Abbreviations
- ADA
adalimumab
- AMSTAR
A Measurement Tool to Assess Systematic Reviews
- CD
Crohn’s disease
- CDAI
Crohn’s Disease Activity Index
- CI
Confidence interval
- CrI
Credible interval
- HR
Hazard ratio
- IFX
infliximab
- IV
Intravenous
- MA
Meta-analysis
- NMA
Network meta-analysis
- NR
Not reported
- OR
Odds ratio
- RCT
Randomized controlled trial
- RR
Risk ratio
- SC
Subcutaneous
- SR
Systematic review
- TNFi
Tumour necrosis factor inhibitor
- VEDO
vedolizumab
Context and Policy Issues
In 2018, of the approximate 270,000 Canadians diagnosed with inflammatory bowel disease, 135,000 Canadians were living with Crohn’s disease (CD).1 With a total direct cost of about $1.28 billion in 2018, roughly 42% is allocated to prescription drug use for individuals with inflammatory bowel disease.2 As an incurable chronic disease with alternating periods of relapse and remission, CD is characterized by symptoms such as weight loss, diarrhea, and abdominal pain.3
To induce remission, conventional pharmacotherapy (e.g., corticosteroids, purine analogues, methotrexate) can be used.4 For patients with refractory CD, biologic therapy (e.g., tumour necrosis factor inhibitors [TNFi], non-TNFi biologics) are available.4 Furthermore, about 75% of individuals suffering from CD will undergo surgical resection at least once.3 However, postoperative endoscopic and clinical recurrence rates can be as high as 61% after 6 months and 86% after 5 years, respectively.3 The aforementioned pharmacotherapy options can be used to lengthen the duration of postoperative remission.3 Nonetheless, uncertainty remains regarding the comparative effectiveness of these pharmacotherapy interventions.3
Biologics such as TNFi (e.g., adalimumab [ADA], infliximab [IFX]) and non-TNFi (e.g., vedolizumab [VEDO], an anti-integrin) have been shown by randomized placebo controlled trials to be effective for individuals with moderate to severe CD that is refractory to conventional treatment options.5 Due to the scarcity of head-to-head trials comparing one biologic to another, the choice of first and second-line biologic therapy has been dependent on clinician experience, patient preference, and/or drug coverage.6 Public and private drug payers have implemented tiered coverage policies as a cost control measure.7 In cases where there is a lack of evidence that one option is more effective than another, these policies require patients to trial inexpensive alternatives first before applying for special authorization to use a higher cost option.8
The objective of this report is to review and summarize the relevant literature regarding the clinical effectiveness of ADA versus IFX or VEDO in adult patients with CD.
Research Question
What is the clinical effectiveness of adalimumab versus infliximab or vedolizumab in adult patients with Crohn’s Disease?
Key Findings
Seven systematic reviews (two with meta-analyses and five with network meta-analyses) and four relevant head-to-head primary studies contained within the systematic reviews were identified regarding the clinical effectiveness of adalimumab compared to infliximab or vedolizumab in adults with Crohn’s disease. The seven systematic reviews were generally well-conducted, but there were methodological limitations in their included primary studies which provided low to moderate strength evidence.
The identified literature revealed mixed conclusions regarding the clinical effectiveness of adalimumab compared to infliximab or vedolizumab in adults with Crohn’s disease. Specifically, in two primary studies and three network meta-analyses comparing adalimumab to infliximab, no treatment was favoured in effectiveness (e.g., disease recurrence) and safety (e.g., hospitalization due to serious infection) outcomes. However, significant findings from two primary studies and one network meta-analysis favoured the use of infliximab in terms of risk of abdominal surgery, early treatment termination, or induction of clinical response, while findings from one network meta-analysis favoured the use of adalimumab in terms of withdrawals due to adverse events.
In three network meta-analyses comparing adalimumab to vedolizumab, no treatment was favoured in effectiveness (e.g., induction of clinical remission) and safety (e.g., withdrawals due to adverse events) outcomes. However, findings from one of the aforementioned network meta-analyses favoured adalimumab over vedolizumab in the maintenance of clinical remission.
The limitations of the included primary literature (e.g., lack of blinding of participants and health care professionals, heterogeneity of outcome measures, variation in prior abdominal surgery history, inconsistencies in study findings) should be considered when interpreting these results. Additionally, since two network meta-analyses involved studies of post-surgical patients, the findings from this report may not be generalizable to all patients living with Crohn’s disease.
Methods
Literature Search Methods
A limited literature search was conducted by an information specialist on key resources including PubMed, the Cochrane Library, the University of York Centre for Reviews and Dissemination (CRD) databases, the websites of Canadian and major international health technology agencies, as well as a focused internet search. The search strategy was comprised of both controlled vocabulary, such as the National Library of Medicine’s MeSH (Medical Subject Headings), and keywords. The main search concepts were adalimumab and Crohn’s Disease. Search filters were applied to limit retrieval to health technology assessments, systematic reviews, meta-analyses, or network meta-analyses, and randomized controlled trials or controlled clinical trials. Where possible, retrieval was limited to the human population. The search was also limited to English language documents published between January 01, 2010 and March 27, 2020.
Selection Criteria and Methods
One reviewer screened citations and selected studies. In the first level of screening, titles and abstracts were reviewed and potentially relevant articles were retrieved and assessed for inclusion. The final selection of full-text articles was based on the inclusion criteria presented in .
Exclusion Criteria
Articles were excluded if they did not meet the selection criteria outlined in , they were duplicate publications, or were published prior to 2010.
Critical Appraisal of Individual Studies
The included systematic reviews (SRs) were critically appraised by one reviewer using A MeaSurement Tool to Assess systematic Reviews 2 (AMSTAR 2)9 and network meta-analyses (NMA) were critically appraised using the ISPOR “Questionnaire to assess the relevance and credibility of a network meta-analysis.”10 Summary scores were not calculated for the included studies; rather, the strengths and limitations of each included study were described narratively.
Summary of Evidence
Quantity of Research Available
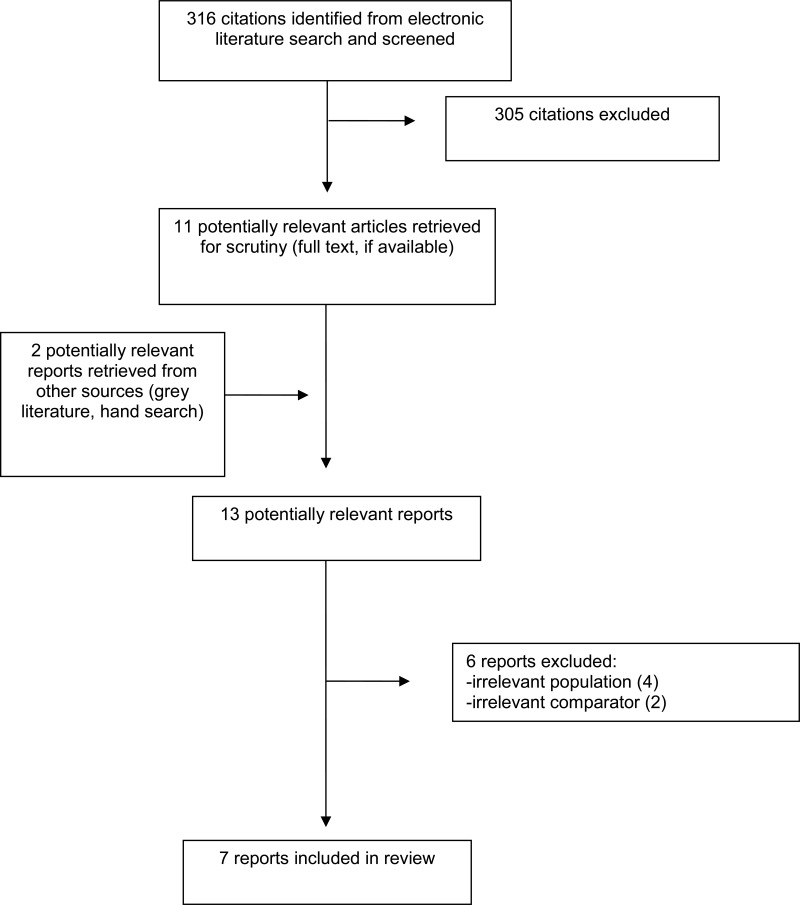
A total of 316 citations were identified in the literature search. Following screening of titles and abstracts, 305 citations were excluded and 11 potentially relevant reports from the electronic search were retrieved for full-text review. Two potentially relevant publications were retrieved from the grey literature search for full-text review. Of these potentially relevant articles, six publications were excluded for various reasons, and seven publications met the inclusion criteria and were included in this report. These comprised seven SRs3,5,6,11–14 (two with meta-analyses [MA]13,14 and five with NMA3,5,6,11,12). Appendix 1 presents the PRISMA15 flowchart of the study selection. Additional references of potential interest are provided in Appendix 6.
Summary of Study Characteristics
Seven SRs3,5,6,11–14 (two with MA13,14 and five with NMA3,5,6,11,12) were identified for inclusion in this review. Additional details regarding the characteristics of included publications are provided in Appendix 2.
The seven included SRs3,5,6,11–14 had broader inclusion criteria than the current report. In addition to five NMAs that conducted indirect comparisons and two MAs, the study characteristics and results of four relevant primary studies found within the included SRs directly comparing ADA to IFX or VEDO are described and summarized in this report.
Study Design
Seven SRs3,5,6,11–14 (two with MA13,14 and five with NMA3,5,6,11,12) were included in this report.
With no language restriction, the two SRs with NMA authored by Bakouny et al. (2019)11 and Iheozor-Ejiofor et al. (2019)3 consisted of studies published from database inception to August 5, 2017 and January 15, 2019, respectively. With study design restricted to interventional comparative randomized or nonrandomized trials, Bakouny et al. (2019)11 included a total of 9 studies; one open-label, randomized study16 was relevant for the current report. With study design restricted to RCTs, Iheozor-Ejiofor et al. (2019)3 included a total of 35 studies; the same open-label, randomized study16 was relevant for the current report. Bakouny et al. (2019)11 performed a NMA using the frequentist approach, while Iheozor-Ejiofor et al. (2019)3 used the Bayesian approach. A table of primary study overlap between the included network meta-analyses is provided in Appendix 5.
Three other SRs with NMA5,6,12 met the inclusion criteria for this report; however, none identified any eligible primary studies. With no language restriction5,6 or language restriction not reported,12 all three SRs with NMA restricted study design to RCTs. Singh et al. (2018),6 Hazlewood et al. (2015),12 and Singh et al. (2014)5 conducted database searches from inception to May 31, 2017, January 2007 to June 2014, and January 1, 1985 to September 30, 2013, respectively. Singh et al. (2018)6 performed a NMA using the frequentist approach, while Hazlewood et al. (2015)12 and Singh et al. (2014)5 used the Bayesian approach.
The two SRs with MA authored by Singh et al. (2020)13 and Kawalec et al. (2013)14 consisted of studies published from database inception to March 18, 2018 and November 2012, respectively. With publication language restricted to English, French, German or Polish and study design restricted to controlled clinical trials (randomized or non-randomized), Kawalec et al. (2013)14 included a total of 19 studies; one open-label, randomized prospective study17 was relevant for the current report. With publication language restricted to English and study design restricted to cohort studies, Singh et al. (2020)13 included a total of 15 studies; two retrospective cohort studies18,19 were relevant for the current report.
