NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010-.
A fast-track chemistry effort was initiated to evaluate the structure-activity relationship of the 3- and 6-positions of the pyrazolo[1,5-a]pyrimidine scaffold of the known bone morphogenetic protein (BMP) inhibitors. This medicinal chemistry effort led to the identification of a potent and selective compound for ALK2 versus ALK3. The potency contributions of several 3-position substituents were evaluated with subtle structural changes leading to significant changes in potency. From these studies, a novel 5-quinoline molecule was identified and designated an MLPCN probe molecule, ML347, which shows >300-fold selectivity for ALK2 and presents the community with a selective molecular probe for further biological evaluation.
Assigned Assay Grant #: R01 HL104040-02
Screening Center Name & PI: A Medicinal Chemistry FastTrack
Chemistry Center Name & PI: Vanderbilt Specialized Chemistry Center for Accelerated Probe Development, Craig W. Lindsley
Assay Submitter & Institution: Charles H. Hong, Vanderbilt University
PubChem Summary Bioassay Identifier (AID): 652288
Probe Structure & Characteristics
| CID/ML# | Target Name | IC50/(nM) [SID, AID] | Anti-target Name(s) | IC50 (μM) [SID, AID] | Fold Selective | Secondary Assay(s) Name: IC50/EC50 (nM) [SID, AID] |
|---|---|---|---|---|---|---|
| CID 44577753/ML347 | BMP4, ALK2 (ACVR1) | 152 nM [SID 136349469; AID 652282]; 32 nM [SID 136349469; AID 652276] | ALK3 ALK4, ALK5, ALK6 BMPR2, TGFBR2, AMPK, KDR/VEGFR2 | 10.8 μM >100 μM >100 μM 9.8 μM >100 μM >100 μM >100 μM >100 μM [AID 686923] | 337-fold >3,000-fold >3,000-fold 306-fold >3,000-fold >3,000-fold >3,000-fold >3,000-fold | ALK3 ALK4, ALK5, ALK6, BMPR2, TGFBR2, AMPK, KDR/VEGFR2 |
1. Recommendations for Scientific Use of the Probe
ML347 (CID 44577753) is a potent inhibitor when tested in a cell-based BMP-responsive luciferase reporter assay (BMP4) (IC50 = 152 nM). In order to further explore the selectivity of ML347 against ALK1, ALK2, ALK3 and ALK6 we further evaluated the compound against a panel of kinases and were able to show that ML347 is selective for ALK2 (32 nM) versus ALK3 (10,800 nM) and ALK6 (9830 nM). This selectivity for ALK2 over ALK3 is unprecedented in the primary literature and should make ML347 an invaluable research tool in order to interrogate the biology of BMP signaling at the subtype resolution. ML347 was also evaluated in a Tier 1 DMPK panel and it was shown to be highly cleared in human and mouse liver microsomes and moderate/highly cleared in rat. The compound was shown to have ∼1% free fraction in plasma in all three species tested. Thus, the compound can be used for both in vitro and in vivo studies provided the route of administration is non-oral (IP, for example).
2. Materials and Methods
BMP-responsive luciferase reporter assays. BMP-responsive C2C12BRA cells, stably transformed with the Id1 promoter-firefly luciferase reporter; kind gift of D, Rifkin, NYU Medical Center) were seeded in 96-well plates, and incubated overnight with the compounds and BMP4 (50 ng/mL). The cells were then lysed, and cell extracts were then subjected to the firefly luciferase assay using Steady-Glo luciferase assay kit (Promega). The results were normalized to cell titers, as measured using Cell Titer-Glo luminescence assay (Promega).
