Abbreviations
- BADL
Basic Activities of Daily Living
- CHF
chronic heart failure
- CI
confidence interval
- COI
conflict of interest
- HADS
Hospital Anxiety and Depression Scale
- IADL
Instrumental Activities of Daily Living
- ICU
Intensive Care Unit
- INR
international normalized ratio
- MMSE
Mini Mental State Examination
- NIR-BD
near-infrared electromagnetic radiation-based devices
- OR
odds ratio
- RCT
randomized controlled trial
- SOC
standard of care
- SV
supraventricular
- VAS
visual analogue scale
Context and Policy Issues
Vascular access, for blood withdrawal or administration of intravenous medications, in acute and long-term care settings is an essential component of many treatment procedures. In addition to treatment delays, patients can experience additional pain and trauma associated with repeated vascular access attempts when prolonged or repeated attempts are required. Repeated attempts at vascular access may also increase the incidence of complications including blockage of arteries, bleeding outside blood vessels (hematoma), blood clots, and damage to blood vessels and nerves.1
Among the most important predictors of a difficult venipuncture are advanced age, dehydration, previous hospitalizations, previous failed attempts, and a history of hypertension or diabetes mellitus.2,3 Adult populations of advanced age often have age-related changes to subcutaneous tissue that can make vein puncture difficult.4
The standard procedure for vascular access is initiated by localization of a suitable vein by visual inspection and palpation. Vascular illumination devices aim to aid in vein localization, facilitating accurate needle placement by the device operator and thereby decreasing the time and attempts required to obtain vascular access.2,4 These devices use near infrared light capable of penetrating several centimeters of tissue to produce a 2D image of blood-filled structures superimposed upon the surface of the skin. Vein illumination devices can be made portable, lightweight, and cordless.5 The clinical benefit of these devices for some populations remains unclear.6,7
The purpose of this report is to retrieve and review the existing evidence on the clinical effectiveness and cost-effectiveness of the use of vascular illumination devices for adult patients, including elderly patients, in acute or long-term care settings. Additionally, this report aims to retrieve and review the evidence-based guidelines on the use of vascular illumination devices for this population.
Research Questions
What is the clinical effectiveness of vein illumination devices for vascular access procedures for adult patients in an acute care and/or long-term care settings?
What is the cost-effectiveness of vein illumination devices for vascular access in adult patients in acute care and/or long-term settings?
What are the evidence-based guidelines for use of vascular access imaging devices for adult patients in acute care and/or long term care settings?
Key Findings
This report identified limited quality evidence to evaluate the clinical efficacy of vein illumination devices for adult patients in acute or long-term care. Evidence from one randomized controlled pilot trial with significant methodological limitations found no difference in outcomes of time and attempts required for vascular access using a vein illumination device as compared to a standard vascular access protocol in a population of elderly acute care patients. This study did identify a significantly lower frequency of hematoma complications as well as less anxiety and depression in the vein illumination device treatment group at a time point following venous access. Further high-quality research is required to make definitive conclusions regarding the clinical efficacy and safety of vein illumination devices in this population. No relevant cost-effective evidence or evidence-based guidelines were identified.
Methods
Literature Search Methods
A limited literature search was conducted by an information specialist on key resources including Ovid Medline, the Cochrane Library, the University of York Centre for Reviews and Dissemination (CRD) databases, the websites of Canadian and major international health technology agencies, as well as a focused internet search. The search strategy was comprised of both controlled vocabulary, such as the National Library of Medicine’s MeSH (Medical Subject Headings), and keywords. The main search concepts were vein illumination devices and long term or acute care. No filters were applied to limit the retrieval by study type. Where possible, retrieval was limited to the human population. The search was also limited to English language documents published between January 1, 2010 and March 24, 2020.
Selection Criteria and Methods
One reviewer screened citations and selected studies. In the first level of screening, titles and abstracts were reviewed and potentially relevant articles were retrieved and assessed for inclusion. The final selection of full-text articles was based on the inclusion criteria presented in .
Exclusion Criteria
Articles were excluded if they did not meet the inclusion criteria outlined in , were duplicate publications, or were published prior to 2010.