Country of Origin
The first authors of the SRs were from Canada,12 Lebanon,11 Poland,14 UK,3 and US.5,6,13 The four relevant primary studies were conducted in Belgium,17 Denmark,19 Italy,16 and US.18
Patient Population
The SRs authored by Bakouny et al. (2019)11 and Iheozor-Ejiofor et al. (2019)3 included studies involving adult patients (≥ 18 years old) diagnosed with CD who have had prior resection (removal of all or part of a section of the gastrointestinal tract). Although Iheozor-Ejiofor et al. (2019) also included studies that recruited patients ≥ 16 years of age, most of the 35 included studies recruited adult patients ≥ 18 years old.3 The total number of participants included in the NMA conducted by Bakouny et al. (2019)11 and Iheozor-Ejiofor et al. (2019)3 were 571 and 3249, respectively. From these two SRs,3,11 one overlapping RCT16 was identified to be relevant for this report. In this relevant RCT, the median ages of participants receiving ADA (n = 10) and IFX (n = 10) were 34.5 and 30.5 years old, respectively.16 The number of participants who have previously used IFX in the ADA and IFX groups were four (40%) and five (50%), respectively.16
The SR authored by Kawalec et al. (2013) included studies involving adult patients (≥ 18 years old) diagnosed with moderate to severe CD or fistulizing CD.14 Within the one relevant RCT involving adult patients diagnosed with CD who have had continued response to at least 6 months of IFX, the number of participants randomized to switch to ADA or continue IFX were 36 and 37, respectively.17 The median ages of participants receiving ADA and IFX were 38 and 37 years old, respectively.17 The percentage of participants with previous abdominal surgery was not reported.17
The SR authored by Singh et al. (2020) included studies involving patients diagnosed with CD or ulcerative colitis (unspecified age group).13 Within the two relevant retrospective cohort studies involving biologic-naïve adult patients (≥ 18 years old) diagnosed with CD, the number of participants receiving ADA and IFX, respectively, were 315 and 51219 or 1248 and 1427.18 The mean ages of participants receiving ADA and IFX, respectively, were 34.9 and 33.619 or 40 and 41 years old.18 The percentage of participants with prior abdominal surgery receiving ADA and IFX, respectively, were 37.1% and 36.7%19 or 5% and 4%.18
Three other SRs5,6,12 included studies involving adult patients diagnosed with moderate to severe CD. In the NMA conducted by Singh et al. (2018),6 1458 biologic-naïve and 1606 biologic-exposed participants were included in the analyses for the induction of clinical remission and response, while 1854 participants were included in the analysis for the maintenance of clinical remission. The total number of participants included in the NMA conducted by Singh et al. (2014) was 2530.5 Hazlewood et al. (2015) did not report the total number of participants included in their NMA.12 No primary studies were identified to be relevant for this report from these three aforementioned SRs.
Interventions and Comparators
All seven included SRs3,5,6,11–14 included primary studies that compared a wider array of pharmacological treatment options for CD (e.g., TNFi, non-TNFi biologics, corticosteroids, 5-aminosalicylic acid, methotrexate, sulfasalazine, purine analogues, antibiotics, and/or probiotics) to no treatment, placebo, or other active treatments. All five included SRs with NMA3,5,6,11,12 allowed the indirect comparison of ADA and IFX, while three SRs with NMA5,6,12 allowed the indirect comparison of ADA and VEDO.
Both relevant RCTs16,17 compared ADA to IFX with treatment durations of one year. In the study authored by Tursi et al. (2014),16 participants were randomized to receive ADA (160 mg subcutaneously [SC], followed by 80 mg 2 weeks later, and then 40 mg every 2 weeks) or IFX (5 mg/kg intravenously [IV] at 0, 2, and 6 weeks, and then every 8 weeks). In the study authored by Van Assche et al. (2012),17 participants were randomized to receive ADA (80 mg subcutaneously followed by 40 mg every other week) or continue IFX (5 mg/kg).
Within the two relevant retrospective cohort studies identified from the SR authored by Singh et al. (2020),13 one study19 compared ADA and IFX, while the other study18 compared ADA, IFX, and certolizumab pegol. While dosing regimens were not reported in either primary studies, the median follow-up period was 2.3 years19 and 19 months.18
Outcomes
The authors of five SRs3,6,11,12,14 (four with NMA3,6,11,12) investigated disease recurrence and adverse events as outcomes, the authors of one SR with MA13 focused only on the risk of serious infections, and the authors of one SR with NMA focused only on disease recurrence.5
The authors of one RCT primary study16 evaluated endoscopic recurrence, histological activity, clinical recurrence, and adverse events as outcomes. The authors of another RCT primary study17 assessed early treatment termination, dose intensification, clinical disease activity, quality of life, and adverse events as outcomes.
The two retrospective cohort primary studies18,19 identified in the SR authored by Singh et al. (2020)13 included CD-related abdominal surgery, CD-related hospitalization, and hospitalization for serious infections as outcomes.
Summary of Critical Appraisal
Additional details regarding the strengths and limitations of included publications are provided in Appendix 3.
Systematic Reviews
The seven included SRs3,5,6,11–14 were generally well conducted as per AMSTAR II criteria. All seven SRs3,5,6,11–14 had clearly stated objectives, inclusion/exclusion criteria, stated key search terms, provided search strategies, searched multiple databases, provided a list of included studies, and evaluated the risk of bias in included primary studies with appropriate techniques. Additionally, all seven SRs3,5,6,11–14 conducted data extraction in duplicate, which decreases the risk for inconsistencies. The literature search strategies of six SRs3,5,11–14 explicitly stated that grey literature searches were conducted, which decreases the risk of missing relevant, non-indexed studies. Four SRs3,5,6,13 were conducted by following an a priori study protocol. The authors of three SRs3,11,12 provided a list of excluded studies. The two SRs with MA used random effects statistical models and statistical methods to assess for heterogeneity (I2 statistics13,14 and X2 test 14), which ranged from low to considerable14 or low to moderate.13 Finally, the SR authors stated that they have nothing to disclose11 or did not have any funding or affiliations that would constitute conflicts of interest.3,5,12,13,19
In terms of methodological limitations, the authors of one SR6 did not report conducting a grey literature search, which could increase the risk of missing relevant, non-indexed studies. The use of an a priori study protocol was not reported in three SRs.11,12,14 Authors of four SRs5,6,13,14 provided reasons for excluding studies, but did not provide a list of excluded articles. Although authors of four SRs3,5,6,13 assessed the risk of publication bias using funnel plots, they were unable to rule out publication bias due to the limited number of studies. Kawalec et al. (2013) did not disclose funding sources and potential conflicts of interest.14 Finally, since the four head-to-head primary studies were conducted in Belgium,17 Denmark,19 Italy,16 and US,18 the findings may not be generalizable to the Canadian setting.
Due to the observational nature of retrospective cohort studies, methodological limitations of these two administrative claims-based primary studies18,19 were lack of randomization, lack of blinding of participants and health care professionals, and evaluation of dose modifications in response to therapeutic drug monitoring. Since a double-dummy administration of ADA and IFX was not performed,16,17 an important methodological limitation of the two primary RCTs was lack of blinding of participants and health care professionals. This lack of blinding may have had an effect on the results of clinical and endoscopic assessments.16,17 Finally, the small sample sizes (n = 2016 and n = 7317) may have resulted in insufficient statistical power to detect significant differences. Inherent quality issues from the primary studies would cause uncertainty in the findings presented in the systematic reviews.
Network Meta-analyses
All five SRs with NMA3,5,6,11,12 contained relevant populations, interventions, and effectiveness outcomes forming the basis of the NMA; reported individual study results; assessed direct and indirect comparisons; provided graphical and/or tabular representations of the evidence networks; and reported conclusions that appeared to be fair and balanced. Three SRs with NMA3,11,12 evaluated for consistency between direct and indirect comparisons, and consistency was observed between direct and indirect comparisons. To assess for consistency, Iheozor-Ejiofor et al. (2019)3 used a node-splitting approach, while Bakouny et al. (2019)11 and Hazlewood et al. (2015)12 compared the overlap in treatment effect CIs between direct and indirect comparisons. Three SRs with NMA3,5,6 provided a ranking of the interventions; however, to minimize misleading inferences, treatment rankings were not included in this report.
There was variability in the assessed outcomes. Two NMAs3,11 evaluated post-surgical disease recurrence, while three NMAs5,6,12 assessed induction and maintenance of clinical remission (Crohn’s Disease Activity Index [CDAI] < 150). The NMAs included studies conducted in Australia,3,11,12 Belgium,3 Canada,3,5,12 Germany,3 Israel,3,11,12 Italy,3,11 Japan,3,5,6,12 Spain,3 UK,3 and US;3,5,6,11,12 therefore, NMA findings may not be entirely generalizable to the Canadian setting. Bakouny et al. (2019) included non-randomized studies.11 Furthermore, authors of two SRs with NMA did not evaluate safety outcomes in their indirect comparison.5,11 Finally, there was a lack of closed loops in two NMAs,5,6 which should be taken into consideration when generalizing study conclusions.
Summary of Findings
The overall findings of the included studies are highlighted below. Seven SRs3,5,6,11–14 (two with MA13,14 and five with NMA3,5,6,11,12) met the inclusion criteria for this report. Within these SRs, four relevant primary studies were identified.16–19 Detailed summaries of the main findings are available in Appendix 4. Three SRs with NMA5,6,12 met the inclusion criteria for this report; however, none identified any eligible primary studies.
