NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Guideline Centre (UK). Emergency and acute medical care in over 16s: service delivery and organisation. London: National Institute for Health and Care Excellence (NICE); 2018 Mar. (NICE Guideline, No. 94.)

Emergency and acute medical care in over 16s: service delivery and organisation.
Show details7. Primary care access to laboratory investigations
7.1. Introduction
General practitioners working within the NHS will refer patients to secondary care (AMU/ED) urgently when following clinical assessment and the patient is deemed at risk of an acute medical emergency. A proportion of these patients will be discharged and reassured following an initial screen, either within an AMU or ED, following initial laboratory investigations. This review question seeks to further explore whether the provision of additional “point of care”, or rapid biochemical/ haematological testing by the general practitioner at the first point of contact can have a positive impact upon clinical outcomes, and reduce the burden on the AME pathway, whilst improving patient and/or carer satisfaction.
The guideline committee discussed the generic issue of point-of-care testing for acute illness in primary care, and chose to focus on 2 acute conditions prioritised as important by family doctors in 3 European countries, including the UK: respiratory infection and inflammatory illnesses and heart failure.30 For the former group, respiratory illness was taken as representing a common and important issue for general practice; the committee decided to focus the review on tests for C-Reactive Protein (CRP) as this test is available and gives rapid results.
7.2. Review question: Does primary care access to laboratory investigations with same day results improve outcomes?
For full details see review protocol in Appendix A.
Table 1
PICO characteristics of review question.
7.3. Clinical evidence
We searched for randomised trials comparing GP access to laboratory investigations with same day results to usual care.
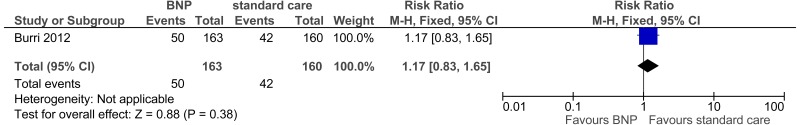
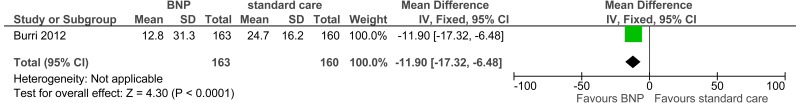
Nine studies were included in the review;3,6,10,14,17,21,22,38,44 these are summarised in Table 2 below. We have updated 1 Cochrane review3 that initially included 6 RCTs6,14,17,22,38,44 with 2 additional RCTs.10,21 All included studies used C-reactive protein (CRP) testing as an intervention except for 1 study10 which used B-type natriuretic peptide (BNP) testing.
Table 2
Summary of studies included in the review.
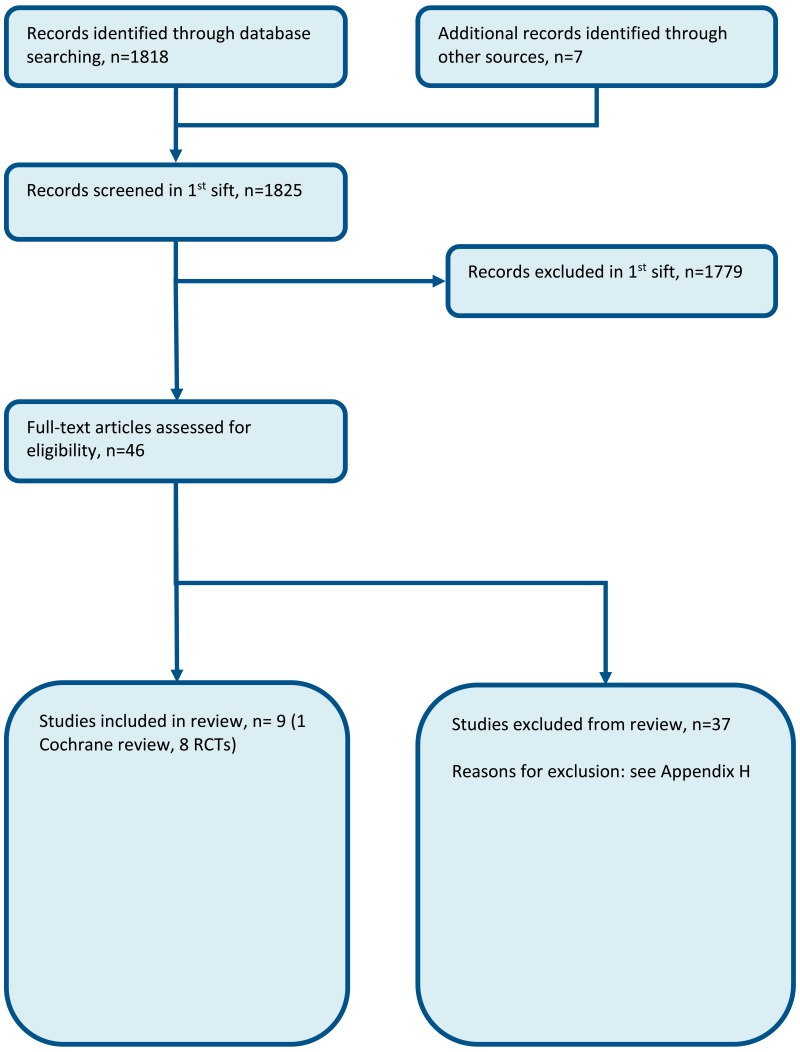
Evidence from these studies is summarised in the GRADE clinical evidence profile/clinical evidence summary below (Table 3, Table 4). See also the study selection flow chart in Appendix B, study evidence tables in Appendix D, forest plots in Appendix C, GRADE tables in Appendix F and excluded studies list in Appendix G.
Table 3
Clinical evidence summary: point of care CRP testing compared to standard care.
Table 4
Clinical evidence summary: point of care BNP testing compared to standard care.
7.4. Economic evidence
Published literature
Three economic evaluations were identified with the relevant comparison and have been included in this review.10,32,58 These are summarised in the economic evidence profile below (Table 5) and the economic evidence tables in Appendix E. A further study was selectively excluded since it was less applicable than the included studies13 – see Appendix H.
Table 5
Economic evidence profile: GP access to laboratory investigations versus usual care.
The economic article selection protocol and flow chart for the whole guideline can found in the guideline’s Appendix 41A and Appendix 41B.