Kinase assay. All kinase assays were conducted by Reaction Biology Corp (Malvern, PA), as previously reported. In brief, compounds were tested at 10 concentrations by 3-fold serial dilutions starting at 100 mM. In vitro kinase reactions were carried out in the presence of 10 μM (33P)gATP. Eleven human kinases tested were the BMP type-I receptors ALK-1/ACVRL1, ALK2/ACRV1, ALK3/BMPR1A and ALK6/BMPR1B, the TGFb type-I receptors ALK4/ACVR1B and ALK5/TGFbR1, the BMP type-II receptor (BMPR2), the TGFb type-II receptor (TGFbR2), VEGF type-II receptor (KDR/VEGFR2), AMP-activated protein kinase (AMPK-A1/B1/G1) and the human platelet-derived growth factor receptor-β (PDGFRβ).
DMPK Methods. In vitro: The metabolism of ML347 was investigated in human, rat and mouse hepatic microsomes (BD Biosciences, Billerica, MA) using substrate depletion methodology (% test article remaining). A potassium phosphate-buffered reaction mixture (0.1 M, pH 7.4) of test article (1 μM) and microsomes (0.5 mg/mL) was pre-incubated (5 min) at 37°C prior to the addition of NADPH (1 mM). The incubations, performed in 96-well plates, were continued at 37 °C under ambient oxygenation and aliquots (80 μL) were removed at selected time intervals (0, 3, 7, 15, 25 and 45 min). Protein was precipitated by the addition of chilled acetonitrile (160 μL), containing glyburide as an internal standard (50 ng/mL), and centrifuged at 3000 rpm (4°C) for 10 min. Resulting supernatants were transferred to new 96-well plates in preparation for LC/MS/MS analysis. The in vitro half-life (t1/2, min, Eq. 1), intrinsic clearance (CLint, mL/min/kg, Eq. 2) and subsequent predicted hepatic clearance (CLhep, mL/min/kg, Eq. 3) were determined employing the following equations:
Plasma Protein Binding. Protein binding of ML347 was determined in human, rat and mouse plasma via equilibrium dialysis employing Single-Use RED Plates with inserts (ThermoFisher Scientific, Rochester, NY). Briefly plasma (220 μL) was added to the 96 well plate containing test article (5 μL) and mixed thoroughly. Subsequently, 200 μL of the plasma-test article mixture was transferred to the cis chamber (red) of the RED plate, with an accompanying 350 μL of phosphate buffer (25 mM, pH 7.4) in the trans chamber. The RED plate was sealed and incubated 4 h at 37 °C with shaking. At completion, 50 μL aliquots from each chamber were diluted 1:1 (50 μL) with either plasma (cis) or buffer (trans) and transferred to a new 96 well plate, at which time ice-cold acetonitrile (2 volumes) was added to extract the matrices. The plate was centrifuged (3000 rpm, 10 min) and supernatants transferred to a new 96 well plate. The sealed plate was stored at −20 °C until LC/MS/MS analysis.
Liquid Chromatography/Mass Spectrometry Analysis. In vitro experiments. ML347 was analyzed via electrospray ionization (ESI) on an AB Sciex API-4000 (Foster City, CA) triple-quadrupole instrument that was coupled with Shimadzu LC-10AD pumps (Columbia, MD) and a Leap Technologies CTC PAL auto-sampler (Carrboro, NC). Analytes were separated by gradient elution using a Fortis C18 2.1 × 50 mm, 3.5 μm column (Fortis Technologies Ltd, Cheshire, UK) thermostated at 40 °C. HPLC mobile phase A was 0.1% NH4OH (pH unadjusted), mobile phase B was acetonitrile. The gradient started at 30% B after a 0.2 min hold and was linearly increased to 90% B over 0.8 min; held at 90% B for 0.5 min and returned to 30% B in 0.1 min followed by a re-equilibration (0.9 min). The total run time was 2.5 min and the HPLC flow rate was 0.5 mL/min. The source temperature was set at 500°C and mass spectral analyses were performed using multiple reaction monitoring (MRM) utilizing a Turbo-Ionspray® source in positive ionization mode (5.0 kV spray voltage). LC/MS/MS analysis was performed employing a TSQ QuantumULTRA that was coupled to a ThermoSurveyor LC system (Thermoelectron Corp., San Jose, CA) and a Leap Technologies CTC PAL auto-sampler (Carrboro, NC). Chromatographic separation of analytes was achieved with an Acquity BEH C18 2.1 × 50 mm, 1.7 μm column (Waters, Taunton, MA).
