Context and Policy Issues
Diabetes mellitus is a group of metabolic disorders that result from deficiencies in insulin secretion, sensitivity, or both.1 Type 2 diabetes mellitus ranges from predominant insulin resistance with relative insulin secretory deficiency, to insulin resistance with a predominant insulin secretory deficiency as the disease progresses.1–3 There are several risk factors for type 2 diabetes, including a history of pre-diabetes, usually defined as having impaired fasting glucose (6.1 to 6.9 mmol/L), impaired glucose tolerance (noted by an oral glucose tolerance test results of 7.8 to 11 mmol/L), or an elevated glycated hemoglobin A1c level (6% to 6.4%).2
In 2017, it was estimated there were 123,085 Canadians (95% confidence interval [CI], 109,119 to 137,118) newly diagnosed with type 2 diabetes,4 raising the national burden of disease to an estimated 2,553,158 (95% CI, 2,295,152 to 2,857,046) prevalent cases.5 Furthermore, it was estimated that 2.12% of total Canadian deaths (95% CI, 2.0% to 2.25%) in 2017 were attributable to type 2 diabetes.6 This translates to an estimated 83,603 years of life lost (95% CI, 76,847 to 90,036) for 2017.7
The goals of therapy in type 2 diabetes are aimed at achieving stringent glycemic control within the normal range as early as possible.2 In addition to diet and lifestyle measures, several classes of antidiabetic agents are approved in Canada: insulins, sulphonylureas (SUs), α-glucosidase inhibitors, biguanides, glucagon-like peptide-1 (GLP-1) receptor agonists, meglitinides (MEGs), thiazolidinediones (TZDs), sodium-glucose cotransporter 2 inhibitors, dipeptidyl peptidase-4 (DPP-4) inhibitors, and a combination of these may often be necessary for optimal treatment.1–3,8 This report will focus on a particular drug of the TZD class, pioglitazone (PIO), which is often considered as a therapeutic option when glycemic targets are not achieved with first-line drugs, such as metformin.2 PIO works by binding to the peroxisome proliferator-activated receptor-γ, which is primarily located on adipose and vascular cells,1 increasing their insulin sensitivity.9
In addition to its hypoglycemic effect, PIO has been shown to have favourable effects on reducing major adverse cardiovascular events (e.g., all-cause mortality, non-fatal myocardial infarction [MI], stroke).10,11 Nevertheless, and as is the case with any drug therapy, the benefits associated with PIO ought to be weighed against possible risks to the patient. Because of previously reported concerns about adverse events (AEs) such as bladder cancer,12–16 heart failure (HF),10,17,18 edema,10,19,20 fractures,8,21 weight gain,10 and ovulation in anovulatory women,22 there remains uncertainty around the overall safety profile of PIO.
Previous CADTH reports on this topic include a 2010 comparison of the safety of PIO and rosiglitazone (ROS) for patients with type 2 diabetes.23 The objective of the present report is to investigate the clinical evidence regarding the safety of PIO for patients with pre-diabetes or type 2 diabetes.
Research Question
What is the clinical evidence regarding the safety of pioglitazone for patients with type 2 diabetes or pre-diabetes?
Key Findings
Five relevant systematic reviews (four with meta-analysis and one with network meta-analysis), two randomized controlled trials, and six non-randomized studies were identified regarding the safety of pioglitazone for patients with pre-diabetes or type 2 diabetes.
In patients with pre-diabetes, evidence from one non-randomized study suggested that pioglitazone was associated with an increased likelihood of weight gain and edema when compared to placebo, while studies evaluating other safety outcomes generally found no significant differences between pioglitazone and comparators. Results in patients with type 2 diabetes were mixed, though there were often no significant differences from systematic reviews regarding several safety outcomes when comparing pioglitazone to other treatments for type 2 diabetes. However, the body of evidence was largely of low to moderate quality. As such, there remains some uncertainty around the overall safety profile of pioglitazone.
The limitations of the included studies (e.g., heterogeneity of the literature, and lack of blinding to treatment), should be considered when interpreting the results.
Methods
Literature Search Methods
A limited literature search was conducted by an information specialist on key resources including PubMed, the Cochrane Library, the University of York Centre for Reviews and Dissemination (CRD) databases, the websites of Canadian and major international health technology agencies, as well as a focused Internet search. The search strategy was comprised of both controlled vocabulary, such as the National Library of Medicine’s MeSH (Medical Subject Headings), and keywords. The main search concepts were pioglitazone and type II diabetes. Search filters were applied to limit retrieval to health technology assessments, systematic reviews (SRs), meta-analyses (MAs), network meta-analyses (NMAs), randomized controlled trials (RCTs), or safety data. Where possible, retrieval was limited to the human population. The search was also limited to English language documents published between January 1, 2010 and May 12, 2020.
