavirin exerts its antiviral activity in vitro against flaviviruses and paramyxoviruses is mediated by inhibition of IMP dehydrogenase. J Virol. 2005;79(3):1943–7. [PMC free article: PMC544097] [PubMed: 15650220]
- 5.
- Virazole drug information.
- 6.
- Hall CB, McBride JT, Walsh EE, Bell DM, Gala CL, Hildreth S, Ten Eyck LG, Hall WJ. Aerosolized ribavirin treatment of infants with respiratory syncytial viral infection. A randomized double-blind study. N Engl J Med. 1983;308:1443–7. [PubMed: 6343860]
- 7.
- Hall CB, Walsh EE, Hruska JF, Betts RF, Hall WJ. Ribavirin treatment of experimental respiratory syncytial viral infection. A controlled double-blind study in young adults. JAMA. 1983;249:2666–70. [PubMed: 6341640]
- 8.
- Hruska JF, Bernstein JM, Douglas RG Jr, Hall CB. Effects of ribavirin on respiratory syncytial virus in vitro. Antimicrob Agents Chemother. 1980;17:770–5. [PMC free article: PMC283873] [PubMed: 7396465]
- 9.
- Hruska JF, Morrow PE, Suffin SC, Douglas RG Jr. In vivo inhibition of respiratory syncytial virus by ribavirin. Antimicrob Agents Chemother. 1982;21:125–30. [PMC free article: PMC181839] [PubMed: 7044296]
- 10.
- Taber LH, Knight V, Gilbert BE, McClung HW, Wilson SZ, Norton HJ, Thurson JM, Gordon WH, Atmar RL, Schlaudt WR. Ribavirin aerosol treatment of bronchiolitis associated with respiratory syncytial virus infection in infants. Pediatrics. 1983;72:613–8. [PubMed: 6356005]
- 11.
- Douglas JL. In search of a small-molecule inhibitor for respiratory syncytial virus. Expert Rev. Anti-infect Ther. 2004;2:625–639. [PubMed: 15482225]
- 12.
- Huntley CC, Weiss WJ, Gazumyan A, Buklan A, Feld B, Hu W, Jones TR, Murphy T, Nikitenko AA, O’Hara B, Prince G, Quartuccio S, Raifeld YE, Wyde P, O’Connell JF. RFI-641, a Potent Respiratory Syncycial Virus Inhibitor. Antimicrob Agents Chemother. 2002;46:841–847. [PMC free article: PMC127488] [PubMed: 11850270]
- 13.
- Razinkov V, Gazumyan A, Nikitenko A, Ellestad G, Krishnamurthy G. RFI-641 inhibits entry of respiratory syncytial virus via interactions with fusion protein. Chem Biol. 2001;8:645–59. [PubMed: 11451666]
- 14.
- Douglas JL, et al. Small Molecules VP-14637 and JNJ-2408068 Inhibit Respiratory Syncytial Virus Fusion by Similar Mechanisms. Antimicrobial Agents and Chemotherapy. 2005;49:2460–2466. [PMC free article: PMC1140497] [PubMed: 15917547]
- 15.
- Wyde PR, Laquerre S, Chetty SN, Gilbert BE, Nitz TJ, Pevear DC. Antiviral efficacy of VP14637 against respiratory syncycial virus in vitro and in cotton rats following delivery by small droplet aerosol. Antiviral Res. 2005;68:18–26. [PubMed: 16112208]
- 16.
- Douglas JL, Panis ML, Ho E, Lin KY, Krawczyk SH, Grant DM, Cai R, Swaminathan S, Cihlar T. Inhibition of respiratory syncytial virus fusion by the small molecule VP-14637 via specific interactions with F protein. J Virol. 2003;77:5054–64. [PMC free article: PMC153948] [PubMed: 12692208]
- 17.
- Carter MC, et al. 1,4-Benzodiazepines as inhibitors of respiratory syncytial virus. J. Med. Chem. 2006;49:2311–2319. 2006. [PubMed: 16570927]
- 18.
