Context and Policy Issues
Aplastic anemia (AA) is a rare, life-threatening disorder due to either inherited or acquired bone marrow failure to produce blood cells, leading to progressive pancytopenia.1 AA affects patients of all ages, with an incidence ranging from 0.6 to 6.1 cases per million population in North America.2,3 Treatment for AA is determined by a number of factors including AA severity, age of the patient, stem cell donor availability, and access to optimal therapies. For newly diagnosed severe AA, bone marrow transplant is usually pursued in all pediatric and younger adult patients when a matched donor is available.4,5 In older adult patients and in all patients lacking a matched donor, immunosuppressive therapy (IST) with antithymocyte globulin and cyclosporine A has been the front line therapy.4,5
Eltrombopag, a thrombopoietin (TPO) agonist, has been found effective for the treatment of immune thrombocytopenia and has been emerging as a treatment option for severe AA that is not responding to immunosuppression with antithymocyte globulin and cyclosporine.6-12
This Rapid Response report aims to review the comparative clinical and cost-effectiveness of eltrombopag versus other options for the treatment AA.
Research Question
What is the clinical effectiveness of eltrombopag in the treatment of aplastic anemia?
What is the cost-effectiveness of eltrombopag in the treatment of aplastic anemia?
Key Findings
There were no studies that met the pre-specified criteria regarding the comparative clinical effectiveness and cost-effectiveness of eltrombopag for the treatment of aplastic anemia.
Methods
A limited literature search was conducted on key resources including Medline in Ovid, Embase in Ovid, PubMed, the Cochrane Library, University of York Centre for Reviews and Dissemination (CRD) databases, Canadian and major international health technology agencies, as well as a focused Internet search. No filters were applied to limit the retrieval by study type. Where possible, retrieval was limited to the human population. The search was also limited to English language documents published between January 1, 2008 and June 14, 2018.
Rapid Response reports are organized so that the evidence for each research question is presented separately.
Selection Criteria and Methods
One reviewer screened citations and selected studies. In the first level of screening, titles and abstracts were reviewed and potentially relevant articles were retrieved and assessed for inclusion. The final selection of full-text articles was based on the inclusion criteria presented in .
Exclusion Criteria
Articles were excluded if they did not meet the selection criteria outlined in , they were duplicate publications, or were published prior to 2008. Studies that examined the clinical effectiveness of eltrombopag without a comparator were excluded.
Critical Appraisal of Individual Studies
Critical appraisal was not performed as no eligible studies were identified.
Summary of Evidence
Quantity of Research Available
