The authors conducted meta-analyses of a subset of included studies reporting SBP, DBP and HbA1c:
SBP (8 studies)
MD: −5.28 (95% CI: −8.20 to −2.36), I2=61%, p=0.012
DBP (7 studies)
MD: −2.15 (95% CI: −3.65 to −0.64), I2=55%, p=0.037
HbA1c (6 studies)
MD: −0.56 (95% CI: −0.90 to −0.22), I2=79%, p=0.000
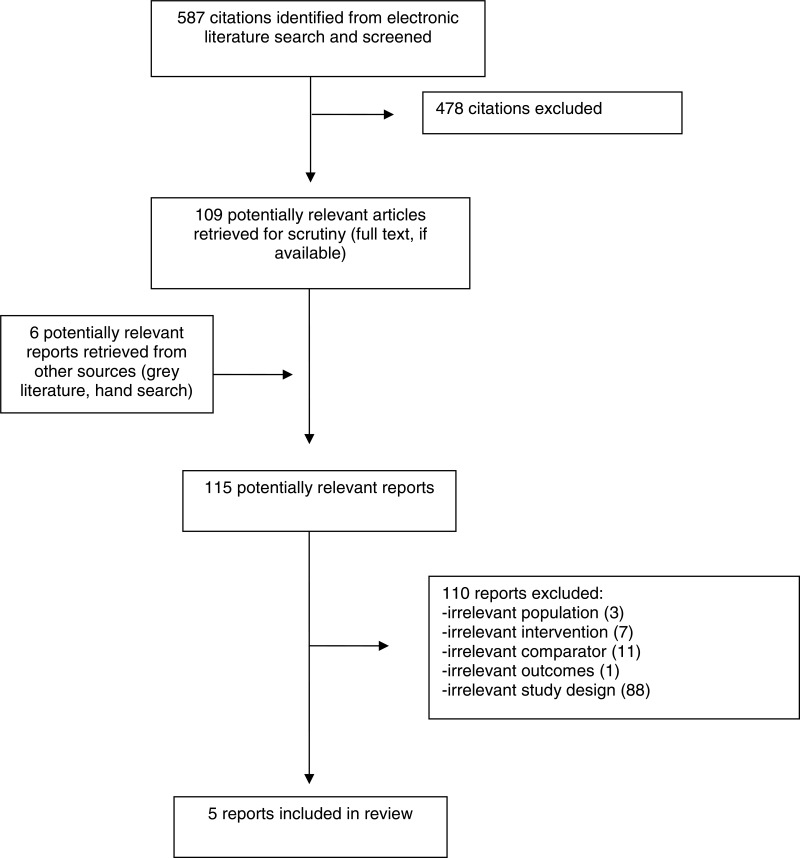
Additional primary outcomes were reported in other studies, but the results were not pooled. Full study-level summaries of these outcomes are available in the paper’s .
Characteristics of Included studies. However, completeness of reporting in this table was poor: the statistical significance of study findings were reported in few studies. Those in which interpretation was possible are summarized here:
Two studies found a positive effect of CPS on medical adherence; One study found a positive effect of CPS on mean asthma control; One study found a positive effect of CPS on ADR; One study found a positive effect of CPS on decreasing hospitalization rates.
| “The review suggested that CPS has significantly positive effect in improving SBP, DBP and HbA1c.” (pe581)
“In most included studies, CPS had a positive effect on the primary clinical outcomes, such as HbA1c, triglycerides, BP, cholesterol, HDL, LDL, blood glucose, weight, BMI, peak expiratory rate and rates of ADRs (47/52 [included studies])” (pe581)
“Positive effects were more often seen in the management of chronic conditions…” (pe581) |
Primary outcomes
Percentage outside blood pressure range (18 RCTs)
Patients in the intervention group were less likely to have blood pressure outside the target range (OR 0.40, 95% CI: 0.29 to 0.55, I2=81%, P<0.00001).