Critical Appraisal of Individual Studies
The included randomized study was critically appraised by one reviewer using the Downs and Black checklist.8 Summary scores were not calculated for the included studies; rather, the strengths and limitations of each included study were described narratively.
Summary of Evidence
Quantity of Research Available
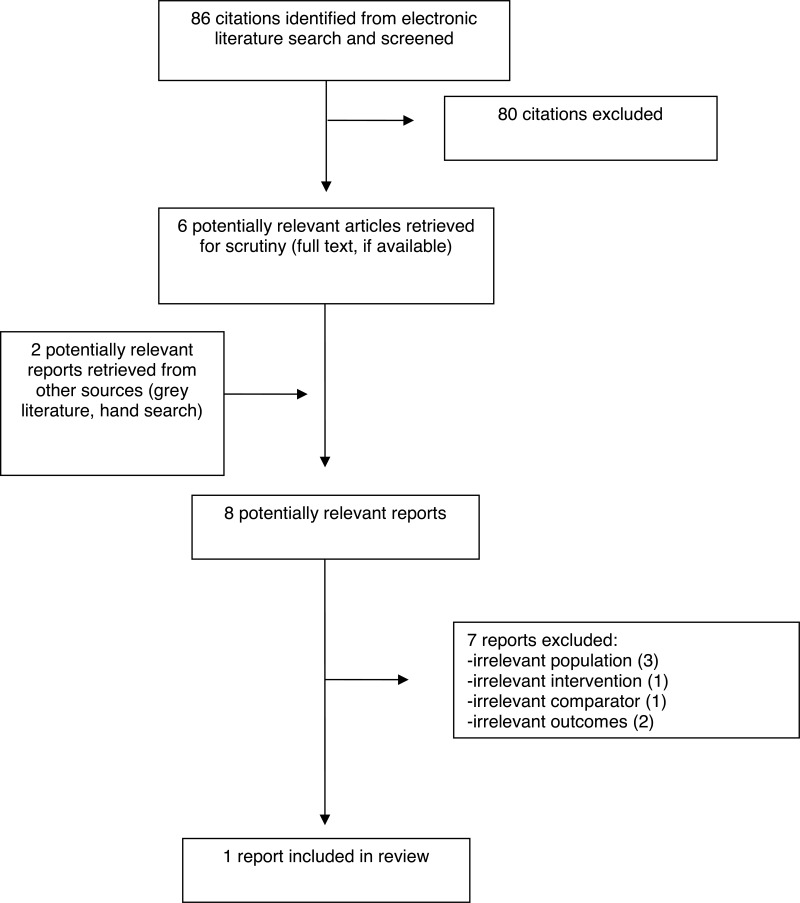
A total of 86 citations were identified in the literature search. Following screening of titles and abstracts, 80 citations were excluded and six potentially relevant reports from the electronic search were retrieved for full-text review. Two potentially relevant publications were also retrieved from the grey literature search for full text review for a total of eight full-text publications examined for inclusion in this report. Of these potentially relevant articles, three were excluded for examining an irrelevant population, two for examining irrelevant outcomes, one for examining an irrelevant intervention, and one for examining an irrelevant comparator. One publication, a randomized study, met the inclusion criteria and was included in this report.4
Appendix 1 presents the PRISMA flowchart of the study selection.9
Summary of Study Characteristics
Additional details regarding the characteristics of the included publication are provided in Appendix 2.