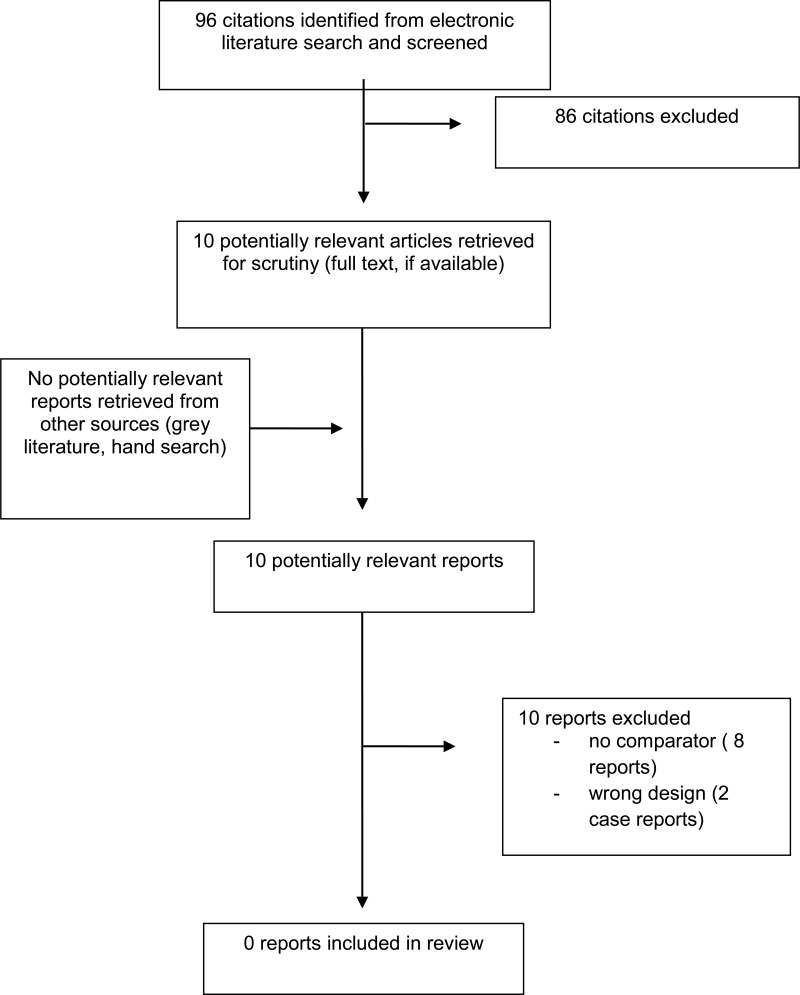
A total of 96 citations were identified in the literature search. Following screening of titles and abstracts, 86citations were excluded and 10 potentially relevant reports from the electronic search were retrieved for full-text review. No potentially relevant publication was retrieved from the grey literature search. Of these potentially relevant articles, 10 publications were excluded for various reasons, and no publications met the inclusion criteria and were included in this report. Appendix 1 presents the PRISMA flowchart of the study selection.
Summary of Findings
No relevant HTAs, SRs, MAs, RCTs, or non-RCTs on the clinical effectiveness or cost-effectiveness of eltrombopag compared to other pharmacological treatments were identified.
Conclusions and Implications for Decision or Policy Making
There were no studies that met the pre-specified criteria on the comparative clinical effectiveness or the cost-effectiveness of eltrombopag in the treatment of aplastic anemia.
In patients with refractory and severe AA, many uncontrolled observational studies consistently found that eltrombopag, alone or in combination with immunosuppressive therapies, was effective to improve hematologic response, including persistent increase of plasma TPO, with few side effects, and reduced transfusion independence.13-18 Despite being generally well-tolerated, eltrombopag may be associated with dose-dependent cutaneous toxicity19 and the presence of high eltrombopag concentrations in blood samples may cause interference in routine chemistry testing such as total cholesterol levels.20 This should be interpreted with caution, as the results are from non-comparative studies that were not eligible for inclusion in this review.
Randomized controlled trials comparing eltrombopag with other immunosuppressive therapies and stem cell transplantation are needed to reduce uncertainty regarding its clinical effectiveness. Cost-effectiveness evaluations on eltrombopag in a Canadian context in patients with severe AA are also needed.
References
- 1.
- 2.
Aplastic Anemia & Myelodysplasia Association of Canada. What are aplastic anemia and myelodysplastic syndrome and PNH?
2012;
https://www.aamac.ca/e/a_31main.cfm. Accessed 2018 Jun 29.
- 3.
- 4.
Miano
M, Dufour
C. The diagnosis and treatment of aplastic anemia: a review.
Int J Hematol. 2015;101(6):527–535. [
PubMed: 25837779]
- 5.
Willis
L, Rexwinkle
A, Bryan
J, Kadia
TM. Recent developments in drug therapy for aplastic anemia.
Ann Pharmacother. 2014;48(11):1469–1478. [
PubMed: 25184310]
- 6.
Eltrombopag: drug information. In: Post
TW, ed.
UpToDate. Waltham (MA): UpToDate; 2018:
www.uptodate.com. Accessed 2018 Jun 29.
- 7.
Scheinberg
P. Developing role of eltrombopag in the treatment of aplastic anemia. Expert Opinion on Orphan Drugs. 2018;6(3):231–235.
- 8.
Scheinberg
P. Recent advances and long-term results of medical treatment of acquired aplastic anemia: are patients cured?
Hematology/Oncology Clinics of North America. 2018. [
PubMed: 30047414]
- 9.
