Abbreviations
- AGREE II
Appraisal of Guidelines for Research and Evaluation II
- AMSTAR-2
A MeaSurement Tool to Assess systematic Reviews
- BL-M
Beta-lactam/macrolide combinations
- BL-FQ
Beta-lactam/fluoroquinolone combination
- CAP
Community-acquired pneumonia
- CRD
Centre for Reviews and Dissemination
- CI
Confidence Interval
- COPD
Chronic Obstructive Pulmonary Disease
- FQs
Fluoroquinolones
- GRADE
Grading of Recommendations Assessment, Development, and Evaluation
- HAP
Hospital-acquired pneumonia
- ICER
Incremental cost-effectiveness ratio
- ICU
Intensive care unit
- ITT
Intention-to-treat
- MA
Meta-analysis
- MSSA
Methicillin-sensitive S. aureus
- NICE
National Institute for Health and Care Excellence
- OR
Odds Ratio
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RCT
Randomized controlled trial
- RR
Risk Ratio
- SR
Systematic Review
- SUCRA
surface under the cumulative ranking
- UK
United Kingdom
- US
United States
- VA/DoD
Veterans Affairs and Department of Defense
- VAP
Ventilator-associated pneumonia
Context and Policy Issues
Fluoroquinolones (FQs) are a popular class of antibiotics used to treat a variety of infections, such as respiratory tract infections, and include medications such as ciprofloxacin, levofloxacin, moxifloxacin, ofloxacin and delafloxacin.1 The use of FQs is associated with common adverse events, such as gastrointestinal and central nervous system toxicities, as well as other adverse events, including rashes and other allergic reactions, tendinitis and tendon rupture, QT prolongation, hypoglycemia and hyperglycemia, and hematologic toxicity. Notably, several FQs have been withdrawn from the market due to adverse events;1 for instance, grepafloxacin was withdrawn from the worldwide market in 1999 due to seven fatal cardiovascular events; trovafloxacin was withdrawn from European markets and the United States (US) Food and Drug Administration (FDA) heavily restricted its use in 1999 due to reports of liver failure; gatifloxacin was removed from the market in 2006 following a study published on dysglycemia side effects; temafloxacin was withdrawn from the American and some European markets shortly following its approval in 1992 due to severe adverse reactions, including hemolytic anemia, acute renal failure, hepatotoxicity and three deaths; sparfloxacin was withdrawn from American markets in 2001 due to QT prolongation and photoxicity; alatrofloxacin was withdrawn worldwide in 2006 due to associations with liver toxicity and death.2
The serious nature of these adverse events has garnered much attention and the use of FQs for treatment of uncomplicated infections has been revisited by a number of countries. For instance, in 2016, the FDA stated that the risk of serious side effects that may be disabling and potentially permanent, although uncommon, generally outweighed the benefits for patients with acute sinusitis, acute bronchitis, and uncomplicated urinary tract infections, who have other treatment options.3 Following the actions of the FDA, Health Canada undertook a review of FQs (including ciprofloxacin, levofloxacin, moxifloxacin, norfloxacin and ofloxacin), which examined the reported persistent and disabling side effects, such as tendonitis, peripheral neuropathy, and central nervous system disorders. In January 2017, Health Canada posted their Summary Safety Review, which concluded that these adverse events may be persistent and disabling in rare cases, and that Health Canada would work with the manufacturers to update product safety information to reflect this potential risk.4 In addition, the United Kingdom (UK) Medicines & Healthcare products Regulatory Agency noted new restrictions and use for FQs due to the rare reports of these disabling and potentially long-lasting side effects; FQs are authorized only for use in serious, life-threatening bacterial infections and treatment should be discontinued at the first sign of tendinitis.5 In light of these recent concerns, questions remain around the use of FQs for various indications.
This report aims to identify and synthesize the evidence describing the clinical effectiveness, cost-effectiveness, and evidence-based guidelines for the treatment of respiratory tract infections, including pneumonia and chronic obstructive pulmonary disease (COPD), using FQs.
Research Questions
What is the clinical effectiveness of fluoroquinolones for the treatment of respiratory tract infections?
What is the cost-effectiveness of fluoroquinolones for the treatment of respiratory tract infections?
What are the evidence-based guidelines for the use of fluoroquinolones for the treatment of respiratory tract infections?
Key Findings
Overall, 15 publications met the eligibility criteria and were included in this report. Ten of the included publications were systematic reviews/meta-analyses; nine of which examined fluoroquinolone use in patients with pneumonia, while one included systematic review/meta-analysis examined fluoroquinolone use in patients with chronic obstructive pulmonary disease. The systematic reviews examining pneumonia were of variable quality and reported inconsistent findings in terms of mortality and clinical response or clinical failure. The systematic review examining chronic obstructive pulmonary disease reported that fluoroquinolones had high clinical cure rates with median rates of adverse effects, although the limited evidence describing this indication is an important consideration.
One cost-effectiveness study related to pneumonia was identified and no evidence regarding the cost-effectiveness of fluroquinolone use in chronic obstructive pulmonary disease exacerbations was identified. The one included cost-effectiveness study concluded that beta-lactam monotherapy is the preferred empirical treatment for patients hospitalized with community acquired pneumonia in the Netherlands; however the limited volume and generalizability of this evidence is an important consideration.
Four guidelines were identified; two informing the treatment of pneumonia and two informing the treatment of chronic obstructive pulmonary disease. The pneumonia-related guidelines stated the following recommendations: health professionals should not routinely offer patients with low-severity community-acquired pneumonia a fluoroquinolone or a dual antibiotic therapy, and a regimen including levofloxacin, among other antibiotics, that has coverage for methicillin-sensitive S. aureus, is recommended for the treatment of clinically suspected ventilator-associated pneumonia and hospital-acquired pneumonia. The chronic obstructive pulmonary disease related guidelines recommended that fluoroquinolones should be used for specific populations of patients such as those who are critically ill, or at higher risk of treatment failure. Low quality evidence was generally utilized in the formulation of the guideline recommendations.
The clinical effectiveness, cost-effectiveness, and appropriate guidelines for use of fluoroquinolones in respiratory tract infections, such as pneumonia and chronic obstructive pulmonary disease, remain unclear due to the quality, quantity, and variable results of the identified evidence.
Methods
Literature Search Methods
A limited literature search was conducted on key resources including OVID Medline, the Cochrane Library, University of York Centre for Reviews and Dissemination (CRD) databases, Canadian and major international health technology agencies, as well as a focused Internet search. Methodological filters were applied to limit retrieval to health technology assessments, systematic reviews, meta-analyses, randomized controlled trials, non-randomized studies, economic studies, and guidelines. For randomized control trials and non-randomized studies, the search was focused to main concepts appearing in the title or subject heading. Where possible, retrieval was limited to the human population. The search was also limited to English language documents published between January 1, 2014 and March 28, 2019.
Selection Criteria and Methods
One reviewer screened citations and selected studies and guidelines. In the first level of screening, titles and abstracts were reviewed and potentially relevant articles were retrieved and assessed for inclusion. The final selection of full-text articles was based on the inclusion criteria presented in .
Exclusion Criteria
Articles were excluded if they did not meet the selection criteria outlined in , they were duplicate publications, or were published prior to 2014. Guidelines with unclear methodology were also excluded.
Critical Appraisal of Individual Studies
The included systematic reviews were critically appraised by one reviewer using A MeaSurement Tool to Assess systematic Reviews (AMSTAR-2),6 economic studies were assessed using the Drummond checklist,7 and guidelines were assessed with the Appraisal of Guidelines for Research and Evaluation II (AGREE II) instrument.8 Summary scores were not calculated for the included studies; rather, a review of the strengths and limitations of each included study were described narratively.
Summary of Evidence
Quantity of Research Available
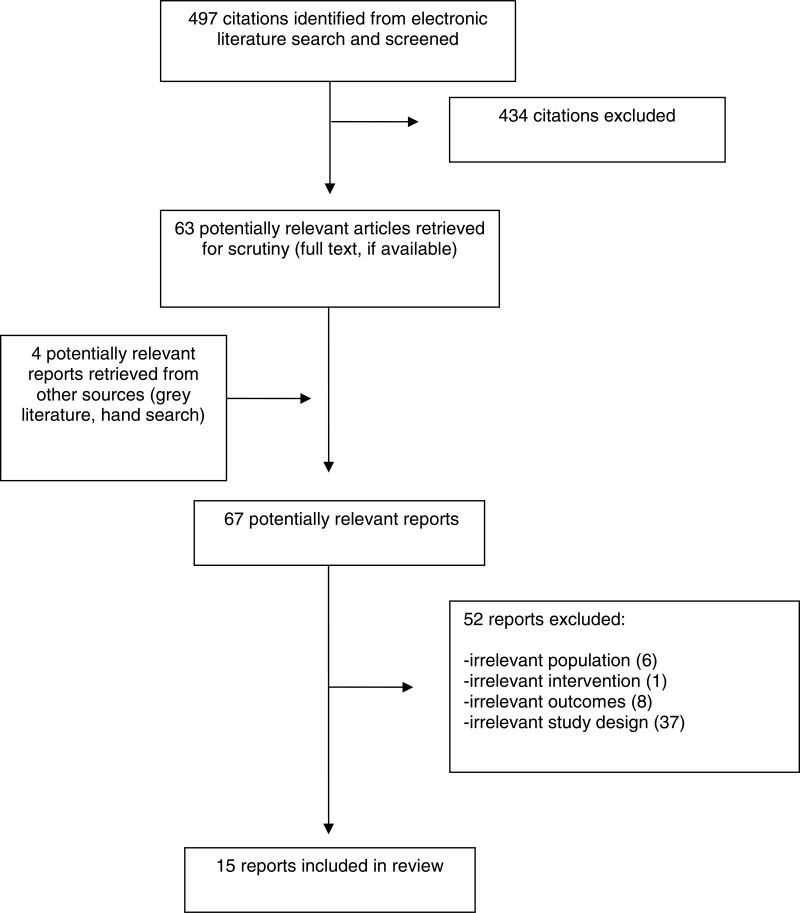
A total of 497 citations were identified in the literature search. Following screening of titles and abstracts, 434 citations were excluded and 63 potentially relevant reports from the electronic search were retrieved for full-text review. Four potentially relevant publications were retrieved from the grey literature search for full text review. Of these 67 potentially relevant articles, 52 publications were excluded for various reasons, and 15 publications met the eligibility criteria and were included in this report. Appendix 1 presents the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)9 flowchart of the study selection.
Additional references of potential interest are provided in Appendix 6.
Summary of Study Characteristics
Overall, 15 publications met the eligibility criteria and were included in this report: ten systematic reviews,10–19 one economic evaluation,20 and four guidelines.21–24
Additional details regarding the characteristics of included publications are provided Appendix 2.
Study Design
Systematic Reviews & Meta-Analysis
Ten systematic reviews/meta-analyses (SR/MAs) were eligible for inclusion in this report. Nine of these examined study populations with pneumonia, and one examined study populations with acute exacerbations of COPD. Two of the SR/MAs were published in 2014,18,19 one in 2015;17 five were published in 2017,12–16 one was published in 201811 and one was published in 2019.10
Pneumonia
The SR/MA of the efficacy and safety of nemonoxacin versus levofloxacin searched for relevant RCTs until September 2018, and three RCTs published between 2010 and 2017 were included.10
A SR/MA comparing atypical coverage (a FQ or combination of a macrolide/doxycycline with a beta-lactam) to a regimen without atypical antibiotic coverage (beta-lactam monotherapy), conducted a search of RCTs that was completed without a date restriction through to December 2016. Five RCTs were included in the SR/MA, and these were published between 1998 and 2014.12
A SR/MA comparing beta-lactam/macrolide combination (BL-M) and beta-lactam/fluoroquinolone combination (BL-FQ) treatments searched articles published prior to December 2015, and included eight trials: seven observational cohort studies, and one RCT. The included studies were published between 1994 and 2013.13
In a SR from the Cochrane Collaboration, RCTs examining the efficacy and safety of various antibiotic treatments (antibiotic versus placebo, and antibiotic versus antibiotic) were sought. The search was an update of a 2009 Cochrane Review, and included publications from 2009 up to March 2014.18
A SR/MA comparing FQs or macrolides alone versus in combination with beta-lactam sought and included RCTs. The authors conducted a literature search with no date restrictions until December 2014. Sixteen RCTs were included in the SR/MA, which were published between 1996 and 2015.17
A SR/MA examined macrolide versus nonmacrolide antibiotics, using a search with no date restriction through to May 2013. The study selection included both RCTs and observational cohort study designs. No RCTs were included; however, 28 observational cohort studies were included in the SR/MA.19
Another SR/MA compared macrolides, beta-lactams, or FQs, either used as monotherapy or in combination. The authors searched PubMed and Scopus until November 2015, and included 50 studies of various designs, such as prospective studies, retrospective studies and RCTs. The included papers were published between 1999 and 2015.14
A SR/MA compared the effectiveness of FQs and macrolides in combination with beta-lactams (BL-FQ versus BL-M) utilizing observational cohort studies, non-randomized clinical trials and RCTs. The authors searched PubMed, Scopus and Cochrane Library databases until November 2015, and 17 studies were identified (11 retrospective and six prospective studies). The papers included in this SR/MA were published between 2001 and 2015.15
Another SR/MA compared ceftriaxone combination therapy to respiratory FQ monotherapy. The authors searched PubMed, EMBASE and Cochrane Central Register of Controlled Trials to identify studies published before September 2017. The authors included nine RCTs published between 2002 and 2013.11
COPD
One SR/MA examined the use of antibiotics for acute exacerbations of COPD. The authors searched PubMed, EMBASE, and Cochrane databases to identify studies published until September 2016. Overall, 19 RCTs were included that assessed 17 types of antibiotics, including FQs.16
The overlap of included studies between the SRs is detailed in Appendix 5.