2.1. Assays
- 2.1.1.
AID 652288 (ML347 Summary Assay BMP Inhibitors)
- 2.1.2.
AID 652284 (Discovery and structure-activity relationship of BMP Inhibitors, in-vivo SAR, zebrafish embryo 24-well plate)
- 2.1.3.
AID 652276 (Discovery and structure-activity relationship of BMP Inhibitors, Secondary in-citro ALK2 Assay)
- 2.1.4.
AID 652282 (Discovery and structure-activity relationship of BMP Inhibitors)
- 2.1.5.
AID 686923 (In vitro counter assay against key known off-targets for BMP4 Inhibitors)
- 2.1.6.
AID 686924 (ML347 Eurofin Panel Assay for BMP Inhibitor (Probe Compound)
2.2. Probe Chemical Characterization
Probe compound ML347 (CID 44577753, SID 136349469) was prepared according to the above scheme and had the following characterization. 5-(6-(4-methoxyphenyl)pyrazolo[1,5-a]pyrimidin-3-yl)quinolone. LCMS: RT = 0.586 min, >98% @ 215 and 254 nM, m/z = 352.7 [M + H]+. 1H NMR (300 MHz, d-DMSO): δ 9.52 (s, 1H), 8.99-8.94 (m, 2H), 8.57 (s, 1H), 8.41 (d, J = 8.0 Hz, 1H), 8.06 (d, J = 8.0 Hz, 1H), 7.90-7.80 (m, 4H), 7.53 (dd, J = 8.1, 4.0 Hz, 1H), 7.11 (d, J = 8.5 Hz, 2H), 3.92 (s, 3H). HRMS, calc'd for C22H17N4O [M + H]+, 353.1402; found 353.1403.
Solubility. Solubility for ML347 in PBS was determined to be 4 μM, which is ∼26-fold higher than the cellular IC50 for BMP inhibition.
Stability. Stability was determined for ML337 at 23 °C in PBS (no antioxidants or other protectorants and DMSO concentration below 0.1%). After 48 hours, ∼29% of the initial concentration of ML347 remained in solution. The loss is most likely due to limited solubility in buffer.
| Percent Remaining (%) | ||||||
|---|---|---|---|---|---|---|
| Compound | 0 min | 15 min | 30 min | 90 min | 24 Hour | 48 Hour |
| ML347, CID 44577753 | 100 ± 0.0 | 94.0 ± 1.2 | 93.3 ± 2.7 | 91.0 ± 1.7 | 31.3 ± 1.0 | 28.6 ± 0.5 |