- Sudo K, Miyazaki Y, Kojima N, Kobayashi M, Suzuki H, Shintani M, Shimizu Y. YM-53403, a unique anti-respiratory syncytial virus agent with a novel mechanism of action. Antiviral Res. 2005;65:125–31. Related structure: YM-53403. [PubMed: 15708639]
- 19.
- Andries K, et al. Substituted benzimidazoles with nanomolar activity against respiratory syncytial virus. Antiviral Res. 2003;60:209–219. [PubMed: 14638397]
- 20.
- Bonfanti J-F, et al. Selection of a respiratory syncytial virus fusion inhibitor clinical candidate, part 1: Improving the pharmacokinetic profile using the structure property relationship. J Med Chem. 2007;50:4572–4584. [PubMed: 17722899]
- 21.
- Yu KL, Zhang Y, Civiello RL, et al. Respiratory syncycial virus inhibitors. Part 2. Benzimidazole-2-one derivatives. Bioorg Med Chem Lett. 2004;14:1133–1137. [PubMed: 14980651]
- 22.
- Cianci C, Yu KL, Combrink K, Sin N, et al. Orally active fusion inhibitors of syncycial virus. Antimicrob Agents Chemother. 2004;48:413–422. [PMC free article: PMC321540] [PubMed: 14742189]
- 23.
- Cianci C, Genovesi EV, Lamb L, Medina I, et al. Oral efficacy of a respiratory syncycial virus inhibitor in rodent models of infection. Antimicrob Agents Chemother. 2004;48:2448–2454. [PMC free article: PMC434195] [PubMed: 15215093]
- 24.
- Bonavia A, Franti M, Keaney EP, Kuhen K, Seepersaud M, Radetich B, Shao J, Honda A, Dewhurst J, Monroe J, Wolff K, Osborne C, Lanieri L, Hoffmaster K, Amin J, Markovits J, Broome M, Skuba E, Cornella-Taracido I, Joberty G, Bouwmeester T, Hamann L, Tallarico JA, Tommasi R, Compton T, Sushell SM. Organic Synthesis Toward Small-Molecule Probes and Drugs Special Feature: Identification of broad-spectrum antiviral compounds and assessment of the druggability of their target for efficacy against respiratory syncytial virus (RSV). PNAS. 2011:6739–6744. [PMC free article: PMC3084118] [PubMed: 21502533]
- 25a.
- Moore BP, Chung D-H, Matharu DS, Golden JE, Maddox C, Rasmussen L, Noah JW, Sosa MI, Ananthan S, Tower NA, White EL, Jia F, Prisinzano TE, Aubé J, Jonsson CB, Severson WE. (S)-N-(2, 5-Dimethylphenyl)-1-(Quinoline-8-ylsulfonyl) Pyrrolidine-2-Carboxamide as a Small Molecule Inhibitor Probe for the Study of Respiratory Syncytial Virus Infection. J Med Chem. 2012;5(20):8582–8587. [PMC free article: PMC3506029] [PubMed: 23043370]
- 25b.
- Noah JW, Severson W, Chung DH, Moore B, Jia F, Xu X, Maddox C, Lynn Rasmussen L, Sosa MI, Tower NA, Ananthan S, White EL, Jonsson C, Matharu DS, Golden JE, Prisinzano TE, Aubé J. Probe Report from the NIH Molecular Libraries Program. Bethesda (MD): National Center for Biotechnology Information (US); 2011. A Cell Based HTS Approach for the Discovery of New Inhibitors of RSV. [Internet] Probe ML232.
- 26.
- Solubility and stability data assessment was outsourced to and data was collected by the Sanford-Burnham Center, under the direction of Dr. Layton Smith.
- 27.
- Gopinathan S, Nouraldeen A, Wilson AGE. Development and application of a highthroughput formulation screening strategy for oral administration in drug discovery. Future Med. Chem. 2010;2(9):1391–1398. [PubMed: 21426133]
- 28.
- Ibrahim F, El-Din MK, Eid MI, Wahba ME. Validated stability-indicating spectrofluorimetric methods for the determination of ebastine in pharmaceutical preparations. Chem Cent J. 2011;5(1):11. [PMC free article: PMC3061886] [PubMed: 21385439]
- 29.