Cost-Effectiveness
Pneumonia
One study compared the cost-effectiveness of beta-lactam monotherapy, BL-M, and FQ monotherapy in adult patients hospitalized to non-intensive care unit wards with CAP.20 Both cost-minimization and cost-effectiveness analyses were applied to the data from a cluster-randomized cross-over trial using 30 and 90 day time horizons. Three perspectives were employed, including third payer (both reduced and full), and societal perspectives.20
Guidelines
Two guidelines for the treatment of pneumonia and two guidelines for the treatment of COPD exacerbations are included in this report.
Pneumonia
The National Institute for Health and Care Excellence (NICE) 2014 Pneumonia: Diagnosis and management of community- and hospital-acquired pneumonia in adults guideline is intended to be relevant to the management of most patients with CAP or hospital-acquired pneumonia (HAP). Systematic literature searches were undertaken according to the NICE guidelines manual 2012, and various study designs, such as RCTs, observational studies, diagnostic and prognostic studies, and qualitative studies were included. Included studies were critically appraised and, where appropriate, Grading of Recommendations Assessment, Development, and Evaluation (GRADE) evidence assessments were applied. The recommendations were drafted based on the interpretation of evidence by the Guideline Development Group and the guideline was subject to a six-week consultation and feedback process.
The Management of Adults with Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society guideline is intended for use by the healthcare professionals who care for patients at risk for HAP and ventilator-associated pneumonia (VAP). Systematic literature searches to answer predefined research questions that were deemed to be of importance to the guideline committee were conducted. The recommendations were labeled as ‘weak’ or ‘strong’ according to the GRADE approach, and recommendations were made according to the available evidence. In cases where there was lower-quality evidence, strong recommendations were sometimes made when panelists believed that most individuals and well-informed clinicians would agree with a course of action. The guideline underwent external peer review.
COPD
The NICE 2018 Chronic obstructive pulmonary disease (acute exacerbation): antimicrobial prescribing guideline is intended for health professionals and people with COPD and their families and carers. Specifically, the recommendations are geared towards adults with COPD and acute exacerbations. A systematic literature search identified the evidence that was of top priority to the guideline committee, including SRs and RCTs. Although the GRADE methodology was used, the recommendations were not accompanied by strength ratings and the methods used to develop and evaluate the recommendations were not included in the report. It should be noted that while an Evidence Summary document was publicly available, a full guideline document was not available. At the time of this report, NICE is reviewing their recommendations relating to FQ antibiotic use in pneumonia due to safety concerns.21
The Veterans Affairs and Department of Defense (VA/DoD) Clinical Practice Guideline for the Management of Chronic Obstructive Pulmonary Disease guideline is intended for use by primary care providers for the treatment of COPD in adults. The guideline was based on a SR addressing key questions and included other SRs, RCTs, and cohort studies. Recommendations were developed during a face-to-face meeting of members and the recommendations were assessed according to GRADE methodology; recommendations were labelled as ‘strong for,’ ‘weak for,’ ‘weak against,’ and ‘strong against’ with the weak options representing conditional or qualified recommendations.
Country of Origin
Among the included SRs, the country of origin for the first authors included: Taiwan,10 Saudi Arabia,12 Korea,13, Canada,18,19 Israel,17 China11,16 and Greece.14,15 The included cost-effectiveness study was conducted in the Netherlands.20 The included guidelines are intended for practice in the UK,21,22 and the US.23,24
Patient Population
Systematic Reviews & Meta-Analysis
Pneumonia
Four SR/MAs specified that the included studies examined patients with CAP who had been hospitalized.12,14,15,19 The SR/MA comparing atypical coverage (a FQ or combination of a macrolide/doxycycline with a beta-lactam) to a regimen without atypical antibiotic coverage (beta-lactam monotherapy) included studies of hospitalized adult patients with CAP; as reported by the authors, the identified studies were comprised of patients with mild-moderate pneumonia and moderately severe pneumonia.12 The SR/MA examining factors associated with mortality included studies that enrolled hospitalized patients with CAP who were treated with either macrolides, beta-lactams, or FQs (either as monotherapy or in combination).14 Similarly, another SR/MA included studies which enrolled adult patients hospitalized with CAP who had been treated with a BL-M combination or a BL-FQ combination.15 The SR/MA comparing 30-day mortality in macrolides and nonmacrolides included critically ill patients (e.g. those who had been admitted to an ICU) with CAP. The average age range of patients was 58–78 years, and 14–49% were women. The authors reported that the included observational cohort studies tended to be smaller and multicenter.19
Other SR/MAs reported that the included studies enrolled patients from various settings. The SR/MA comparing BL-M and BL-FQ treatments identified observational cohort studies and RCTs that included adult patients with severe CAP who had been exposed to BL-M or BL-FQ therapy. The identified studies looked at a variety of patients diagnosed with CAP, including patients admitted to the intensive care unit (ICU), patients with severe CAP, intubated patients, severe bacteremic CAP, and elderly patients.13 A SR/MA comparing FQs or macrolides alone versus in combination with beta-lactams included studies that enrolled adults with CAP from inpatient and outpatient settings.17
Whether patients were treated as inpatients or outpatients was not specified in other SR/MAs. The SR/MA that examined the efficacy and safety of nemonoxacin versus levofloxacin identified only RCTs which treated patients with CAP. All of the included studies were double-blind, multicenter studies with adult patients with CAP. The three included studies had study sites in South Africa/Taiwan, China, and China/Taiwan.10 The SR/MA comparing ceftriaxone combination therapy to respiratory FQ monotherapy included studies which enrolled adults over the age of 18 with CAP. The authors noted that among the identified studies, the majority of patients had moderate and/or severe CAP.11
Finally, the Cochrane Review of antibiotic treatments for CAP included 11 RCTs with both adolescent and adult outpatients with CAP. Eight of the included RCTs examined adult patients, while three reported the inclusion of adolescents.18
COPD
The SR/MA of antibiotic use for acute exacerbations of COPD included patients with COPD, however patients with stable COPD or those who underwent treatment for prophylactic exacerbations were not included. The authors reported that more men than women were included in the study populations, although one of the included studies did not mention the ratio of men to women.16
Cost-Effectiveness
Pneumonia
The cost-minimization and cost-effectiveness study included in this report examined adult patients recruited for a trial who were hospitalized to non-intensive care unit wards with a clinical diagnosis of CAP in the Netherlands.20
Guidelines
Pneumonia
Of the guidelines related to pneumonia, the NICE 2014 guideline is intended for various healthcare providers for the diagnosis and management of CAP or HAP in adults over the age of 18 years,21 and the Infectious Diseases Society of America and American Thoracic Society guideline is intended for various healthcare providers for the diagnosis and management of adults with HAP and VAP.23
COPD
Of the guidelines related to COPD, the NICE 2018 guideline is intended for health professionals, patients with COPD and acute exacerbations, and their families and carers,22 and the VA/DoD 2014 guideline is intended for primary care providers treating adult patients with COPD who are eligible for care in the VA or DoD healthcare delivery systems.
Only the recommendations related to antibiotic treatment, and specifically treatment with FQs, are relevant for this Rapid Response.
Interventions and Comparators
Systematic Reviews & Meta-Analysis
Pneumonia
The nine included SR/MAs that examined patients with CAP identified studies that examined a variety of antibiotics, including monotherapy and combination therapies.10–15,17–19
Four of the included SR/MAs compared various antibiotic treatments used as monotherapies.10,11,18,19 The SR/MA of the efficacy and safety of nemonoxacin versus levofloxacin identified three RCTs: two compared nemonoxacin 750 mg, nemonoxacin 500 mg, and levofloxacin 500 mg while the other study compared nemonoxacin 500 mg or levofloxacin 500 mg for 7–10 days.10 The Cochrane Review of antibiotic treatments for CAP examined the efficacy and safety of various antibiotic treatments (antibiotic versus placebo, and antibiotic versus antibiotic); all included studies compared antibiotics (no placebo studies were included in the review). The antibiotics studied varied, and included: clarithromycin versus amoxicillin, erythromycin versus clarithromycin, azithromycin and levofloxacin, clarithromycin versus azithromycin microspheres, clarithromycin versus telithromycin, azithromycin microspheres versus levofloxacin, telithromycin versus levofloxacin, cethromycin versus clarithromycin, solithromycin versus levofloxacin, and nemonoxacin versus levofloxacin.18 The SR/MA comparing macrolides and nonmacrolides and short term mortality in critically ill patients with CAP included studies that utilized a macrolide antibiotic compared to a nonmacrolide antibiotic. Though few details on the interventions were provided, subgroup analyses explored monotherapy as well as combination therapies (BL-M and BL-FQ).19 Another SR/MA of CAP compared the safety and efficacy of ceftriaxone combination therapy to respiratory FQ monotherapy, with levofloxacin and moxifloxacin.11
Two of the included SR/MAs compared antibiotics alone versus in combination with beta-lactam.14,17 In the SR/MA comparing FQs or macrolides alone versus in combination with beta-lactams, nine of the included studies compared FQ monotherapy to BL-M, three trials compared FQ monotherapy to a BL-FQ, and four trials compared macrolide monotherapy to BL-M.17 The SR/MA examining factors associated with mortality of hospitalized patients with CAP, who were treated with either macrolides, beta-lactams, or FQs (either as monotherapy or in combination) listed the number of patients in each study treated with a monotherapy (beta-lactam, macrolide or FQ) or combination therapy (BL-M or BL-FQ) did not provide details (e.g. dosing) of the interventions utilized in the included studies.14
Finally, three of the included SR/MAs looked at combination therapies, where FQs were used in combination with a beta-lactam.12,13,15 One SR/MA compared atypical coverage (a FQ or combination of a macrolide/doxycycline with a beta-lactam) to a regimen without atypical antibiotic coverage (beta-lactam monotherapy).12 The remaining two SR/MAs compared BL-M and BL-FQ treatments13,15
COPD
The SR/MA related to COPD examined the use of 17 antibiotics utilized for acute exacerbations including: amoxicillin, amoxicillin-clavulanic acid, ampicillin-sulbactam, azithromycin, cefaclor, cefuroxime, ciprofloxacin, clarithromycin, dirithromycin, doxycycline, levofloxacin, moxifloxacin, ofloxacin, prulifloxacin, sparfloxacin, trimethoprim-sulfamethoxazole, and zabofloxacin. Authors utilized a network-meta analysis to compared antibiotic regimens.16
Cost-Effectiveness
Pneumonia
The cost-minimization and cost-effectiveness study compared three treatment arms: beta-lactam monotherapy (which was considered to be the preferred treatment in the Netherlands), BL-M, and FQ monotherapy. Further detail on the dosing and duration of treatment was not available.20
Guidelines
Pneumonia
The NICE 2014 guideline considered pharmacological interventions such as antibiotic treatment, including when to start treatment, which antibiotic or combination of antibiotics, and duration of treatment.21 The Infectious Diseases Society of America and American Thoracic Society guideline considered interventions such as various diagnosis methods and treatment of VAP and HAP, including treatment regimens, dosing, and length of treatment.23
COPD
The NICE 2018 guideline sets out an antimicrobial prescribing strategy for acute exacerbations of COPD, including antibiotic choice.22 The VA/DoD 2014 guideline covered interventions such as inhaled and systemic pharmacologic treatments and non-pharmacologic treatments used in acute management and maintenance management of COPD.24
Outcomes
Systematic Reviews & Meta-Analysis
Pneumonia
In five of the included SR/MAs the primary outcome was mortality.13–15,17,19 The primary outcomes of the SR/MA comparing BL-M and BL-FQ treatment regimens for patients with severe CAP were mortality and length of stay.13 In the SR/MA comparing FQs or macrolides alone versus in combination with beta-lactams the primary outcome was 30-day all-cause mortality; the secondary outcomes reported were clinical failure, treatment discontinuation, microbiological failure, any adverse events, and diarrhea. 17 The primary outcome of the SR/MA comparing macrolides and nonmacrolides in critically ill patients with CAP was short term (in-hospital, ICU, 28 or 30-day) mortality.19 One SR/MA examined factors associated with mortality of hospitalized patients with CAP, who were treated with macrolides, beta-lactams, or FQs (either as monotherapy or in combination).14 Another SR/MA examined mortality of hospitalized patients with CAP, who were treated with either a BL-M or a BL-FQ.15
The other four SR/MAs examined clinical cure or clinical failure.10–12,18 The primary outcome in the SR/MA of the efficacy and safety of nemonoxacin versus levofloxacin was the overall clinical cure rate, and secondary outcomes were microbiologic response rate and adverse events.10 The primary outcome in the SR/MA comparing atypical coverage (a FQ or combination of a macrolide/doxycycline with a beta-lactam) to a regimen without atypical antibiotic coverage (beta-lactam monotherapy) was clinical failure; secondary outcomes included mortality, bacteriologic failure, and adverse events.12 In the Cochrane Review of antibiotic treatments for CAP the primary outcome of interest was test-of-clinical cure/clinical response. Secondary outcomes included radiologic response, bacteriologic response, adverse events, hospitalization and mortality.18 The SR/MA comparing ceftriaxone combination therapy to respiratory FQ monotherapy examined the safety and efficacy of these treatments. The primary outcome was treatment success, defined at the test-of-cure visit based on clinically evaluable and intention-to-treat (ITT) populations. Secondary outcomes included drug-related adverse events and microbiological treatment success.11
COPD
The SR/MA that examined antibiotic use in patients with acute exacerbations of COPD examined clinical cure rate and rate of adverse events as primary outcomes. Secondary outcomes included microbiological response rate, relapse of exacerbations, and mortality.16
Cost-Effectiveness
Pneumonia
The reported outcomes of the cost-effectiveness study included crude average costs (cost of illness), and incremental cost-effectiveness ratio (ICER).20
Guidelines
Pneumonia
The NICE 2014 guideline considered outcomes such as mortality, hospital admission, length of hospital stay, clinical cure, health-related quality of life, hospital readmission, C. difficile, withdrawal due to adverse events, and complications for intervention-related research questions.21 The Infectious Diseases Society of America and American Thoracic Society guideline did not explicitly state the guideline committee’s various outcomes of interest, although it was clearly stated that outcomes of interest were identified a priori and were agreed upon and rated for importance for decision making.23
COPD
The NICE 2018 guideline listed various outcomes of interest, however the following outcomes were identified as critically important by the guideline committee: time to clinical cure, reduction in symptoms, rate of complications, health and social care utilization, thresholds for antimicrobial treatment.22 The VA/DoD 2014 guideline considered outcomes such as quality of life, morbidity, dyspnea, functional capacity, exacerbation rate and/or severity, mortality, harms, healthcare utilization, and diagnostic test accuracy.24
Summary of Critical Appraisal
Critical appraisal was completed for each of the included publications in this report. Additional details regarding the critical appraisal of included publications are provided Appendix 3.