Study Design
The included primary study was conducted as a pilot RCT, published in 2017.4
Country of Origin
The included pilot RCT took place at a single intensive care unit (ICU) in Florence, Italy.4
Patient Population
Patients in the included study were enrolled as a consecutively admitted patient cohort to the ICU that required venous puncture and consisted of 103 patients. No exclusion criteria were defined. This enrollment resulted in a cohort of elderly critically ill patients with a mean age of 74 ± 12 years and consisted of 59.2% males. Reported characteristics of the treatment groups included blood counts, concomitant medications, comorbidities, and main diagnosis. The most common main diagnoses were acute coronary syndromes, chronic heart failure, and supraventricular (SV) arrhythmias. Additionally, Basic (BADL) and Instrumental (IADL) Activities of Daily Living scores, and Mini Mental State Examination (MMSE) scores were reported at baseline.4
Interventions and Comparators
Patients were randomized to standard of care (SOC) for vascular access, or a vein illumination device that was referred to as a near-infrared electromagnetic radiation-based device (NIR-BD). The protocol defined the SOC for vascular access as veins pricked following a tightened tourniquet, visual inspection and palpation. The intervention group was the same as the SOC protocol except visual inspection of the area of interest was conducted with NIR-BD (EasyVein, InSono, Calenzano, Florence, Italy). The device displayed veins detected with near-infrared superimposed upon a visible light image of the area of interest to the user on an LCD screen. The authors reported that the device users, three nurses, were trained in its use. By protocol, patients could crossover to the other treatment group in the case of failure of the first attempts.4
Outcomes
The clinical outcomes reported by Fumagalli et al. were pain as measured by patients on a visual analogue scale (VAS), Hospital Anxiety and Depression Scale (HADS), complications (hematoma), time required for vascular access, consecutive attempts for vascular access, and number of crossovers to the alternative treatment group. Pain was assessed immediately after the procedure on a VAS where the patient indicated a higher number with more perceived pain. The VAS scale itself was not defined. Anxiety and depression were independently assessed by HADS on a scale from 1 to 21, with greater numbers indicating greater anxiety and depression.10 The exact timing of HADS assessment was unclear but was assessed after blood sample drawing or peripheral vein catheter insertion without baseline assessments. Analysis also reported patient risk factors for hematoma. The crossover outcome did not provide a defined number of ‘first attempts’ before patients were permitted to crossover to the alternative treatment group.4
Summary of Critical Appraisal
This study had significant strengths including a comprehensive list of patient characteristics, inclusive patient criteria that increased external validity, and a comment regarding device training and a discussion on the limitations of the study design were provided. The study also did not have any loss-to-follow-up. As a pilot RCT, Fumagalli et al.,4 did not provide a statistical power calculation to determine sample size requirements; however, the authors cited a lack of data for this patient population to provide a basis for such a calculation. Additional important limitations of this RCT included a lack of allocation concealment and a lack of clear randomization methodology, a lack of blinding, restricted reporting for pain outcomes (limited to reporting the number of patients with VAS > 1), an incomplete outcome definition for crossover events, and a lack of definition for hematoma complication events. The authors reported that the observed frequency of hematoma complications in the SOC group was much higher (28.6% vs 5.5%), than comparable literature values and suggested that it was probably a result of difference in sensitivity criteria. Two authors were employees of the device manufacturer which represented a conflict of interest. Overall, the limitations of this study as outlined in this paragraph reduce the confidence in the findings of this study.
Additional details regarding the strengths and limitations of included publications are provided in Appendix 3.
Summary of Findings
Clinical Effectiveness of Vein Illumination Devices
Statistically significant differences (P < 0.05) in clinical effectiveness parameters examined by Fumagalli et al. consisted of fewer complications of hematoma, and lower anxiety and depression as measured by HADS when the vein illumination device, EasyVein, was used as compared to the SOC.4 While this study had inclusive patient criteria that increased the external validity of these results, a definition of hematoma complication was not provided in the methodology so it is not clear how consistently this outcome was reported. Importantly, a baseline HADS measure was not taken in this study which meant that the degree to which the level of anxiety and depression was affected by the intervention and comparator was not clear, nor did the authors provide information on what would be a clinically important difference in HADS measures. The authors suggested that, based on a lack of differences in other patient characteristics at baseline, significant baseline differences in HADS measures were unlikely.
This relatively small study (n = 103) did not detect a statistically significant difference in outcomes of pain, time required, crossovers, or number of consecutive events for vascular access between SOC and NIR-BD. It is possible that this pilot RCT was underpowered to detect significant differences in these outcomes. Pain was reported only as percent of patients self-reporting pain as VAS ≥ 1, which may not accurately represent the range of pain experienced by the elderly study participants. The number of vascular access attempts prior to switching patients to the alternative treatment group was also left undefined so there is some lack of clarity on the comparability of crossover event outcomes.