Selection Criteria and Methods
One reviewer screened citations and selected studies. In the first level of screening, titles and abstracts were reviewed and potentially relevant articles were retrieved and assessed for inclusion. The final selection of full-text articles was based on the inclusion criteria presented in .
Exclusion Criteria
Articles were excluded if they did not meet the selection criteria outlined in , or they were duplicate publications. Articles published prior to 2019 were excluded due to the volume of relevant evidence identified from the literature search. SRs in which all relevant studies were captured in other more recent or more comprehensive SRs were excluded. Primary studies retrieved by the search were excluded if they were captured in one or more included SRs.
Critical Appraisal of Individual Studies
The included publications were critically appraised by one reviewer using the following tools as a guide: A MeaSurement Tool to Assess systematic Reviews 2 (AMSTAR 2)24 for SRs, the “Questionnaire to assess the relevance and credibility of a network meta-analysis”25 for NMAs, and the Downs and Black checklist26 for randomized and non-randomized studies (NRSs). Summary scores were not calculated for the included studies; rather, the strengths and limitations of each included publication were described narratively.
Summary of Evidence
Quantity of Research Available
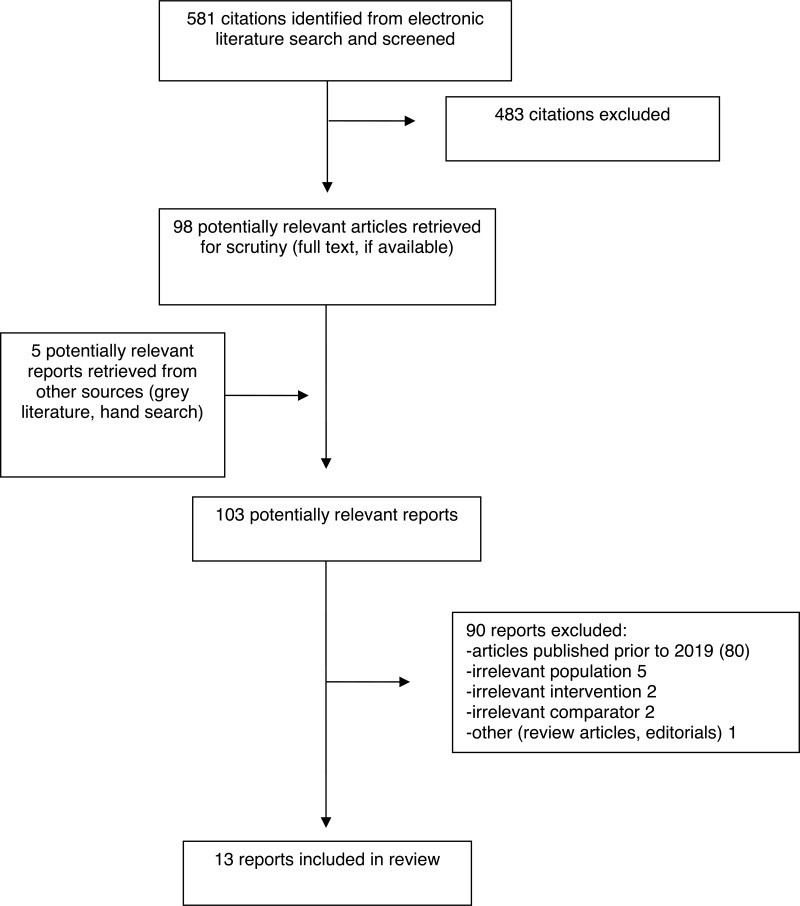
A total of 581 citations were identified in the literature search. Following screening of titles and abstracts, 483 citations were excluded and 98 potentially relevant reports from the electronic search were retrieved for full-text review. Five potentially relevant publications were retrieved from the grey literature search for full-text review. Of these potentially relevant articles, 90 publications were excluded for various reasons, and 13 publications met the inclusion criteria and were included in this report. These comprised five SRs, two RCTs, and six NRSs. Appendix 1 presents the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)27 flowchart of the study selection.
Additional references of potential interest are provided in Appendix 5.
Summary of Study Characteristics
Five SRs28–32 (four with MAs28,30–32 and one with NMA29), two RCTs,33,34 and six NRSs35–40 were identified and included in this review. Additional details regarding the characteristics of included publications are provided in Appendix 2, , and .
Three28,29,32 SRs had broader inclusion criteria than the present review. Specifically, one SR32 included studies in patients with all endocrine and metabolic disorders and the other two SRs28,29 included studies where additional interventions and comparators were involved. Only the characteristics and results of the subset of relevant studies are described in this report.