Systematic Reviews and Meta-analyses
A number of strengths of the SR/MAs were identified through the critical appraisal process. The research questions and inclusion criteria were clearly stated in all studies,10–19 and study selection and data extraction were performed in duplicate for the majority of included studies,10,12–15,17–19 although it was not stated if the study selection process11 or the data extraction process16 was completed in duplicate for two studies. For the majority of SR/MAs, authors performed risk of bias assessments10–13,15,17–19 and provided a methodological description for the meta-analyses.10,12–15,17,19 In addition, four studies examined potential publication bias,12,14,15,19 two reported the funding of included studies,12,18 and one study clearly stated that a written protocol was established prior to the conduct of the review.19
The following limitations of the included SR/MAs were identified through critical appraisal: eight studies did not explicitly state that a protocol was developed prior to the conduct of the review, 10–17 eight studies did not discuss the selection of study designs for inclusion,10–13,15,17–19 eight studies did not provide the list of excluded studies,10,11,13–17,19 and eight studies did not provide funding details for included studies.10,11,13–15,17,19 One study did not report if a risk of bias assessment was conducted,14 and similarly four studies did not account for risk of bias when interpreting the results.13,14,17,19
Cost-Effectiveness
The critical appraisal of the included pneumonia-related cost-effectiveness study found that the use of multiple perspectives, appropriate discounting of productivity losses, and the potential generalizability of results were strengths of the study. Limitations included the use of self-reported data, the potential introduction of uncertainty related to missing data, and cost differences in favor of FQs that may have been inflated by physician tendency to begin with oral FQ therapy.20
Guidelines
All four of the included guidelines clearly defined the objectives, questions, and populations of interest, as well as the target users of the guidelines.21–24 All guidelines presented recommendations that were specific and easily identifiable, and clearly presented options for the management of the condition.21–24 The rigour of development for the reviews used to inform the guidelines was well reported, including details on the search methods, criteria for selecting evidence, and strengths and limitations of the body of evidence. Similarly, all guidelines considered the benefits and risks when formulating decisions and explicit links between the recommendations and supporting evidence. In addition to the above identified strengths, the following limitations were also identified. The applicability of the guidelines was not well described, with the majority of guidelines not reporting barriers to application, providing tools or advice on putting recommendations into practice, considering the resource implications of doing so, and presenting monitoring or auditing criteria.
Summary of Findings
The summary of findings below is presented according to the research questions posed by this report. Appendix 4 presents a table of the main study findings and authors’ conclusions.
Clinical Effectiveness of Fluoroquinolones
Nine SR/MAs were identified which examined the clinical effectiveness and safety of FQs in the treatment of patients with pneumonia;10–15,17–19 and one systematic review was identified which examined the clinical effectiveness and safety of FQs in the treatment of patients with COPD.16
Pneumonia
The SR/MA comparing BL-M and BL-FQ found that among patients with severe CAP, BL-M combination therapy may be more effective in reducing overall mortality (overall rates of morality in the BL-M and BL-FQ groups were 19.4% versus 26.8%, respectively (Odds Ratio (OR) 0.68; 95% CI: 0.49 to 0.94, P=0.02) and length of stay in the hospital (mean difference −3.05 days; 95% CI: −6.01 to −0.09, P=0.04); however, the authors note that these results are limited by the high risk of bias in the identified studies due to methodological limitations.13
In the SR/MA comparing monotherapy (FQs or macrolides alone) versus BL-M or BL-FQ in there were no statistically significant differences reported in the primary outcome, all-cause 30-day mortality, across the comparators. For the secondary outcomes, FQ monotherapy resulted in significantly fewer clinical failures (RR 0.72; 95% CI: 0.57 to 0.91), treatment discontinuations (RR 0.65; 95% CI: 0.54 to 0.78), and diarrhea (RR 0.13; 95% CI: 0.05–0.34) compared to BL-M combinations. The authors noted that the addition of a beta-lactam to FQ did not improve outcomes.17
The SR/MA comparing macrolides and nonmacrolides (i.e. BL-FQ) and 30-day mortality in critically ill patients with CAP found that risk of mortality was lower with macrolide use compared to nonmacrolide use (21% versus 24%; RR 0.82; 95% CI: 0.70–0.79, P= 0.02); however the authors note there was substantial heterogeneity (I2 = 63%). In a subgroup analysis the authors reported reduced mortality in patients treated with a BL-M compared to patients treated with a BL-FQ (20% versus 23%; RR 0.83; 95% CI: 0.67–1.03, P = 0.09.19
The SR/MA examining factors associated with mortality of hospitalized patients with CAP, who were treated with either macrolides, beta-lactams, or FQs (either as monotherapy or in combination) concluded that, due to considerable heterogeneity, specific recommendations for the use of one specific antibiotic over another could not be formulated However, the authors reported that any monotherapy was not significantly associated with higher mortality as compared to any combination therapy (RR 1.14; 95% CI: 0.99 to 1.32). Beta-lactam monotherapy was associated with higher mortality than BL-M combination in the primary analysis (RR 1.32; 95% CI: 1.12 to 1.56,) and in most sensitivity analyses, while there was no statistically significant difference in mortality between FQ monotherapy and BL-M combination (RR 0.98; 95% CI: 0.78 to 1.23).14
An SR/MA which examined mortality of hospitalized patients with CAP, who were treated with either a BL-M combination or a BL-FQ combination, noted that in the absence of RCT data no recommendations could be made for or against the studied regimens based on the low quality evidence from the included retrospective and prospective studies, the authors reported a non-significant difference between the two regimens.15
The authors of the SR/MA that examined the efficacy and safety of two FQs, nemonoxacin versus levofloxacin, found that overall nemonoxacin had a clinical cure rate similar to levofloxacin in the treatment of CAP (OR 1.05; 95% CI: 0.67 to 1.64).10 The authors also reported that both nemonoxacin versus levofloxacin had similar clinical responses against Streptococcus pneumoniae, Haemophilus spp., Staphylococcus aureus, and atypical pathogens. When examining the microbiologic response rate, the authors reported that nemonoxacin and levofloxacin had similar microbiologic response rates, with no significant differences between the two drugs or at different doses. Likewise, no significant differences in treatment-emergent adverse events were noted by the authors; however, in a subgroup analysis the authors reported that the 750mg dose of nemonoxacin had a higher risk of adverse events when compared to the 500mg dose (OR 1.38; 95% CI: 0.49 to 3.95). The authors concluded from the evidence that nemonoxacin can be recommended as an appropriate treatment for CAP, given that the clinical and microbiologic efficacy as well as tolerability is the same as that of levofloxacin.10
The SR/MA comparing atypical coverage (a FQ or combination of a macrolide/doxycycline with a beta-lactam) to a regimen without atypical antibiotic coverage (beta-lactam monotherapy) found a benefit for atypical coverage in the treatment of hospitalized adult patients with CAP, where the authors report a statistically significant decrease in clinical failure (approximately 15%). The authors reported no significant differences across the two types of treatment regimens for secondary outcomes, which included mortality, bacteriologic failure or adverse events.12
The Cochrane Review of antibiotic treatments for CAP provided an update to a 2009 Cochrane Review, and sought to compare the efficacy and safety of different antibiotic treatments (including five studies that compared FQs and other antibiotics) for CAP in participants older than 12 years treated in outpatient settings as measured using clinical, radiological and bacteriological outcomes. The authors concluded that the individual study results did not demonstrate differences in efficacy outcomes across antibiotics or antibiotic groups, but did note there were differences for adverse events. Among the studies which included a FQ, the following adverse events were noted in the review: nemonoxacin resulted in higher gastrointestinal (nausea, diarrhea) and nervous system (dizziness, headache) adverse events compared to levofloxacin; gastritis and diarrhea were more common in the high-dose amoxicillin group (1g three times a day) when compared to the other three antibiotic groups (clarithromycin, azithromycin and levofloxacin).18
The SR/MA comparing ceftriaxone combination therapy to respiratory FQ monotherapy showed that the efficacy was similar among patients hospitalized with CAP, and that ceftriaxone combination therapy was associated with lower drug-related adverse events. A meta-analysis of all studies, based on a clinically evaluable population, showed no statistically significant difference in treatment success between ceftriaxone combination therapy and respiratory FQ monotherapy (pooled RR 0.96; 95% CI: 0.92 to 1.01). Results of five studies which provided data on the intention-to-treat population showed that ceftriaxone combination therapy was slightly more effective compared to respiratory FQ (pooled RR 0.93; 95% CI: 0.88 to 0.99). In terms of secondary outcomes, no statistically significant differences were reported for microbiological treatment success (pooled RR 0.99; 95% CI: 0.90 to 1.09). Drug-related adverse events were found to be significantly lower with ceftriaxone combination therapy than respiratory FQ monotherapy (pooled RR 1.27; 95% CI: 1.04 to 1.55).11
COPD
The single SR/MA identified for the treatment of acute exacerbations of COPD used network meta-analyses and cluster ranking. In terms of efficacy specifically, ofloxacin performed significantly better than doxycycline (logOR 2.05; 95% CI: 0.26 to 3.83). The authors conducted a rank-order of treatments based on surface under the cumulative ranking (SUCRA) probability scores and found that in terms of efficacy (clinical cure rate), ofloxacin (79.1%) was most likely to be the best antibiotic in acute exacerbation of COPD treatment, followed by ciprofloxacin (70.4%); however, dirithromycin (88.4%) was deemed to be the best drug in terms of tolerability.16
Cost-Effectiveness
One study was identified which reported on the cost-effectiveness of antibacterial treatment of CAP in adult patients hospitalized in non-intensive ward units in the Netherlands.20
Pneumonia
The cost-effectiveness study reported similar crude average costs within 90 days from the reduced third payer perspective: €4,294, €4,392, and €4,002 per patient for the beta-lactam monotherapy, BL-M combination, and FQ monotherapy strategy, respectively. The ICER was not statistically significant between the treatment strategies and similar results were found with all perspectives. Overall, the authors concluded that the results supported the use of beta-lactam monotherapy as the preferred empirical treatment for patients hospitalized with CAP in the Netherlands.20
Guidelines
Four guidelines were included in report, including two pneumonia-related guidelines and two COPD-related guidelines.