- Pharmacology profiling services provided by: Ricerca Biosciences, LLC. Taiwan; www
.ricerca.com; Study number: AB08686, Quote #: 27797-1, Compound Code: 53301904 (1156014)
APPENDIX. NMR, LCMS, HRMS and HPLC Data for Probe ML275 SID 124756536 CID 53301904
Figure A1A. Proton data for ML275, SID 124756536, CID 53301904
Figure A1B. Carbon data for ML275, SID 124756536, CID 53301904
Figure A1C. LCMS purity data at 214 nm for ML275 SID 124756536, CID 53301904
Figure A1D. HRMS data for ML275 SID 124756536 CID 53301904
Ricerca LeadProfiling® Report for ML275
STUDY OBJECTIVE
To evaluate, in Radioligand Binding assays, the activity of compound 53301904 (UK-5, PT# 1156014)
METHODS
Methods employed in this study have been adapted from the scientific literature to maximize reliability and reproducibility Reference standards were run as an integral part of each assay to ensure the validity of the results obtained. Assays were performed under conditions described in the accompanying “Methods” section of this report.
Where presented. IC50 values were determined by a non-linear, least squares regression analysis using MathlQ™ (ID Business Solutions Ltd., UK). Where inhibition constants (Ki), are presented, the Ki, values were calculated using the equation of Cheng and Prusoff (Cheng, Y., Prusoff. W.H., Biochem. Pharmacol 22:3099–3108,1973) using the observed IC50 of the tested compound, the concentration of radioligand employed in the assay, and the historical values for the KD of the ligand (obtained experimentally at Ricerca Biosciences, LLC) Where presented, the Hill coefficient (nH),. defining the slope of the competitive binding curve, was calculated using MathlQ™. Hill coefficients significantly different than 1.0, may suggest that the binding displacement does not follow the laws of mass action with a single binding site. Where IC50, Ki, and/or nH data are presented without Standard Error of the Mean (SEM). data are insufficient to be quantitative, and the values presented (Ki, IC50, nH) should be interpreted with caution.
Detailed methods for any of the listed assays can be found by searching by the catalog number for the assay (listed) at: https://pharmacology.ricerca.com/Catalog/AssayCatalog/AssayCatalog.aspx
| Cat # | Assay Name | Batch* | Spec. | Rep. | Conc. | % Inh. |
|---|---|---|---|---|---|---|
| Compound: 53301904, PT#: 1156014 | ||||||
| 200510 | Adenosine A1 | 305599 | hum | 2 | 10 μM | 4 |
| 200610 | Adenosine A2A | 305600 | hum | 2 | 10 μM | −3 |
| 200720 | Adenosine A3 | 305602 | hum | 2 | 10 μM | 44 |
| 203100 | Adrenergic α1A | 305613 | rat | 2 | 10 μM | 22 |
| 203200 | Adrenergic α1B | 305614 | rat | 2 | 10 μM | 4 |
| 203400 | Adrenergic α1D | 305615 | hum | 2 | 10 μM | 14 |
| 203620 | Adrenergic α2A | 305616 | hum | 2 | 10 μM | 16 |
| 204010 | Adrenergic β1 | 305603 | hum | 2 | 10 μM | 22 |
| 204110 | Adrenergic β2 | 305604 | hum | 2 | 10 μM | 1 |
| 285010 | Androgen (Testosterone) AR | 305773 | rat | 2 | 10 μM | 34 |
| 212510 | Bradykinin B1 | 305625 | hum | 2 | 10 μM | 3 |
| 212620 | Bradykinin B2 | 305626 | hum | 2 | 10 μM | 12 |