Study Design
Three SRs28–30 (one with NMA29) were published in 2020, and two SRs31,32 were published in 2019. All of them searched multiple electronic databases for eligible RCTs published over pre-specified periods ranging from database inception to August 2019, except one28 in which search period was unspecified. In the four SRs with MAs,28,30–32 there were two,28 23,30 16,31 and four32 relevant primary studies. The SR with NMA29 included eight primary studies,41–48 of which two studies41,42 directly involved PIO as intervention or comparator. The NMA used frequentist methods and a random effects model. Overall, the publications included 53 unique primary studies, with no overlap in primary studies included in each SR.
The two RCTs33,34 were randomized open label parallel design trials, one which followed patients for 26 weeks33 and the other for 52 weeks.34
Three35,36,38 of the NRSs included in this report were retrospective cohort studies. A fourth NRS40 was a secondary analysis of clinical trial data from an international multicenter double blinded trial.11 The latter11 was also included in a SR30 retained in this review. A fifth NRS37 was a cohort study using prospectively collected data, while the sixth37 was a retrospective and prospective cohort study.39 Three NRSs conducted a propensity-score matched analysis of the cohorts to address potential confounding variables,35,36,39 while two used an analysis of variance,38,40 and one did not report on such analyses.37
Country of Origin
The primary authors of the SRs were from China28,30,32, Japan29 and Malaysia.31 The primary authors of the RCTs and NRSs were from Canada,40 China,38 Denmark,37 Iran,34 Korea,33 Taiwan35,36 and the United States of America.39
Among the two RCTs, one was a multicenter study conducted at eight sites in Korea,33 and the other34 was a single center Iranian study.
Patient Population
Five SRs28–32 included studies of patients with type 2 diabetes. Two SRs also included studies of individuals with pre-diabetes30 or with impaired glucose tolerance.32 One SR31 only included studies involving patients with type 2 diabetes without any comorbid diseases or diabetes associated complications. The number of patients in the analytical sample in the SRs ranged from 58528 to 19,607.30
Two RCTs33,34, and five NRSs35–39 enrolled patients with type 2 diabetes. The Kim et al. (2020) RCT,33 enrolled 135 patients with hemoglobin A1C levels from 7.5% to 10% and with a body mass index (BMI) of 18.5 to 35 kg/m2, and the baseline characteristics were balanced between groups.
The Khaloo et al. (2019) RCT34 recruited 250 eligible patients (125 in each group) between the ages of 25 and 70 years. The patients in the PIO group had statistically significant differences in four baseline characteristics: sex (PIO: 55.5% females vs. sitagliptin [SIT]: 44.5%; P value = 0.04), disease duration (PIO: 14.3 years vs. SIT: 11.3 years; P value = 0.001), systolic blood pressure (SBP) (PIO: 129.1 mmHg vs. SIT: 135.7; P value = 0.001), and mean weight (PIO: 74.2 kg vs. SIT: 78.3 kg; P value 0.019).
One NRS40 analysed patients with pre-diabetes from the participants of a multicenter RCT who were at least 40 years of age and had a transient ischemic attack (TIA) or stroke during the six months prior to randomization.11 Pre-diabetes was defined as hemoglobin A1c levels of 6.0% to 6.4% at baseline as per Diabetes Canada guidelines.49 Among the 1,410 patients (PIO, n = 709; placebo, n= 701) included in the intention-to-treat (ITT) analysis relevant to the current report, mean age (PIO = 64.1 years; Placebo = 64.5 years), sex (PIO = 65.2 % males; placebo = 63.6% males) and co-morbidities were similar across the study arms.
Two NRSs35,36 identified newly diagnosed type 2 diabetes patients from the National Health Insurance Database in Taiwan, through overlapping periods of enrollment. They enrolled 5,15835 and 10,19036 patients each, with mean age of 6235 and 5936 years respectively. The study by Cid Ruzafa and colleagues37 identified eligible patients from the Danish health registers into an incident cohort (PIO, n = 80; comparator, n = 17,699) and a prevalent cohort (PIO, n=140; comparator, n=13,183) during the period of August 2011 to December 2015. Median age of the patients in each cohort arm ranged from 62.4 years to 67.2 years.37
The Miao et al. (2019) study,38 conducted from July 2005 to June 2017, enrolled over 70,000 patients from the clinical data repository of a Chinese hospital, among which 13% were prescribed PIO at least once (PIO, n = 8,226).38 The patients in the PIO group had statistically significant differences in several baseline characteristics (e.g., mean age, concurrent antidiabetic medications, and likelihood to have comorbidities; P value < 0.001 for each between group comparison).38
Interventions and Comparators
In one SR,28 the antidiabetic drug aleglitazar (ALE) (not currently approved in Canada) was compared to PIO. In the NMA by Ida et al. (2020)29 PIO was compared to placebo, conventional treatment, and other oral antidiabetic drugs (OAD) (e.g., liraglutide [LIR], exenatide [EXE], SIT, linagliptin [LIN], ROS, voglibose (not currently approved in Canada), and glimepiride [GLIM]) regardless of the use of dietary or exercise therapy. The SR by Alam and colleagues31 compared PIO monotherapy with other FDA approved antidiabetic medications (e.g., metformin [MET], SUs, DPP-4 inhibitors, acarbose [ACAR], MEG). Lastly two SRs compared the effects of PIO to placebo32 and any control (e.g., placebo, active comparator, usual care)30 respectively.