7.5. Evidence statements
Clinical
Point of care CRP testing
- Nine studies comprising 4950 people evaluated the role of point of care CRP testing for improving outcomes in adults and young people at risk of an AME, or with a suspected or confirmed AME. The evidence suggested that point of care CRP testing may provide a benefit in reduced antibiotics prescribed at index consultation (7 studies, very low quality), antibiotics prescribed within 28 days (5 studies, moderate quality) and improved patient satisfaction (2 studies, low quality). The evidence suggested there was no effect on clinical recovery at day 7 (3 studies, moderate quality), clinical recovery at day 28 (3 studies, low quality) and hospitalisation (1 study, very low quality) for point of care CRP testing compared to standard care.
Point of care BNP testing
- One study comprising 323 people evaluated the role of point of care BNP testing for improving outcomes in adults and young people at risk of an AME, or with a suspected or confirmed AME. The evidence suggested that point of care BNP testing may provide a benefit for reduced time to initiation of appropriate therapy (moderate quality). However, the evidence suggested there was no effect either on hospitalisation within 3 months or hospitalisation within 12 months (low quality). The evidence was graded moderate to low quality for all outcomes due to imprecision and indirectness.
Economic
- One cost–utility analysis found that point of care CRP testing was dominant (less costly and more effective) compared to usual care for people with a suspected AME. This analysis was assessed as directly applicable with minor limitations.
- Another cost–utility analysis found that point of care CRP testing was cost effective compared to usual care for people with a suspected AME (ICER: £7,500 per QALY gained). This analysis was assessed as partially applicable with potentially serious limitations.
- One cost-consequences analysis found that point of care BNP testing for patients presenting with new onset or clearly worsening dyspnoea was more costly (£317 per patient), had more hospitalisations (0.04 per patient) and greater diagnostic certainty (+13%) compared to usual care. This analysis was assessed as partially applicable with potentially serious limitations.
7.6. Recommendations and link to evidence
| Recommendations |
|
| Research recommendation | - |
| Relative values of different outcomes | Quality of life, patient and/or carer satisfaction, avoidable adverse events and ED attendance were considered by the committee to be critical outcomes. Antibiotic usage and lab/ diagnostic turn around for result to GP were considered by the committee to be important outcomes. |
| Trade-off between benefits and harms |
There was evidence from 9 RCTs for this review question; 8 RCTs compared same day point of care CRP testing with standard care and 1 RCT compared same day point of care BNP testing with standard care, in primary care. Point of care CRP testing: The evidence from the review comparing CRP testing and standard care in patients with lower respiratory tract infections suggested that point of care CRP testing may provide a benefit in reduced antibiotics prescribed at index consultation, antibiotics prescribed within 28 days and improved patient satisfaction. The evidence suggested there was no effect on clinical recovery at day 7, clinical recovery at day 28 and hospitalisation. One study reported no serious adverse events; indicating that the reduction in antimicrobial use associated with CRP-POC testing was not harmful. No evidence was available for the outcomes quality of life, lab/diagnostic turn around for result to GP and ED attendance. Point of care BNP testing: The evidence from the review comparing BNP testing with standard care in patients presenting with dyspnoea suggested that there may be a benefit in reduced time to initiation of appropriate therapy. However, the evidence suggested there was no effect either on hospitalisation within 3 months or hospitalisation within 12 months. No evidence was available for the outcomes: antibiotic usage, avoidable adverse events, quality of life, patient and/or carer satisfaction and lab/ diagnostic turn around for result to GP and ED attendance. The outcomes of hospitalisation and time to appropriate therapy were non-protocol outcomes and these were considered as surrogate outcomes for ED attendance and lab/ diagnostic turn around for result to GP respectively. Overall: The committee agreed that the evidence for CRP testing in adult patients with lower respiratory tract infections was quite clear in demonstrating reduction in antibiotic prescription and increase in patient and/or carer satisfaction without a difference in serious adverse events. Therefore, the committee recommended CRP testing at point of care for patients with suspected lower respiratory tract infections. The committee also agreed that this recommendation fits with national strategy to reduce antibiotic prescribing for people with lower respiratory tract infections. The vast majority of respiratory infections are caused by viruses, against which antibiotics are ineffective and unnecessary and also there is a concern that antibiotics may cause side effects and are directly associated with antibiotic resistance in common bacteria, causing treatment failure and complications, including death. 3 The committee noted that all the evidence was from studies of tests conducted at point-of care within practice hours and no evidence was available for tests conducted out-of-hours. The committee acknowledged that there was some benefit of BNP testing on achieving drug therapy. However, they did not feel that there were sufficient data available on which to base a recommendation for primary care, particularly given the small size of the study. Studies of the diagnostic utility of BNP in the emergency department were not relevant for this review.55,64 Given the lack of evidence for BNP testing in primary care, and the strong evidence for CRP, the committee formulated a recommendation solely for CRP-POC testing. |
| Trade-off between net effects and costs |
The cost of point of care c-reactive protein testing is likely to be offset by a subsequent reduction in respiratory infections and antibiotic prescribing. Two economic evaluations were included evaluating GP access to CRP results through same day point of care testing compared to usual care. Both studies included cost-utility analysis, including 1 from a UK perspective, which was considered directly applicable and with only minor limitations. They both found that GP same day point of care testing would be cost-effective at the £20,000 per QALY threshold. The studies found that the intervention reduced the number of antibiotic prescriptions. Reducing unnecessary antibiotic prescription to avoid antimicrobial resistance has an uncertain, potentially large, economic benefit on top of any cost per QALY.63 It is not clear whether point of care CRP testing will have a net increase or decrease in overall cost but it appears to be cost effective. There was one economic evaluation of point of care BNP testing. It found an increase in cost that was partly due to an increase in the average number of hospitalisations. This could be where admission to hospital based on earlier laboratory results could have a clinical benefit. An increase in diagnostic accuracy within the study provides potential evidence to support this. However, the study was not designed to evaluate whether the clinical benefits were large enough to justify the increased cost. In conclusion, there was cost effectiveness evidence to support CRP point of care testing but no evidence to support other tests. |