Pneumonia
The NICE 2014 guideline clearly states one recommendation of relevance to this report. For low-severity CAP, the Guideline Development Committee stated that health professionals should not routinely offer a FQ or dual antibiotic therapy. Low quality evidence and safety concerns were cited as considerations weighed by the Guideline Development Committee. It should be noted that this recommendation applies to both respiratory and non-respiratory FQs.21
The 2016 Infectious Diseases Society of America and American Thoracic Society guideline clearly states two recommendations of relevance to this report. In terms of antibiotics for the empiric treatment of clinically suspected VAP, the guideline suggests a regimen including levofloxacin, among other antibiotics, that has coverage for methicillin-sensitive S. aureus (MSSA). Similarly, in terms of antibiotics for the empiric treatment of clinically suspected HAP, the guideline suggests prescribing an antibiotic with activity against MSSA, such as levofloxacin or others. Both recommendations are considered ‘weak’ by the committee and are based on very low-quality evidence.23
COPD
The NICE 2018 guideline specifies one recommendation of relevance to this report. In terms of choice of antibiotic for an acute exacerbation of COPD in adults, it is recommended that FQs (and levofloxacin in particular) should be used as an alternative oral antibiotic for people who may be at higher risk of treatment failure (guided by susceptibilities when available). While the strength of this recommendation is not specified, it is explicitly stated that the committee was aware of safety concerns related to FQs, such as side effects involving the muscles, tendons, bones, and the nervous system. It should also be noted that as of March 2019, NICE is reviewing recommendations related to FQs in light of restrictions and precautions for use issued by the Medicines and Healthcare products Regulatory Agency in the UK.22
The VA/DoD 2014 guideline states one recommendation of relevance to this report. For the management of patients with acute exacerbations of COPD, it recommends that FQs be reserved for specific patients such as critically ill patients in intensive care units, patients with recent history of resistance or treatment failure, or patients with risk factors for healthcare-associated infections. The rating is ‘weak for’ this recommendation based on a lack of head-to-head studies adequately designed to show superiority of one antibiotic over another. Specifically, it was stated that FQs should be reserved in order to conserve the activity of this class of antibiotics and to reduce the development of resistant strains.24
Limitations
There are several limitations that should be noted. For the included SR/MAs, authors noted potential limitations in findings due to a high risk of bias among the studies included. While nine SR/MAs were identified for the treatment of pneumonia, only one study was identified addressing the clinical efficacy and safety of FQ treatment for exacerbations of COPD. Overall, the generalizability of the SR/MA findings is limited by variability of included study design, interventions, and comparators, contradictory or inconclusive findings, and in the case of COPD limited volume.
A limited volume of cost-effectiveness evidence was identified for pneumonia (i.e., one study) and no cost-effectiveness evidence was identified for COPD. While the authors of the identified study claim that the results of the included cost-effectiveness study may be generalizable to the Netherlands, generalizability to other jurisdictions, such as Canada, is unclear.
The included guidelines were generally well developed methodologically; however, low-quality evidence was utilized to formulate recommendations. Overall, the guidelines recommended the use of FQs for limited populations. The identified guidelines are intended for practice in the UK and US, and thus recommendations specific to the Canadian population were not captured in this report.
Conclusions and Implications for Decision or Policy Making
Fifteen publications describing clinical effectiveness, cost-effectiveness, and evidence-based guidelines for the use of FQs in patients with pneumonia and COPD exacerbations were identified in this report; of these, ten are systematic reviews, one is an economic evaluation, and four are evidence-based guidelines. Specific findings were as follows:
The nine SR/MAs describing patients with pneumonia compared a variety of antibiotics and antibiotic combinations. Five of these studies examined mortality among patients with CAP; however, some authors noted that their findings were limited by the studies included (e.g. high risk of bias or lack of RCTs). Findings from three of these SR/MAs suggested that alternative antibiotic regimens may be more effective in reducing mortality compared to FQ-containing regimens.13,17,19 Two of these SR/MAs did not draw any conclusions due to variability in the treatment regimens used and study designs of included evidence.14,15
The remaining four SR/MAs describing patients with pneumonia examined the efficacy of antibiotics using clinical cure or clinical failure. One study reported that treatment with a FQ resulted in lower clinical failure than treatment with beta-lactam monotherapy.12 A study comparing two FQs reported similar responses and adverse events,10 while another study reported similar efficacy between FQs and ceftriaxone; though, adverse events were significantly lower in the ceftriaxone group.11 Finally, another SR/MA noted that no conclusions could be drawn due to variability in treatment regimens.18
The SR/MA that examined treatment for acute exacerbations of COPD found that while FQs were superior in terms of efficacy, dirithromycin was found to be superior in tolerability according to the authors’ findings.16
The cost-effectiveness study that examined pneumonia treatments found that crude average costs were similar between beta-lactam monotherapy, BL-M and FQ therapy, the ICER was not statistically significant between treatment strategies, and authors concluded that beta-lactam monotherapy was the preferred treatment in the Netherlands.20
The two pneumonia-related guidelines, intended for use in the UK and US, both cited low quality evidence as a consideration when implementing their recommendations. It is recommended that for low-severity CAP, FQs should not routinely be offered,21 and for VAP and HAP levofloxacin should be considered as an approach to cover MSSA.23 The two COPD-related guidelines for acute exacerbations, also intended for practice in the UK and US, similarly recommended the use of FQs for particular patient populations only, including those at higher risk of treatment failure, patients in the ICU, and those with risk factors for other infections.22,24 It should be noted that the FQ-related recommendations in the NICE 2018 guideline for COPD exacerbations is currently under review due to safety concerns.22
Overall, variable findings and methodological limitations in the body of evidence identified to inform this report limit generalizability and warrant caution in its interpretation. The clinical effectiveness, cost-effectiveness, and recommendations for FQs remain unclear. Further evidence, particularly in the Canadian context, is needed to provide guidance on the appropriate use of FQs for pneumonia and exacerbations of COPD.
References
- 1.
Hooper
D. Fluoroquinolones. In: Post
TW, ed. Waltham (MA): UptoDate; 2019:
www.uptodate.com. Accessed 2019 May 6.
- 2.
Qureshi
ZP, Seoane-Vazquez
E, Rodriguez-Monguio
R, Stevenson
KB, Szeinbach
SL. Market withdrawal of new molecular entities approved in the United States from 1980 to 2009.
Pharmacoepidemiol Drug Saf. 2011;20(7):772–777. [
PubMed: 21574210]
- 3.
US Food & Drug Administration. FDA drug safety communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together. 2016:
https://www.fda.gov/Drugs/DrugSafety/ucm500143.htm. Accessed 2019 May 6.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
Liberati
A, Altman
DG, Tetzlaff
J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration.
J Clin Epidemiol. 2009;62(10):e1–e34. [
PubMed: 19631507]
- 10.
Chang
SP, Lee
HZ, Lai
CC, Tang
HJ. The efficacy and safety of nemonoxacin compared with levofloxacin in the treatment of community-acquired pneumonia: a systemic review and meta-analysis of randomized controlled trials.
Infect. 2019;12:433–438. [
PMC free article: PMC6388749] [
PubMed: 30863126]
- 11.
Zhang
YQ, Zou
SL, Zhao
H, Zhang
MM, Han
CL. Ceftriaxone combination therapy versus respiratory fluoroquinolone monotherapy for community-acquired pneumonia: a meta-analysis.
Am J Emerg Med. 2018;36(10):1759–1765. [
PubMed: 29499898]
- 12.
Eljaaly
K, Alshehri
S, Aljabri
A, et al. Clinical failure with and without empiric atypical bacteria coverage in hospitalized adults with community-acquired pneumonia: a systematic review and meta-analysis.
BMC Infect Dis. 2017;17(1):385. [
PMC free article: PMC5457549] [
PubMed: 28576117]
- 13.
Lee
JH, Kim
HJ, Kim
YH. Is beta-lactam plus macrolide more effective than beta-lactam plus fluoroquinolone among patients with severe community-acquired pneumonia?: a systemic review and meta-Analysis.
J Korean Med Sci. 2017;32(1):77–84. [
PMC free article: PMC5143302] [
PubMed: 27914135]
- 14.
Vardakas
KZ, Trigkidis
KK, Apiranthiti
KN, Falagas
ME. The dilemma of monotherapy or combination therapy in community-acquired pneumonia.
Eur J Clin Invest. 2017;47(12). [
PubMed: 29027205]
- 15.
Vardakas
KZ, Trigkidis
KK, Falagas
ME. Fluoroquinolones or macrolides in combination with beta-lactams in adult patients hospitalized with community acquired pneumonia: a systematic review and meta-analysis.
Clin Microbiol Infect. 2017;23(4):234–241. [
PubMed: 27965070]
- 16.
Zhang
HL, Tan
M, Qiu
AM, Tao
Z, Wang
CH. Antibiotics for treatment of acute exacerbation of chronic obstructive pulmonary disease: a network meta-analysis.
BMC Pulm Med. 2017;17(1):196. [
PMC free article: PMC5727987] [
PubMed: 29233130]
- 17.
Raz-Pasteur
A, Shasha
D, Paul
M. Fluoroquinolones or macrolides alone versus combined with beta-lactams for adults with community-acquired pneumonia: systematic review and meta-analysis.
Int J Antimicrob Agents. 2015;46(3):242–248. [
PubMed: 26092096]
- 18.
Pakhale
S, Mulpuru
S, Verheij
TJ, Kochen
MM, Rohde
GG, Bjerre
LM. Antibiotics for community-acquired pneumonia in adult outpatients.
Cochrane Database Syst Rev. 2014(10):CD002109. [
PMC free article: PMC7078574] [
PubMed: 25300166]
- 19.
Sligl
WI, Asadi
L, Eurich
DT, Tjosvold
L, Marrie
TJ, Majumdar
SR. Macrolides and mortality in critically ill patients with community-acquired pneumonia: a systematic review and meta-analysis.
Crit Care Med. 2014;42(2):420–432. [
PubMed: 24158175]
- 20.
van Werkhoven
CH, Postma
DF, Mangen
MJ, Oosterheert
JJ, Bonten
MJ, Cap-Start study group. Cost-effectiveness of antibiotic treatment strategies for community-acquired pneumonia: results from a cluster randomized cross-over trial.
BMC Infect Dis. 2017;17(1):52. [
PMC free article: PMC5223446] [
PubMed: 28068956]
- 21.
- 22.
National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease (acute exacerbation): antimicrobial prescribing. (
NICE guideline NG114). 2018:
https://www.nice.org.uk/guidance/ng114. Accessed 2019 May 6.
- 23.
Kalil
AC, Metersky
ML, Klompas
M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society.
Clin Infect Dis. 2016;63(5):e61–e111. [
PMC free article: PMC4981759] [
PubMed: 27418577]
- 24.