In one RCT,33 PIO 15 mg per day was compared to GLIM 2 mg per day along with existing MET and alogliptin (ALO) treatment in both groups. The dose of PIO and GLIM in both groups could be increased after 12 weeks based on investigator’s decision. The RCT by Khaloo et al.34 compared PIO 30 mg per day to SIT 100 mg daily. MET 500 mg four times daily and gliclazide (GLIC) 80 mg thrice daily was given to patients in both groups.
Five NRSs, compared PIO use to PIO non-use (e.g., drugs other than PIO, drugs other than TZD, oral drugs other than PIO),35,36,38 with insulin37 and with LIN.39 In the sixth NRS,40 PIO (15 mg/day increased to 45 mg/day over three months, with patients given the highest tolerable dose) was compared to placebo.
Outcomes
The outcomes considered in the SRs were: all-cause mortality,30 bladder cancer,31 body weight,28,32 cardiovascular events,30,31 hospitalization for HF,30 hypoglycemia,28 left ventricular diastolic function,29 peripheral edema,28,31 abnormal liver function,31 blood pressure,31 bone mineral density (BMD),32 BMI,32 bone fracture,32 laboratory findings (e.g., serum creatinine, albumin to creatinine ratio, urinary protein excretion),28,30 musculoskeletal disorders (e.g., arthralgia, back pain, musculoskeletal pain),31 upper respiratory tract infection,31 and vascular disorders (arterial thrombosis and aortic stenosis).31
The outcomes of interests in the RCTs were: body weight,34 blood pressure,34 BMI,34 waist circumference,34 hip circumference,34 and other AEs.33,34
The NRSs sought outcomes on: all-cause mortality,35,40 cancer (e.g., hepatocellular carcinoma, bladder cancer, any cancer),36,37,40 HF,37,38 MI,38,39 stroke,38,39 unstable angina,39 coronary revascularization,39 cirrhosis,36 esophageal varices,36 hepatic failure,36 body weight,40 cardiovascular events,40 hospitalization for HF,40 edema,40 bone fracture,40 and haematuria.37
Summary of Critical Appraisal
Additional details regarding the strengths and limitations of included publications are provided in Appendix 3, , and .
SRs
Strengths of all SRs28–32 (four with MAs28,30–32 and one with NMA29) included: clear objectives and inclusion criteria, reporting of key search terms and search strategies, and authors included statements on conflicts of interest.
While all SRs28–32 performed a quality assessment of included studies using an appropriate tool, three SRs included high quality, low risk of bias studies in their analyses,28,30,32 and two SRs included studies with high or unclear risk of bias in relation to random sequence generation and blinding of participants and personnel.29,31
While all studies performed study selection in duplicate, data extraction was reported to have been performed in duplicate for three SRs.30–32 Three SRs did not report having an a priori protocol for their review.28,29,32 These weaknesses of reporting decrease the confidence in the findings and the reproducibility of the SRs. None of the SRs provided a list of excluded studies; therefore, the accuracy and comprehensiveness of the exclusions could not be assessed. One SR29 did not report exploring publication bias; therefore, it is unclear if the direction or strength of the findings are biased. Four SRs28,29,31,32 did not adequately report the primary study results; therefore, the accuracy of data reporting and interpretation cannot be assessed.
The MA of one SR31 had a small sample size (n = 2,681) and included nine (out of 16) studies that were assessed as having a high risk of bias in relation to their open-label study designs and selective reporting, decreasing overall confidence in the results.
With respect to the NMA,29 a network diagram of the included primary studies was reported. There were no significant inconsistencies between direct and indirect comparisons in the design by treatment interaction model.29 Systematic differences in treatment effect modifiers (e.g., BMI, age, number of comorbidities) were present between the different indirect treatment comparisons. These imbalances were not compared across the included studies and were not addressed in the analysis. Despite the presence of heterogeneity, authors did not perform additional analyses (e.g., subgroup analysis, meta-regression) to explore its origins.29 While it was appropriate to have selected a random-effect NMA model,29 authors did not justify its use.
RCTs
Strengths of all RCTs33,34 included: clear descriptions of objectives, interventions, main outcomes, population characteristics, and eligibility criteria; and the major findings were described in a way that allowed verification of analyses and conclusions. Estimates of random variability were reported, and the data analyses were planned at the outset.