| Quality of evidence |
The RCT evidence was moderate to very low quality. This was primarily due to risk of bias and imprecision. The outcomes hospitalisation and time to initiation of appropriate therapy were further downgraded for indirectness, as these outcomes were surrogates for ED attendance and lab/diagnostic turn around for result to GP respectively. One of the CRP economic evaluations was assessed as directly applicable with minor limitations. The other was partially applicable with potentially serious limitations as it was set in Scandinavia and based on observational evidence. The economic evaluation of BNP testing was assessed as partially applicable with potentially serious limitations as it was set in Scandinavia and based on observational evidence. |
| Other considerations |
A review of CRP-POC testing reports good acceptance by doctors and patients; 50% of GP practices report minimal impact on workload.20 It should be noted that CRP does not distinguish bacterial from viral infections, the latter not being susceptible to antimicrobial treatment, so a high level of CRP is not necessarily an indication for antimicrobial treatment. Adjunctive tests such as procalcitonin which may distinguish bacterial from viral infections have yet to show utility. This recommendation fits with the national strategy to reduce antibiotic prescribing for people with lower respiratory tract infections. The committee wished to note 3 other related NICE guidelines in this area: Pneumonia in adults: diagnosis and management,50 Antimicrobial stewardship: systems and processes for effective antimicrobial medicine use52 and Respiratory tract infections (self-limiting): prescribing antibiotics.54 For further guidance on BNP testing please see: Acute heart failure: diagnosis and management49 and the Chronic heart failure in adults: management.46 The committee agreed that all same day point of care tests must be subject to quality assurance. The committee recognised that other point of care tests in acute illness were available for use in primary care, including (but not limited to) creatinine to screen for acute kidney injury (Acute kidney injury: prevention, detection and management48), D-dimer for venous thrombosis or pulmonary embolism (Venous thromboembolic diseases: diagnosis, management and thrombophilia testing47); Pulmonary embolism,53 and troponin for myocardial infarction.51 Other tests could also become available with the development of new technologies. These tests have the potential to guide primary care-delivered treatments, rule out or refer serious illness or refine existing treatments. The utility of these tests is usually established in secondary care, for example, in the emergency department. Their utility in primary and community care requires independent evaluation to take into account the differing clinical contexts. The committee wished to note that the recommendation does not exclude services being set up to provide testing in a centralised manner. In cities this could be provided in hubs and in rural areas it may be achieved using kits within the healthcare setting. This testing may occur within GP practices, walk in centres, urgent care centres and other health care providers. The sampling processing times should be sufficiently rapid to provide results without delaying patient management. Results should be available within a few minutes. |
References
- 1.
- Uses of serologic tests in the EPI. EPI Newsletter. 1984; 6(2):6–8 [PubMed: 12313078]
- 2.
- RCT of point of care C-reactive protein test and enhanced communication skills for managing acute cough due to lower respiratory tract infection in general practice: cost effectiveness and effect on diagnostic testing, antibiotic prescribing and recovery. 2005. Available from: http://erj
.ersjournals .com/content/40/Suppl_56/P720 .full.pdf+html - 3.
- Aabenhus R, Jensen Jens-Ulrik S, Jørgensen KJ, Hróbjartsson A, Bjerrum L. Biomarkers as point-of-care tests to guide prescription of antibiotics in patients with acute respiratory infections in primary care. Cochrane Database of Systematic Reviews. 2014; Issue 11:CD010130. DOI:10.1002/14651858.CD010130.pub2 [PubMed: 25374293] [CrossRef]
- 4.
- Andersen T, Hvid M, Johansen C, Stengaard-Pedersen K, Hetland ML, Horslev-Petersen K et al. Interleukin-23 in early disease development in rheumatoid arthritis. Scandinavian Journal of Rheumatology. 2015; 44(6):438–442 [PubMed: 26087654]
- 5.
- Andreeva E, Melbye H. The usefulness of point-of-care-testing for C-reactive protein in lower respiratory tract infection/acute cough. European Respiratory Journal. 2012; 40:(Suppl 56):117s
- 6.
- Andreeva E, Melbye H. Usefulness of C-reactive protein testing in acute cough/respiratory tract infection: an open cluster-randomized clinical trial with C-reactive protein testing in the intervention group. BMC Family Practice. 2014; 15:80 [PMC free article: PMC4016668] [PubMed: 24886066]
- 7.
- Bjerrum L, Cots JM, Llor C, Molist N, Munck A. Effect of intervention promoting a reduction in antibiotic prescribing by improvement of diagnostic procedures: a prospective, before and after study in general practice. European Journal of Clinical Pharmacology. 2006; 62(11):913–918 [PubMed: 16967300]
- 8.
- Bjerrum L, Gahrn-Hansen B, Munck AP. C-reactive protein measurement in general practice may lead to lower antibiotic prescribing for sinusitis. British Journal of General Practice. 2004; 54(506):659–662 [PMC free article: PMC1326065] [PubMed: 15353050]
- 9.
- Bjerrum L, Munck A, Gahrn-Hansen B, Hansen MP, Jarbol DE, Cordoba G et al. Health Alliance for prudent antibiotic prescribing in patients with respiratory tract infections (HAPPY AUDIT) - impact of a non-randomised multifaceted intervention programme. BMC Family Practice. 2011; 12:52 [PMC free article: PMC3146837] [PubMed: 21689406]
- 10.
- Burri E, Hochholzer K, Arenja N, Martin-Braschler H, Kaestner L, Gekeler H et al. B-type natriuretic peptide in the evaluation and management of dyspnoea in primary care. Journal of Internal Medicine. 2012; 272(5):504–513 [PubMed: 22550938]
- 11.
- CADTH. Point of care testing compared to laboratory testing for the assessment of white blood cell counts and differentials: a review of the clinical effectiveness, diagnostic precision and accuracy, cost-effectiveness, and guidelines. Canadian Agency for Drugs and Technologies in Health (CADTH), 2013. Available from: https://www
.cadth.ca /sites/default/files /pdf/htis/nov-2013/RC0489 %20POC%20WBC%20Final.pdf - 12.
- Cals J, Butler C, Hopstaken R, Hood K, Dinant G-J. Effect of C-reactive protein point of care testing and clinical communication skills training on antibiotic use and patient recovery in lower respiratory tract infections: a cluster randomised trial. European Respiratory Society Annual Congress, Berlin, Germany, October 4-8. 2008;3500
- 13.
- Cals JWL, Ament AJHA, Hood K, Butler CC, Hopstaken RM, Wassink GF et al. C-reactive protein point of care testing and physician communication skills training for lower respiratory tract infections in general practice: economic evaluation of a cluster randomized trial. Journal of Evaluation in Clinical Practice. 2011; 17(6):1059–1069 [PubMed: 20666881]
- 14.
- Cals JWL, Butler CC, Hopstaken RM, Hood K, Dinant GJ. Effect of point of care testing for C reactive protein and training in communication skills on antibiotic use in lower respiratory tract infections: cluster randomised trial. BMJ. 2009; 338:b1374 [PMC free article: PMC2677640] [PubMed: 19416992]
- 15.