Appendix 1. Selection of Included Studies
Appendix 2. Characteristics of Included Publications
Table 2Characteristics of Included Systematic Reviews and Meta-Analyses
View in own window
| First Author, Publication Year, Country | Study Designs and Numbers of Primary Studies Included | Population Characteristics | Intervention and Comparator(s) | Clinical Outcomes, Length of Follow-Up |
|---|
| Chang et al, 2019, Taiwan10 | SR/MA 3 included RCTs
| Adult patients with CAP | Nemonoxacin vs. levofloxacin | Primary Outcome:
Secondary Outcomes:
|
| Zhang et al, 2018, China11 | SR/MA 9 included RCTs
| Adults aged ≥ 18 years of age with CAP | Ceftriaxone combination therapy vs. respiratory FQ monotherapy | Primary Outcome:
Secondary Outcomes:
Microbiological treatment success |
| Eljaaly et al, 2017, Saudi Arabia12 | SR/MA 5 included RCTs
| Hospitalized adult patients with CAP | Atypical coverage (a respiratory FQ or combination of a macrolide/doxycycline with a beta-lactam) vs. a regimen without atypical antibiotic coverage (beta-lactam monotherapy) were identified and included FQs included levofloxacin, moxifloxacin, and gemifloxacin Macrolides included azithromycin, clarithromycin, or erythromycin Beta-lactam agents with >85% coverage against S. pneumoniae were allowed and this included amoxicillin, amoxicillin/clavulanate, ampicillin, ampicillin/sulbactam, piperacillin, piperacillin/tazobactam, cefuroxime, cefpodoxime, cefdinir, cefditorin, cefotaxime, ceftriaxone, cefepime, ceftaroline, imipenem, meropenem, and ertapenem | Primary Outcome:
Secondary Outcomes:
Mortality Bacteriologic failure Adverse events
|
| Lee et al, 2017, Korea13 | SR/MA 8 included studies
| Severe CAP adult patients over 18 years of age | BL-M vs. BL-FQ combination therapy | Clinical outcomes:
Mortality (total, inhospital, ICU or 30-day) Length of stay (hospital or ICU)
|
| Vardakas et al, 2017a, Greece14 | SR/MA 50 included studies
RCT, n = 11 Prospective, n = 15 Retrospective, n = 23
Retrospective/Prospective, n = 1 | Adult patients hospitalized with CAP | Any comparison of a beta-lactam, a FQ or a macrolide, either alone or in combination | Primary Outcome:
|
| Vardakas et al, 2017b, Greece15 | SR/MA 17 included studies
| Adult patients with CAP requiring hospitalization | BL-M vs BL-FQ | Primary Outcome:
|
| Zhang et al, 2017 China16 | SR/MA 19 included RCTs
| Patients with exacerbations of COPD but not stable COPD or prophylactic treatment for exacerbations | Amoxicillin, amoxicillin-clavulanic acid, ampicillin-sulbactam, azithromycin, cefaclor, cefuroxime, ciprofloxacin, clarithromycin, dirithromycin, doxycycline, levofloxacin, moxifloxacin, ofloxacin, prulifloxacin, sparfloxacin, trimethoprimsulfamethoxazole, and zabofloxacin | Primary Outcome:
Secondary Outcomes:
|
| Raz-Pasteur et al, 2015, Israel17 | SR/MA 16 included RCTs
| RCTs if adults aged >18 years with CAP treated in the hospital or in the community. | Any respiratory FQ or any macrolide administered as monotherapy vs. BL-M or BL-F The included trials compared quinolone monotherapy versus BL-M (9 trials), quinolone monotherapy vs. BL-FQ (3 trials), and macrolide monotherapy vs. BL-FQ (4 trials) | Primary Outcome:
Secondary Outcomes:
|
| Pakhale et al, 2014, Canada18 | SR/MA 11 included RCTs
| CAP outpatients ≥ 12 years of age | Antibiotics vs. placebo, as well as antibiotics vs. another antibiotic | Primary Outcome:
Secondary Outcomes:
Radiologic Response Bacteriologic Response Adverse Events Hospitalization Mortality
|
| Sligl et al, 2014, Canada19 | SR/MA 28 included observational studies
| Critically ill adult patients with CAP | Macrolide antibiotic vs. nonmacrolide antibiotic | Primary Outcome:
|
BL-FQ = beta-lactam/fluoroquinolone combination; BL-M = beta-lactam/macrolide combination; CAP = community-acquired pneumonia; COPD = chronic obstructive pulmonary disease; FQ = fluroquinolones; ICU = intensive care unit; RCT = randomized controlled trial
Table 3Characteristics of Included Economic Evaluation
View in own window
| First Author, Publication Year, Country | Type of Analysis, Time Horizon, Perspective | Decision Problem | Population Characteristics | Intervention and Comparator(s) | Approach | Clinical and Cost Data Used in Analysis | Main Assumptions |
|---|
| Van Werkhoven et al, 2017, Netherlands20 | Cost-minimization and cost-effectiveness analysis 30 and 90 day time horizons were utilized Third payer (reduced and full), and societal perspectives utilized | To conduct a cost-minimization analysis of different antibiotic strategies, and a cost-effectiveness analysis from a third payer and a social perspective | Adult patients hospitalized to non-intensive care unit wards with a clinical diagnosis of CAP | Beta-lactam monotherapy vs. BL-M vs. FQ monotherapy | Friction and Human capital approaches utilized Trial-based analysis was utilized | Data from a cluster-randomized cross-over trial of antibiotic treatment strategies was used | Not reported |
BL-M = beta-lactam/macrolide combination; CAP = community-acquired pneumonia; FQ = fluoroquinolone
Table 4Characteristics of Included Guidelines
View in own window
| Organization, Publication, Year, Country | Intended Users, Target Population | Intervention and Practice Considered | Major Outcomes Considered | Evidence Collection, Selection, and Synthesis | Evidence Quality Assessment | Recommendations Development and Evaluation | Guideline Validation |
|---|
| Pneumonia |
|---|
| NICE, 2014, UK21 | The guideline Pneumonia: Diagnosis and management of community- and hospital-acquired pneumonia in adults is “expected to be relevant to the management of most (~80%) patients with community-acquired pneumonia (CAP) or hospital-acquired pneumonia (HAP)” (pg. 17) Intended users are A & E, acute, general, respiratory, elderly care, intensive care, GP and infectious disease physicians, as well as microbiologists, biochemists and nurses | Key clinical issues covered:
Diagnostic investigations Microbiological investigations Severity assessment tools to guide referral Pharmacological interventions (antibiotic treatment, glucocorticostero id treatment)
|
Mortality Hospital admission Length of hospital stay Clinical cure (success or improvement, clinical stability [opposite direction] as surrogates) Health-related quality-of life Hospital readmission C. difficile-associated diarrhea Withdrawal due to treatment-related adverse events Complications
| Systematic literature searches were undertaken according to the NICE guidelines manual 2012 The steps include determining research question, writing a review protocol, search strategy, sifting results for inclusion, including using full papers, adapting Cochrane reviews, assessing risk of bias, extracting data, analyzing results, assessing evidence quality (GRADE), interpreting evidence. | “Relevant studies were critically appraised using the appropriate checklist as specified in the guidelines manual 2012” (p32) | “Recommendations were drafted on the basis of the Guideline Development Group interpretation of the available evidence, taking into account the balance of benefits, harms and costs between different courses of action” (p48) | “This guidance is subject to a 6-week public consultation and feedback as part of the quality assurance and peer review of the document. All comments received from registered stakeholders are responded to in turn and posted on the NICE website when the prepublication check of the full guideline occurs” (p48) |
| Kalil, 2016, US23 | Intended users are “healthcare professionals who care for patients at risk for HAP and VAP, including specialists in infectious diseases, pulmonary diseases, critical care, and surgeons, anesthesiologists, hospitalists and any clinicians and healthcare providers caring for hospitalized patients with nosocomial pneumonia” (pg. e61) | Evidence and recommendations are provided for various diagnosis methods, and treatment of VAP and HAP (including regimens, dosing, length of treatment) | The outcomes of interest were not reported, however, it was clearly stated that outcomes of interest were identified a priori and the guideline committee rated their importance for decision making | Clinical questions were approved by the whole guideline committee, and literature searches were designed to address questions. Titles and abstracts were screened and potentially relevant citations were subjected to full-text review, using predefined inclusion and exclusion criteria. Evidence summaries were prepared using the GRADE approach. | Recommendations were labeled as “strong” or “weak” (conditional according to the GRADE approach | Recommendations were made according to evidence; in cases where there was lower-quality evidence, strong recommendations were sometimes made when panelists believed that most individuals would desire the recommended course of action, and that most well-informed clinicians would agree. All panel members participated in the guideline preparation and approved the recommendations | Feedback was obtained from external peer reviewers |
| COPD |
|---|
| NICE, 2018, UK22 | This guideline is intended for health professionals and people with COPD, their families and carers and the intended population is adults with COPD and acute exacerbations | The guideline sets out an antimicrobial prescribing strategy for acute exacerbations of COPD | Critical outcomes
| A literature review was conducted, and titles and abstracts, followed by full text references were assessed for relevance. | The GRADE approach was utilized; however the strength of recommendations was not provided | Not reported | Not reported |
| VA/DoD, 2014, US24 | This clinical practice guideline is intended to assist primary care providers in treating adult patients with COPD who are eligible for care in the VA or DoD healthcare delivery systems | Interventions covered include inhaled and systemic pharmacologic treatments and non-pharmacologic treatments used in acute and maintenance management of COPD | Outcomes considered included QoL, morbidity, dyspnea, functional capacity, exacerbation rate and/or severity, mortality, harms, health care utilization, and diagnostic test accuracy | A systematic review was undertaken to answer Key Questions developed by the guideline Champions. | The overall quality of evidence was assessed using GRADE and ratings of “high,” “moderate,” “low,” and “very low” were applied | “At a three and one-half day face-to-face meeting, the CPG Champions and Work Group members, with support from the Team, drew on the body of evidence to develop recommendations. During this process, they took into account the GRADE rating for the strength of the evidence, as well as a number of other factors (balance of desirable and undesirable outcomes, values and preferences, and other considerations), to rate the strength of the recommendation as “Strong For,” “Weak For,” “Strong Against,” or “Weak Against.”” (p8) | Not reported |
A & E = accident and emergency department; CAP = community-acquired pneumonia; COPD = chronic obstructive pulmonary disease; CPG = Clinical Practice Guideline; DoD = Department of Defense; GP = general practitioner; GRADE = Grading of Recommendations Assessment, Development and Evaluation; HAP = hospital-acquired pneumonia; NICE = National Institute for Health and Care Excellence; QoL = quality of life; RCT = randomized controlled trial; VA = Veterans Affairs; VAP = ventilator-associated pneumonia
Appendix 3. Critical Appraisal of Included Publications
Table 5Strengths and Limitations of Systematic Reviews and Meta-Analyses using AMSTAR-26
View in own window
| Strengths | Limitations |
|---|
| Pneumonia |
|---|
| Chang, 201910 |
|---|
The research question and inclusion criteria of the review included population, intervention, comparator, and outcomes of interest. The authors searched at least two databases and provided key words searched. Study selection and data extraction were performed in duplicate. The authors described the populations, interventions (including doses), comparators (including doses), outcomes and research designs of included studies. The authors assessed risk of bias for the included studies. The authors provided methodological details for the meta-analysis. The authors noted that most domains of the included studies were classified as low risk of bias. The authors provided results for the heterogeneity in the meta-analysis. The authors reported no conflicts of interests.
|
The review did not provide an explicit statement that the review methods/protocol were established prior to the conduct of the review. The authors did not explain their selection of study designs for inclusion in the review. A list of excluded studies was not provided. The authors did not report the sources of funding for the included studies. The authors did not investigate publication bias or possible impact on the results of the review.
|
| Zhang, 201811 |
|---|
The research question and inclusion criteria of the review included population, intervention, comparator, and outcomes of interest The authors searched at least two databases and provided key search terms. References of selected studies were also searched Data extraction was performed in duplicate; however, authors did not state whether Study Selection was performed in duplicate. The authors described the populations, interventions (including dosing), comparators (including dosing), outcomes and research designs of included studies. The authors assessed risk of bias for the included studies. The authors noted that the low quality of included studies may impact the findings. The authors reported no conflicts of interests.
|
The review did not provide an explicit statement that the review methods/protocol were established prior to the conduct of the review. The authors did not explain their selection of study designs for inclusion in the review. A list of excluded studies was not provided. The authors did not report the sources of funding for the included studies. Only limited methodological details of the meta-analysis were included. The authors did not carry out an investigation of publication bias. The authors did not provide a discussion of heterogeneity in the results or discussion.
|
| Eljaaly, 201712 |
|---|
The research question and inclusion criteria of the review included population, intervention, comparator, and outcomes of interest. The authors searched at least two databases and provided key their search strategy. Reference lists of the included studies and trial registries were also searched. Study selection and data extraction were performed in duplicate. The authors provided a list of excluded studies and justified the exclusions. The authors described the populations, interventions (including doses), comparators (including doses), outcomes and research designs of included studies. The authors assessed risk of bias for the included studies. The authors provided methodological details for the meta-analysis. The authors reported the sources of funding for the included studies. The authors noted that most domains of the included studies were classified as low risk of bias. The authors provided results for the heterogeneity in the meta-analysis. The authors carried out an investigation of publication bias. The authors reported no conflicts of interests.
|
The review did not provide an explicit statement that the review methods/protocol were established prior to the conduct of the review. The authors did not explain their selection of study designs for inclusion in the review.
|
| Lee, 201713 |
|---|
The research question and inclusion criteria of the review included population, intervention, comparator, and outcomes of interest. The authors searched at least two databases and provided key their search strategy. Reference lists of relevant reviews were also searched. Study selection and data extraction were performed in duplicate. The authors described the populations, interventions, comparators, outcomes and research designs of included studies. The authors assessed risk of bias for the included studies. The authors provided methodological details for the meta-analysis. The authors reported no conflicts of interests.
|
The review did not provide an explicit statement that the review methods/protocol were established prior to the conduct of the review. The authors did not explain their selection of study designs for inclusion in the review. A list of excluded studies was not provided. The authors did not report the sources of funding for the included studies. The authors did not account for the risk of bias in the individual studies when interpreting/discussing the results. The authors noted heterogeneity but did not provide an explanation or discussion of the heterogeneity. The authors did not carry out an investigation of publication bias due to the low volume of RCTs.
|
| Vardakas, 2017a14 |
|---|
The research question and inclusion criteria of the review included population, intervention, comparator, and outcomes of interest The authors searched at least two databases and provided key search terms. References of selected studies were also searched. Study selection and data extraction were performed in duplicate. The authors described the populations, interventions, comparators, outcomes and research designs of included studies. The authors provided methodological details for the meta-analysis. The authors provided results for the heterogeneity in the meta-analysis. The authors carried out an investigation of publication bias. The authors reported no conflicts of interests.
|
The review did not provide an explicit statement that the review methods/protocol were established prior to the conduct of the review. The authors did not explain their selection of study designs for inclusion in the review. A list of excluded studies was not provided. The authors did not assess risk of bias for the included studies. The authors did not report the sources of funding for the included studies.
|
| Vardakas, 2017b15 |
|---|
The research question and inclusion criteria of the review included population, intervention, comparator, and outcomes of interest The authors searched at least two databases and provided key search terms. References of selected studies were also searched. Study selection and data extraction were performed in duplicate. The authors described the populations, interventions, comparators, outcomes and research designs of included studies. The authors assessed risk of bias for the included studies. The authors provided methodological details for the meta-analysis. The authors provided results for the heterogeneity in the meta-analysis. The authors carried out an investigation of publication bias. The authors reported no conflicts of interests or described their funding sources and conflicts of interest.
