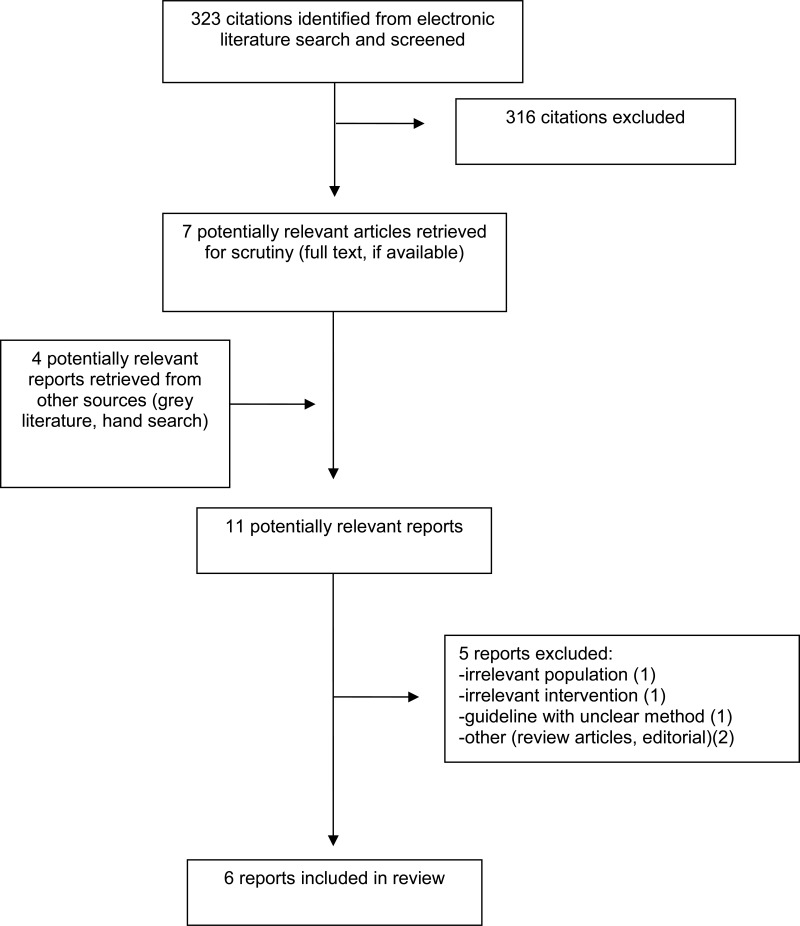
| NICE,11 2019, UK |
|---|
Intended users: Health care professionals; People with acute cough, their families and care-givers Target population: Patients with acute cough | Mucolytics (including NAC), beta-2-agonists, corticosteroids, antibiotics, and self care | Productive cough, dyspnea, lung function, and adverse events | The guideline document did not specifically report on the methodology used to develop the guidelines. However details regarding the guideline development process and GDG are available in the NICE guideline development manual.17 According to the manual, a systematic review is conducted to identify relevant evidence, the quality of the evidence is assessed using GRADE, and recommendations are formulated using formal consensus methods such as Delphi and nominal group techniques, and consensus development conferences. Also, according to the manual, GDG comprises practitioners (specialist and generalists), service or care providers, and at least two lay members such as patients or their caregivers. |
| CTS (Bourbeau),12 2017, Canada |
|---|
Intended users: Respirologist, internist, primary care physician, pharmacist, nurse practitioner, respiratory educator, and healthcare decision maker. Target population: Patients with stable COPD as well as those with concomitant asthma. | Mucolytics (including NAC), and other agents such as beta-2-agonists, antimuscarinics (or anticholinergics), corticosteroids, and phosphodiesterase-4-inhibitors. | Dysnea, exercise tolerance, physical activity, health status, exacerbations | The method used for the development of the guideline was based on the CTS guideline production methodology.19 A systematic literature review was undertaken. | Recommendations were classified using GRADE, as adapted for use by CHEST (details in CHEST/CTS [Criner],15 shown below) | Recommendations were based on scientific evidence and expert-informed opinion and were formulated based on majority consensus The GDG comprised 10 respirologists, two primary care physicians, one pharmacist, and one patient with COPD | Externally reviewed; published in a peer-reviewed journal |
| ERS/ATS (Wedzicha),13 2017, UK |
|---|
Intended users: Not mentioned explicitly; appear to be individuals involved with prevention of COPD exacerbations Target population: Patients with COPD | Mucolytics and other agents such as beta-2-agonists, antimuscarinics, roflumilast, and fluroquinolones | Exacerbation, hospitalization, quality of life, adverse events, mortality, sputum production, lung function | A systematic literature review was undertaken | Recommendations were classified as conditional or strong. A conditional recommendation for an intervention indicates that the panel was uncertain that the desirable consequences of the intervention outweigh the undesirable consequences. A strong recommendation for an intervention indicates that the panel was certain that the desirable consequences of the intervention outweigh the undesirable consequences. | Recommendations were based on iterative consensus GDG comprised 11 clinicians with experience in COPD management and research, two methodologists, and one clinician-methodologist | Reviewed and approved by all panel members before submission |
| AARC (Strickland),14 2015, US |