- Cals JWL, de Bock L, Beckers PJ, Francis NA, Hopstaken RM, Hood K et al. Enhanced communication skills and C-reactive protein point-of-care testing for respiratory tract infection: 3.5-year follow-up of a cluster randomized trial. Annals of Family Medicine. 2013; 11(2):157–164 [PMC free article: PMC3601394] [PubMed: 23508603]
- 16.
- Cals JWL, Hopstaken RM, Butler CC, Hood K, Severens JL, Dinant GJ. Improving management of patients with acute cough by C-reactive protein point of care testing and communication training (IMPAC3T): study protocol of a cluster randomised controlled trial. BMC Family Practice. 2007; 8:15 [PMC free article: PMC1847819] [PubMed: 17394651]
- 17.
- Cals JWL, Schot MJC, de Jong SAM, Dinant GJ, Hopstaken RM. Point-of-care C-reactive protein testing and antibiotic prescribing for respiratory tract infections: a randomized controlled trial. Annals of Family Medicine. 2010; 8(2):124–133 [PMC free article: PMC2834719] [PubMed: 20212299]
- 18.
- Chandrajay D, Narayanan D, Barth JH. Evaluation of the effect of clinical validation of out of hours critical laboratory results. Annals of Clinical Biochemistry. 2016; 53(Pt 2):274–278 [PubMed: 26092980]
- 19.
- Cook EJ, Randhawa G, Guppy A, Large S. A study of urgent and emergency referrals from NHS Direct within England. BMJ Open. 2015; 5(5):e007533 [PMC free article: PMC4431129] [PubMed: 25968002]
- 20.
- Cooke J, Butler C, Hopstaken R, Dryden MS, McNulty C, Hurding S et al. Narrative review of primary care point-of-care testing (POCT) and antibacterial use in respiratory tract infection (RTI). BMJ Open Respiratory Research. 2015; 2(1):e000086 [PMC free article: PMC4426285] [PubMed: 25973210]
- 21.
- Dahler-Eriksen BS, Lauritzen T, Lassen JF, Lund ED, Brandslund I. Near-patient test for C-reactive protein in general practice: assessment of clinical, organizational, and economic outcomes. Clinical Chemistry. 1999; 45(4):478–485 [PubMed: 10102907]
- 22.
- Diederichsen HZ, Skamling M, Diederichsen A, Grinsted P, Antonsen S, Petersen PH et al. Randomised controlled trial of CRP rapid test as a guide to treatment of respiratory infections in general practice. Scandinavian Journal of Primary Health Care. 2000; 18(1):39–43 [PubMed: 10811042]
- 23.
- Do NTT, Ta NTD, Tran NTH, Than HM, Vu BTN, Hoang LB et al. Point-of-care C-reactive protein testing to reduce inappropriate use of antibiotics for non-severe acute respiratory infections in Vietnamese primary health care: a randomised controlled trial. Lancet Global Health. 2016; 4(9):e633–e641 [PMC free article: PMC4985565] [PubMed: 27495137]
- 24.
- Engel MF, Paling FP, Hoepelman AIM, van der Meer V, Oosterheert JJ. Evaluating the evidence for the implementation of C-reactive protein measurement in adult patients with suspected lower respiratory tract infection in primary care: a systematic review. Family Practice. 2012; 29(4):383–393 [PubMed: 22159030]
- 25.
- Grodzinsky E, Wirehn AB, Fremner E, Haglund S, Larsson L, Persson LG et al. Point-of-care testing has a limited effect on time to clinical decision in primary health care. Scandinavian Journal of Clinical and Laboratory Investigation. 2004; 64(6):547–551 [PubMed: 15370459]
- 26.
- Hanrahan CF, Clouse K, Bassett J, Mutunga L, Selibas K, Stevens W et al. The patient impact of point-of-care vs. laboratory placement of Xpert() MTB/RIF. International Journal of Tuberculosis and Lung Disease. 2015; 19(7):811–816 [PMC free article: PMC4869324] [PubMed: 26056107]
- 27.
- Holm A, Pedersen SS, Nexoe J, Obel N, Nielsen LP, Koldkjaer O et al. Procalcitonin versus C-reactive protein for predicting pneumonia in adults with lower respiratory tract infection in primary care. British Journal of General Practice. 2007; 57(540):555–560 [PMC free article: PMC2099638] [PubMed: 17727748]
- 28.
- Hopstaken RM, Butler CC, Muris JW, Knottnerus JA, Kester AD, Rinkens PE et al. Do clinical findings in lower respiratory tract infection help general practitioners prescribe antibiotics appropriately? An observational cohort study in general practice. Family Practice. 2006; 23(2):180–187 [PubMed: 16326800]
- 29.
- Hopstaken RM, Muris JW, Knottnerus JA, Kester AD, Rinkens PE, Dinant GJ. Contributions of symptoms, signs, erythrocyte sedimentation rate, and C-reactive protein to a diagnosis of pneumonia in acute lower respiratory tract infection. British Journal of General Practice. 2003; 53(490):358–364 [PMC free article: PMC1314594] [PubMed: 12830562]
- 30.
- Howick J, Cals JW, Jones C, Price CP, Pluddemann A, Heneghan C et al. Current and future use of point-of-care tests in primary care: an international survey in Australia, Belgium, The Netherlands, the UK and the USA. BMJ Open. 2014; 4(8):e005611 [PMC free article: PMC4127935] [PubMed: 25107438]
- 31.
- Huang Y, Chen R, Wu T, Wei X, Guo A. Association between point-of-care CRP testing and antibiotic prescribing in respiratory tract infections: a systematic review and meta-analysis of primary care studies. British Journal of General Practice. 2013; 63(616):e787–e794 [PMC free article: PMC3809432] [PubMed: 24267862]
- 32.
- Hunter R. Cost-effectiveness of point-of-care C-reactive protein tests for respiratory tract infection in primary care in England. Advances in Therapy. 2015; 32(1):69–85 [PMC free article: PMC4311066] [PubMed: 25620538]
- 33.
- Jakobsen KA, Melbye H, Kelly MJ, Ceynowa C, Molstad S, Hood K et al. Influence of CRP testing and clinical findings on antibiotic prescribing in adults presenting with acute cough in primary care. Scandinavian Journal of Primary Health Care. 2010; 28(4):229–236 [PMC free article: PMC3444795] [PubMed: 20704523]
- 34.