|
The review did not provide an explicit statement that the review methods/protocol were established prior to the conduct of the review. The authors did not explain their selection of study designs for inclusion in the review. A list of excluded studies was not provided. The authors did not report the sources of funding for the included studies.
|
| Raz-Pasteur, 201517 |
|---|
The research question and inclusion criteria of the review included population, intervention, comparator, and outcomes of interest. The authors searched at least two databases and provided key words searched. Study selection and data extraction were performed in duplicate. The authors described the populations, interventions (including doses), comparators (including doses), outcomes and research designs of included studies. The authors assessed risk of bias for the included studies. The authors provided methodological details for the meta-analysis. The authors provided results for the heterogeneity in the meta-analysis. The authors reported no conflicts of interests.
|
The review did not provide an explicit statement that the review methods/protocol were established prior to the conduct of the review. The authors did not explain their selection of study designs for inclusion in the review. A list of excluded studies was not provided. The authors did not report the sources of funding for the included studies. The authors did not account for the risk of bias in the individual studies when interpreting/discussing the results. The authors did not carry out an investigation of publication bias.
|
| Pakhale, 201418 |
|---|
The research question and inclusion criteria of the review included population, intervention, comparator, and outcomes of interest. The review contains information on the study protocol and explained deviations from the protocol. The authors used a comprehensive literature search strategy. Study selection and data extraction were performed in duplicate. The authors provided a list of excluded studies and justified the exclusions. The authors described the populations, interventions (including doses), comparators (including doses), outcomes and research designs of included studies. The authors assessed risk of bias for the included studies and accounted for risk of bias when interpreting/discussing the results of the review. The authors reported the sources of funding for the included studies. The authors reported any potential sources of conflict of interest.
| |
| Sligl, 201419 |
|---|
The research question and inclusion criteria of the review included population, intervention, comparator, and outcomes of interest. The authors state that they had a written protocol for a search strategy that was established prior to the conduct of the review. The authors searched at least two databases and provided key their search strategy. Trial/Study registries and conferences were hand searched; the authors consulted content experts in the field. Study selection and data extraction were performed in duplicate. The authors described the populations, interventions, comparators, outcomes and research designs of included studies. The authors assessed risk of bias for the included studies. The authors provided methodological details for the meta-analysis. The authors provided results for the heterogeneity in the meta-analysis. The authors carried out an investigation of publication bias. The authors reported no conflicts of interests.
|
The authors did not explain their selection of study designs for inclusion in the review. A list of excluded studies was not provided. The authors did not report the sources of funding for the included studies. The authors did not account for the risk of bias in the individual studies when interpreting/discussing the results.
|
| COPD |
|---|
| Zhang, 201716 |
|---|
The research question and inclusion criteria of the review included the population, intervention, comparator group, and outcome. Study selection was performed in duplicate; however, authors did not state whether data extraction was performed I duplicate. Included studies were described in adequate detail. The authors assessed risk of bias for the included studies. There was no significant heterogeneity in the results.
|
The authors did not explicitly state that the review methods/protocol were established prior to conducting the review, however the research questions, search strategy, inclusion/exclusion criteria, and risk of bias assessment were discussed in detail. The authors did not provide a list of excluded studies and justification for the exclusions. The authors did not report the sources of funding for the included studies. The authors did not report sources of conflict of interest.
|
COPD = chronic obstructive pulmonary disease; RCT = randomized controlled trial
Table 6Strengths and Limitations of Economic Studies using the Drummond Checklist7
View in own window
| Strengths | Limitations |
|---|
| Van Werkhoven, 201720 |
|---|
Multiple perspectives were utilized and were clearly stated Well-defined research question Appropriate alternatives were examined Effectiveness established through RCT Productivity losses were appropriately discounted Authors commented on the generalizability of results
|
Missing data was a potential limitation that may have introduced uncertainty Cost differences in favor of FQs may have been inflated by physician tendency to start with oral FQ Unclear if capital costs included Self-reported data was used
|
FQ = fluoroquinolone; RCT = randomized control trial;
Table 7Strengths and Limitations of Guidelines using AGREE II8
View in own window
| Item | Guideline |
|---|
| NICE, 201421 | Kalil, 201623 | *NICE, 201822 | VA/DoD, 201424 |
|---|
| Domain 1: Scope and Purpose |
|---|
| 1. The overall objective(s) of the guideline is (are) specifically described. | 7 | 7 | 7 | 7 |
| 2. The health question(s) covered by the guideline is (are) specifically described. | 7 | 7 | 7 | 7 |
| 3. The population (patients, public, etc.) to whom the guideline is meant to apply is specifically described. | 7 | 7 | 7 | 7 |
| Domain 2: Stakeholder Involvement |
|---|
| 4. The guideline development group includes individuals from all relevant professional groups. | 7 | 7 | 1 | 7 |
| 5. The views and preferences of the target population (patients, public, etc.) have been sought. | 2 | 1 | 1 | 7 |
| 6. The target users of the guideline are clearly defined. | 7 | 7 | 7 | 7 |
| Domain 3: Rigour of Development |
|---|
| 7. Systematic methods were used to search for evidence. | 7 | 7 | 7 | 7 |
| 8. The criteria for selecting the evidence are clearly described. | 7 | 7 | 7 | 7 |
| 9. The strengths and limitations of the body of evidence are clearly described. | 7 | 7 | 7 | 7 |
| 10. The methods for formulating the recommendations are clearly described. | 7 | 7 | 1 | 7 |
| 11. The health benefits, side effects, and risks have been considered in formulating the recommendations. | 7 | 7 | 7 | 7 |
| 12. There is an explicit link between the recommendations and the supporting evidence. | 7 | 7 | 7 | 7 |
| 13. The guideline has been externally reviewed by experts prior to its publication. | 7 | 7 | 1 | 1 |
| 14. A procedure for updating the guideline is provided. | 7 | 2 | 5 | 2 |
| Domain 4: Clarity of Presentation |
|---|
| 15. The recommendations are specific and unambiguous. | 7 | 7 | 7 | 7 |
| 16. The different options for management of the condition or health issue are clearly presented. | 7 | 7 | 7 | 7 |
| 17. Key recommendations are easily identifiable. | 7 | 7 | 7 | 7 |
| Domain 5: Applicability |
|---|
| 18. The guideline describes facilitators and barriers to its application. | 1 | 1 | 1 | 7 |
| 19. The guideline provides advice and/or tools on how the recommendations can be put into practice. | 1 | 1 | 7 | 7 |
| 20. The potential resource implications of applying the recommendations have been considered. | 1 | 1 | 7 | 1 |
| 21. The guideline presents monitoring and/or auditing criteria. | 1 | 1 | 7 | 1 |
| Domain 6: Editorial Independence |
|---|
| 22. The views of the funding body have not influenced the content of the guideline. | 7 | 7 | 7 | 7 |
| 23. Competing interests of guideline development group members have been recorded and addressed. | 7 | 7 | 1 | 7 |
- *
A full guideline document was not publicly available and the responses to the AGREE II tool are based on the evidence summary document
Note: Responses for each domain are graded on a scale from 1 (Strongly Disagree) to 7 (Strongly Agree)
Appendix 4. Main Study Findings and Authors’ Conclusions
Table 8Summary of Findings Included Systematic Reviews and Meta-Analyses
View in own window
| Main Study Findings | Authors’ Conclusion |
|---|
| Pneumonia |
|---|
| Chang, 201910 |
|---|
Clinical Efficacy
Nemonoxacin had a clinical cure rate similar to levofloxacin in the treatment of CAP (OR 1.05; 95% CI: 0.67 to 1.64, I2=0%) All studies found no significant differences in the clinical cure rates of patients treated with nemonoxacin 500 mg and levofloxacin 500 mg (OR 1.01; 95% CI: 0.62 to 1.65, I2=0%). Two included studies compared the clinical cure rates of nemonoxacin 750 mg and levofloxacin 500 mg and no significant difference was found (OR 1.09; 95% CI: 0.58 to 2.05, I2=0%) Nemonoxacin and levofloxacin exhibited similar clinical responses
Streptococcus pneumoniae (OR 1.20; 95% CI: 0.21 to 6.75, I2=0%), Haemophilus spp. (OR 0.77; 95% CI: 0.16 to 3.63, I2=0%), Staphylococcus aureus (OR 2.29; 95% CI: 0.12 to 41.98, I2=0%), Atypical pathogens (OR 0.80; 95% CI: 0.17 to 1.92, I2=0%)
Microbiologic Response
Nemonoxacin had a microbiologic response rate similar to levofloxacin in the treatment of CAP (OR 0.89; 95% CI: 0.44 to 1.81, I2=0%) Three included studies compared the microbiologic response of nemonoxacin 500 mg with levofloxacin 500 mg and found no significant differences (OR 0.83; 95% CI: 0.39 to 1.77, I2=0%). Two included studies compared the microbiologic response between nemonoxacin 750 mg and levofloxacin 500 mg and found no significant difference (OR 0.98; 95% CI: 0.33 to 2.90, I2=0%).
Adverse Events
No significant differences were found for treatment-emergent AEs in overall and subgroup comparisons
nemonoxacin at 500 or 750 mg vs levofloxacin 500 mg: OR 1.08; 95% CI: 0.81 to 1.43, I2=0%, nemonoxacin at 500 mg vs levofloxacin 500 mg: OR 0.95; 95% CI: 0.71 to 1.28, I2=0%; nemonoxacin at 750 mg vs levofloxacin 500 mg: OR 1.46; 95% CI: 0.92 to 2.31, I2=0%
Subgroup analysis (nemonoxacin 750 vs 500 mg)
There were no significant differences in the clinical cure rate (OR 0.99; 95% CI: 0.33 to 2.99, I2=57%) and microbiologic response rate (OR 1.38; 95% CI: 0.49 to 3.95, I2=0%) between different doses of nemonoxacin (750 and 500 mg). The 750 mg dosage of nemonoxacin had a higher risk of treatment-emergent AEs than the 500 mg dose (OR 1.63; 95% CI: 1.03 to 2.58, I2=0%).
| “In conclusion, based on the findings of this meta-analysis of three RCTs, the clinical and microbiologic efficacy of nemonoxacin is as good as levofloxacin in the treatment of CAP, and this antibiotic is as well tolerated as levofloxacin. Thus, nemonoxacin can be recommended as an appropriate antibiotic therapy for CAP.” (p437) |
| Zhang, 201811 |
|---|
Treatment Success
Analysis of the nine studies revealed that there was no difference in treatment success between ceftriaxone combination therapy and respiratory FQ monotherapy (pooled RR 0.96; 95% CI: 0.92 to 1.01, P = 0.669) based on clinically evaluable populations. In five studies which reported the ITT population ceftriaxone combination therapy was slightly more effective than respiratory FQ monotherapy (pooled RR 0.93; 95% CI: 0.88 to 0.99, P = 0.831)
Drug-Related Adverse Events
The most common AEs were gastrointestinal disturbances, including diarrhea, vomiting, and other GI complaints. ceftriaxone combination therapy was associated with fewer AEs (pooled RR 1.27; 95% CI: 1.04 to 1.55, P = 0.553; six studies).