Both RCTs33,34 performed safety endpoint analyses based on their ITT populations; however, one33 did not specify how missing data were handled, while the other34 indicated that missing data were handled using the “last observation carried forward” method. Furthermore, they both lacked the characterization of patients who withdrew or were lost to follow up. Both RCTs33,34 were open label, meaning that patients and investigators were aware of their treatment group allocation. This may have introduced observer biases for certain outcomes like cancer or cardiovascular events; however, this unlikely to introduce bias for objectively measurable outcomes like death or fracture.
Patients in the Khaloo et al. (2019) RCT had statistically significant differences in four baseline characteristics: sex, disease duration, systolic blood pressure (SBP), and mean weight.34 A lack of adequate adjustment for confounders that were not balanced at baseline may have introduced a bias in the analysis from which the main findings were drawn.
While the allocation of patients to the treatments groups was randomized in one RCT,33 authors did not indicate their randomization method; therefore, the accuracy and comprehensiveness of the process could not be assessed. Also, the safety conclusions of the study were based on the analysis of treatment (i.e., per protocol) rather than ITT, meaning the comparisons among treatment groups may have been biased due to dropouts.33
NRSs
Several strengths were identified in all NRSs35–40 including: clear descriptions of objectives, interventions, main outcomes, population characteristics, eligibility criteria. Propensity score matching was used in three studies,35,36,39 minimizing the influence of principal confounders between groups; however, one study did not match by hemoglobin A1c values,35 while another did not match by duration of disease.36 Four studies utilised a retrospective cohort design,35–38 suffering no losses to follow up.
In one NRS,37 the effects of the main confounders were not investigated nor were adjustments made in the final analyses; therefore it is unclear what impact, if any, the confounding variables had on the results. One study39 performed multiple observations over time, yet no statistical adjustments were made for multiplicity.
Summary of Findings
A detailed summary of findings is provided in Appendix 4, , and .
Clinical evidence regarding the safety of pioglitazone for patients with pre-diabetes
Death
Information regarding death outcomes with PIO in patients with pre-diabetes was available from one NRS.40
Among patients with pre-diabetes (using the American Diabetes Association [ADA] criteria),50 authors reported no difference in all-cause mortality among PIO users compared with placebo.40
Cardiovascular outcomes
Information regarding cardiovascular outcomes with PIO in patients with pre-diabetes was available from one SR,30 and one NRS.40
The SR30 reported on major adverse cardiovascular events (i.e., the composite of non-fatal MI, non-fatal stroke, and cardiovascular death), non-fatal MI, non-fatal stroke, as well as hospitalization for HF among patients with pre-diabetes at baseline, with a history of established atherosclerotic cardiovascular disease (CVD) at baseline, reported no statistical differences between PIO and comparator groups (i.e., placebo, and not PIO). Similarly, results were not statistically significant in the group without a history of established atherosclerotic CVD at baseline between PIO and placebo.30
The NRS40 was a secondary analysis of clinical trial data from a study11 that was included in the SR30 above. Authors reported statistically significant results favouring PIO over placebo among patients with pre-diabetes (using the World Health Organisation [WHO]/Diabetes Canada criteria),49 for the outcomes of stroke or MI (ITT analysis; hazard ratio [HR] = 0.70, 95% CI, 0.51 to 0.95; P value = 0.02), and stroke (ITT analysis; HR = 0.68; 95% CI, 0.48 to 0.97; P value = 0.03).40 However, among patients with pre-diabetes (using the ADA criteria),50 authors reported no difference for the outcomes of HF causing hospitalization or death.40 Similarly, in patients with pre-diabetes (using the WHO/Diabetes Canada criteria),49 there were no differences in acute coronary syndrome, stroke, MI, or hospitalised HF.40
BMD
Information regarding BMD outcomes with PIO in patients with pre-diabetes was available from one SR.32
The SR32 reported a mean difference in the change from baseline of BMD of the lumbar spine, favouring the comparators (−1.08; 95% CI, −2.04 to 0.13; P value = 0.03 [values as reported in the article]).
Fractures
Information regarding fracture outcomes with PIO in patients with pre-diabetes was available from one SR,32 and one NRS.40
The SR reported on odds ratio of fractures and found there was no statistically significant difference between PIO and placebo.32
The NRS40 reported statistically significant results favouring placebo over PIO among patients with pre-diabetes (using the ADA criteria),50 for the outcomes of bone fracture causing hospitalization, surgery, or procedure (ITT analysis; PIO, n = 71 (4.9%) vs. placebo, n = 46 (3.2%); P value = 0.02; number needed to harm [NNH] = 59).