- Joshi A, Perin DP, Gehle A, Nsiah-Kumi PA. Feasibility of using C-reactive protein for point-of-care testing. Technology and Health Care. 2013; 21(3):233–240 [PubMed: 23792796]
- 35.
- Kavanagh KE, O’Shea E, Halloran R, Cantillon P, Murphy AW. A pilot study of the use of near-patient C-reactive protein testing in the treatment of adult respiratory tract infections in one Irish general practice. BMC Family Practice. 2011; 12:93 [PMC free article: PMC3175160] [PubMed: 21880122]
- 36.
- Kind P, Hardman G, and Macran S. UK population norms for EQ-5D. University of York, 1999. Available from: http://www
.york.ac.uk /media/che/documents /papers/discussionpapers /CHE%20Discussion%20Paper%20172.pdf - 37.
- Leber W, McMullen H, Anderson J, Marlin N, Santos AC, Bremner S et al. Promotion of rapid testing for HIV in primary care (RHIVA2): a cluster-randomised controlled trial. The Lancet HIV. 2015; 2(6):e229–e235 [PubMed: 26423195]
- 38.
- Little P, Stuart B, Francis N, Tonkin-Crine S, Douglas E, Anthierens S. The effect of web-based training in communication skills and an interactive patient booklet and the use of a CRP point of care test in acute respiratory tract infection (RTI): a multi-national cluster randomised factorial controlled trial. The Lancet. 2015; 382(9899):1175–1182 [PMC free article: PMC3807804] [PubMed: 23915885]
- 39.
- Llor C, Hernandez S, Cots JM, Bjerrum L, Gonzalez B, Garcia G et al. Physicians with access to point-of-care tests significantly reduce the antibiotic prescription for common cold. Revista Espanola De Quimioterapia. 2013; 26(1):12–20 [PubMed: 23546457]
- 40.
- Llor C, Bjerrum L, Arranz J, Garcia G, Cots JM, Gonzalez Lopez-Valcarcel B et al. C-reactive protein testing in patients with acute rhinosinusitis leads to a reduction in antibiotic use. Family Practice. 2012; 29(6):653–658 [PubMed: 22447979]
- 41.
- Llor C, Bjerrum L, Munck A, Hansen MP, Cordoba GC, Strandberg EL et al. Predictors for antibiotic prescribing in patients with exacerbations of COPD in general practice. Therapeutic Advances in Respiratory Disease. 2013; 7(3):131–137 [PubMed: 23325784]
- 42.
- Llor C, Cots JM, Lopez-Valcarcel BG, Arranz J, Garcia G, Ortega J et al. Interventions to reduce antibiotic prescription for lower respiratory tract infections: Happy Audit study. European Respiratory Journal. 2012; 40(2):436–441 [PubMed: 22183489]
- 43.
- Llor C, Cots JM, Hernandez S, Ortega J, Arranz J, Monedero MJ et al. Effectiveness of two types of intervention on antibiotic prescribing in respiratory tract infections in Primary Care in Spain. Happy Audit Study. Atencion Primaria. 2014; 46(9):492–500 [PMC free article: PMC6985636] [PubMed: 24768657]
- 44.
- Melbye H, Aaraas I, Fleten N, Kolstrup N, Mikalsen JI. The value of C-reactive protein testing in suspected lower respiratory tract infections. A study from general practice on the effect of a rapid test on antibiotic research and course of the disease in adults. Tidsskrift for Den Norske Laegeforening : Tidsskrift for Praktisk Medicin, Ny Raekke. 1995; 115(13):1610–1615 [PubMed: 7778075]
- 45.
- Mueller C, Laule-Kilian K, Scholer A, Frana B, Rodriguez D, Schindler C et al. Use of B-type natriuretic peptide for the management of women with dyspnea. American Journal of Cardiology. 2004; 94(12):1510–1514 [PubMed: 15589006]
- 46.
- National Clinical Guideline Centre. Chronic heart failure: the management of chronic heart failure in adults in primary and secondary care. NICE clinical guideline 108. London. National Clinical Guideline Centre, 2010. Available from: http://guidance
.nice.org.uk/CG108/ - 47.
- National Clinical Guideline Centre. Venous thromboembolic diseases: the management of venous thromboembolic diseases and the role of thrombophilia testing. NICE clinical guideline 144. London. National Clinical Guideline Centre, 2012. Available from: http://guidance
.nice.org.uk/CG144 - 48.
- National Clinical Guideline Centre. Acute kidney injury: prevention, detection and management of acute kidney injury up to the point of renal replacement therapy. NICE clinical guideline 169. London. National Clinical Guideline Centre, 2013. Available from: http://guidance
.nice.org.uk/CG169 - 49.
- National Clinical Guideline Centre. Acute heart failure: diagnosing and managing acute heart failure in adults. NICE clinical guideline 187. London. National Clinical Guideline Centre, 2014. Available from: http://guidance
.nice.org.uk/CG187 - 50.
- National Clinical Guideline Centre. Pneumonia: Diagnosis and management of community- and hospital-acquired pneumonia in adults. NICE clinical guideline CG191. London. National Clinical Guideline Centre, 2014. Available from: http://guidance
.nice.org.uk/CG191 - 51.
- National Institute for Health and Care Excellence. Myocardial infarction (acute): Early rule out using high-sensitivity troponin tests (Elecsys Troponin T high-sensitive, ARCHITECT STAT High Sensitive Troponin-I and AccuTnI+3 assays). NICE diagnostic guidance 15. London. National Institute for Health and Care Excellence (NICE), 2014. Available from: http://guidance
.nice.org.uk/DG15 - 52.
- National Institute for Health and Care Excellence. Antimicrobial stewardship: systems and processes for effective antimicrobial medicine use. NICE guideline 15. Manchester. National Institute for Health and Care Excellence, 2015. Available from: https://www
.nice.org.uk/guidance/ng15 - 53.
- National Institute for Health and Care Excellence and Clinical Knowledge Summaries. Pulmonary embolism, 2015. Available from: https://cks
.nice.org .uk/pulmonary-embolism - 54.
- National Institute for Health and Clinical Excellence. Respiratory tract infections - antibiotic prescribing: prescribing of antibiotics for self-limiting respiratory tract infections in adults and children in primary care, 2008. Available from: https://www
.nice.org .uk/guidance/cg69/resources /guidance-respiratory-tract-infections-antibiotic-prescribing-pdf [PubMed: 21698847] - 55.