Microbiological Treatment Success
| “The findings of this meta-analysis suggest that ceftriaxone combination therapy was as efficacious as respiratory fluoroquinolones monotherapy, but the drug-related AEs were lower in the ceftriaxone combination therapy regimen. Given the limitation of quantity and quality of these included studies, more high-quality RCTs are required to explore the conclusion further.” (p1762) |
| Eljaaly, 201712 |
|---|
Clinical Failure
Mortality
No statistically significant differences were reported by the authors. RR 0.549 (95% CI: 0.259 to 1.165, P = 0.118), I2 = 61.434%; Q = 9.635 (P = 0.022)
Bacteriologic Failure
No statistically significant differences were reported by the authors RR 0.816 (95% CI: 0.523 to 1.272, P = 0.369), I2 = 0%; Q = 0.47 (P = 0.79)
Adverse Events
No statistically significant differences were reported by the authors Total AEs: RR 0.982 (95% CI: 0.697 to 1.383, P = 0.918, I2 = 69.011%); Q = 5.722 (P = 0.057) Diarrhea: RR 0.746 (95% CI: 0.311 to 1.790, P = 0.512, I2 = 69.011%); Q = 5.722 (P = 0.057) AEs requiring antibiotic discontinuation: RR 0.83 (95% CI: 0.542 to 1.270, P = 0.39, I2 = 65.048%); Q = 6.454 (P = 0.04))
| “Our restricted but targeted meta-analysis of RCTs was able to define a significant reduction (approximately 15%) in clinical failure with the inclusion of atypical coverage in hospitalized adults with CAP. No significant differences were found in terms of secondary outcomes including mortality, bacteriologic failure and adverse events. Our meta-analysis provides supports for the current recommendations of the major CAP guidelines. However, some of the difference noted may be due to differences in typical coverage between treatment arms.” (p6) |
| Lee, 201713 |
|---|
Mortality
Overall mortality rates were 19.4% for the BL-M group vs 26.8% for the BL-FQ group. A random effect model showed that BL-M therapy was significantly associated with reduced overall mortality (OR 0.68; 95% CI: 0.49 to 0.94; P = 0.02; I2 = 58.0%) (8 studies)
Length of Stay
BL-M therapy was significantly associated with shorter length of hospital stay (mean difference −3.05 days; 95% CI: −6.01 to −0.09, P = 0.04; I2 = 65.0%) There was no significant difference between BL-M and BL-FQ for length of ICU stay.
| “In conclusion, our systemic review and meta-analysis revealed that BL-M combination therapy compared to BL-F combination therapy for severe CAP may be more effective in reducing overall mortality and length of hospital stay. However, the methodological limitations of the included trials and the scarcity of available clinical studies prevented a definitive conclusion. Accordingly, further large-scale, well-designed RCTs are needed to clarify which regimen is more effective for severe CAP.”(p83) |
| Vardakas, 2017a14 |
|---|
Monotherapy
Monotherapy (any regimen) was not associated with higher mortality than combination (any regimen, RR 1.14; 95% CI: 0.99 to 1.32, I2 = 84%). Thirty-day, in-hospital and ICU mortality was not significantly different for monotherapy versus combination. BL monotherapy was associated with statistically significantly higher mortality than BL-M combination (38 studies, RR 1.32; 95% CI: 1.12 to 1.56, I2 =85%). No significant difference in mortality between patients receiving FQ and BL-M was observed (27 studies, RR 0.98; 95% CI: 0.78 to 1.23, I2 =73%). There was no difference reported in mortality between BL and BL-FQ (12 studies, RR 0.93; 95% CI: 0.83 to 1.05, I2 =0%). Macrolides (7 studies, RR 0.39; 95% CI: 0.23 to 0.66, I2 24%) and FQ (13 studies, RR 0.75; 95% CI: 0.57 to 0.98, I2 =36%) were associated with statistically significantly lower mortality than BL-FQ.
| “Due to the considerable heterogeneity and inclusion of unadjusted data, it is difficult to recommend a specific antibiotic regimen over another. Specific antibiotic regimens, study design and the characteristics of the population under study seem to influence the reported outcomes.” (p1) |
| Vardakas, 2017b15 |
|---|
In the analysis of unadjusted data, mortality with BL-FQ was statistically significantly higher than with BL-M (RR 1.33; 95% CI: 1.15 to 1.54, P = 0.0001, I2 = 28%) BL-FQ was associated with higher mortality in American but not European studies; no difference was observed in patients with bacteraemia and septic shock. In the meta-analysis of all mortality data, a non-significant difference between the two regimens was observed (eight studies, adjusted RR 1.26: 95% CI: 0.95 to 1.67, I2 =43%). The meta-analysis of adjusted data only showed no statistically significant difference in mortality between the two combination regimens (5 studies, aRR 1.36; 95% CI: 0.93 to1.98)
| “In conclusion, very low quality evidence is available for the comparative effectiveness of BLFQ and BLM for the treatment of hospitalized patients with CAP. These data show a trend towards higher mortality with a BLFQ combination. However, in the absence of RCTs, recommendations cannot be made for or against either of the studied regimens. Furthermore, the subgroup of patients who might not benefit from treatment with BLFQ has not been defined. The finding suggesting higher mortality in the unadjusted analysis with the guideline-recommended BLFQ combination, although inconclusive, may advocate a less frequent use of this combination as empirical treatment for severe CAP. Patients with suspected Pseudomonas aeruginosa CAP (although there is still no strong evidence that a combination with fluoroquinolones would lower mortality) [52], increased probability for multidrug-resistant pathogens or documented severe allergy to macrolides could be more appropriate candidates for the BLFQ regimen until well-designed RCTs comparing the two regimens are performed.” (p238) |
| Raz-Pasteur, 201517 |
|---|
FQ vs. BL-M – RR (95% CI)
All-cause mortality: 0.99 (0.70 to 1.40), I2= 0 Clinical failure: 0.72 (0.57 to 0.91), I2= 0 Clinical failure (subgroup pneumococcal pneumonia): 2.03 (0.94 to 4.38), I2= 22 Treatment discontinuation: 0.65 (0.54 to 0.78), I2= 0 Microbiological failure: 0.93 (0.63 to 1.38), I2= 0 Any AE: 0.90 (0.81 to 1.00), I2= 0 Diarrhea: 0.13 (0.05 to 0.34), I2= 52
FQ vs. BL-FQ – RR (95% CI)
All-cause mortality: 1.00 (0.69 to 1.45), I2= 42 Clinical failure: 1.11 (0.89 to 1.38), I2= 23 Clinical failure (subgroup pneumococcal pneumonia): 0.92 (0.53 to 1.59), I2=0 Treatment discontinuation: Not reported Microbiological failure: 1.15 (0.71 to 1.86), I2= 0 Any AE: 1.02 (0.9 to 1.14), I2= 60 Diarrhea: 2.05 (1.13 to 3.73), I2= not relevant
(Note: RR < 1.0 favors monotherapy) | “In conclusion, we summarise that monotherapy with respiratory FQs is as safe and efficacious as beta—lactam/macrolide combination therapy and results in fewer treatment modifications. Addition of a beta-lactam to the fluroquinolone does not confer further benefit in the treatment of hospitalized CAP among adults. Data are not available to direct the management of patients with CAP in the community and severe CAP in the ICU. Further studies should examine the ecological implications of selecting a policy of beta-lactam monotherapy versus that of a respiratory FQ in the management of CAP in hospitals.” (p247) |
| Sligl, 201419 |
|---|
Macrolide use was associated with a statistically significant lower risk of mortality compared with nonmacrolide antibiotic use (21% vs 24%, RR 0.82; 95% CI: 0.70 to 0.97; P=0.02) When comparing broadly guideline-concordant regimens there was a trend toward improved mortality and heterogeneity was reduced
| “In this systematic review and meta-analysis of observational studies including almost 10,000 patients, we found that macrolide use in the treatment of critically ill patients with CAP was associated with a robust and statistically significant 18% relative (3% absolute) reduction in crude mortality compared with nonmacrolide regiments and an even larger relaive risk reduction in adjusted analyses. In the absence of randomized trial data, we believe this meta-analysis supports the use of macrolides as first-line combination treatment in critically ill patients with severe CAP and reinforces current guidelines for this high-risk population.” (p425) |
| Pakhale et al, 201418 |
|---|
Test of Clinical Cure Overall, success rates were very high, usually ranging from 76% to 89% though they were similar in treatment and comparator arms in individual studies
Solithromycin vs. levofloxacin
Nemonoxacin vs. levofloxacin
Two comparisons reported (difference between the two analyses/comparisons NR); neither showing a statistically significant difference:
- ▪
OR (M-H, Fixed 95% CI): 1.18 (0.55 to 2.53) - ▪
OR (M-H, Fixed 95% CI): 0.76 (0.38 to 1.54)
Azithromycin microspheres vs. levofloxacin
Telithromycin vs. levofloxacin
Radiological Response
Bacteriological Cure
Solithromycin versus levofloxacin
Nemonoxacin versus levofloxacin
Nemonoxacin versus levofloxacin
Azithromycin microspheres versus levofloxacin
Telithromycin versus levofloxacin
Adverse eventsThough the majority of AEs were similar between all antibiotics, nemonoxacin demonstrated higher gastrointestinal and nervous system AEs compared to levofloxacin, while cethromycin demonstrated significantly more nervous system side effects, especially dysgeusia, when compared to clarithromycin. high-dose amoxicillin (1g three times a day) was associated with higher incidence of gastritis and diarrhea compared to clarithromycin, azithromycin and levofloxacin
Solithromycin versus levofloxacin
Nemonoxacin versus levofloxacin
Nemonoxacin versus levofloxacin
Azithromycin microspheres versus levofloxacin
Telithromycin versus levofloxacin
| “Currently available evidence from randomised controlled trials (RCTs) is insufficient to make evidence-based recommendations for the choice of antibiotic to be used in the treatment of community-acquired pneumonia (CAP) in ambulatory patients. At most, it can be stated that individual study results do not reveal significant differences in efficacy between various antibiotics and antibiotic groups” (p15) |
| COPD |
|---|
| Zhang 201716 |
|---|
Meta-analyses
In terms of efficacy (clinical cure rate), ofloxacin was significantly better than both doxycycline (logOR 2.05; 95% CI: 0.26 to 3.83) Ranking of treatments based on SUCRA probability scores found that in terms of efficacy (clinical cure rate), ofloxacin (79.1%) was the most likely to be the best antibiotic in AECOPD treatment followed by ciprofloxacin (70.4%) and trimethoprim-sulfamethoxazole (68.1%). In terms of tolerability, dirithromycin (88.4%) was most likely to be the best drug followed by azithromycin (81.4%) and amoxicillin (68.6%; Fig. 6) (pg. 8) The cluster ranking showed that dirithromycin had a high clinical cure rate with a low rate of adverse effects. Ofloxacin, ciprofloxacin, and trimethoprim-sulfamethoxazole had high clinical cure rates with median rates of adverse effects.
| “Our study indicated that dirithromycin is adequate for improving the clinical cure rate of patients with AECOPD with few adverse effects. Ofloxacin, ciprofloxacin, and trimethoprim-sulfamethoxazole are also recommended for disease treatment. However, caution should still be exercised when using antibiotics to treat AECOPD” (p1) |
AE = adverse event; AECOPD = acute exacerbation of chronic obstructive pulmonary disease; aRR = adjusted Risk Ratio; BL = beta-lactam; BL-FQ = beta-lactam/fluoroquinolone combination; BL-M = beta-lactam/macrolide combination; CAP = community acquired pneumonia; CI = confidence interval; COPD = chronic obstructive pulmonary disease; FQ = fluoroquinolone; GI = gastrointestinal; ICU = intensive care unit; ITT = intention-to-treat; NR = not reported; OR = odds ratio; PSI = Pneumonia Severity Index; RCT = randomized controlled trial; RR = risk ratio; SUCRA = surface under the cumulative ranking
Table 9Summary of Findings of Included Economic Evaluation
View in own window
| Main Study Findings | Authors’ Conclusion |
|---|
| van Werkhoven, 201720 |
|---|
Crude average costs within 90 days from the reduced third payer perspective were €4,294, €4,392, and €4,002 per patient for the beta-lactam monotherapy, BL-M, and FQ monotherapy strategy, respectively. CMA results were €106 (95% CI €−697 to €754) for the BLM strategy and €−278 (95%CI €−991 to €396) for the FQ monotherapy strategy, both compared to the beta-lactam monotherapy strategy. The ICER was not statistically significantly different between the strategies from the reduced third payer perspective. Other perspectives yielded similar results.
| The authors found there were no significant differences in cost-effectiveness of strategies of preferred antibiotic treatment of CAP on non-ICU wards with either beta-lactam monotherapy, beta-lactam/macrolide combination therapy, or FQ monotherapy “These data support the use of beta-lactam monotherapy as preferred empirical treatment for patients hospitalized” with CAP (p7) |
BL-FQ = beta-lactam/fluoroquinolone combination; BL-M = beta-lactam/macrolide combination; CAP = community-acquired pneumonia; CI = confidence interval; FQ = fluoroquinolone; ICER = incremental cost-effectiveness ratio; ICU = intensive care unit;
Table 10Summary of Recommendations in Included Guidelines
View in own window
| Recommendation(s) | Strength of Evidence and Recommendations |
|---|
| NICE, 201421 |
|---|
| Low-severity CAP
| “Very low quality evidence from 2 trials including 150 patients showed that antibiotic therapy with a non-respiratory FQ may improve clinical cure at the end of treatment compared with a macrolide” (p217) “Four studies considered respiratory FQ. The licence for these antibiotics for CAP is currently limited due to safety concerns regarding hepatotoxicity, skin reactions, cardiac arrhythmias and tendon rupture. No clinically important difference was found for any outcomes when respiratory FQ was compared with macrolide or amoxicillin. The GDG agreed that safety concerns outweighed any potential benefit seen in these studies. As such, the GDG concluded that respiratory FQs should not be offered routinely as first-line treatment” (p220) “One cost-effectiveness analysis found that respiratory FQ was dominant (less costly and more effective) compared with cephalosporin for treating high-severity CAP. This study was assessed as partially applicable with very serious limitations” (p240) |
| Kalil, 201623 |
|---|
Antibiotic for empiric treatment of clinically suspected VAP:
“When empiric treatment that includes coverage for MSSA (and not MRSA) Is indicated, we suggest a regimen including piperacillin-tazobactam, cefepime, levofloxacin, imipenem, or meropenem (weak recommendation, very low-quality evidence). Oxacillin, nafcillin, or cefazolin are preferred agents for treatment of proven MSSA, but are not necessary for the empiric treatment of VAP if one of the above agents is used” (pg. e81)
Antibiotics for empiric treatment of clinically suspected HAP:
“For patients with HAP who are being treated empirically and have no risk factors for MRSA infection and are not at high risk of mortality, we suggest prescribing an antibiotic with activity against MSSA. When empiric treatment that includes coverage for MSSA (and not MRSA) is indicated, we suggest a regimen including piperacillin-tazobactam, cefepime, levofloxacin, imipenem, or meropenem. Oxacillin, nafcillin, or cefazolin are preferred for the treatment of proven MSSA, but are not necessary for empiric coverage of HAP if one of the above agents is used (weak recommendation, very low-quality evidence)” (pg. e86)
|
Antibiotic for empiric treatment of clinically suspected VAP: weak recommendation, very low-quality evidence). Antibiotics for empiric treatment of clinically suspected HAP: weak recommendation, very low-quality evidence
|
| NICE, 201822 |
|---|
Choice of antibiotic: When prescribing an antibiotic for an acute exacerbation of COPD, for adults aged 18 year and over: The alternative oral antibiotics for people who may be at a higher risk of treatment failure (guided by susceptibilities when available) include:
| The strength of evidence and recommendations was not commented upon in the Evidence Summary document “The committee was aware of the European Medicines Agency’s Pharmacovigilance Risk Assessment Committee recommendation to restrict the use of FQ antibiotics following a review of disabling and potentially long-lasting side effects mainly involving muscles, tendons and bones and the nervous system. However, they discussed that FQs are appropriate as an alternative option for people who may be at a higher risk of treatment failure. The committee was keen to point out, however, that FQ safety concerns should be taken into account on an individual patient basis.” (p21) |
| VA/DoD, 201424 |
|---|
Management of Patients in Acute Exacerbation of COPD
Despite the paucity of evidence regarding the choice of antibiotics, the guideline suggests reserving broader spectrum antibiotics (e.g., quinolones) for patients with specific indications such as:
Critically ill patients in the intensive care unit (ICU); Patients with recent history of resistance, treatment failure, or antibiotic use; and Patients with risk factors for health care associated infections.