Univariate analysis identified crossover events, time required, and the number of attempts required for venous access as statistically significant independent risk factors for hematoma formation in all study participants. While international normalized ratio (INR), differed between treatment groups (SOC: 1.4 ± 0.5; NIR-BD: 1.2 ± 0.3), the difference did not reach statistical significance (P = 0.05), and multivariate logistic regression analysis did not identify INR as a predictor of hematoma formation in this patient cohort.
Cost-Effectiveness of Vein Illumination Devices
No evidence regarding the cost-effectiveness of NIR-BD for the population of interest was identified.
Guidelines for Vein Illumination Devices
No evidence-based guidelines regarding the use of NIR-BD for adult patients in acute or long-term care settings were identified.
Appendix 4 presents a table of the main study findings and authors’ conclusions.
Limitations
Most significantly, this report is limited by the quantity of identified evidence on the clinical-effectiveness, cost-effectiveness, and guidelines on vein illumination devices for adult patients in acute or long-term care. One pilot RCT authored with an important conflict of interest was identified that provided evidence for the clinical efficacy of a vein illumination device in an elderly ICU population; however, no evidence was identified on elderly patients in a long-term care setting. Aside from the other limitations outlined in the critical appraisal, the applicability of this evidence from a pilot study conducted in Italy to the Canadian health care setting is uncertain. No cost-effectiveness or evidence-based guidelines were identified.
Conclusions and Implications for Decision or Policy Making
A single pilot RCT, published in 2017, was identified that provided clinical efficacy evidence for NIR-BD for an elderly patient population in acute care. Fumagalli et al. found decreased complications of hematoma incidence in addition to lower anxiety and depression following venous access using a vein illumination device as compared to SOC. The study found no statistically significant differences between the vein illumination device and SOC in the time or number of attempts required for venous access, or any statistically significant difference in a patient-reported pain outcome. The authors concluded that in elderly critically ill patients NIR-BD does not require more time or more attempts for venous access while also not increasing pain associated with the procedure.
The purpose of vein illumination devices is to aid in visualizing even particularly thin or hidden veins.4 The clinical importance of increased vein visualization without a correlation to decreased time and number of attempts for venous access was not addressed by the authors; however, it is possible the study was simply underpowered, or perhaps a study on a more specific subpopulation of elderly ICU patients would reveal statistically significant differences in these outcomes. The evidence in this RCT that suggested a decreased incidence of hematoma complications in elderly acute care patients when using vein illumination devices for venous access warrants further study. The authors did not hypothesize a mechanism for decreased hematoma formation using vein illumination, and the higher frequency of hematoma formation in the SOC treatment group (28.6%) as compared to prior literature was not sufficiently discussed. The lower anxiety and depression in the NIR-BD intervention group also suggests superiority of vein illumination devices over SOC for venous access; however, future studies should provide baseline measures of anxiety and depression, in addition to establishing a minimal clinically important difference in HAD outcomes, so that the impact of the intervention can be more definitively quantified.
Two prior CADTH publications on the topic of vein illumination devices identified inconsistent findings of clinical effectiveness for vein illumination devices.6,7 One Rapid Response Report: Summary with Critical Appraisal found evidence from seven adequately powered RCTs and three non-randomized studies that did not support superior clinical effectiveness of VascuLuminator, AccuVein AV300, or VeinViewer as compared to SOC. However, the population of interest in that report was the general pediatric population in acute care and is of uncertain applicability to adult patients in acute or long-term care. The authors of four RCTs identified in this prior CADTH report suggested that future research may identify a role for vein illumination devices in specific patient populations.6 Another Rapid Response Report: Summary of Abstracts published by CADTH on this topic also briefly summarizes evidence of inconsistent findings on the effectiveness of vein illumination devices organized by population of interest however the focus was on neonates in acute care settings and patients in the emergency department, no evidence on adult or elderly patients in acute care or long-term settings was included in this report.7
The evidence retrieved and reviewed in this report is associated with a significant degree of uncertainty and further well-conducted RCTs are required to make clinical efficacy conclusions on vein illumination devices for vascular access in acute or long-term care patients.
References
- 1.
Cuper
NJ, de Graaff
JC, Hartman
BJ, Verdaasdonk
RM, Kalkman
CJ. Difficult arterial cannulation in children: is a near-infrared vascular imaging system the answer?