Clinical Effectiveness of Adalimumab Compared to Infliximab in Adults with Crohn’s Disease
Direct Comparisons in Primary Studies
Endoscopic Recurrence
Evidence regarding the clinical effectiveness of ADA compared to IFX in adults with CD was available from two primary studies16,17 within three SRs.3,11,14 The authors of one relevant RCT16 overlapping in two SRs with NMA3,11 reported endoscopic recurrence as an outcome. In this prospective open-label RCT, 20 patients with CD who had undergone curative ileocolonic resection were randomized to receive ADA or IFX.16 At one year, no significant difference was detected in endoscopic recurrence (defined as Rutgeerts’ score ≥ 2) between the ADA and IFX groups.16
Clinical Disease Activity
The authors of two RCT primary studies reported clinical recurrence (i.e., Harvey-Bradshaw Index score ≥ 8)16 or clinical disease activity (i.e., increase in CDAI of ≥ 100 points from baseline)17 as outcomes. At one year, no significant difference was detected in clinical recurrence between the ADA and IFX groups.16 In the second prospective open-label RCT conducted by Van Assche et al. (2012),17 73 patients with CD and continued response to at least 6 months of IFX treatment were randomized to receive ADA or continue IFX for one year. In this RCT, ten of 36 (28%) and seven of 37 (19%) patients in the ADA and IFX groups, respectively, had an increase in CDAI of ≥ 100 points from baseline (statistical analysis not reported).17
Other Effectiveness Outcomes
The authors of two head-to-head RCTs16,17 reported histological activity, early treatment termination, dose intensification, and/or quality of life as outcomes. At one year, no significant difference was detected in histological disease activity as per the Geboes score between the ADA (n = 2 with moderate histological activity score ≥ 4.1) and IFX (n = 3) groups.16 In the second RCT authored by Van Assche et al. (2012),17 the proportion of participants requiring early treatment termination or dose intensification (P = 0.006), or who had to stop therapy due to intolerance or loss of response (P = 0.003) was significantly greater in the ADA group than the IFX group.17 However, quality of life as per median Inflammatory Bowel Disease Questionnaire scores was comparable between the ADA and IFX groups at baseline and 54 weeks; however, since statistical analysis was not reported, it is unclear whether there were differences.17
Adverse Events
Evidence regarding the safety of ADA compared to IFX in adults with CD was available from four primary studies16–19 within four SRs.3,11,13,14 The authors of one RCT reported that there were no significant adverse events in either group; however, no details of specific adverse events were provided.16 In the second RCT authored by Van Assche et al. (2012),17 eight participants (22%) receiving ADA experienced mild injection reactions, while one participant (3%) receiving IFX experienced an infusion reaction (P = 0.01). Additionally, 30 of 36 (83%) and 27 of 37 (73%) participants in the ADA and IFX groups, respectively, exhibited TNFi-related side effects such as fatigue, skin lesions, upper respiratory tract infections, and/or injection site reactions (statistical analysis not reported).17 Finally, all serious adverse events (i.e., anal abscess, terminal ileitis and secondary ileus, perforation, stenosis, and/or complex fistula) were experienced by five participants (14%) in the ADA group and none in the IFX group.17
Two retrospective cohort studies18,19 identified in the SR authored by Singh et al. (2020)13 reported CD-related abdominal surgery, CD-related hospitalization, and hospitalization for serious infections as outcomes. Based on data extracted from administrative claims databases, these two primary studies included biologic-naïve patients diagnosed with CD who received ADA or IFX.18,19 Authors of one retrospective primary study by Singh et al. (2018) did not detect significant differences between IFX and ADA groups in the incidence of serious infections needing hospitalization, CD-related hospitalization, or CD-related abdominal surgery.19 Although authors of another retrospective primary study by Singh et al. (2016) did not detect a significant difference between IFX and ADA groups in the incidence of serious infections needing hospitalization, they did detect significantly lower risk of CD-related hospitalization (adjusted HR, 0.80; 95% CI, 0.66 to 0.98; P = 0.048) and CD-related abdominal surgery (adjusted HR, 0.76; 95% CI, 0.58 to 0.99; P = 0.029) in patients receiving IFX compared to ADA.18
Indirect Comparisons in NMA
Clinical Effectiveness
Evidence regarding the clinical effectiveness of ADA compared to IFX in adults with CD was available from five NMA with indirect comparisons.3,5,6,11,12 The authors of one NMA6 detected a significant difference (OR, 0.10; 95% CrI, 0.02 to 0.51) favouring IFX over ADA in the induction of clinical response (i.e., achieving a reduction in CDAI of ≥ 100 from baseline). However, the authors of three NMAs5,6,12 did not detect a treatment that was favoured in terms of induction or maintenance of clinical remission (i.e., CDAI < 150) between ADA and IFX groups. Furthermore, two NMAs involving studies of post-surgical patients did not detect a treatment that was favoured in terms of endoscopic and clinical recurrence between the ADA and IFX groups.3,11 Direct comparisons made within these two NMAs3,11 also resulted in no statistically significant differences in endoscopic and clinical recurrence between the two groups.
Adverse Events
Evidence regarding the safety of ADA compared to IFX in adults with CD was available from three NMA with indirect comparisons.3,6,12 The authors of one NMA6 concluded that participants receiving ADA exhibited a significantly greater risk of any infections (OR, 1.78; 95% CrI, 1.04 to 3.03) compared to those receiving IFX. The authors of another NMA12 concluded that ADA was favoured over IFX in terms of withdrawals due to adverse events (OR, 0.18; 95% CrI, 0.09 to 0.34) and no treatment was favoured in total withdrawals for any reason. Furthermore, similar to findings from direct comparisons, the authors of one NMA involving post-surgical patients did not detect a treatment that favoured in terms of withdrawals due to adverse events between ADA and IFX.3
Clinical Effectiveness of Adalimumab Compared to Vedolizumab in Adults with Crohn’s Disease
Direct Comparisons in Primary Studies
No primary studies were identified regarding the clinical effectiveness of ADA compared to VEDO in adults with CD; therefore, no summary can be provided.
Indirect Comparisons in NMA
Clinical Effectiveness
Evidence regarding the clinical effectiveness of ADA compared to VEDO in adults with CD was available from three NMA with indirect comparisons.5,6,12 The authors of one NMA12 concluded that ADA was favoured over VEDO (OR, 0.42; 95% CrI, 0.22 to 0.85) in the maintenance of clinical remission. However, the authors of three NMAs did not detect a treatment that was favoured in the induction5,6,12 or maintenance5,6 of clinical remission, and/or the induction of clinical response6 between ADA and VEDO groups.
Adverse Events
Evidence regarding the safety of ADA compared to VEDO in adults with CD was available from two NMAs with indirect comparisons.6,12 No treatment was favoured in terms of the risk of any infections,6 total withdrawals for any reasons,12 or withdrawals due to adverse events12 between ADA and VEDO groups.
Limitations
Numerous limitations were identified in the critical appraisal (Appendix 3); however, additional limitations exist.
Although the seven included SRs3,5,6,11–14 were generally well-conducted according to AMSTAR II criteria, only four SRs3,11,13,14 included primary studies relevant for this report due to the scarcity of head-to-head trials comparing ADA versus IFX or VEDO.
As the four head-to-head primary studies were conducted in Belgium,17 Denmark,19 Italy,16 and US,18 the findings may not be generalizable to the Canadian setting. These four primary studies were rated by the authors of the four SRs3,11,13,14 to have low to moderate risk of bias. Furthermore, these four primary studies differed in the proportion of participants with prior abdominal surgery, which may be a source of variability in disease progression. Due to the use of data from administrative claims databases, dosing information was not provided in two primary studies.18,19 Additionally, the assessed disease severity scoring scales lacked standardization across primary studies (i.e., Harvey-Bradshaw Index,16 CDAI score17). These sources of heterogeneity may pose a challenge in interpreting global findings across studies.
Two SRs with NMA3,11 involved studies of post-surgical patients with CD; therefore, findings from this report may not be generalizable to all patients living with CD. Furthermore, evidence regarding the clinical effectiveness and safety of ADA compared to VEDO in adults with CD was only identified from indirect comparisons in three NMA.5,6,12
Conclusions and Implications for Decision or Policy Making
This review was comprised of seven SRs3,5,6,11–14 regarding the clinical effectiveness of ADA compared to IFX or VEDO in adults with CD. Previous CADTH reports20–22 did not specifically compare ADA to IFX or VEDO. Of the four identified primary studies16–19 and five NMAs3,5,6,11,12 which compared ADA and IFX, most findings were non-significant except for three studies6,17,18 that favoured IFX and one study12 that favoured ADA. Furthermore, of the three NMAs5,6,12 which compared ADA and VEDO, most findings were non-significant except for one NMA12 that favoured ADA.
Evidence regarding the clinical effectiveness of ADA compared to IFX in adults with CD was identified in two primary studies16,17 and five NMA.3,5,6,11,12 The results of one RCT16 and two NMA3,11 suggested that there is no significant difference in endoscopic and clinical recurrence between study participants receiving ADA and IFX. Furthermore, the authors of this RCT also did not detect a significant difference in histological disease activity between ADA and IFX groups.16 In the second RCT which randomized study participants to continue IFX or change to ADA, it was unclear whether there were differences in quality of life between ADA and IFX groups.17 Compared to the IFX group, significantly more participants in the ADA group required early treatment termination (due to intolerance or loss of response) or required dose intensification.17 Additionally, apart from one NMA6 that favoured IFX over ADA in the induction of clinical response, authors of three NMA5,6,12 did not detect a treatment that was favoured in the induction or maintenance of clinical remission between ADA and IFX.
Evidence regarding the safety of ADA compared to IFX in adults with CD was identified in four primary studies16–19 and three NMA.3,6,12 Findings from two primary retrospective studies and two NMA suggested that between ADA and IFX groups, no treatment was favoured in terms of serious infections requiring hospitalization,18,19 CD-related hospitalization,19 CD-related abdominal surgery,19 total withdrawals for any reason,12 and/or withdrawals due to adverse events.3 However, authors of one retrospective study detected significant findings that favoured IFX in terms of CD-related hospitalization and abdominal surgery.18 Albeit it was unclear whether there were differences between ADA and IFX in the incidence of overall adverse events in two primary RCTs,16,17 authors of one RCT17 detected significant findings that favoured IFX in terms of serious adverse events and injection/infusion reactions. However, authors of another NMA12 detected findings that favoured ADA in terms of withdrawals due to adverse events.
Evidence regarding the clinical effectiveness5,6,12 and safety6,12 of ADA compared to VEDO in adults with CD was identified from three NMAs. Specifically, between ADA and VEDO groups, no treatment was favoured in the induction5,6,12 or maintenance5,6 of clinical remission, induction of clinical response,6 risk of any infections,6 total withdrawals for any reasons,12 and/or withdrawals due to adverse events.12 However, authors of one NMA12 detected findings that favoured ADA over VEDO in the maintenance of clinical remission.
Due to the scarcity of head-to-head trials comparing ADA to IFX or VEDO, only four relevant primary studies were included in this report. Consisting of low (i.e. retrospective studies) to moderate (i.e., RCTs) strength evidence, the limitations of the four primary studies (e.g., lack of blinding of participants and health care professionals, heterogeneity of outcome measures, variation in prior abdominal surgery history, inconsistencies in study findings) should be considered when interpreting these results.
Further research investigating the clinical effectiveness of ADA versus IFX or VEDO, especially with large clinical trials with long-term follow-up and measures to increase methodological quality, would provide additional knowledge base for clinicians providing care to adults living with CD and for drug benefit payers in formulary decision-making.
References
- 1.
- 2.
Kuenzig
ME, Benchimol
EI, Lee
L, et al. The Impact of Inflammatory Bowel Disease in Canada 2018: Direct Costs and Health Services Utilization.
J Can Assoc Gastroenterol. 2019;2:(Suppl 1):S17–s33. [
PMC free article: PMC6512251] [
PubMed: 31294382]
- 3.
Iheozor-Ejiofor
Z, Gordon
M, Clegg
A, et al. Interventions for maintenance of surgically induced remission in Crohn’s disease: a network meta-analysis.
Cochrane Database Syst Rev. 2019;9:CD013210. [
PMC free article: PMC6741529] [
PubMed: 31513295]
- 4.
Panaccione
R, Steinhart
AH, Bressler
B, et al. Canadian Association of Gastroenterology Clinical Practice Guideline for the Management of Luminal Crohn’s Disease.
Clin Gastroenterol Hepatol. 2019;17(9):1680–1713. [
PubMed: 30853616]
- 5.
Singh
S, Garg
SK, Pardi
DS, Wang
Z, Murad
MH, Loftus
EV, Jr.
Comparative efficacy of biologic therapy in biologic-naive patients with Crohn disease: a systematic review and network meta-analysis.
Mayo Clin Proc. 2014;89(12):1621–1635. [
PubMed: 25441399]
- 6.