- Nayer J, Aggarwal P, Galwankar S. Utility of point-of-care testing of natriuretic peptides (brain natriuretic peptide and n-terminal pro-brain natriuretic peptide) in the emergency department. International Journal of Critical Illness and Injury Science. 2014; 4(3):209–215 [PMC free article: PMC4200546] [PubMed: 25337482]
- 56.
- Neumark T, Brudin L, Molstad S. Use of rapid diagnostic tests and choice of antibiotics in respiratory tract infections in primary healthcare-a 6-y follow-up study. Scandinavian Journal of Infectious Diseases. 2010; 42(2):90–96 [PubMed: 19902992]
- 57.
- Oosterheert JJ, Loon AM, Schuurman R, Hoepelman AI, Hak E, Thijsen S et al. Impact of rapid detection of viral and atypical bacterial pathogens by real-time polymerase chain reaction for patients with lower respiratory tract infection. Clinical Infectious Diseases. 2005; 41(10):1438–1444 [PMC free article: PMC7107964] [PubMed: 16231254]
- 58.
- Oppong R, Jit M, Smith RD, Butler CC, Melbye H, Molstad S et al. Cost-effectiveness of point-of-care C-reactive protein testing to inform antibiotic prescribing decisions. British Journal of General Practice. 2013; 63(612):e465–e471 [PMC free article: PMC3693803] [PubMed: 23834883]
- 59.
- Organisation for Economic Co-operation and Development (OECD). Purchasing power parities (PPP), 2007. Available from: http://www
.oecd.org/std/ppp - 60.
- Peters CM, Schouwenaars FM, Haagsma E, Evenhuis HM, Echteld MA. Antibiotic prescribing and C-reactive protein testing for pulmonary infections in patients with intellectual disabilities. British Journal of General Practice. 2013; 63(610):e326–e330 [PMC free article: PMC3635578] [PubMed: 23643230]
- 61.
- Pluddemann A, Heneghan C, Price CP, Wolstenholme J, Thompson M. Point-of-care blood test for ketones in patients with diabetes: primary care diagnostic technology update. British Journal of General Practice. 2011; 61(589):530–531 [PMC free article: PMC3145526] [PubMed: 21801574]
- 62.
- Rebnord IK, Hunskaar S, Gjesdal S, Hetlevik O. Point-of-care testing with CRP in primary care: a registry-based observational study from Norway. BMC Family Practice. 2015; 16:170 [PMC free article: PMC4653870] [PubMed: 26585447]
- 63.
- Smith R, Coast J. The true cost of antimicrobial resistance. BMJ. 2013; 346:f1493 [PubMed: 23479660]
- 64.
- Stokes NR, Dietz BW, Liang JJ. Cardiopulmonary laboratory biomarkers in the evaluation of acute dyspnea. Open Access Emergency Medicine : OAEM. 2016; 8:35–45 [PMC free article: PMC4886298] [PubMed: 27307771]
- 65.
- Strykowski DF, Nielsen ABS, Llor C, Siersma V, Bjerrum L. An intervention with access to C-reactive protein rapid test reduces antibiotic overprescribing in acute exacerbations of chronic bronchitis and COPD. Family Practice. 2015; 32(4):395–400 [PubMed: 25902912]
Appendices
Appendix A. Review protocol
Table 6Review protocol: GP access to laboratory investigations
| Review question | Does primary care access to laboratory investigations with same day results improve outcomes? |
|---|---|
| Guideline condition and its definition | Acute Medical Emergencies. Definition: people with suspected or confirmed acute medical emergencies or at risk of an acute medical emergency. |
| Review population | Adults and young people (16 years and over) with a suspected or confirmed AME. |
| Adults. | |
| Line of therapy not an inclusion criterion. | |
|
Interventions and comparators: generic/class; specific/drug (All interventions will be compared with each other, unless otherwise stated) |
GP access to laboratory investigations within practice hours; GP access to phlebotomy and blood tests with same day results within practice hours including cardiac biomarkers including BNP and/or CRP and/or renal function and/or full blood count and/or LFT. GP access to laboratory investigations out of practice hours; GP access to phlebotomy and blood tests with same day results in out of practice hours including cardiac biomarkers including BNP and/or CRP and/or renal function and/or full blood count and/or LFT. Standard services; as defined in study. No GP access to laboratory investigations. |
| Outcomes |
|
| Study design | Systematic reviews (SRs) of RCTs, RCTs, observational studies only to be included if no relevant SRs or RCTs are identified. |
| Unit of randomisation |
Patient. GP surgeries/practices. |
| Crossover study | Not permitted. |
| Minimum duration of study | Not defined. |