Weight
Information regarding weight change outcomes with PIO in patients with pre-diabetes was available from one NRS.40
The NRS40 reported statistically significant results favouring placebo over PIO among patients with pre-diabetes (using the ADA criteria),50 for the outcomes of weight change of 10% or more from baseline (ITT analysis; PIO, n = 382 (26.2%) vs. placebo, n = 182 (12.7%); P value < 0.001; NNH = 7).40
Edema
Information regarding edema outcomes with PIO in patients with pre-diabetes was available from one NRS.40
The NRS40 reported statistically significant results favouring placebo over PIO among patients with pre-diabetes (using the ADA criteria),50 for the outcomes of self-reported new or worsening edema (ITT analysis; PIO, n = 541 (37.2% vs. placebo, n = 360 (25.2%); P value < 0.001; NNH = 8).40
Other AEs
Information regarding other AEs with PIO in patients with pre-diabetes was available from one NRS.40
The NRS40 reported no statistically significant results when comparing placebo with PIO among patients with pre-diabetes (using the ADA criteria),50 for the outcomes of hospitalization or incident cancer.
Clinical evidence regarding the safety of pioglitazone for patients with type 2 diabetes mellitus
Death
Information regarding death outcomes with PIO in patients with type 2 diabetes was available from one SR,30 and one NRS.35
In the SR,30 authors reported outcomes for patients with type 2 diabetes, without a history of established atherosclerotic CVD at baseline. There was no statistical difference in relative risk (RR) between PIO and comparators (i.e., SUs, not PIO, MET 500 mg, MET 750 mg, MET 850 mg, GLIM, glyburide) on the outcomes of all-cause mortality.30 The same SR30 performed an additional analysis for patients with or at high risk of type 2 diabetes, without a history of established atherosclerotic CVD at baseline. On the outcome of all-cause mortality results were not statistically different between groups.30
Authors of the NRS35 reported that PIO users had a statistically significantly lower risk of all-cause mortality (HR adjusted for sex, age, and baseline comorbidities: 0.47; 95% CI, 0.38 to 0.58; P value < 0.001), as well as lower risk of non-cardiovascular (CV) death (HR adjusted for sex, age, and baseline comorbidities: 0.50; 95% CI, 0.38 to 0.66; P value < 0.001) compared with the use of antidiabetic drugs other than insulin and PIO. However, they reported no statistical differences on the incidence of CV death.35
Cardiovascular outcomes
Information regarding cardiovascular outcomes with PIO in patients with type 2 diabetes was available from three SRs,29–31 two RCTs,33,34 and four NRSs.35,37–39
The first SR29 reported no statistical difference in left ventricular diastolic function in a direct comparison of PIO with ROS, as well as PIO with conventional treatment (not defined). When authors performed an NMA, they reported that PIO was statistically significantly worse than LIR for worsening left ventricular diastolic function (standardised mean difference [SMD] = −1.38; 95% CI, −2.11 to −0.65).29 However, the same NMA found no difference when PIO was compared with placebo, EXE, SIT, or LIN.29
Another SR30 reported outcomes for patients with type 2 diabetes, without a history of established atherosclerotic CVD at baseline. There were no differences in RR between PIO and comparators (i.e. SUs, not PIO, GLIM, glyburide) on the outcomes of: major adverse cardiovascular events (i.e., the composite of non-fatal MI, non-fatal stroke, and cardiovascular death), non-fatal MI, non-fatal stroke, hospitalization for HF, as well as cardiovascular death. The same SR30 performed an additional analysis for patients with or at high risk of type 2 diabetes, without a history of established atherosclerotic CVD at baseline. On the outcomes of major adverse cardiovascular events (i.e., non-fatal MI, non-fatal stroke, or cardiovascular death), non-fatal MI, non-fatal stroke, hospitalization for HF, cardiovascular death, as well as overall effect (i.e., total of major adverse cardiovascular events, non-fatal MI, and non-fatal stroke), results were not statistically different between groups.
The third SR31 reported no statistical difference between PIO and comparators (i.e., ACAR, GLIM, GLIC, and MET) in changes from baseline on outcomes of: blood pressure (systolic, diastolic, and overall), cardiovascular events, as well as vascular disorders.
In the first RCT, authors reported no statistically significant difference between PIO and GLIM in number of patients reporting palpitations.33 While the second RCT34 reported a statistically significant difference between the two treatment groups in increase in SBP from baseline, favouring PIO over SIT (PIO: 2.4 mmHg, standard deviation [SD] = 14.6; SIT 3 mmHg, SD = 15.4; P value < 0.001). However, it is important to highlight that baseline characteristics of patients with regards to their SBP were not balanced between groups (P value = 0.001); it is unclear whether this may have impacted these results. The same RCT34 found no difference in diastolic blood pressure changes between groups.
The first NRS35 reported no statistical differences between PIO users and non-users (i.e., use of antidiabetic drugs other than insulin and PIO) on the incidence of: hospitalized coronary artery disease (CAD), hospitalized stroke, and HF. A second NRS37 reported an incidence of less than five cases of HF among 77 incident PIO users (incidence of nine per 1,000 person-years [95% CI, 2 to 34]) and less than five cases of HF among 133 prevalent PIO users (incidence of two per 1,000 person-years [95% CI, 0 to 13]) during the follow-up period.