Singh
S, Fumery
M, Sandborn
WJ, Murad
MH. Systematic review and network meta-analysis: first- and second-line biologic therapies for moderate-severe Crohn’s disease.
Aliment Pharmacol Ther. 2018;48(4):394–409. [
PubMed: 29920733]
- 7.
- 8.
Green
CJ, Maclure
M, Fortin
PM, Ramsay
CR, Aaserud
M, Bardal
S. Pharmaceutical policies: effects of restrictions on reimbursement.
The Cochrane database of systematic reviews. 2010;2010(8):CD008654–CD008654. [
PMC free article: PMC6791298] [
PubMed: 20687098]
- 9.
- 10.
Jansen
JP, Trikalinos
T, Cappelleri
JC, et al. Indirect treatment comparison/network meta-analysis study questionnaire to assess relevance and credibility to inform health care decision making: an ISPOR-AMCP-NPC Good Practice Task Force report.
Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2014;17(2):157–173. [
PubMed: 24636374]
- 11.
Bakouny
Z, Yared
F, El Rassy
E, et al. Comparative Efficacy of Anti-TNF Therapies For The Prevention of Postoperative Recurrence of Crohn’s Disease: A Systematic Review and Network Meta-Analysis of Prospective Trials.
J Clin Gastroenterol. 2019;53(6):409–417. [
PubMed: 29517709]
- 12.
Hazlewood
GS, Rezaie
A, Borman
M, et al. Comparative effectiveness of immunosuppressants and biologics for inducing and maintaining remission in Crohn’s disease: a network meta-analysis.
Gastroenterology. 2015;148(2):344–354.e345; quiz e314–345. [
PubMed: 25448924]
- 13.
Singh
S, Facciorusso
A, Dulai
PS, Jairath
V, Sandborn
WJ. Comparative Risk of Serious Infections With Biologic and/or Immunosuppressive Therapy in Patients With Inflammatory Bowel Diseases: A Systematic Review and Meta-Analysis.
Clin Gastroenterol Hepatol. 2020;18(1):69–81.e63. [
PMC free article: PMC8011651] [
PubMed: 30876964]
- 14.
Kawalec
P, Mikrut
A, Wisniewska
N, Pilc
A. Tumor necrosis factor-alpha antibodies (infliximab, adalimumab and certolizumab) in Crohn’s disease: systematic review and meta-analysis.
Arch. 2013;9(5):765–779. [
PMC free article: PMC3832823] [
PubMed: 24273556]
- 15.
Liberati
A, Altman
DG, Tetzlaff
J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration.
J Clin Epidemiol. 2009;62(10):e1–e34. [
PubMed: 19631507]
- 16.
Tursi
A, Elisei
W, Picchio
M, et al. Comparison of the effectiveness of infliximab and adalimumab in preventing postoperative recurrence in patients with Crohn’s disease: an open-label, pilot study.
Tech Coloproctol. 2014;18(11):1041–1046. [
PubMed: 24915941]
- 17.
Van Assche
G, Vermeire
S, Ballet
V, et al. Switch to adalimumab in patients with Crohn’s disease controlled by maintenance infliximab: prospective randomised SWITCH trial.
Gut. 2012;61(2):229–234. [
PubMed: 21948942]
- 18.
Singh
S, Heien
HC, Sangaralingham
LR, et al. Comparative Effectiveness and Safety of Anti-Tumor Necrosis Factor Agents in Biologic-Naive Patients With Crohn’s Disease.
Clin Gastroenterol Hepatol. 2016;14(8):1120–1129.e1126. [
PMC free article: PMC4955682] [
PubMed: 27058635]
- 19.
Singh
S, Andersen
NN, Andersson
M, Loftus
EV, Jr., Jess
T. Comparison of infliximab with adalimumab in 827 biologic-naive patients with Crohn’s disease: a population-based Danish cohort study.
Aliment Pharmacol Ther. 2018;47(5):596–604. [
PubMed: 29239001]
- 20.
- 21.
- 22.
- 23.
Hayden
JA, van der Windt
DA, Cartwright
JL, Cote
P, Bombardier
C. Assessing bias in studies of prognostic factors.
Ann Intern Med. 2013;158(4):280–286. [
PubMed: 23420236]
- 24.
Higgins
JPT, Altman
DG, Gøtzsche
PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials.
BMJ (Clinical research ed). 2011;343:d5928–d5928. [
PMC free article: PMC3196245] [
PubMed: 22008217]
- 25.
Schoenfeld
P, Cook
D, Hamilton
F, Laine
L, Morgan
D, Peterson
W. An evidence-based approach to gastroenterology therapy.
Gastroenterology. 1998;114(6):1318–1325. [
PubMed: 9609770]
- 26.
Jadad
AR, Moore
RA, Carroll
D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?
Controlled clinical trials. 1996;17(1):1–12. [
PubMed: 8721797]
- 27.
- 28.
Fukushima
K, Sugita
A, Futami
K, et al. Postoperative therapy with infliximab for Crohn’s disease: a 2-year prospective randomized multicenter study in Japan.
Surgery Today. 2018;48(6):584–590. [
PubMed: 29383596]
- 29.
Regueiro
M, Feagan
BG, Zou
B, et al. Infliximab Reduces Endoscopic, but Not Clinical, Recurrence of Crohn’s Disease After Ileocolonic Resection.
Gastroenterology. 2016;150(7):1568–1578. [
PubMed: 26946343]
- 30.
De Cruz
P, Kamm
MA, Hamilton
AL, et al. Efficacy of thiopurines and adalimumab in preventing Crohn’s disease recurrence in high-risk patients - a POCER study analysis.
Aliment Pharmacol Ther. 2015;42(7):867–879. [
PubMed: 26314275]
- 31.
Scapa
E, Maharshak
N, Kariv
Y, et al. Sa1150 Early Initiation of Adalimumab Significantly Diminishes Post-Operative Crohn’s Disease Recurrence, and Is Superior to Immunomodulator Therapy. Preliminary Results From the POPART Trial. Gastroenterology. 2015;148(4, Supplement 1):S-240–S-241.
- 32.
Sands
BE, Feagan
BG, Rutgeerts
P, et al. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed.
Gastroenterology. 2014;147(3):618–627.e613. [
PubMed: 24859203]
- 33.
Sandborn
WJ, Feagan
BG, Rutgeerts
P, et al. Vedolizumab as Induction and Maintenance Therapy for Crohn’s Disease.
New England Journal of Medicine. 2013;369(8):711–721. [
PubMed: 23964933]
- 34.
Savarino
E, Bodini
G, Dulbecco
P, et al. Adalimumab is more effective than azathioprine and mesalamine at preventing postoperative recurrence of Crohn’s disease: a randomized controlled trial.
Am J Gastroenterol. 2013;108(11):1731–1742. [
PubMed: 24019080]
- 35.
Rutgeerts
P, Van Assche
G, Sandborn
WJ, et al. Adalimumab induces and maintains mucosal healing in patients with Crohn’s disease: data from the EXTEND trial.
Gastroenterology. 2012;142(5):1102–1111.e1102. [
PubMed: 22326435]
- 36.
Yoshida
K, Fukunaga
K, Ikeuchi
H, et al. Scheduled infliximab monotherapy to prevent recurrence of Crohn’s disease following ileocolic or ileal resection: a 3-year prospective randomized open trial.
Inflamm Bowel Dis. 2012;18(9):1617–1623. [
PubMed: 22081474]
- 37.
Watanabe
M, Hibi
T, Lomax
KG, et al. Adalimumab for the induction and maintenance of clinical remission in Japanese patients with Crohn’s disease.
J Crohns Colitis. 2012;6(2):160–173. [
PubMed: 22325170]
- 38.
Regueiro
M, Schraut
W, Baidoo
L, et al. Infliximab prevents Crohn’s disease recurrence after ileal resection.
Gastroenterology. 2009;136(2):441–450.e441; quiz 716. [
PubMed: 19109962]
- 39.
Feagan
BG, Greenberg
GR, Wild
G, et al. Treatment of active Crohn’s disease with MLN0002, a humanized antibody to the alpha4beta7 integrin.
Clin Gastroenterol Hepatol. 2008;6(12):1370–1377. [
PubMed: 18829392]
- 40.
Colombel
JF, Sandborn
WJ, Rutgeerts
P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial.
Gastroenterology. 2007;132(1):52–65. [
PubMed: 17241859]
- 41.
- 42.
Sandborn
WJ, Rutgeerts
P, Enns
R, et al. Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial.
Ann Intern Med. 2007;146(12):829–838. [
PubMed: 17470824]
- 43.
Hanauer
SB, Sandborn
WJ, Rutgeerts
P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial.
Gastroenterology. 2006;130(2):323–333; quiz 591. [
PubMed: 16472588]
- 44.
Lémann
M, Mary
JY, Duclos
B, et al. Infliximab plus azathioprine for steroid-dependent Crohn’s disease patients: a randomized placebo-controlled trial.
Gastroenterology. 2006;130(4):1054–1061. [
PubMed: 16618399]
- 45.
Hanauer
SB, Feagan
BG, Lichtenstein
GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial.
Lancet. 2002;359(9317):1541–1549. [
PubMed: 12047962]
- 46.
Rutgeerts
P, D’Haens
G, Targan
S, et al. Efficacy and safety of retreatment with anti-tumor necrosis factor antibody (infliximab) to maintain remission in Crohn’s disease.
Gastroenterology. 1999;117(4):761–769. [
PubMed: 10500056]
- 47.
Targan
SR, Hanauer
SB, van Deventer
SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group.