Br J Anaesth. 2012;109(3):420–426. [
PubMed: 22735300]
- 2.
Perry
AM, Caviness
AC, Hsu
DC. Efficacy of a near-infrared light device in pediatric intravenous cannulation: a randomized controlled trial.
Pediatr Emerg Care. 2011;27(1):5–10. [
PubMed: 21178814]
- 3.
Lapostolle
F, Catineau
J, Garrigue
B, et al. Prospective evaluation of peripheral venous access difficulty in emergency care.
Intensive Care Med. 2007;33(8):1452–1457. [
PubMed: 17554524]
- 4.
Fumagalli
S, Torricelli
G, Massi
M, et al. Effects of a new device to guide venous puncture in elderly critically ill patients: results of a pilot randomized study.
Aging Clin Exp Res. 2017;29(2):335–339. [
PubMed: 26914485]
- 5.
Kaddoum
RN, Anghelescu
DL, Parish
ME, et al. A randomized controlled trial comparing the AccuVein AV300 device to standard insertion technique for intravenous cannulation of anesthetized children.
Paediatr Anaesth. 2012;22(9):884–889. [
PubMed: 22694242]
- 6.
- 7.
- 8.
- 9.
Liberati
A, Altman
DG, Tetzlaff
J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration.
J Clin Epidemiol. 2009;62(10):e1–e34. [
PubMed: 19631507]
- 10.
Zigmond
AS, Snaith
RP. The hospital anxiety and depression scale.
Acta Psychiatr Scand. 1983;67(6):361–370. [
PubMed: 6880820]
Appendix 1. Selection of Included Studies
Appendix 2. Characteristics of Included Publications
Table 2Characteristics of Included Primary Clinical Study
View in own window
| First Author, Publication Year, Country | Study Design | Population Characteristics | Intervention and Comparator(s) | Clinical Outcomes |
|---|
| Fumagalli, 20174, Italy | Pilot RCT Single center study examined prospectively enrolled consecutive patients Crossover by protocol in case of failure of either intervention | All patients (74 ± 13 years old) requiring venous puncture. No exclusion criteria defined. (n = 103)
Main diagnoses included ACS, CHF, SV arrhythmias, and others. | EasyVein (InSono, Calenzano, Florence, Italy). An ‘augmented reality’ of visualized veins using near infrared are superimposed on a visible light image and displayed on an LCD screen. (n = 47)
Standard methodology: following tourniquet tightening veins were pricked after inspection and palpation (n = 56) | |
ACS = acute coronary syndromes; CHF = chronic heart failure; RCT = randomized controlled trial; SV = supraventricular; VAS = visual analogue scale.
Appendix 3. Critical Appraisal of Included Publications
Table 3Strengths and Limitations of Clinical Studies using Downs and Black8
View in own window
| Strengths | Limitations |
|---|
| Fumagalli, 20174 |
|---|
Patient characteristics tabulated - comprehensive characteristics and no significant differences between treatment groups Clearly defined patient eligibility and intervention Reported complications Training of device operators mentioned Discussion on study limitations provided No loss-to-follow-up
|
Single center study No allocation concealment or randomization methodology reported Open-label study - no blinding Pilot study - did not include a statistical power calculation to determine sample size requirements COI - Industry funded investigators Complication outcomes left undefined Incomplete outcome reporting SOC results not consistent with historical literature
|
COI = conflict of interest; SOC = standard of care.