A third NRS38 reported a statistically significant difference favouring PIO users compared to non-users (i.e., use of other OADs) in the incidence of MI (RR adjusted for sex and age: 0.55; 95% CI, 0.37 to 0.80; P value = 0.002), as well as the incidence of HF (RR adjusted for sex and age: 0.72; 95% CI, 0.55 to 0.95; P value = 0.021). However, authors reported no difference in incidence of stroke between groups.38
The fourth NRS39 reported no statistical differences between PIO and LIN at last follow-up on outcomes of: MI, stroke, unstable angina, coronary revascularization, and composite (i.e., hospitalization for acute MI, ischaemic or haemorrhagic stroke, unstable angina, or coronary revascularization).
Fractures
Information regarding fracture outcomes with PIO in patients with type 2 diabetes was available from one RCT.33
Authors reported no statistical difference in the number of patients reporting a fracture between PIO and GLIM.33 The number of events in the PIO group was two out of 69 patients (one cuneiform bone of the foot and one intertrochanteric section of femur after falling), while there were zero fractures out of 66 patients in the GLIM group.33
Weight
Information regarding weight change outcomes with PIO in patients with type 2 diabetes was available from one SR,28 and two RCTs.33,34
In the SR,28 authors performed a MA and reported no significant difference in percent weight change from baseline between ALE and PIO. Similarly, one RCT33 reported no significant difference in number of patients reporting weight gain between PIO and GLIM.
While the second RCT34 reported a statistically significant increase in body weight (PIO: 0.9 kg, SD = 1.5; SIT: −0.5 kg, SD = 1.1; P value < 0.001) and hip circumference (PIO: 2 cm, SD = 5.3; SIT: −0.7 cm, SD = 3.1; P value < 0.001), for the PIO group. Furthermore, the PIO group saw nine early study discontinuations, of 125 patients, due to weight gain.34 However, authors reported no significant difference in changes from baseline with regards to BMI and waist circumference, measured at week 52.34
Edema
Information regarding edema outcomes with PIO in patients with type 2 diabetes was available from two SRs,28,31 and two RCTs.33,34
In the first SR,28 authors performed a MA and reported no difference in the odds ratio for edema events between ALE and PIO. Conversely, authors in the second SR with MA31 found a statistically significant difference in the RR of peripheral edema (2.21; 95% CI, 1.48 to 3.31; P value = 0.0001) favouring the comparators (i.e., SIT, vildagliptin [VIL], GLIM, GLIC, repaglinide [REP], and ALO) over PIO.
Authors from one RCT33 reported no significant difference between PIO and GLIM in the number of patients reporting edema. However, the PIO group in the other RCT saw six early study discontinuations, of 125 patients, due to edema, while none of the 125 patients in the SIT group discontinued the study.34
Hypoglycemia
Information regarding hypoglycemia outcomes with PIO in patients with type 2 diabetes was available from two SRs,28,31 and one RCT.33
In the SR,28 authors performed a MA and reported no difference in odds ratio of hypoglycemia events between ALE and PIO. Conversely, authors in the second SR with MA31 found a statistically significant difference in the RR of hypoglycemia (0.51; 95% CI, 0.33 to 0.80; P value = 0.003) favouring PIO over the comparators (i.e., MET, VIL, REP, GLIC, GLIM, SIT).
Similarly, authors of the RCT reported a statistically significant difference in the number of patients reporting hypoglycemia, favouring PIO over GLIM (P value = 0.002).33
Cancer
Information regarding cancer outcomes with PIO in patients with type 2 diabetes was available from two SRs,28,31 and one NRS.37
Authors of the first SR reported the absolute number of malignancy events (type not defined) for ALE (5/945), placebo (0/997), and PIO (1/148).28 While authors of the second SR found no statistical difference in the RR of breast cancer between PIO and SIT, nor the RR of colon cancer between PIO and ALO.31
Authors of the NRS reported no new bladder cancer cases among incident PIO users and fewer than five new bladder cancer cases among prevalent PIO users. New bladder cancer cases among prevalent users of other agents were not reported.37
Renal function
Information regarding renal function outcomes with PIO in patients with type 2 diabetes was available from one SR,28 one RCT,33 and one NRS.37
In the SR,28 authors performed a MA and reported a statistically significant difference between groups in the percent change from baseline in serum creatinine (P value < 0.00001) and estimated glomerular filtration rate (eGFR) (P value < 0.0004), favouring PIO over ALE.