| Subgroup analyses if there is heterogeneity |
|
| Search criteria |
Databases: Medline, Embase, the Cochrane Library. Date limits for search: None. Language: English. |
Appendix B. Clinical article selection
Appendix C. Forest plots
C.1. Point of care CRP testing vs. Standard care
Appendix D. Clinical evidence tables
Download PDF (447K)
Appendix E. Economic evidence tables
Download PDF (522K)
Appendix F. GRADE tables
Table 7Clinical evidence profile: Point of care CRP testing versus standard care
| Quality assessment | No of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | CRP | standard care | Relative (95% CI) | Absolute | ||
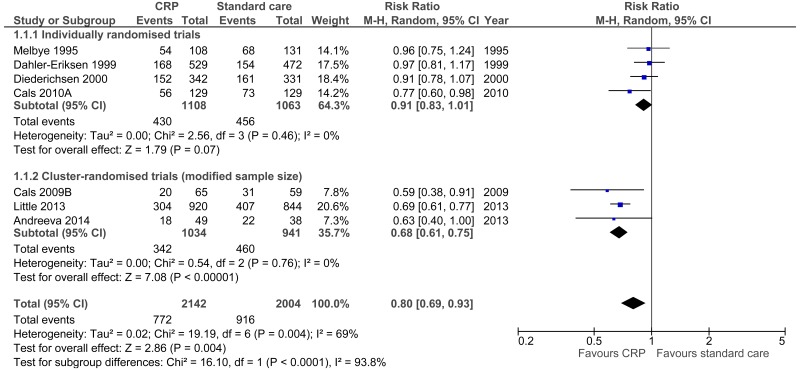
| Antibiotics prescribed at index consultation. All trials (cluster-randomised with modified sample size) 6 | ||||||||||||
| 7 | randomised trials | seriousa | seriousb | no serious indirectness | seriousc | None |
772/2142 (36%) | 51.9% | RR 0.8 (0.69 to 0.93) | 104 fewer per 1000 (from 36 fewer to 161 fewer) |
⨁◯◯◯ VERY LOW | IMPORTANT |
| Antibiotics prescribed at index consultation. All trials - Individually randomised trials | ||||||||||||
| 4 | randomised trials | Seriousa | no serious inconsistency | no serious indirectness | no serious imprecision | None |
430/1108 (38.8%) | 50.3% | RR 0.91 (0.83 to 1.01) | 45 fewer per 1000 (from 86 fewer to 5 more) |
⨁⨁⨁◯ MODERATE | IMPORTANT |
| Antibiotics prescribed at index consultation. Cluster-randomised trials (modified sample size) 6 | ||||||||||||
| 3 | randomised trials | seriousa | no serious inconsistency | no serious indirectness | no serious imprecision | None |
342/1034 (33.1%) | 52.5% | RR 0.68 (0.61 to 0.75) | 168 fewer per 1000 (from 131 fewer to 205 fewer) |
⨁⨁⨁◯ MODERATE | IMPORTANT |
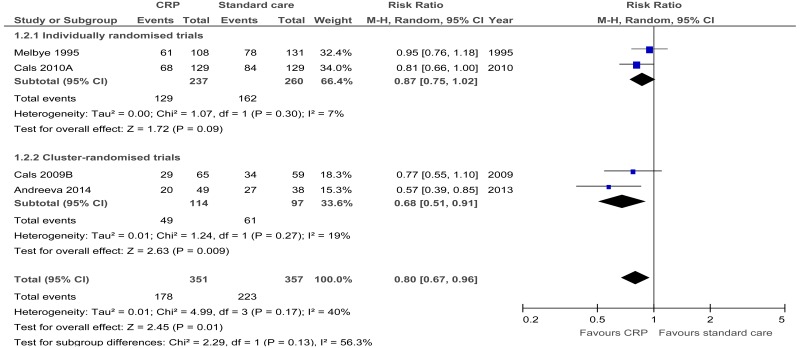
| Antibiotics prescribed within 28 days. All trials (cluster-randomised trials with modified sample size) 6 | ||||||||||||
| 4 | randomised trials | seriousa | no serious inconsistency | no serious indirectness | no serious imprecision | None |
178/351 (50.7%) | 62.3% | RR 0.8 (0.67 to 0.96) | 125 fewer per 1000 (from 25 fewer to 206 fewer) |
⨁⨁⨁◯ MODERATE | IMPORTANT |
| Antibiotics prescribed within 28 days - Individually randomised trials | ||||||||||||
| 2 | randomised trials | seriousa | no serious inconsistency | no serious indirectness | no serious imprecision | None |
129/237 (54.4%) | 62.3% | RR 0.87 (0.75 to 1.02) | 81 fewer per 1000 (from 156 fewer to 12 more) |
⨁⨁⨁◯ MODERATE | IMPORTANT |
| Antibiotics prescribed within 28 days -cluster-randomised trials with modified sample size6 | ||||||||||||
| 2 | randomised trials | seriousa | no serious inconsistency | no serious indirectness | seriousc | None |
49/114 (43%) | 64.3% | RR 0.68 (0.51 to 0.91) | 206 fewer per 1000 (from 58 fewer to 315 fewer) |
⨁⨁◯◯ LOW | IMPORTANT |
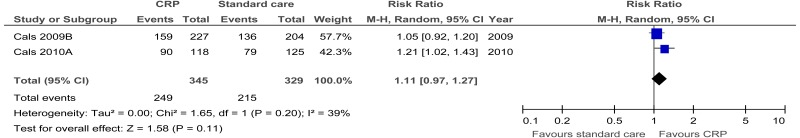
| Patient satisfaction | ||||||||||||
| 2 | randomised trials | seriousa | no serious inconsistency | no serious indirectness | seriousc | None |
249/345 (72.2%) | 64.9% | RR 1.11 (0.97 to 1.27) | 71 more per 1000 (from 19 fewer to 175 more) |
⨁⨁◯◯ LOW | CRITICAL |
| Clinical recovery day 7 (number of patients substantially improved by day 7) | ||||||||||||
| 3 | randomised trials | seriousa | no serious inconsistency | No serious indirectness | no serious imprecision | None |
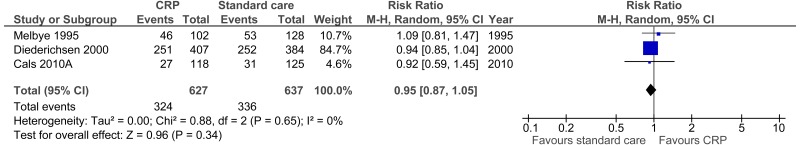
324/627 (51.7%) | 41.4% | RR 0.95 (0.87 to 1.05) | 21 fewer per 1000 (from 54 fewer to 21 more) |
⨁⨁⨁◯ MODERATE | IMPORTANT? |
| Clinical recovery day 28 (number of patients substantially improved at follow-up within 28 days) (cluster-randomised trials with modified sample size)6 | ||||||||||||
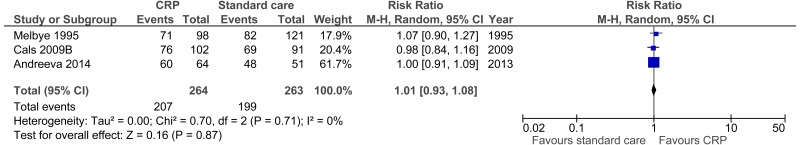
| 3 | randomised trials | seriousa | no serious inconsistency | No serious indirectness | seriousc | None |
207/264 (78.4%) | 75.8% | RR 1.01 (0.93 to 1.08) | 8 more per 1000 (from 53 fewer to 61 more) |
⨁⨁◯◯ LOW | IMPORTANT? |
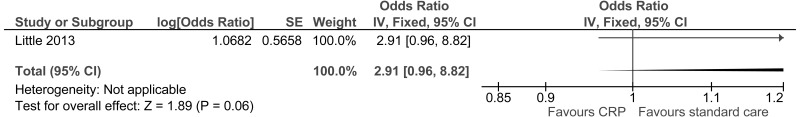
| Serious adverse events | ||||||||||||
| 1 | randomised trials | seriousa | no serious inconsistency | no serious indirectness | no serious imprecision | None |
0/129 (0%) | 0% | not pooled | not pooled |
⨁⨁⨁◯ MODERATE | IMPORTANT |
| Hospitalisation | ||||||||||||
- 1
Downgraded by 1 increment if the majority of the evidence was at high risk of bias, and downgraded by 2 increments if the majority of the evidence was at very high risk of bias.