The New England journal of medicine. 1997;337(15):1029–1035. [
PubMed: 9321530]
Appendix 1. Selection of Included Studies
Appendix 2. Characteristics of Included Publications
Table 2Characteristics of Included Systematic Reviews and Meta-Analyses
View in own window
| First Author, Publication Year, Country | Study Designs and Numbers of Primary Studies Included | Population Characteristics | Intervention and Comparator(s) | Clinical Outcomes, Length of Follow-Up |
|---|
Singh et al., 202013 US | Study design: SR with MA of relevant cohort studies (placebo controlled RCTs were excluded) Literature search strategy: As per an a priori protocol, authors performed literature searches in Ovid Medline, Ovid EMBASE, Scopus, Web of Science, Ovid Cochrane Central Register of Controlled Trials, and Ovid Cochrane Database of Systematic Reviews from inception to March 18, 2018. A grey literature search was also conducted. Number of studies included: Of 15 included studies, two observational studies were relevant for this report Quality assessment tool: Quality in Prognosis Studies tool23 Objective: To assess the comparative risk of serious infections with TNFi, non-TNF biologics, tofacitinib, and immunosuppressants | Patients diagnosed with CD or UC (unspecified age group) | Interventions:
- -
TNFi (ADA, IFX), non-TNFi biologics (VEDO, ustekinumab), tofacitinib, and/or immunosuppressants - -
Interventions relevant to this report were ADA, IFX and VEDO
Comparators:
- -
TNFi, non-TNFi biologics, and/or immunosuppressants
| Relevant Outcomes:
- -
Risk of serious infections
Follow-up: Studies with any follow-up duration ≥ 500 person-years were included |
Bakouny et al., 201911 Lebanon | Study design: SR with NMA (frequentist approach) of relevant prospective interventional comparative randomized or nonrandomized trials Literature search strategy: Authors performed literature searches in PubMed, EMBASE, the Cochrane Central Register of Controlled Trials, and recent AGA meeting abstracts (starting from 2015) from inception to August 4, 2017 Number of studies included: Of 9 included RCTS and non-randomized studies, one open-label, randomized pilot study directly compared drugs relevant for this report Quality assessment tool: Cochrane risk of bias tool24 Objective: To assess the comparative effectiveness and safety of TNFi compared to each other or to non-TNFi agents | Adult patients (≥ 18 years of age) diagnosed with CD who have had resection of small intestine and/or colon | Interventions:
- -
TNFi (ADA, IFX, certolizumab pegol, golimumab, etanercept) - -
Interventions relevant to this report were ADA and IFX
Comparators:
- -
Non-TNFi agents (mesalamine, thiopurine)
Relevant Interventions Included in NMA:
- -
ADA, IFX
| Relevant Outcomes:
- -
Post-surgical endoscopic and/or clinical recurrence - -
Medication discontinuation rate due to adverse events
Follow-up: Studies with any follow-up duration ≥ 6 months were included |
Iheozor-Ejiofor et al., 20193 UK | Study design: SR with NMA (Bayesian approach) of relevant RCTs Literature search strategy: As per an a priori protocol, authors performed literature searches in Cochrane IBD Group Specialized Register, CENTRAL, MEDLINE, and Embase from inception to January 15, 2019. A grey literature search was also conducted (i.e., reference lists of relevant articles, abstracts from major gastroenterology meetings, ClinicalTrials.gov, and the WHO ICTRP). Number of studies included: Of 35 included RCTs, one open-label, randomized pilot study directly compared drugs relevant for this report Quality assessment tool: Cochrane Risk of bias tool24 Objective: To assess the comparative effectiveness and safety of treatment options for maintaining post-operative recurrence in patients with CD | Patients (≥ 16 years of age) diagnosed with CD who were remission after surgery (most studies recruited patients ≥ 18 years of age) | Interventions:
- -
TNFi, non-TNFi biologics, 5-aminosalicylic acid, sulfasalazine, purine analogues, antibiotics, probiotics, oral/topical corticosteroids, or any other pharmaceutical intervention - -
Interventions relevant to this report were ADA and IFXVEDO
Comparators:
- -
No treatment, placebo, or other active treatments
Relevant Interventions Included in NMA:
- -
ADA, IFX
| Relevant Outcomes:
- -
Post-surgical endoscopic and/or clinical recurrence - -
Medication discontinuation rate due to adverse events
Follow-up: Studies with any follow-up duration were included |
Singh et al., 20186 US | Study design: SR with NMA (frequentist approach) of relevant RCTs Literature search strategy: As per an a priori protocol, authors performed literature searches in multiple databases from inception to May 31, 2017 (appendix not available to assess databases searched) Number of studies included: Of 23 included RCTs, no studies were relevant for this report Quality assessment tool: Evidence-Based Gastroenterology Steering Group criteria25 Objective: To assess the comparative effectiveness and safety of first and second-line biologic therapy for patients with moderate to severe CD | Adult patients (> 18 years of age) diagnosed with moderate to severe CD who were biologic-naïve or who have had TNFi previously | Interventions:
- -
TNFi (ADA, IFX, certolizumab pegol), anti-integrin agents (VEDO), or anti-IL12/23 agents (ustekinumab) - -
Interventions relevant to this report were ADA, IFX, and VEDO
Comparators:
- -
Placebo or other biologics
Relevant Interventions Included in NMA:
- -
ADA, IFX, VEDO
| Relevant Outcomes:
- -
Induction of clinical remission (CDAI < 150), maintenance of remission - -
Adverse events
Follow-up: Studies with follow-up duration of up to 54 weeks were included |
Hazlewood et al., 201512 Canada | Study design: SR with NMA (Bayesian approach) of relevant RCTs Literature search strategy: Studies were identified from existing Cochrane SRs and an American Gastroenterology Association report. The authors updated the database search in MEDLINE, Embase, and the Cochrane Central register of controlled trials from January 2007 to June 2014. A grey literature search was conducted (i.e., conference proceedings). Number of studies included: Of 39 included RCTs, no head-to-head studies were relevant for this report Quality assessment tool: Cochrane Risk of bias tool24 Objective: To assess the comparative effectiveness and safety of treatment options for inducing and maintaining remission in patients with CD | Adult patients (≥ 18 years old) diagnosed with moderate to severe CD | Interventions:
- -
ADA, IFX, VEDO, certolizumab, methotrexate, azathioprine/6-mercaptopurine, or combined therapies - -
Interventions relevant to this report were ADA, IFX, and VEDO
Comparators:
- -
Placebo or another active agent
Relevant Interventions Included in NMA:
- -
ADA, IFX, VEDO
| Relevant Outcomes:
- -
Induction and maintenance of clinical remission (CDAI < 150) - -
Medication withdrawal due to adverse events
Follow-up: Induction and maintenance of remission trials had follow-up periods of 4 to 17 weeks and a minimum of 24 weeks, respectively |
Singh et al., 20145 US | Study design: SR with NMA (Bayesian approach) of relevant RCTs Literature search strategy: As per an a priori protocol, authors performed literature searches in Ovid MEDLINE In-Process & Other Non-Indexed Citations, Ovid MEDLINE, Ovid EMBASE, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, Web of Science, and Scopus from January 1, 1985 to September 30, 2013. A grey literature search was conducted (abstracts from major gastroenterology conferences from 2005 to 2013). Number of studies included: Of 17 included RCTs, no studies were relevant for this report Quality assessment tool: Evidence-Based Gastroenterology Steering Group criteria25 Objective: To assess the comparative effectiveness of biologic therapy for patients with moderate to severe CD who are biologic-naïve | Adult patients (specific age cutoff not reported) diagnosed with moderate to severe CD who were biologic-naïve | Interventions:
- -
TNFi (ADA, IFX, certolizumab pegol), anti-integrin agents (natalizumab, VEDO), or anti-IL12/23 agents (ustekinumab) - -
Interventions relevant to this report were ADA, IFX, and VEDO
Comparators:
- -
Placebo or other biologics
Relevant Interventions Included in NMA:
- -
ADA, IFX, VEDO
| Relevant Outcomes:
- -
Induction of clinical remission (CDAI < 150), maintenance of remission
Follow-up: Studies with follow-up duration of up to 60 weeks were included |
Kawalec et al., 201314 Poland | Study design: SR with MA of relevant RCTs and CCTs Literature search strategy: Authors performed literature searches in PubMed, EMBASE, Cochrane Central Register of Controlled Trials, and Cochrane Database of Systematic Reviews from inception to November 2012. A grey literature search was also conducted (i.e., Cochrane IBD/FBD Review Group Specialized Trials Register, British Society of Gastroenterology, European Crohn’s and Colitis Organization, and clinicaltrials.gov). Number of studies included: Of 19 included studies, one open-label, randomized prospective study directly compared drugs relevant for this report Quality assessment tool: RCTs and CCTs were assessed with the Jadad scale26 and NOS scale,27 respectively Objective: To assess the comparative effectiveness and safety of treatment options for maintaining post-operative recurrence in patients with CD | Patients (≥ 18 years of age) diagnosed with moderate to severe active CD or fistulizing CD | Interventions:
- -
ADA, IFX, or certolizumab - -
Interventions relevant to this report were ADA and IFX
Comparators:
- -
Placebo or each other
| Relevant Outcomes:
- -
Change in CDAI CR-70: decrease of ≥ 70 points from baseline CR-100: decrease of ≥ 100 points Remission: decrease of ≤ 150 points - -
Adverse events
Follow-up: Studies with any follow-up duration were included |
ADA = adalimumab; AGA = American Gastroenterological Association; CCT = controlled clinical trial; CD = Crohn’s disease; CDAI = Crohn’s Disease Activity Index; FBD = functional bowel disorders; IBD = inflammatory bowel disease; IFX = infliximab; MA = meta-analysis; NMA = network meta-analysis; NOS = Newcastle-Ottawa Scale; RCT = randomized controlled trial; SR = systematic review; TNFi = tumour necrosis factor inhibitor; UC = ulcerative colitis; VEDO = vedolizumab.