| ‘Weak For’ The confidence in the evidence is low to moderate based on the lack of head-to-head studies adequately designed to show superiority of one antibiotic over another FQs should be reserved for specific patients in order to conserve the activity of this class of antibiotics and reduce the potential development of resistant strains |
CAP = community-acquired pneumonia; COPD = chronic obstructive pulmonary disease; FQ = fluoroquinolone; HAP = hospital acquired pneumonia; ICU = intensive care unit; mg = milligrams; MRSA = Methicillin-resistant Staphylococcus aureus; MSSA = methicillin-sensitive S. aureus; PO = orally; VAP = ventilator-associated pneumonia
Appendix 5. Overlap between Included Systematic Reviews
Table 11Primary Study Overlap between Included Systematic Reviews
View in own window
| Primary Study Citation | Systematic Review Citation |
|---|
| Chang 2019 | Eljaay 2017 | Lee 2017 | Pakhale 2014 | Raz-Pasteur 2015 | Sligl 2014 | Vardakas 2017a | Vardakas 2017b | Zhang 2018 | Zhang 2017 |
|---|
| Liu 2017 | ✕ | | | | | | | | | |
| van Rensburg 2010 (a) | ✕ | | | ✕ | | | | | | |
| Yuan 2017 | ✕ | | | | | | | | | |
| Leophonte et al 2004 | | ✕ | | | | | | | | |
| Petitpretz et al 2001 | | ✕ | | | | | | | | |
| Norrby et al 1998 | | ✕ | | | | | | | | |
| Garin et al 2014 | | ✕ | | | | | ✕ | | | |
| Kalbematter et al 2000 | | ✕ | | | | | | | | |
| Adrie et al 2013 | | | ✕ | | | | ✕ | ✕ | | |
| Bratzler et al 2008 | | | ✕ | | | ✕ | ✕ | ✕ | | |
| Karhu et al 2013 | | | ✕ | | | ✕ | | ✕ | | |
| Martin-Loeches et al 2010 | | | ✕ | | | ✕ | | ✕ | | |
| Mortensen et al 2005 | | | ✕ | | | | | ✕ | | |
| Waterer et al 2001 | | | ✕ | | | | ✕ | ✕ | | |
| Wilson et al 2012 | | | ✕ | | | | | ✕ | | |
| Gaillat et al 1994 | | | ✕ | | | | | | | |
| Anderson et al 1991 | | | | ✕ | | | | | | |
| Chien et al 1993 | | | | ✕ | | | | | | |
| Drehobl 2005 | | | | ✕ | | | | | | |
| D’Ignazio et al 2005 | | | | ✕ | | | | | | |
| English et al 2012 | | | | ✕ | | | | | | |
| Kohno et al 2003 | | | | ✕ | | | | | | |
| Mathers Dunbar et al 2004 | | | | ✕ | | | | | | |
| Oldach et al 2013 | | | | ✕ | | | | | | |
| Udupa 2011 | | | | ✕ | | | | | | |
| Vacarezza et al 2010 | | | | ✕ | | | | | | |
| van Rensburg 2010b | | | | ✕ | | | | | | |
| Asadi et al 2013 | | | | | | | ✕ | | | |
| Aspa et al 2006 | | | | | | | ✕ | | | |
| Blasi et al 2008 | | | | | | | ✕ | | | |
| Brown et al 2003 | | | | | | | ✕ | | | |
| Burgess et al 2000 | | | | | | | ✕ | | | |
| Capelastegui et al 2005 | | | | | | | ✕ | ✕ | | |
| Capelastegui et al 2006 | | | | | | ✕ | ✕ | ✕ | | |
| Charles et al 2008 | | | | | | ✕ | ✕ | ✕ | | |
| Chokshi et al 2007 | | | | | | | ✕ | | | |
| Dwyer et al 2006 | | | | | | | ✕ | | | |
| Frank et al 2002 | | | | | ✕ | | ✕ | | ✕ | |
| Frei et al 2003 | | | | | | | ✕ | | | |
| Frei et al 2006 | | | | | | | ✕ | ✕ | | |
| Garcia et al 2005 | | | | | | | ✕ | | | |
| Gleason et al 1999 | | | | | | | ✕ | | | |
| Houck et al 2001 | | | | | | | ✕ | ✕ | | |
| Leroy et al 2005 | | | | | ✕ | | ✕ | | | |
| Lin et al 2007 | | | | | ✕ | | ✕ | | | |
| Lodise et al 2007 | | | | | | | ✕ | | | |
| Loh et al 2005 | | | | | | | ✕ | | | |
| Lujan et al 2004 | | | | | | | ✕ | | | |
| Marras et al 2004 | | | | | | ✕ | ✕ | | | |
| Martinez et al 2003 | | | | | | | ✕ | | | |
| Mello et al 2011 | | | | | | | ✕ | | | |
| Menendez et al 2002 | | | | | | | ✕ | | | |
| Menendez et al 2012 | | | | | | ✕ | ✕ | ✕ | | |
| Minhas et al 2007 | | | | | | ✕ | ✕ | ✕ | | |
| Mongardon et al 2012 | | | | | | ✕ | ✕ | ✕ | | |
| Mufson et al 2006 | | | | | | | ✕ | | | |
| Naucler et al 2013 | | | | | | | ✕ | ✕ | | |
| Paul et al 2007 | | | | | | | ✕ | | | |
| Piso et al 2013 | | | | | | | ✕ | | | |
| Portier et al 2005 | | | | | ✕ | | ✕ | | | |
| Querol-Ribelles et al 2005 | | | | | | | ✕ | | | |
| Rello et al 2002 | | | | | | ✕ | ✕ | | | |
| Reyes et al 2007 | | | | | | | ✕ | | | |
| Rodrigo et al 2013 | | | | | | ✕ | ✕ | | | |
| Romanelli et al 2002 | | | | | | | ✕ | | | |
| Tessmet et al 2009 | | | | | | | ✕ | | | |
| Torres et al 2008 | | | | | ✕ | | ✕ | | | |
| Vergis et al 2000 | | | | | ✕ | | ✕ | | | |
| Weiss et al 2004 | | | | | | | ✕ | | | |
| Welte et al 2005 | | | | | | | ✕ | | ✕ | |
| Postma et al 2015 | | | | | ✕ | | ✕ | | | |
| Mahboub et al 2015 | | | | | | | ✕ | ✕ | | |
| Zervos et al 2004 | | | | | ✕ | | ✕ | | ✕ | |
| Etemadi et al 2011 | | | | | ✕ | | | | | |
| Lee et al 2012 | | | | | ✕ | | | | ✕ | |
| Portier et al 1996 | | | | | ✕ | | | | | |
| Ramirez et al 2003 | | | | | ✕ | | | | | |
| Xu et al 2006 | | | | | ✕ | | | | | |
| Fogarty et al 2004 | | | | | ✕ | | | | ✕ | |
| Rovira et al 1999 | | | | | ✕ | | | | | |
| Vetter et al 1997 | | | | | ✕ | | | | | |
| Erard et al 2004 | | | | | | | | | ✕ | |
| Katz et al 2004 | | | | | | | | | ✕ | |
| Yi et al 2010 | | | | | | | | | ✕ | |
| Yang etal 2009 | | | | | | | | | ✕ | |
| Lopez-Vejar et al 2013 | | | | | | | | | ✕ | |
| Vardakas et al 2008 | | | | | | | | | ✕ | |
| Restrepo et al 2009 | | | | | | ✕ | | | | |
| Rodriguez et al 2007 | | | | | | ✕ | | | | |
| Aspa et al 2008 | | | | | | ✕ | | | | |
| Cilloniz et al 2011 | | | | | | ✕ | | | | |
| Dambrava et al 2008 | | | | | | ✕ | | | | |
| Grenier et al 2011 | | | | | | ✕ | | | | |
| Kontou et al 2009 | | | | | | ✕ | | | | |
| Le Bris-Tomczak et al 2012 | | | | | | ✕ | | | | |
| Pascual et al 2000 | | | | | | ✕ | | | | |
| Roson et al 2004 | | | | | | ✕ | | | | |
| Song et al 2008 | | | | | | ✕ | | | | |
| Wilson et al 2005 | | | | | | ✕ | | | | |
| Frei et al 2006 | | | | | | ✕ | | | | |
| Shorr et al 2013 | | | | | | ✕ | | | | |
| Sligl et al (in press) | | | | | | ✕ | | | | |
| Wilson et al 2012 | | | | | | ✕ | | | | |
| Arnold et al 2009 | | | | | | ✕ | | | | |
| Giusti 2016 | | | | | | | | | | ✕ |
| Rhee 2015 | | | | | | | | | | ✕ |
| Brusse-Keizer | | | | | | | | | | ✕ |
| Uzun 2014 | | | | | | | | | | ✕ |
| Yoon 2013 | | | | | | | | | | ✕ |
| Blasi 2013 | | | | | | | | | | ✕ |
| Llor 2012 | | | | | | | | | | ✕ |
| Wilson 2012 | | | | | | | | | | ✕ |
| Nouira 2010 | | | | | | | | | | ✕ |
| Daniels 2010 | | | | | | | | | | ✕ |
| Llor 2009 | | | | | | | | | | ✕ |
| Andre-Alves 2007 | | | | | | | | | | ✕ |
| Petitpretz 2007 | | | | | | | | | | ✕ |
| Lode 2004 | | | | | | | | | | ✕ |
| Basyigit 2004 | | | | | | | | | | ✕ |
| Castaldo 2003 | | | | | | | | | | ✕ |
| Nouira 2001 | | | | | | | | | | ✕ |
| Umut 1999 | | | | | | | | | | ✕ |
| Allegra 1996 | | | | | | | | | | ✕ |
Appendix 6. Additional References of Potential Interest
Related CADTH Reports
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
About the Series
CADTH Rapid Response Report: Summary with Critical Appraisal
Funding: CADTH receives funding from Canada’s federal, provincial, and territorial governments, with the exception of Quebec.
Suggested citation:
Fluoroquinolones for the treatment of respiratory tract infections. Ottawa: CADTH; 2019 May. (CADTH rapid response report: summary with critical appraisal).
Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services.
While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up-to-date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. The views and opinions of third parties published in this document do not necessarily state or reflect those of CADTH.
CADTH is not responsible for any errors, omissions, injury, loss, or damage arising from or relating to the use (or misuse) of any information, statements, or conclusions contained in or implied by the contents of this document or any of the source materials.
This document may contain links to third-party websites. CADTH does not have control over the content of such sites. Use of third-party sites is governed by the third-party website owners’ own terms and conditions set out for such sites. CADTH does not make any guarantee with respect to any information contained on such third-party sites and CADTH is not responsible for any injury, loss, or damage suffered as a result of using such third-party sites. CADTH has no responsibility for the collection, use, and disclosure of personal information by third-party sites.
Subject to the aforementioned limitations, the views expressed herein are those of CADTH and do not necessarily represent the views of Canada’s federal, provincial, or territorial governments or any third party supplier of information.
This document is prepared and intended for use in the context of the Canadian health care system. The use of this document outside of Canada is done so at the user’s own risk.
This disclaimer and any questions or matters of any nature arising from or relating to the content or use (or misuse) of this document will be governed by and interpreted in accordance with the laws of the Province of Ontario and the laws of Canada applicable therein, and all proceedings shall be subject to the exclusive jurisdiction of the courts of the Province of Ontario, Canada.