|---|
Intended users: Not mentioned explicitly; appear to be individuals prescribing airway clearance therapy Target population: Hospitalized patients without CF; patients with NMD, respiratory muscle weakness, or impaired cough; and post-operative patients | Mucolytics (including NAC), and other agents such as beta agonists, anticholinergics, saline (normal and hypertonic) | Airway clearance, oxygenation, hospital stay, sputum properties, quality of life, atelectasis, adverse events | Recommendations were based on a systematic review and clinical experience. | Recommendations were not graded. | As high-quality evidence was not available, a formal guideline development process was not used. Composition of GDG was not reported | Externally reviewed; published in a peer-reviewed journal |
| CHEST/CTS (Criner),15 2015, Canada |
|---|
Intended users: Not mentioned explicitly; appear to be individuals involved with prevention of COPD exacerbations Target population: Patients with COPD | Mucolytics (including NAC); and other agents such as beta agonists, anticholinergics, and non pharmacological therapies and vaccinations | Exacerbations, emergency department visits, hospital admission, unscheduled physician visit | A systematic literature review was undertaken The recommendation and its associated grade were based on information from the evidence review. | Recommendations were graded using the CHEST grading system. Grades 1A, 1B, and 1C indicate strong recommendations based on high-quality, moderate-quality, and low- or very-low-quality evidence respectively. Grades 2A, 2B, and 2C indicate weak recommendations based on high-quality, moderate-quality, and low- or very-low-quality evidence respectively. | Recommendations were drafted by three panelists, sent to all the panelists and finalized by voting. The expert panel comprised experts in pulmonology and respiratory therapy (a chair from CHEST, a vice-chair from CTS, eight panelists from CHEST and nine from CTS). A methodologist was involved in study selection, data extraction, and quality review. | Externally reviewed; published in a peer-reviewed journal |
| VA/DoD,16 2014, US |
|---|
Intended users: Primary care providers Target population: Patients with COPD | NAC, and other agents such as beta-2-agonists, antimuscarinics, corticosteroids, and phosphodiesterase-4-inhibitors, and non-pharmacological therapies and vaccination | Exacerbation, dyspnea, hospitalization, lung function, adverse events. | It was stated that the methodology used for developing the guidelines followed the process described in the VA/DoD Guideline for Guidelines.20 A systematic review was conducted. | When applicable, recommendations were classified based on the GRADE system as described in VA/DoD Guideline for Guidelines.20 | Recommendations were based on consensus Strength of the recommendations were stated where applicable GDG comprised of Guideline Champions (Champions), and subject matter experts from within the VA and DoD (Work group). Further details were not presented; however a participant list was included in the Appendix and comprised individuals with credentials such as MD, PhD, PharmD, MPH, RN. | The final drafts of VA/DoD guidelines are submitted for independent review (according to the VA/DoD Guideline methods) |