Appendix 3. Critical Appraisal of Included Publications
Table 3Strengths and Limitations of Systematic Reviews and Meta-Analyses using AMSTAR 29 and ISPOR Checklist10
View in own window
| Strengths | Limitations |
|---|
| Singh et al., 202013 |
|---|
The objectives and inclusion/exclusion criteria were clearly stated, and timeframe for follow-up was stipulated Components of PICO that were described were population, intervention, comparator, and outcome Multiple databases were searched (Ovid Medline, Ovid EMBASE, Scopus, Web of Science, Ovid Cochrane Central Register of Controlled Trials, and Ovid Cochrane Database of Systematic Reviews) Grey literature search was conducted Search terms (contained in Supplementary Material document) and time frames were provided (inception to March 18, 2018) An a priori study protocol was followed The details of study selection and extraction were explicitly reported and performed by two reviewers The choice of included study designs was justified A list of included studies was provided, and the characteristics of included studies were described in detail The quality of included studies was assessed using the Quality in Prognosis Studies tool Conducted a random effects meta-analysis Assessed for heterogeneity using I2 statistics Publication bias was assessed using funnel plots when more than 10 studies were compared The authors disclosed their funding source (NIH) for this SR
|
The patient age component of PICO was not explicitly stated The exclusion of non-English publications was not justified Apart from listing the exclusion criteria, a list of excluded studies was not provided Two authors received industry funding The two relevant primary studies were conducted in Denmark and US; findings may not be generalizable to the Canadian setting
|
| Bakouny et al., 201911 |
|---|
The objectives and inclusion criteria were clearly stated, and timeframe for follow-up was stipulated Components of PICO that were described were population, intervention, comparator, and outcome Multiple databases were searched (PubMed, EMBASE, the Cochrane Central Register of Controlled Trials, and recent AGA meeting abstracts) Grey literature search was conducted Search terms and time frames were provided (inception to August 4, 2017) Selective reporting was assessed for each included study The details of study selection and extraction were explicitly reported and performed by two reviewers A list of included studies was provided, and the characteristics of included studies were described in detail A list of excluded studies was provided No language restriction was applied The quality of included studies was assessed using the Cochrane risk of bias tool The authors stated that they have nothing to disclose
|
The exclusion criteria were not explicitly stated Despite following PRISMA guidelines, an a priori study protocol was not developed No justification was provided for the choice of included study designs The one relevant head-to-head primary study was conducted in Italy; findings may not be generalizable to the Canadian setting
|
| Strengths and Limitations of NMA by Bakouny et al., 201911 |
|---|
The populations, interventions, and effectiveness outcomes forming the basis of the NMA were relevant There were closed loops in the NMA (i.e., multiple treatment comparison) Selective reporting was assessed for each included study Consistency between direct and indirect comparisons were evaluated Both direct and indirect comparisons were included in the NMA Assessed for heterogeneity using the inverse variance heterogeneity method A graphical and tabular representation of the evidence network was provided with information pertaining to the number of RCTs per direct comparison The individual study results were reported The results of direct and indirect comparisons were reported separately All pairwise comparisons were reported along with measures of uncertainty The authors’ conclusions appear to be fair and balanced
|
NMA was not conducted for safety outcomes due to incomplete reporting of adverse events within studies The 9 included studies in the NMA were conducted in Australia, Israel, Italy, and US; findings may not be generalizable to the Canadian setting Two of 9 included trials were non-randomized studies, which could lead to bias The use of statistical methods to preserve within-study randomization was not reported Baseline patient characteristics were not assessed amongst included studies The effect of important patient characteristics on treatment effects was not reported
|
| Iheozor-Ejiofor et al., 20193 |
|---|
The objectives and inclusion/exclusion criteria were clearly stated, and timeframe for follow-up was stipulated Components of PICO that were described were population, intervention, comparator, and outcome Multiple databases were searched (Cochrane IBD Group Specialized Register, CENTRAL, MEDLINE, and Embase) Grey literature search was conducted Search terms and time frames were provided (inception to January 15, 2019) An a priori study protocol was followed No language restriction was applied The details of study selection and extraction were explicitly reported and performed by two reviewers A list of included studies was provided, and the characteristics of included studies were described in detail A list of excluded studies was provided The quality of included studies was assessed using the Cochrane risk of bias tool Publication bias was assessed using funnel plots The authors assessed funding and conflicts of interest in the included studies The authors disclosed personal funding sources
|
|
| Strengths and Limitations of NMA by Iheozor-Ejiofor et al., 20193 |
|---|
The populations, interventions, and effectiveness outcomes forming the basis of the NMA were relevant The authors attempted to identify and include all relevant RCTs There were closed loops in the NMA (i.e., multiple treatment comparison) Baseline patient characteristics were assessed amongst included studies Statistical methods were used to preserve within-study randomization Consistency between direct and indirect comparisons were evaluated Both direct and indirect comparisons were included in the NMA Both random-effects and fixed-effects models were used to compare which model had a good fit Assessed for heterogeneity using forest plots, X2, and I2 statistics A graphical and tabular representation of the evidence network was provided with information pertaining to the number of RCTs per direct comparison The individual study results were reported The results of direct and indirect comparisons were reported separately All pairwise comparisons were reported along with measures of uncertainty The authors’ conclusions appear to be fair and balanced
|
The 35 included RCTs in the NMA were conducted in Australia, Belgium, Canada, Germany, Israel, Italy, Japan, Spain, UK, and US; findings may not be entirely generalizable to the Canadian setting
|
| Singh et al., 20186 |
|---|
The objectives and inclusion/exclusion criteria were clearly stated, and timeframe for follow-up was stipulated Components of PICO that were described were population, intervention, comparator, and outcome Multiple databases were searched (appendix not available to assess databases searched) Search terms and time frames were provided (inception to May 31, 2017) An a priori study protocol was followed No language restriction was applied The details of study selection and extraction were explicitly reported and performed by two reviewers A list of included studies was provided, and the characteristics of included studies were described in detail The quality of included studies was assessed using Evidence-Based Gastroenterology Steering Group criteria Publication bias was assessed using funnel plots The authors disclosed that they had no funding sources for this SR
|
Apart from listing the exclusion criteria, a list of excluded studies was not provided No justification was provided for the choice of included study designs Authors did not state if grey literature was searched Multiple authors received industry funding
|
| Strengths and Limitations of NMA by Singh et al., 20186 |
|---|
The populations, interventions, and effectiveness outcomes forming the basis of the NMA were relevant The authors attempted to identify and include all relevant RCTs Baseline patient characteristics were assessed amongst included studies Both direct and indirect comparisons were included in the NMA Assessed for heterogeneity using I2 testA graphical and tabular representation of the evidence network was provided with information pertaining to the number of RCTs per direct comparison The individual study results were reported The results of direct and indirect comparisons were reported separately All pairwise comparisons were reported along with measures of uncertainty The authors’ conclusions appear to be fair and balanced
|
The 23 included RCTs in the NMA were conducted in other countries such as France, Japan, and US; findings may not be entirely generalizable to the Canadian setting There was a lack of closed loops in the NMA (i.e., multiple treatment comparison) A random-effects and fixed-effects models were used (no rationale was provided) The use of statistical methods to preserve within-study randomization was not reported The evaluation of consistency between direct and indirect comparisons was not reported
|
| Hazlewood et al., 201512 |
|---|
The objectives and inclusion/exclusion criteria were clearly stated, and timeframe for follow-up was stipulated Components of PICO that were described were population, intervention, comparator, and outcome Multiple databases were searched (MEDLINE, Embase, and the Cochrane Central register of controlled trials) A grey literature search was conducted (conference proceedings) Search terms and time frames were provided (January 2007 to June 2014) The details of study selection and extraction were explicitly reported and performed by two reviewers A list of included studies was provided, and the characteristics of included studies were described in detail A list of excluded studies was provided in a supplementary table The quality of included studies was assessed using Cochrane Risk of bias tool The authors disclosed their funding source (The Alberta IBD Consortium) for this SR
|
The use of an a priori study protocol was not reported No justification was provided for the choice of included study designs Language restriction was not reported Assessment of publication bias was not reported Multiple authors received industry funding
|
| Strengths and Limitations of NMA by Hazlewood et al., 201512 |
|---|
The populations, interventions, and effectiveness outcomes forming the basis of the NMA were relevant The authors attempted to identify and include all relevant RCTs There were closed loops in the NMA (i.e., multiple treatment comparison) Baseline patient characteristics were assessed amongst included studies Consistency between direct and indirect comparisons were evaluated Both direct and indirect comparisons were included in the NMA A random-effects model was preferred by the authors due to the clinical heterogeneity of included studies. A fixed-effects model was used to assess safety outcomes due to scarcity of events. Heterogeneity was assessed by the between-study standard deviation in log odds ratio A graphical and tabular representation of the evidence network was provided with information pertaining to the number of RCTs per direct comparison The individual study results were reported The results of direct and indirect comparisons were reported separately All pairwise comparisons were reported along with measures of uncertainty The authors’ conclusions appear to be fair and balanced
|
The 39 included RCTs in the NMA were conducted in other countries such as Australia, Canada, Europe, Japan, Israel, South Africa, and US; findings may not be entirely generalizable to the Canadian setting The use of statistical methods to preserve within-study randomization was not reported
|
| Singh et al., 20145 |
|---|
The objectives and inclusion/exclusion criteria were clearly stated, and timeframe for follow-up was stipulated Components of PICO that were described were population, intervention, comparator, and outcome Multiple databases were searched (Ovid MEDLINE In-Process & Other Non-Indexed Citations, Ovid MEDLINE, Ovid EMBASE, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, Web of Science, and Scopus) A grey literature search was conducted (abstracts from major gastroenterology conferences) Search terms and time frames were provided (January 1, 1985 to September 30, 2013) An a priori study protocol was followed No language restriction was applied The details of study selection and extraction were explicitly reported and performed by two reviewers A list of included studies was provided, and the characteristics of included studies were described in detail The quality of included studies was assessed using Evidence-Based Gastroenterology Steering Group criteria Publication bias was assessed using funnel plots The authors disclosed their funding sources (Mayo Clinic, NIH) for this SR
|
Apart from listing the exclusion criteria, a list of excluded studies was not provided No justification was provided for the choice of included study designs One author received industry funding
|
| Strengths and Limitations of NMA by Singh et al., 20145 |
|---|
The populations, interventions, and effectiveness outcomes forming the basis of the NMA were relevant The authors attempted to identify and include all relevant RCTs Baseline patient characteristics were assessed amongst included studies Both direct and indirect comparisons were included in the NMA Assessed for heterogeneity using I2 statistics A tabular representation of the evidence network was provided with information pertaining to the number of RCTs per direct comparison The individual study results were reported The results of direct and indirect comparisons were reported separately All pairwise comparisons were reported along with measures of uncertainty The authors’ conclusions appear to be fair and balanced
|
The 17 included RCTs in the NMA were conducted in other countries such as Canada, France, Japan, and US; findings may not be entirely generalizable to the Canadian setting There was a lack of closed loops in the NMA (i.e., multiple treatment comparison) A random-effects model was used (no rationale was provided) The use of statistical methods to preserve within-study randomization was not reported The evaluation of consistency between direct and indirect comparisons was not reported
|
| Kawalec et al., 201314 |
|---|
The objectives and inclusion criteria were clearly stated, and timeframe for follow-up was stipulated Components of PICO that were described were population, intervention, comparator, and outcome Multiple databases were searched (PubMed, EMBASE, Cochrane Central Register of Controlled Trials, and Cochrane Database of Systematic Reviews) Grey literature search was conducted Search terms and time frames were provided (inception to November 2012) The details of study selection and extraction were explicitly reported and performed by two reviewers A list of included studies was provided, and the characteristics of included studies were described in detail To minimized risk of bias, the quality of included studies was assessed using the Jadad and NOS scales Conducted a random effects meta-analysis Assessed for heterogeneity using the X2 test
|
The exclusion criteria were not explicitly stated, and a list of excluded studies was not provided No justification was provided for the choice of included study designs Despite following PRISMA guidelines, an a priori study protocol was not used The assessment of publication bias was not reported The language restriction to English, French, German, or Polish was not justified The one relevant primary study was conducted in Belgium; findings may not be generalizable to the Canadian setting The authors did not disclose funding sources and potential conflicts of interest
|
AGA = American Gastroenterological Association; IBD = inflammatory bowel disease; NIH = National Institutes of Health; NMA = network meta-analysis; PICO = population, intervention, comparator, and outcome; RCT = randomized controlled trial; SR = systematic review.