Appendix 4. Main Study Findings and Authors’ Conclusions
Table 4Summary of Findings of Included Primary Clinical Study
View in own window
| Main Study Findings | Authors’ Conclusion |
|---|
| Fumagalli, 20174 |
|---|
| Hematoma | “In elderly critically ill patients, the utilization of a new NIR-BD technology to make easier the visualization and puncture of veins does not require more time and attempts than the traditional one. Patients do not experience more pain. However, the new approach is associated to a significantly reduced incidence of hematoma formation and to a lower burden of anxiety and depressive symptoms. The results of this pilot study could represent the basis of further trials aimed at confirming the advantages of this approach and identifying subjects most likely to benefit from it.” (p. 338) |
| Incidence (P = 0.012) |
| NIR-BD (n = 47) | 8.5% |
| SOC (n = 56) | 28.6% |
| HADS - lower score indicates less anxiety/depression |
| Anxiety (P = 0.038) |
| NIR-BD (n = 47) | 5.8 |
| SOC (n = 56) | 7.7 |
| Depression (P = 0.037) |
| NIR-BD (n = 47) | 5.7 |
| SOC (n = 56) | 7.5 |
| Pain (VAS ≥ 1) - lower score indicates less pain |
| Before puncture (P = 1.000) |
| NIR-BD (n = 47) | 10.6% |
| SOC (n = 56) | 12.5% |
| End of procedure (P = 0.557) |
| NIR-BD (n = 47) | 51.1% |
| SOC (n = 56) | 44.6% |
| Time needed (minutes) (P = 0.173) |
| NIR-BD (n = 47) | 8.0 ± 5.8 |
| SOC (n = 56) | 7.0 ± 3.9 |
| Number of consecutive attempts (P = 0.361) |
| NIR-BD (n = 47) | 1.2 ± 0.6 |
| SOC (n = 56) | 1.3 ± 0.6 |
| Crossover to other technique (P = 0.081) |
| NIR-BD (n = 47) | 6.4% |
| SOC (n = 56) | 19.6% |
| Univariate risk factors for hematoma formation |
| Time needed (minutes) (P = 0.003) |
| Hematoma (n = 20) | 10.3 ± 4.0 |
| No hematoma (n = 83) | 6.8 ± 4.8 |
| Number of consecutive attempts (P = 0.001) |
| Hematoma (n = 20) | 1.8 ± 0.7 |
| No hematoma (n = 83) | 1.2 ± 0.5 |
| Crossover to other technique (P < 0.001) |
| Hematoma (n = 20) | 45.0% |
| No hematoma (n = 83) | 6.0% |
| Multivariate risk factors for hematoma formation |
| Number of consecutive attempts |
| Overall (P < 0.001) |
| ORper attempt (95% CI) | 5.86 (2.23 to 15.41) |
| NIR-BD group (P = 0.022) |
| ORper attempt (95% CI) | 0.21 (0.05 to 0.80) |
| INR values, time needed, and crossover to other technique were not statistically significant. |
CI = confidence interval; HADS = Hospital Anxiety and Depression Scale; INR = international normalized ratio; NIR-BD = near-infrared electromagnetic radiation based device; OR = odds ratio; SOC = standard of care; VAS = visual analogue scale.
About the Series
CADTH Rapid Response Report: Summary with Critical Appraisal
Funding: CADTH receives funding from Canada’s federal, provincial, and territorial governments, with the exception of Quebec.
Suggested citation:
Vein Illumination Devices in Long-Term and Acute Care Settings: A Review of Clinical Effectiveness, Cost-Effectiveness and Guidelines. Ottawa: CADTH; 2020 Apr. (CADTH rapid response report: summary with critical appraisal).
Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services.
While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up-to-date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. The views and opinions of third parties published in this document do not necessarily state or reflect those of CADTH.
CADTH is not responsible for any errors, omissions, injury, loss, or damage arising from or relating to the use (or misuse) of any information, statements, or conclusions contained in or implied by the contents of this document or any of the source materials.
This document may contain links to third-party websites. CADTH does not have control over the content of such sites. Use of third-party sites is governed by the third-party website owners’ own terms and conditions set out for such sites. CADTH does not make any guarantee with respect to any information contained on such third-party sites and CADTH is not responsible for any injury, loss, or damage suffered as a result of using such third-party sites. CADTH has no responsibility for the collection, use, and disclosure of personal information by third-party sites.
Subject to the aforementioned limitations, the views expressed herein are those of CADTH and do not necessarily represent the views of Canada’s federal, provincial, or territorial governments or any third party supplier of information.
This document is prepared and intended for use in the context of the Canadian health care system. The use of this document outside of Canada is done so at the user’s own risk.
This disclaimer and any questions or matters of any nature arising from or relating to the content or use (or misuse) of this document will be governed by and interpreted in accordance with the laws of the Province of Ontario and the laws of Canada applicable therein, and all proceedings shall be subject to the exclusive jurisdiction of the courts of the Province of Ontario, Canada.