Percentage outside HbA1c range (5 RCTs)
No significant difference between the intervention and control groups (OR 0.29, 95% CI 0.04 to 2.22, I2=92%, P <00001)
Hospital attendance/admission (14 RCTs)
No significant difference between the intervention and control groups (OR 0.85, 95% CI: 0.65 to 1.11, I2=44%, P =0.04)
ADE (3 RCTs)
No significant difference between the intervention and control groups (OR 1.65, 95% CI 0.84 to 3.24, I2=52%, P =0.12)
SF-36 physical functioning (7 RCTs)
Patients in the intervention group had significantly improved physical functioning compared to the control group (MD: 5.84, 95% CI: 1.21 to 10.48, I2=84%, P <00001)
Mortality (9 trials)
No significant difference between the intervention and control groups (OR 0.79, 95% CI: 0.56 to 1.12, I2=13%, P =0.33)
Secondary outcomes
Other effects for HbA1c (15 RCTs)
Mean HbA1c was lower among the intervention group compared to the control group (MD: −0.77, 95% CI: −0.97 to −0.58, I2=77%, P <0.00001)
Continuous measures of blood pressure (31 RCTs for DBP and 32 RCTs for SPB)
DBP and SBP was reduced among intervention group compared to control (MD DBP: −3.50, 95% CI: −5.44 to −1.56, I2=94%, P <0.00001, MD SBP: −5.96, 95% CI: −7.35 to −4.57, I2=74%, P <0.00001)
Lipids (7 RCTs)
Patients in the intervention group had lower total cholesterol compared to patients in the control group (MD: −0.35, 95% CI: −0.56 to −0.13, I2=77%, P =0.00017). No significant difference in LDL cholesterol between intervention and control groups (MD: −0.14, 95% CI: −0.30 to 0.02, I2=56%, P =0.087)
Respiratory function (3 RCTs for FEV1, 2 RCTs for peak flow, and 2 RCTs for dyspnoea)
No significant difference in FEV1 (MD: 0.11, 95% CI: −0.01 to 0.23, I2=0%, P =0.81), peak flow (MD: 3.36, 95% CI: −0.36 to 7.09, I2=0%, P =0.54) or dyspnoea (OR 0.90, 95% CI: 0.68 to 1.20, I2=0%, P =0.49) between the intervention and control groups.
| “Compared with usual care, we are uncertain whether pharmacist services improved the percentage of patients outside the glycolated haemoglobin target range (very low-certainty evidence). Pharmacist services may make little or no difference to hospital attendance Pharmacist services for non-hospitalised patients or readmission (moderate-certainty evidence) or to adverse drug effects (low-certainty evidence). Pharmacist services may, however, reduce the percentage of patients whose blood pressure is outside the target range (low-certainty evidence) and may also slightly improve physical functioning (low-certainty evidence).” (p16)
“As expected, we detected substantial heterogeneity in most of the meta-analyses undertaken, possibly due to variation in interventions tested and definitions used. Using GRADE, we downgraded all outcomes to moderate certainty due to high risks of bias, with some outcomes being further downgraded due to high levels of heterogeneity.” (p16)
“The pharmacist services were poorly described and thus limit the ability to replicate these interventions for future trials or for service delivery.” (p16) |
A meta-analysis was not completed due to heterogeneity in interventions and outcomes.
One study found a positive effect of community pharmacists undertaking clinical medication reviews on MAI scores (−2.0 versus −3.0, P <0.001).23 The study also found a positive effect of the intervention on the change in number of medicines used (less in the intervention group) and dose reductions, as well as statistical improvements in SF-36 emotional role and social functioning among the control group.
A second study found significant improvement in MAI score in the intervention group (sustained clinical pharmacist interventions for elderly outpatients with polypharmacy) compared to the control (−4.9 compared to −0.9, P <0.0001).24 There was no significant difference between intervention and control for SF-36, medication compliance, medication knowledge, general healthcare satisfaction, or pharmacy-related healthcare satisfaction. A beneficial effect of the intervention on MAI was observed, but the authors note that the clinical significance of this change is unknown.
A third study found no difference in appropriateness of prescribing among older patients in the intervention group (pharmaceutical care shared between community pharmacists and general practitioners) compared to the control group.25
A fourth study found a positive effect of the intervention (medication review and educational meeting with physicians) on MAI.26 However, no significant association between intervention and SF-36, but significant improvements in hospitalization and ED visits, blood pressure control, HbA1c control, LDL and INR, medication compliance and medication knowledge. A beneficial effect of the intervention on MAI was observed, but the authors note that the clinical significance of this change is unknown.
And a fifth study found significant reductions in PIP among intervention (computerized tool that flagged potentially inappropriate medicines among patients ≥65 years) compared to control, and significant difference in dispensing rates of amitriptyline and diazepam.27 | “Three of the five studies reported an improvement in the MAI score in the intervention group compared to the control group.” (p. 7), “…however, the effect sizes are small which highlights the need for further research to assess the impact of pharmacist-led interventions in primary care.” (p9)
“…the methodological quality of the studies was poor overall, limiting the generalisability…” (p9) |