Dhillon
S, McCormack
PL. Eltrombopag in severe aplastic anaemia: a guide to its use in the EU. Drugs and Therapy Perspectives. 2016;32(6):232–237.
- 10.
- 11.
McCormack
PL. Eltrombopag: a review of its use in patients with severe aplastic anaemia.
Drugs. 2015;75(5):525–531. [
PubMed: 25700916]
- 12.
- 13.
- 14.
Lengline
E, Drenou
B, Peterlin
P, et al. Nationwide survey on the use of eltrombopag in patients with severe aplastic anemia: a report on behalf of the French Reference Center for Aplastic Anemia.
Haematologica. 2018;103(2):212–220. [
PMC free article: PMC5792265] [
PubMed: 29170252]
- 15.
Hwang
YY, Gill
H, Chan
TSY, Leung
GMK, Cheung
CYM, Kwong
YL. Eltrombopag in the management of aplastic anaemia: real-world experience in a non-trial setting.
Hematology. 2018:1–6. [
PubMed: 29303047]
- 16.
- 17.
Bart-Smith
EE, Kordasti
S, Kulasekararaj
AG, Richardson
D, Mufti
GJ, Marsh
JC. Successful treatment of aplastic anaemia associated with HIV infection with eltrombopag: implications for a possible immunomodulatory role.
AIDS. 2014;28(18):2786–2788. [
PubMed: 25333665]
- 18.
Olnes
MJ, Scheinberg
P, Calvo
KR, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia.[Erratum appears in N Engl J Med. 2012 Jul 19;367(3):284].
N Engl J Med. 2012;367(1):11–19. [
PMC free article: PMC3422737] [
PubMed: 22762314]
- 19.
Kazemi
T, Martin
S, Worswick
S. Morbilliform eruption related to eltrombopag: Emerging data on the cutaneous toxicity of thrombopoietin receptor agonists.
Dermatol Online J. 2016;22(6). [
PubMed: 27617608]
- 20.
Gounden
V, Zhao
Z. Eltrombopag interference in routine chemistry testing.
Ann Clin Biochem. 2016;53(Pt 5):611–614. [
PubMed: 26491115]
Appendix 1. Selection of Included Studies
About the Series
CADTH Rapid Response Report: Summary with Critical Appraisal
Funding: CADTH receives funding from Canada’s federal, provincial, and territorial governments, with the exception of Quebec.
Suggested citation:
Eltrombopag for the treatment of aplastic anemia: A review of clinical and cost-effectiveness. Ottawa: CADTH; 2018 July. (CADTH rapid response report: summary with critical appraisal).
Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services.
While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up-to-date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. The views and opinions of third parties published in this document do not necessarily state or reflect those of CADTH.
CADTH is not responsible for any errors, omissions, injury, loss, or damage arising from or relating to the use (or misuse) of any information, statements, or conclusions contained in or implied by the contents of this document or any of the source materials.
This document may contain links to third-party websites. CADTH does not have control over the content of such sites. Use of third-party sites is governed by the third-party website owners’ own terms and conditions set out for such sites. CADTH does not make any guarantee with respect to any information contained on such third-party sites and CADTH is not responsible for any injury, loss, or damage suffered as a result of using such third-party sites. CADTH has no responsibility for the collection, use, and disclosure of personal information by third-party sites.
Subject to the aforementioned limitations, the views expressed herein are those of CADTH and do not necessarily represent the views of Canada’s federal, provincial, or territorial governmentsor any third party supplier of information.
This document is prepared and intended for use in the context of the Canadian health care system. The use of this document outside of Canada is done so at the user’s own risk.
This disclaimer and any questions or matters of any nature arising from or relating to the content or use (or misuse) of this document will be governed by and interpreted in accordance with the laws of the Province of Ontario and the laws of Canada applicable therein, and all proceedings shall be subject to the exclusive jurisdiction of the courts of the Province of Ontario, Canada.