Compounds added to the SMR collection (MLS#s): MLS004797119 (ML347, CID 44577753, 25 mg); MLS004797120 (CID 60182382, 8.5 mg); MLS004797121 (CID 60182399, 6.3 mg); MLS004797122 (CID 60182380, 6.05 mg); MLS004797123 (CID 60182372, 6.26 mg); MLS004797124 (CID 60182397, 7.4 mg)
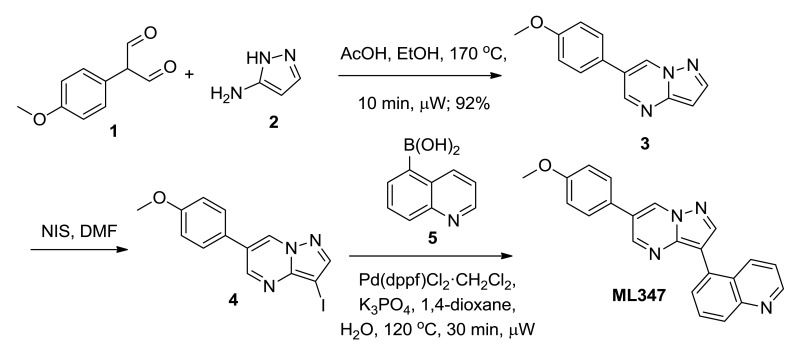
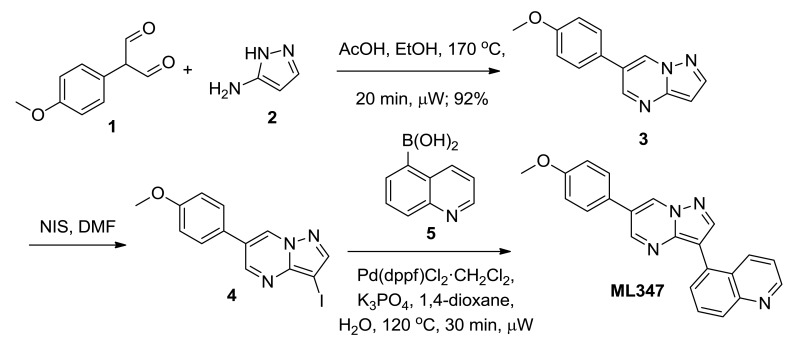
2.3. Probe Preparation
Probe compound ML347 (CID 44577753, SID 136349469) was prepared according to the above scheme and had the following characterization. 5-(6-(4-methoxyphenyl)pyrazolo[1,5-a]pyrimidin-3-yl)quinoline.
6-(4-methoxyphenyl)pyrazolo[1,5-a]pyrimidine (3). Equally divided amongst two 20 mL μwave vials, to a solution of 1H-pyrazol-3-amine, 2, (1.17 g, 14.0 mmol, 1.0 eq) in 5% AcOH/EtOH (12 mL/ vial) was added 2-(4-methoxyphenyl)malonaldehyde, 1, (2.5 g, 14 mmol, 1.0 eq). The reaction was heated to 170 °C for 20 min under microwave irradiation. Upon cooling, a precipitate formed. Filtration of the reaction provided an off white solid (2.9 g, 92% yield). LCMS: RT = 0.620 min, >98% @ 215 and 254 nM, m/z = 226.1 [M + H]+.
3-iodo-6-(4-methoxyphenyl)pyrazolo[1,5-a]pyrimidine (4). To a solution of 6-(4-methoxyphenyl)pyrazolo[1,5-a]pyrimidine (2.90 g, 12.9 mmol, 1.0 eq) in DMF (30 mL) at rt was added N-iodosuccinimide (3.05 g, 13.5 mmol, 1.05 eq). After 16 h, the brown slurry was diluted with H2O (100 mL) and Brine (15 mL). The mixture was extracted with EtOAc (100 mL). The aqueous layer was re-extracted with EtOAc (100 mL) and the collected organic layers were washed with H2O (2 × 30 mL), 10% sodium thiosulfate (20 mL), Brine (30 mL) and dried (MgSO4). After filtration, the solution was concentrated to afford 3-iodo-6-(4-methoxyphenyl)pyrazolo[1,5-a]pyrimidine, 4, as an off-white solid. The material was taken through without further purification. LCMS: RT = 0.781 min, >98% @ 215 and 254 nM, m/z = 352.0 [M + H]+.