| 214510 | Calcium Channel L-Type, Benzothiazepine | 305776 | rat | 2 | 10 μM | 52 |
| 214600 | Calcium Channel L-Type, Dihydropyridine | 305794 | rat | 2 | 10 μM | 70 |
| 216000 | Calcium Channel N-Type | 305795 | rat | 2 | 10 μM | 0 |
| 217030 | Cannabinoid CB1 | 305621 | hum | 2 | 10 μM | 86 |
| 219500 | Dopamine D1 | 305797 | hum | 2 | 10 μM | 26 |
| 219700 | Dopamine D2s | 305798 | hum | 2 | 10 μM | 9 |
| 219800 | Dopamine D3 | 305799 | hum | 2 | 10 μM | 19 |
| 219900 | Dopamine D4.2 | 305800 | hum | 2 | 10 μM | 5 |
| 224010 | Endothelin ETA | 305622 | hum | 2 | 10 μM | 9 |
| 224110 | Endothelin ETB | 305623 | hum | 2 | 10 μM | −4 |
| 225510 | Epidermal Growth Factor (EGF) | 305638 | hum | 2 | 10 μM | 11 |
| 226010 | Estrogen ERα | 305716 | hum | 2 | 10 μM | 6 |
| 226600 | GABAA, Flunitrazepam, Central | 305802 | rat | 2 | 10 μM | −20 |
| 226500 | GABAA, Muscimol, Central | 305801 | rat | 2 | 10 μM | −4 |
| 228610 | GABAB1A | 305888 | hum | 2 | 10 μM | 3 |
| 232030 | Glucocorticoid | 305774 | hum | 2 | 10 μM | 21 |
| 232700 | Glutamate, Kainate | 305634 | rat | 2 | 10 μM | −3 |
| 232810 | Glutamate, NMDA, Agonism | 305632 | rat | 2 | 10 μM | 2 |
| 232910 | Glutamate, NMDA, Glycine | 305635 | rat | 2 | 10 μM | 10 |
| 233000 | Glutamate, NMDA, Phencyclidine | 305803 | rat | 2 | 10 μM | −2 |
| 239610 | Histamine H1 | 305804 | hum | 2 | 10 μM | 11 |
| 239710 | Histamine H2 | 305805 | hum | 2 | 10 μM | 9 |
| 239820 | Histamine H3 | 305806 | hum | 2 | 10 μM | 11 |
| 241000 | Imidazoline I2, Central | 305807 | rat | 2 | 10 μM | 12 |
| 243520 | Interleukin IL-1 | 305639 | mouse | 2 | 10 μM | 15 |
| 250460 | Leukotriene, Cysteinyl CysLT1 | 305750 | hum | 2 | 10 μM | 22 |
| 251600 | Melatonin MT1 | 305752 | hum | 2 | 10 μM | 26 |
| 252610 | Muscarinic M1 | 305608 | hum | 2 | 10μM | 0 |
| 252710 | Muscarinic M2 | 305609 | hum | 2 | 10 μM | 4 |
| 252810 | Muscarinic M3 | 305610 | hum | 2 | 10 μM | 19 |
| 257010 | Neuropeptide Y Y1 | 305636 | hum | 2 | 10 μM | 13 |
| 257110 | Neuropeptide Y Y2 | 305637 | hum | 2 | 10 μM | 3 |
| 258590 | Nicotinic Acetylcholine | 305811 | hum | 2 | 10 μM | -47 |
| 258700 | Nicotinic Acetylcholine α Bungarotoxin | 305812 | hum | 2 | 10 μM | -2 |
| 260130 | Opiate δ1 (OP1, DOP) | 305631 | hum | 2 | 10 μM | 13 |
| 260210 | Opiate κ(OP2, KOP) | 305988 | hum | 2 | 10 μM | 2 |
| 260410 | Opiate μ(OP3, MOP) | 305815 | hum | 2 | 10 μM | 27 |
| 264500 | Phorbol Ester | 305816 | mouse | 2 | 10 μM | -1 |
| 265010 | Platelet Activating Factor (PAF) | 305687 | hum | 2 | 10 μM | 43 |
| 265600 | Potassium Channel [KATP] | 305817 | ham | 2 | 10 μM | -2 |
| 265900 | Potassium Channel hERG | 305818 | hum | 2 | 10 μM | 26 |
| 268420 | Prostanoid EP4 | 305819 | hum | 2 | 10 μM | 25 |
| 268700 | Purinergic P2x | 305713 | rabbit | 2 | 10 μM | 2 |
| 268810 | Purinergic P2Y | 305714 | rat | 2 | 10 μM | 5 |
| 270000 | Rolipram | 305820 | rat | 2 | 10 μM | 23 |
| 271110 | Serotonin (5-Hydroxytryptamine) 5-HT1A | 305821 | hum | 2 | 10 μM | -12 |
| 271700 | Serotonin (5-Hydroxytryptamine) 5-HT2B | 305822 | hum | 2 | 10 μM | 54 |