Appendix 4. Main Study Findings and Authors’ Conclusions
Table 4Summary of Findings Included Systematic Reviews and Meta-Analyses
View in own window
| Main Study Findings | Authors’ Conclusion |
|---|
| Singh et al., 202013 |
|---|
SR with MA that assessed the comparative risk of serious infections with TNFi, non-TNFi biologics, tofacitinib, and immunosuppressants in patients (unspecified age group) diagnosed with CD or UC. Findings of Relevant Primary Studies: Singh et al., 201819
- -
The authors of this retrospective administrative claims database study assessed the comparative effectiveness and safety of ADA (n = 315) and IFX (n = 512) in biologic-naïve adult patients (mean age [ADA, IFX] = 34.9, 33.6; P = 0.18) diagnosed with CD (dosing regimens were not reported) - -
The percentage of participants who have had prior abdominal surgery were 37.1% and 36.7% for patients receiving ADA and IFX, respectively - -
With a median follow-up of 2.3 years and after propensity-score matching, the authors did not detect a significant difference in the incidence of serious infections needing hospitalization (adjusted HR, 1.06; 95% CI, 0.26 to 4.21; P = 0.94) between IFX and ADA patients - -
Furthermore, between IFX and ADA groups, there were no significant differences in the incidence of CD-related hospitalization (adjusted HR, 0.81; 95% CI, 0.55 to 1.20; P = 0.30) or CD-related abdominal surgery (adjusted HR, 1.24; 95% CI, 0.66 to 2.33; P = 0.50)
Singh et al., 201618
- -
This retrospective propensity-matched cohort study extracted real-world data from a US administrative claims database to assess the comparative effectiveness and safety of TNFi agents (ADA n = 1248; IFX n = 1427; certolizumab pegol n = 530) in biologic-naïve adult patients (mean age [ADA, IFX] = 40, 41; P = 0.39) diagnosed with CD (dosing regimens were not reported) - -
The percentage of participants who have had prior abdominal surgery were 5% and 4% for patients receiving ADA and IFX, respectively - -
With a median follow-up period of 19 months, the authors did not detect a significant difference in the risk of serious infections needing hospitalization (adjusted HR, 0.88; 95% CI, 0.48 to 1.64; P = 0.690) between IFX and ADA patients - -
Compared to ADA, patients receiving IFX exhibited significantly lower rates of CD-related hospitalization (adjusted HR, 0.80; 95% CI, 0.66 to 0.98; P = 0.048) and CD-related abdominal surgery (adjusted HR, 0.76; 95% CI, 0.58 to 0.99; P = 0.029)
| “We did not identify any full-text articles comparing the risk of serious infections between patients treated with TNFi vs non-TNFi biologic agents. [ ] in patients with Crohn’s disease, there was no significant difference in risk of serious infections in infliximab- vs adalimumab-treated patients (4 cohorts: OR, 0.91; 95% CI, 0.49 to 1.70), with moderate heterogeneity (I2 = 40%).” (pp76) |
| Bakouny et al., 201911 |
|---|
SR with NMA (frequentist approach) that assessed the comparative effectiveness and safety of TNFi compared to each other or to non-TNFi agents in adult patients (≥ 18 years of age) diagnosed with CD who have had resection of small intestine and/or colon. Findings of Relevant Head-to-Head Primary Study: Tursi et al., 201416
- -
In this prospective, open-label, randomized pilot trial, 20 consecutive patients with CD who had undergone curative ileocolonic resection were randomized to receive ADA (160 mg SC, followed by 80 mg 2 weeks later, and then 40 mg every 2 weeks) or IFX (5 mg/kg at 0, 2, and 6 weeks, and then every 8 weeks) for one year - -
There were no differences in baseline characteristics between the groups receiving ADA (n = 10; 6 female; median age [range] = 34.5 years [22 to 39]; 4 previously used IFX) and IFX (n = 10; 5 female; median age [range] = 30.5 years [20 to 33]; 5 previously used IFX) - -
CIs were not reported in this primary study
Endoscopic Recurrence
- -
At one year, there was no significant difference in endoscopic recurrence (defined as Rutgeerts’ score ≥ 2) between the ADA (n = 1; score of 2) and IFX (n = 2; scores of 2 and 4) groups (P = 1.0)
Histological Activity
- -
There was no significant difference in histological disease activity as per the Geboes score between the ADA (n = 2 with moderate histological activity score ≥ 4.1) and IFX (n = 3) groups (P = 1.0)
Clinical Recurrence
- -
There was no significant difference in clinical recurrence (defined as HBI score ≥ 8) between the ADA (n = 1; score of 10) and IFX (n = 1; score of 9) groups (P = 1.0)
Adverse Events
- -
The authors reported that there were no significant adverse events - -
No other details regarding adverse events were provided in this study
Overall Findings from NMA:
- -
Network comparisons between ADA and IFX were calculated and reported as OR - -
571 patients were included in this NMA - -
ADA dose: 40 mg every 2 weeks - -
IFX dose: 5 mg/kg (unspecified intervals) - -
No rankings of treatments were reported in this NMA
Endoscopic and Clinical Recurrence
- -
By indirect comparisons between ADA and IFX via NMA, no significant differences were detected in endoscopic recurrence (OR, 0.92; 95% CI, 0.18 to 4.75) and clinical recurrence (OR, 1.0; 95% CI, 0.2 to 6.0)
Adverse Events
- -
NMA of adverse events was not possible due to incomplete reporting in primary studies
| “Only 1 prospective study previously compared the efficacy of infliximab and adalimumab in the prevention of postoperative recurrence of CD.16 This study was included in the current NMA and provided the sole direct comparison between the 2 agents. The conclusions of the current NMA are similar to those of Tursi and colleagues study in that both studies concluded that adalimumab and infliximab have similar treatment effects in the prevention of postoperative CD recurrence. However, the addition of indirect comparisons to Tursi and colleagues results allowed a more precise estimation of treatment effects, which was reflected by the narrower CIs for the endoscopic and clinical recurrence outcomes of the NMA compared with Tursi et al’s results16 [ ].” (pp414) |
| Iheozor-Ejiofor et al., 20193 |
|---|
SR with NMA (Bayesian approach) that assessed the comparative effectiveness and safety of treatment options for maintaining post-operative recurrence in patients (≥ 16 years of age) diagnosed with CD. Findings of Relevant Head-to-Head Primary Study: Tursi et al., 201416
- -
Due to overlap, the findings of this primary study are described above under the SR authored by Bakouny et al., 201911
Overall Findings from NMA:
- -
Network comparisons between ADA and IFX were calculated and reported as HR - -
3249 patients were included in this NMA - -
ADA dose: 160 mg SC, followed by 80 mg 2 weeks later, and then 40 mg every 2 weeks - -
IFX dose: 5 mg/kg every 8 weeks - -
Rankings for treatments were reported in this NMA, but not summarized in this report
Endoscopic and Clinical Recurrence
- -
By indirect comparisons between ADA and IFX via NMA, no treatment was favoured in terms of endoscopic recurrence (HR, 2.81; 95% CrI, 0.13 to 13.41) and clinical recurrence (HR, 4.40; 95% CrI, 0.20 to 23.93)
Adverse Events
- -
By indirect comparisons via NMA, no treatment was favoured in terms of withdrawals due to adverse events between ADA and IFX (HR, 12.53; 95% CrI, 0.00 to 19.28)
| “We ranked the treatments based on effectiveness and the certainty of the evidence. For clinical relapse, the five most highly ranked treatments were adalimumab, infliximab, budesonide, 5-ASA, and purine analogues. Due to low-certainty evidence in the networks, we are unable to draw conclusions on which treatment is most effective for preventing clinical relapse and endoscopic relapse. Evidence on the safety of the interventions was inconclusive, however cases of pancreatitis and leukopenia from purine analogues were evident in the studies. Larger trials are needed to further understand the effect of the interventions on endoscopic relapse.” (pp2) |
| Singh et al., 20186 |
|---|
SR with NMA (frequentist approach) that assessed the comparative effectiveness and safety of first and second-line biologic therapy for adult patients (> 18 years of age) diagnosed with moderate to severe CD. Overall Findings from NMA:
- -
Network comparisons between ADA versus IFX and ADA versus VEDO were calculated and reported as OR - -
1458 biologic-naïve and 1606 biologic-exposed patients were included in the NMA for the induction of clinical remission (i.e., CDAI < 150) and clinical response (i.e., achieving a reduction in CDAI of ≥ 100 from baseline) - -
1854 patients were included in the NMA for the maintenance of clinical remission - -
ADA dose: 160 mg/80 mg, 80 mg/40 mg; or 40 mg/20 mg SC at weeks 0 and 2; or 40 mg SC every 2 weeks - -
IFX dose: 5 mg/kg IV at weeks 0, 2, 6; 5/10 mg/kg IV at weeks 2, 6, every 8 weeks; or 10 mg/kg IV every 8 weeks - -
VEDO dose: 300 mg IV at weeks 0, 2, 6; or 300 mg IV every 4 or 8 weeks - -
Prior history of abdominal surgery NR - -
Rankings for treatments were reported in this NMA, but not summarized in this report
Induction of Clinical Remission & Response and Maintenance of Clinical Remission: ADA versus IFX
- -
Biologic-naïve: induction of clinical remission (OR, 0.64; 95% CrI, 0.22 to 1.88) and induction of clinical response (OR, 0.10; 95% CrI, 0.02 to 0.51) - -
Biologic-exposed: no findings on induction of clinical remission or response - -
Maintenance of clinical remission (OR, 1.54; 95% CrI, 0.75 to 3.17)
ADA versus VEDO
- -
Biologic-naïve: induction of clinical remission (OR, 0.71; 95% CrI, 0.25 to 1.98) and induction of clinical response (OR, 0.75; 95% CrI, 0.33 to 1.74) - -
Biologic-exposed: induction of clinical remission (OR, 0.43; 95% CrI, 0.15 to 1.20) and induction of clinical response (OR, 0.74; 95% CrI, 0.27 to 2.00) - -
Maintenance of clinical remission (OR, 0.52; 95% CrI, 0.26 to 1.07)
Adverse Events: ADA versus IFX
- -
Risk of any infections (OR, 1.78; 95% CrI, 1.04 to 3.03)
ADA versus VEDO
- -
Risk of any infections (OR, 0.76; 95% CrI, 0.43 to 1.33)
| “Indirect comparisons suggest that infliximab or adalimumab may be preferred first-line agents, and ustekinumab a preferred second-line agent, for induction of remission in patients with moderate-severe CD. Head-to-head trials are warranted.” (pp394) |
| Hazlewood et al., 201512 |
|---|
SR with NMA (Bayesian approach) that assessed the comparative effectiveness and safety of treatment options for inducing and maintaining clinical remission for adult patients (≥ 18 years old) diagnosed with CD. Overall Findings from NMA:
- -
Network comparisons between ADA versus IFX and ADA versus VEDO were calculated and reported as OR - -
Total number of patients included in the NMA and prior history of abdominal surgery NR - -
ADA dose: 160 mg/80 mg, 80 mg/40 mg, or 40 mg/20 mg SC at weeks 0 and 2; or 40 mg SC every 2 weeks - -
IFX dose: 5/10/20 mg/kg IV at week 0; 5/10 mg/kg IV every 8 weeks - -
VEDO dose: 300 mg IV at weeks 0, 2, 6; 300 mg IV every 4 or every 8 weeks; or 0.5mg/2 mg/kg IV at weeks 0 and 4 - -
Rankings for treatments NR
Induction and Maintenance of Clinical Remission: ADA versus IFX
- -
Induction of clinical remission (OR, 1.0; 95% CrI, 0.32 to 2.4) and maintenance of clinical remission (OR, 1.8; 95% CrI, 0.94 to 3.4)
ADA versus VEDO
- -
Induction of clinical remission (OR, 0.67; 95% CrI, 0.33 to 1.5) and maintenance of clinical remission (OR, 0.42; 95% CrI, 0.22 to 0.85)
Adverse Events: ADA versus IFX
- -
Total withdrawals for any reason (OR, 0.60; 95% CrI, 0.27 to 1.3) and withdrawals due to adverse events (OR, 0.18; 95% CrI, 0.09 to 0.34)
ADA versus VEDO
- -
Total withdrawals for any reason (OR, 2.1; 95% CrI, 1.0 to 4.6) and withdrawals due to adverse events (OR, 1.4; 95% CrI, 0.72 to 2.8)
| |
| Singh et al., 20145 |
|---|