5-(6-(4-methoxyphenyl)pyrazolo[1,5-a]pyrimidin-3-yl)quinoline (ML347). In a μwave vial, 3-iodo-6-(4-methoxyphenyl)pyrazolo[1,5-a]pyrimidine, 4, (1.0 g, 2.9 mmol, 1.0 eq), quinolin-5-ylboronic acid (0.54 g, 3.1 mmol, 1.1 eq), and Pd(dppf)Cl2•CH2Cl2 (116 mg, 0.143 mmol, 0.05 eq) were added. The solid mixture was evacuated under vacuo and purged with Argon (3x). To the mixture was added 1,4-dioxane (13 mL), followed by a solution of K3PO4 (1.2 g, 5.7 mmol, 2.0 eq) in H2O (5 mL). The reaction was heated to 120 °C for 30 min under microwave irradiation. The reaction was added to EtOAc:H2O (1:1, 200 mL). The organic layer was separated, washed with H2O (3 × 25 mL), Brine (25 mL), dried (MgSO4), filtered and concentrated. The material was purified by reverse-phase HPLC (20-55% acetonitrile: H2O w/0.1% TFA) to provide 5-(6-(4-methoxyphenyl)pyrazolo[1,5-a]pyrimidin-3-yl)quinoline (ML347) (0.47 g, 47% yield). LCMS: RT = 0.586 min, >98% @ 215 and 254 nM, m/z = 352.7 [M + H]+. 1H NMR (300 MHz, DMSO-d6): δ 9.52 (s, 1H), 8.99-8.94 (m, 2H), 8.57 (s, 1H), 8.41 (d, J = 8.0 Hz, 1H), 8.06 (d, J = 8.0 Hz, 1H), 7.90-7.80 (m, 4H), 7.53 (dd, J = 8.1, 4.0 Hz, 1H), 7.11 (d, J = 8.5 Hz, 2H), 3.92 (s, 3H). HRMS, calc'd for C22H17N4O [M + H]+, 353.1402; found 353.1403.
3. Results
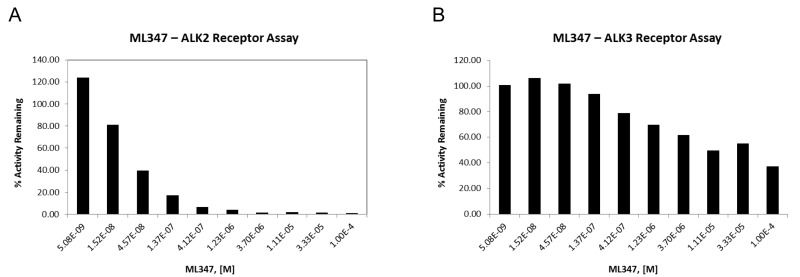
3.1. Dose Response Curves for Probe
Figure 3In vitro molecular pharmacology characterization of ML347
Concentration-response curves of for ML347 in ALK2 kinase assay (A) and ALK3 kinase assay (B).
ML347 is an extraordinarily selective compound which exhibits >3,000 fold selectivity for ALK2 over ALK4, ALK5, BMPR2, TGFBR2, AMPK and KDR/VERGR2, and over 300 fold selectivity for ALK2 over ALK2 and ALK6.
3.2. Cellular Activity
The primary screening assay (BMP4) is a cell-based assays, indicating that ML347 can gain access to its molecular target when applied to cells. The compound did not exhibit acute toxicity in cell based assays at concentrations up to 30 μM.
3.3. Profiling Assays
To more fully characterize this potent, selective ALK2 inhibitor ML347 was tested using EuroFin's (formerly MDS Pharma's) Lead Profiling Screen (binding assay panel of 68 GPCRs, ion channels and transporters screened at 10 μM). Included in the EuroFin screening panel are a number of ion channels (Calcium Channel, L-Type and N-Type; Potassium channel [KATP]; Potassium channel [hERG]) and class A GPCRs (D1-5, H1-3, etc…). ML347 did not inhibit any of the targets conducted (inhibition of radio ligand binding > 50% at 10 μM), highlighting a clean ancillary pharmacology profile.
4. Discussion
4.1. Comparison to Existing Art and How the New Probe is an Improvement
ML347 (CID 44577753) is a potent BMP4 receptor inhibitor (IC50 = 152 nM) and is the most selective compound for ALK2 (32 nM) reported to date with >300-fold selectivity vs. ALK2 and ALK6 and inactive against a panel of other closely related kinases. The existing art, represented by 1-3, are inferior to the probe ML347 in terms of ALK3 and ALK6 selectivity. ML347 can be used both in vitro and in vivo to study the role of selective ALK2 inhibitors. ML347 can be used for basic biochemical and electrophysiological experiments as well as to help establish potential utility for BMP receptor inhibitors. Finally, ML347 is free from IP constraints, which will allow the MLPCN to freely provide this probe to the biomedical research community.