| 271910 | Serotonin (5-Hydroxytryptamine) 5-HT3 | 305720 | hum | 2 | 10 μM | 28 |
| 278110 | Sigma σ1 | 305823 | hum | 2 | 10 μM | 4 |
| 255520 | Tachykinin NK1 | 305810 | hum | 2 | 10 μM | -11 |
| 285900 | Thyroid Hormone | 305624 | rat | 2 | 10 μM | 29 |
| 220320 | Transporter, Dopamine (DAT) | 305619 | hum | 2 | 10 μM | 27 |
| 226400 | Transporter, GABA | 305882 | rat | 2 | 10 μM | 2 |
| 204410 | Transporter, Norepinephrine (NET) | 305618 | hum | 2 | 10 μM | 54 |
| 274030 | Transporter, Serotonin (5-Hydroxytryptamine) (SERT) | 305620 | hum | 2 | 10 μM | 9 |
Note: Items meeting criteria for significance (≥50% stimulation or inhibition) are highlighted.
- *
Batch: Represents compounds tested concurrently in the same assay(s).
R=See Remarks (if any) at end of this section.
ham=Hamster; hum=Human
- PMCPubMed Central citations
- PubChem BioAssay for Chemical ProbePubChem BioAssay records reporting screening data for the development of the chemical probe(s) described in this book chapter
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review A Cell Based HTS Approach for the Discovery of New Inhibitors of RSV.[Probe Reports from the NIH Mol...]Review A Cell Based HTS Approach for the Discovery of New Inhibitors of RSV.Noah JW, Severson W, Chung DH, Moore B, Jia F, Xu X, Maddox C, Rasmussen L, Sosa MI, Tower NA, et al. Probe Reports from the NIH Molecular Libraries Program. 2010
- A cell based high-throughput screening approach for the discovery of new inhibitors of respiratory syncytial virus.[Virol J. 2013]A cell based high-throughput screening approach for the discovery of new inhibitors of respiratory syncytial virus.Chung DH, Moore BP, Matharu DS, Golden JE, Maddox C, Rasmussen L, Sosa MI, Ananthan S, White EL, Jia F, et al. Virol J. 2013 Jan 10; 10:19. Epub 2013 Jan 10.
- A Phenotypic High-Throughput Screen with RSV-Infected Primary Human Small Airway Epithelial Cells (SAECs).[J Biomol Screen. 2015]A Phenotypic High-Throughput Screen with RSV-Infected Primary Human Small Airway Epithelial Cells (SAECs).Gobel J, Gartland M, Gurley SH, Kadwell S, Gillie D, Moore C, Goetz A. J Biomol Screen. 2015 Jul; 20(6):729-38. Epub 2015 Apr 10.
- Prophylaxis in RSV infection (Palivizumab)--is it worthwhile?[Ir Med J. 2000]Prophylaxis in RSV infection (Palivizumab)--is it worthwhile?Hashmi NA, Cosgrove JF, MacMahon P. Ir Med J. 2000 Dec; 93(9):284.
- Review Respiratory syncytial virus disease: update on treatment and prevention.[Expert Rev Anti Infect Ther. 2...]Review Respiratory syncytial virus disease: update on treatment and prevention.Krilov LR. Expert Rev Anti Infect Ther. 2011 Jan; 9(1):27-32.
- Identification of a Series of Quinazolinediones as Potent, Selective, Post-Entry...Identification of a Series of Quinazolinediones as Potent, Selective, Post-Entry Inhibitors of Human Respiratory Syncytial Virus (hRSV) via a Cell-Based High Throughput Screen and Chemical Optimization - Probe Reports from the NIH Molecular Libraries Program
Your browsing activity is empty.
Activity recording is turned off.
See more...