- 2
Downgraded by 1 or 2 increments because the point estimate varies widely across studies, unexplained by subgroup analysis.
- 3
Downgraded by 1 increment if the confidence interval crossed 1 MID or by 2 increments if the confidence interval crossed both MIDs.
- 4
Downgraded by 1 or 2 increments because the point estimate varies widely across studies, unexplained by subgroup analysis.
- 5
Downgraded by 1 increment because majority of evidence had indirect outcomes, and downgraded by 2 increments if the majority of the evidence had very indirect outcomes (this is a surrogate outcome for ED attendance).
- 6
The unit of analysis was the individual patient. For cluster-RCTs the Cochrane review authors adjusted the unit of analysis by calculating the design effect to modify sample sizes and inflate confidence intervals (CIs) accordingly
Table 11Clinical evidence profile: Point of care BNP testing versus standard care
| Quality assessment | No of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | BNP | standard care | Relative (95% CI) | Absolute | ||
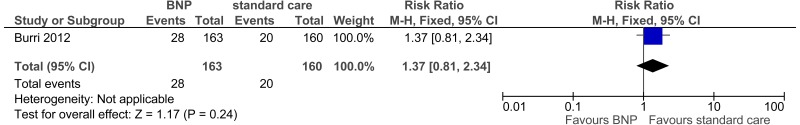
| Hospitalisation within 3 months | ||||||||||||
| 1 | randomised trials | no serious risk of bias | no serious inconsistency | serious indirectnessd | seriousa | None |
28/163 (17.2%) | 12.5% | RR 1.37 (0.81 to 2.34) | 46 more per 1000 (from 24 fewer to 167 more) |
⨁⨁◯◯ LOW | IMPORTANT |
| Hospitalisation within 12 months | ||||||||||||
| 1 | randomised trials | no serious risk of bias | no serious inconsistency | serious indirectnessd | seriousa | None |
50/163 (30.7%) | 26.3% | RR 1.17 (0.83 to 1.65) | 45 more per 1000 (from 45 fewer to 171 more) |
⨁⨁◯◯ LOW | IMPORTANT |
| Time to initiation of appropriate therapy (days) (Better indicated by lower values) | ||||||||||||
| 1 | randomised trials | no serious risk of bias | no serious inconsistency | seriousb,c | no serious imprecision | None | 163 | 160 | - | MD 11.9 lower (17.32 to 6.48 lower) |
⨁⨁⨁◯ MODERATE | IMPORTANT |
- 1
Downgraded by 1 increment if the confidence interval crossed 1 MID or by 2 increments if the confidence interval crossed both MIDs.
- 2
Downgraded by 1 increment because majority of evidence had indirect outcomes, and downgraded by 2 increments if the majority of the evidence had very indirect outcomes (this outcome was used as a surrogate outcome for lab/diagnostic turn around for result to GP).
- 3
Result not reported as a hazard ratio.
- 4
Downgraded by 1 increment because majority of evidence had indirect outcomes, and downgraded by 2 increments if the majority of the evidence had very indirect outcomes (this is a surrogate outcome for ED attendance)
Appendix G. Excluded clinical studies
Table 8Studies excluded from the clinical review
| Study | Exclusion reason |
|---|---|
| Andersen 2015 4 | Incorrect intervention. The study investigated the levels of interleukin (IL)-23 in patients with early rheumatoid arthritis and the effect of anti-tumour necrosis factor treatment on IL-23 levels. |
| Andreeva 20125 | Abstract |
| Anon 19841 | Incorrect interventions. Narrative paper. Use of serological tests in the EPI. |
| Anon 20052 | Article not in English |
| Bjerrum 20048 | Observational study |
| Bjerrum 20067 | Before-After study |
| Bjerrum 20119 | Before-After audit based study |
| Cadth 201311 | Incorrect interventions. A review of the clinical effectiveness of point of care testing technologies compared with central laboratory methods to assess patients’ white blood cell counts. |
| Cals 200716 | Study protocol |
| Cals 200812 | Study protocol |
| Cals 201315 | No outcomes of interest |
| Chandrajay 2016 18 | Incorrect study design- prospective cohort study (RCT evidence available). Incorrect intervention- evaluation of the effect of clinical validation of out of hours critical laboratory results |
| Cook 2015A 19 | Narrative review of primary care point-of-care testing and anti-bacterial use in respiratory tract infection. RCTs included in this review have already been included in our evidence review. |
| Do 201623 | Incorrect setting- primary health care centres in the community |
| Engel 201224 | Systematic review- screened for relevant references |
| Grodzinsky 200425 | Observational study (RCT data available) |
| Hanrahan 2015 26 | Incorrect intervention. The study evaluated the effect of Xpert (MTB/RIF assay to diagnose TB rapidly) either at point of care or at an off-site laboratory for diagnosis of pulmonary TB. Tests for diagnosis of TB not included intervention of interest in our protocol. |
| Holm 2007 27 | Observational study (RCT evidence available) |
| Hopstaken 2003 29 | Observational study (RCT evidence available) |
| Hopstaken 2006 28 | Observational study |
| Huang 201331 | Systematic review- screened for relevant references |
| Jakobsen 201033 | Observational study (RCT evidence available) |
| Joshi 201334 | Review paper checked for references |
| Kavanagh 201135 | Observational study (RCT evidence available) |
| Leber 2015 37 | Incorrect intervention. This study assessed rapid HIV testing which was not included as an intervention of interest in our protocol. |
| Llor 201240 | Before-After audit based study |
| Llor 201242 | Before-After audit based study |
| Llor 201341 | Cross-sectional study |
| Llor 201339 | Observational study |
| Llor 201443 | Before- After audit based study |
| Mueller 200445 | Incorrect setting (patients in Emergency department) |
| Neumark 201056 | Observational study (RCT evidence available) |
| Oosterheert 200557 | Incorrect intervention and setting. Intervention is real time polymerase chain reaction (PCR) and setting is University hospital. |
| Peters 201360 | Case control study |
| Pluddemann 201161 | Review article |
| Rebnord 2015 62 | Incorrect study design- observational study (RCT evidence available) |
| Strykowski 2015 65 | Incorrect study design- before and after study (RCT evidence available) |
Appendix H. Excluded economic studies
Table 9Studies excluded from the health economic review
- GP access to laboratory investigations - Emergency and acute medical care in ove...GP access to laboratory investigations - Emergency and acute medical care in over 16s: service delivery and organisation
Your browsing activity is empty.
Activity recording is turned off.
See more...