Mechanism of Action Studies
ML347 is an ATP-competitive antagonist of the BMP type-I receptor ALK2 (activin receptor like kinase-2). Based on crystallographic data of the parent compound and ALK2, ML347 is predicted to associate with the catalytic active site of ALK2.1
Efficacy in Cell-Based Assays
The primary BMP4 receptor assay is a cell based functional assay. ML347 displays no cytotoxicity in any assays.
5. References
- 1.
- Chaikuad A, Alfano I, Shrestha B, Muniz JRC, Petrie K, Fedorov O, Phillips C, Bishop S, Mahajan P, Pike ACW, von Delft F, Arrowsmith CH, Edwards AM, Weigelt J, Bountra C, Knapp S, Bullock A. Structure of the bone morphogenetic protein receptor ALK2 and implications for fibrodysplasia ossificans progressiva. J. Biol. Chem. 2012;287:36990–36998. [PMC free article: PMC3481300] [PubMed: 22977237]
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Synthesis and structure-activity relationships of a novel and selective bone morphogenetic protein receptor (BMP) inhibitor derived from the pyrazolo[1.5-a]pyrimidine scaffold of dorsomorphin: the discovery of ML347 as an ALK2 versus ALK3 selective MLPCN probe.[Bioorg Med Chem Lett. 2013]Synthesis and structure-activity relationships of a novel and selective bone morphogenetic protein receptor (BMP) inhibitor derived from the pyrazolo[1.5-a]pyrimidine scaffold of dorsomorphin: the discovery of ML347 as an ALK2 versus ALK3 selective MLPCN probe.Engers DW, Frist AY, Lindsley CW, Hong CC, Hopkins CR. Bioorg Med Chem Lett. 2013 Jun 1; 23(11):3248-52. Epub 2013 Apr 11.
- Review Discovery and characterization of ML398, a potent and selective chiral morpholine based antagonist of the dopamine 4 (D(4)) receptor.[Probe Reports from the NIH Mol...]Review Discovery and characterization of ML398, a potent and selective chiral morpholine based antagonist of the dopamine 4 (D(4)) receptor.Berry CB, Locuson CW, Daniels JS, Lindsley CW, Hopkins CR. Probe Reports from the NIH Molecular Libraries Program. 2010
- ALK2 R206H mutation linked to fibrodysplasia ossificans progressiva confers constitutive activity to the BMP type I receptor and sensitizes mesenchymal cells to BMP-induced osteoblast differentiation and bone formation.[J Bone Miner Res. 2010]ALK2 R206H mutation linked to fibrodysplasia ossificans progressiva confers constitutive activity to the BMP type I receptor and sensitizes mesenchymal cells to BMP-induced osteoblast differentiation and bone formation.van Dinther M, Visser N, de Gorter DJ, Doorn J, Goumans MJ, de Boer J, ten Dijke P. J Bone Miner Res. 2010 Jun; 25(6):1208-15.
- ALK2 functions as a BMP type I receptor and induces Indian hedgehog in chondrocytes during skeletal development.[J Bone Miner Res. 2003]ALK2 functions as a BMP type I receptor and induces Indian hedgehog in chondrocytes during skeletal development.Zhang D, Schwarz EM, Rosier RN, Zuscik MJ, Puzas JE, O'Keefe RJ. J Bone Miner Res. 2003 Sep; 18(9):1593-604.
- Review Bone morphogenetic proteins.[Growth Factors. 2004]Review Bone morphogenetic proteins.Chen D, Zhao M, Mundy GR. Growth Factors. 2004 Dec; 22(4):233-41.
- Development of a potent and ALK2 selective bone morphogenetic protein receptor (...Development of a potent and ALK2 selective bone morphogenetic protein receptor (BMP) inhibitor - Probe Reports from the NIH Molecular Libraries Program
Your browsing activity is empty.
Activity recording is turned off.
See more...