SR with NMA (Bayesian approach) that assessed the comparative effectiveness of biologic therapy for adult patients (specific age cutoff not reported) with moderate to severe CD who are biologic-naïve. Overall Findings from NMA:
- -
Network comparisons between ADA versus IFX and ADA versus VEDO were calculated and reported as RR - -
2530 biologic-naïve patients were included in the NMA for the induction and maintenance of clinical remission (i.e., CDAI < 150) - -
ADA dose: 160 mg/80 mg or 80 mg/40 mg SC at weeks 0 and 2; or 40 mg SC every week or every 2 weeks - -
IFX dose: 5/10/20 mg/kg IV at week 0; or 5/10 mg/kg IV at weeks 0, 2, 6, and every 8 weeks - -
VEDO dose: 300 mg IV at weeks 0 and 2; or 300 mg IV every 4 or 8 weeks; or 0.5mg/2 mg/kg IV at weeks 0 and 4 - -
Prior history of abdominal surgery NR - -
Rankings for treatments were reported in this NMA, but not summarized in this report
Induction and Maintenance of Clinical Remission: ADA versus IFX
- -
Induction of clinical remission (RR, 0.49; 95% CrI, 0.11 to 1.85) and maintenance of clinical remission (RR, 1.56; 95% CrI, 0.26 to 8.92)
ADA versus VEDO
- -
Induction of clinical remission (RR, 0.47; 95% CrI, 0.13 to 1.75) and maintenance of clinical remission (RR, 0.43; 95% CrI, 0.05 to 3.36)
Adverse Events:
- -
Adverse events NR
| “Indirect comparisons suggest that infliximab or adalimumab may be preferred first-line agents, and ustekinumab a preferred second-line agent, for induction of remission in patients with moderate-severe CD. Head-to-head trials are warranted.” (pp394) |
| Kawalec et al., 201314 |
|---|
SR with MA that assessed the comparative effectiveness and safety of treatment options for maintaining post-operative recurrence in adult patient (≥ 18 years of age) diagnosed with moderate to severe active CD or fistulizing CD. Findings of Relevant Primary Study: Van Assche et al., 201217
- -
In this prospective, open-label, randomized trial, 73 patients with CD and continued response to at least 6 months of IFX treatment were randomized to receive ADA (n = 36; 80 mg sc followed by 40 mg every other week) or continue IFX (n = 37; 5 mg/kg) for one year - -
There were no differences in baseline characteristics between the groups receiving ADA (n = 36; 50% female; median age [range] = 38 years [27 to 47]) and IFX (n = 37; 46% female; median age [range] = 37 years [29 to 42]) - -
Percentage of patients with previous abdominal surgery was not reported - -
CIs were not reported in this primary study
Early Treatment Termination or Dose Intensification
- -
There was a statistically significant difference in the proportion of patients who required early treatment termination or dose intensification between the ADA (n = 17) and IFX (n = 6) groups (P = 0.006) - -
There was a statistically significant difference in the proportion of patients who had to stop therapy due to intolerance or loss of response between the ADA (n = 10) and IFX (n = 1) groups (P = 0.003)
Clinical Disease Activity
- -
Ten and seven patients in the ADA and IFX groups, respectively, had an increase in CDAI of ≥ 100 points from baseline (statistical analysis NR)
Quality of Life
- -
Median Inflammatory Bowel Disease Questionnaire scores were comparable between the ADA (week 0: 197 [IQR 181–212], week 54: 193 [IQR 160–214]) and IFX (week 0: 191 [IQR 172–203], week 54: 188 [IQR 170–204]) groups at baseline and 54 weeks (statistical analysis NR)
Adverse Events
- -
Overall, 30 and 27 patients in the ADA and IFX groups, respectively, experienced TNFi-related side effects including fatigue, skin lesions, upper respiratory tract infections, and/or injection site reactions (statistical analysis NR) - -
Eight patients receiving ADA experienced mild injection reactions, while one patient receiving IFX experienced an infusion reaction (P = 0.01) - -
All serious adverse events (i.e., anal abscess, terminal ileitis and secondary ileus, perforation, stenosis, and/or complex fistula) were experienced by five patients receiving ADA
| “In study17 the results for adalimumab therapy (after switching from infliximab) were statistically inferior when compared to the continued use of infliximab with regard to the need for dose intensification, risk of early treatment termination (RR = 0.34, 95% CI: 0.15–0.77, p = 0.01) and risk of treatment discontinuation due to loss of response or intolerance (RR = 0.10, 95% CI: 0.01–0.72, p = 0.02). Both important and inconsistent with the clinical results, significantly more patients preferred adalimumab over infliximab at most time points throughout the study. Using the data available from study17 as a basis, there was no statistically significant difference detected in the risk of any AEs related to the anti-TNF therapy with infliximab when compared to adalimumab (RR = 0.88, 95% CI: 0.69–1.12, p = 0.29).” (pp773–775) |
ADA = adalimumab; CCT = controlled clinical trial; CD = Crohn’s disease; CDAI = Crohn’s Disease Activity Index; CI = confidence interval; CrI = credible interval; HR = hazard ratio; IBD = inflammatory bowel disease; IFX = infliximab; IV = intravenous; IQR = interquartile range; MA = meta-analysis; NMA = network meta-analysis; OR = odds ratio; RCT = randomized controlled trial; SC = subcutaneous; SR = systematic review; TNFi = tumour necrosis factor inhibitor; UC = ulcerative colitis; VEDO = vedolizumab.
Appendix 5. Overlap between Included Systematic Reviews
Table 5Primary Study Overlap between Included Network Meta-analyses
View in own window
| Primary Study Citation | Network Meta-analyses Citation |
|---|
| Bakouny et al., 201911 | Iheozor-Ejiofor et al., 20193 | Singh et al., 20186 | Hazlewood et al., 201512 | Singh et al., 20145 |
|---|
| Fukushima et al., 201828 | | ▪ | | | |
| Regueiro et al., 201629 | ▪ | ▪ | | | |
| De Cruz et al., 201530 | ▪ | | | | |
| Scapa et al., 201531 | ▪ | | | | |
| Sands et al., 201432 | | | ▪ | ▪ | |
| Tursi et al., 201416 | ▪ | ▪ | | | |
| Sandborn et al., 201333 | | | ▪ | ▪ | ▪ |
| Savarino et al., 201334 | ▪ | | | | |
| Rutgeerts et al., 201235 | | | | ▪ | |
| Yoshida et al., 201236 | ▪ | ▪ | | | |
| Watanabe et al., 201237 | | | ▪ | ▪ | ▪ |
| Regueiro et al., 200938 | ▪ | ▪ | | | |
| Feagan et al., 200839 | | | | ▪ | ▪ |
| Colombel et al., 200740 | | | ▪ | ▪ | ▪ |
| Sandborn et al., 2007 41 | | | ▪ | ▪ | ▪ |
| Sandborn et al., 200742 | | | ▪ | ▪ | |
| Hanauer et al., 200643 | | | ▪ | ▪ | ▪ |
| Lemann et al., 200644 | | | ▪ | | ▪ |
| Hanauer et al., 200245 | | | ▪ | ▪ | ▪ |
| Rutgeerts et al., 199946 | | | ▪ | ▪ | ▪ |
| Targan et al., 199747 | | | ▪ | ▪ | ▪ |
▪ = the primary study was included in the network meta-analysis.
Appendix 6. Additional References of Potential Interest
Systematic Reviews and Meta-analyses – Alternative Indication
Zhou
HY, Guo
B, Lufumpa
E, et al. Comparative of the Effectiveness and Safety of Biological Agents, Tofacitinib, and Fecal Microbiota Transplantation in Ulcerative Colitis: Systematic Review and Network Meta-Analysis.
Immunol Invest. 2020
Feb
02:1–15.
PubMed: PM32009472 [
PubMed: 32009472]
Systematic Reviews and Meta-analyses – Alternative Population
Mocko
P, Kawalec
P, Pilc
A. Safety profile of biologic drugs in the therapy of Crohn disease: A systematic review and network meta-analysis.
Pharmacol Rep. 2016
Dec;68(6):1237–1243.
PubMed: PM27686963 [
PubMed: 27686963]
Hutfless
S, Lau
BD, Wilson
LM, Lazarev
M, Bass
EB. Pharmacological Management of Crohn’s Disease: Future Research Needs: Identification of Future Research Needs From Comparative Effectiveness Review No. 131 [Internet].
Agency for Healthcare Research and Quality (US). 2014
02;13(14):02.
PubMed: PM24783272 [
PubMed: 24783272]
Thaler
KJ, Gartlehner
G, Kien
C, et al. Drug Class Review: Targeted Immune Modulators: Final Update 3 Report [Internet].
Oregon Health & Science University. 2012
03;03:03.
PubMed: PM23166955 [
PubMed: 23166955]
Systematic Reviews and Meta-analyses – Alternative Comparator
Cholapranee
A, Hazlewood
GS, Kaplan
GG, Peyrin-Biroulet
L, Ananthakrishnan
AN. Systematic review with meta-analysis: comparative efficacy of biologics for induction and maintenance of mucosal healing in Crohn’s disease and ulcerative colitis controlled trials.
Aliment Pharmacol Ther. 2017
May;45(10):1291–1302.
PubMed: PM28326566 [
PMC free article: PMC5395316] [
PubMed: 28326566]
About the Series
CADTH Rapid Response Report: Summary with Critical Appraisal
Version: 2.0 Corrected Version
Correction Notice
The original report, published on April 27, 2020, misrepresented the statistical significance of findings from Bayesian network meta-analyses. Due to differences from network meta-analyses performed through a frequentist approach, references to statistically significant results from Bayesian network meta-analyses have been rephrased in the Key Findings, Summary of Findings, and Conclusions sections in this version of the report (see sample amendment below). These wording amendments do not affect the analyses and conclusions made in this report.
Original:
“In three network meta-analyses comparing adalimumab to vedolizumab, no statistically significant differences were detected in effectiveness (e.g., induction of clinical remission) and safety (e.g., withdrawals due to adverse events) outcomes.”
Amendment:
“In three network meta-analyses comparing adalimumab to vedolizumab, no treatment was favoured in effectiveness (e.g., induction of clinical remission) and safety (e.g., withdrawals due to adverse events) outcomes.”
Finally, the critical appraisal of network meta-analyses (see Appendix 3) has been updated to clarify or elaborate on some items (e.g., analysis of heterogeneity, presence of closed loops).
Funding: CADTH receives funding from Canada’s federal, provincial, and territorial governments, with the exception of Quebec.
Suggested citation:
Adalimumab for Adult Patients with Crohn’s Disease: A Review of Clinical Effectiveness. Ottawa: CADTH; 2020 April. (CADTH rapid response report: summary with critical appraisal).
Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services.
While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up-to-date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. The views and opinions of third parties published in this document do not necessarily state or reflect those of CADTH.
CADTH is not responsible for any errors, omissions, injury, loss, or damage arising from or relating to the use (or misuse) of any information, statements, or conclusions contained in or implied by the contents of this document or any of the source materials.
This document may contain links to third-party websites. CADTH does not have control over the content of such sites. Use of third-party sites is governed by the third-party website owners’ own terms and conditions set out for such sites. CADTH does not make any guarantee with respect to any information contained on such third-party sites and CADTH is not responsible for any injury, loss, or damage suffered as a result of using such third-party sites. CADTH has no responsibility for the collection, use, and disclosure of personal information by third-party sites.
Subject to the aforementioned limitations, the views expressed herein are those of CADTH and do not necessarily represent the views of Canada’s federal, provincial, or territorial governments or any third party supplier of information.
This document is prepared and intended for use in the context of the Canadian health care system. The use of this document outside of Canada is done so at the user’s own risk.
This disclaimer and any questions or matters of any nature arising from or relating to the content or use (or misuse) of this document will be governed by and interpreted in accordance with the laws of the Province of Ontario and the laws of Canada applicable therein, and all proceedings shall be subject to the exclusive jurisdiction of the courts of the Province of Ontario, Canada.