Authors of the RCT,33 reported no statistical difference in the number of patients reporting acute pyelonephritis between PIO and GLIM. Authors of the NRS, also reported no statistical difference between incident PIO users and prevalent PIO users in the number of uninvestigated cases of macroscopic haematuria (i.e., patients with a recording of haematuria, but without a subsequent laboratory urine assessment, or other investigation) during the follow-up period.37
Other AEs
Information regarding other AEs with PIO in patients with type 2 diabetes was available from one SR,31 one RCT,33 and one NRS.36
In the SR,31 authors reported no statistically significant differences between PIO and comparators for the outcomes of: upper respiratory tract infections (comparators: REP, ALO, VIL, and SIT), nervous system disorders (comparators: SIT, REP, VIL, ALO), diarrhea (comparators: SIT, REP), musculoskeletal and connective tissues disorders (comparators: REP, ALO), asthenia (comparator: VIL), abnormal liver function parameters (comparator: GLIC), nausea (comparator: SIT), vomiting (comparator: SIT), and non-cardiac chest pain (comparator: ALO).
The RCT authors reported no statistically significant differences between PIO and GLIM for the outcomes of: upper respiratory infection, dizziness, headache, dyspepsia, diarrhea, itching, abdominal pain, ache, and myalgia.33
Authors of the NRS36 reported a statistically significant difference in the incidence of cirrhosis favouring PIO users compared with non-TZD users (HR adjusted for sex, age, and baseline comorbidities: 0.35; 95% CI, 0.15 to 0.85; P value < 0.05).
Limitations
A number of limitations were identified in the critical appraisal as shown in Appendix 3, , and ; however, additional limitations exist. The main limitations of this review are related to the heterogeneity of the study populations and the generalizability of the findings.
Heterogeneity was apparent in the baseline patient characteristics of primary studies included in the SRs,28–32 and among the RCTs33,34 and NRSs35–40 included in this report (e.g., duration of type 2 diabetes, baseline hemoglobin A1c, controlled or uncontrolled diabetes, number of comorbidities, number of concurrent antidiabetic medications). As PIO is generally not considered a first-line treatment in diabetes,2 its use in more severe cases of diabetes brings with it additional confounders that should be considered, particularly with NRSs.
Another heterogenous aspect, affecting the pre-diabetes literature, was the lack of a standard definition of pre-diabetes (e.g., American definition, WHO definition, Canadian definition, homeostatic model assessment of insulin resistant [HOMA-IR]). As such, this reduces the ability to compare study findings.
Of note in the SRs, primary study data were often available only at the study characteristic level. Heterogeneity existed and was likely the result of differences in baseline characteristics of participants, sample size, or combination treatments. Although all the trials included in the SRs were randomized, minimising potential biases introduced by these limitations, it remains unclear whether any differences between outcomes were due to differences in these characteristics. An additional source of variability was the follow-up period (weeks33,34,39 to years35,36,40) of the primary studies included in the SRs. As some outcomes such as cancer, HF, and fractures require months to years to develop, the reader should be mindful of the study durations when interpreting results of this report. Additionally, the doses of PIO interventions as well as those of comparator drugs varied from study to study. Therefore, caution should be exercised in interpreting and generalizing their findings.
Two SRs,28,31 contained comparator drugs (e.g., VIL, ALE, currently not available in Canada, limiting the applicability of their findings to Canadian settings. One RCT33,34 was of 26 week duration, which may not have been long enough to detect longer term outcomes such as fractures and cancer.
One study40 was a secondary analysis of a subgroup of patients enrolled in a previous clinical trial that used the HOMA-IR score to measure insulin resistance. The use of the HOMA-IR score is not common in Canadian clinical settings; therefore, this study’s results may have limited generalisability and application in clinical practice.
Except for one NRS,40 participant adherence with treatment was not reported which introduces uncertainty with regards to the magnitude of effects reported. Furthermore, five NRSs35–39 queried large prescription databases for data on PIO dispensing, which does not necessarily corelate with patients actively taking the medication. Information bias, affecting the accuracy of outcome measurements, may have resulted from relying on dispensing information and the inability to ascertain the actual drug intake. Furthermore, depending on the comprehensiveness of administrative databases, some confounding factors that may change risk of outcomes such as CVD, cancer, and mortality (e.g., lipid profile, hemoglobin A1c, renal function, hypoglycemia, diet and lifestyle, BMI, tobacco use) may not have been consistently available for inclusion in analyses, resulting in an underestimation of the reported risk.
The small number of PIO users in some included studies, resulted in high uncertainty around treatment effects, precluding meaningful inferences. In addition, while RCTs are a robust study design for establishing the safety and efficacy of pharmaceuticals, they generally exclude considerable portions of the potentially treatable population, thus limiting the generalizability of their findings.
Other potential safety issues of PIO (e.g., macular edema, ovulation in anovulatory women) were not examined in this report due to a lack of relevant data. This gap would suggest the need for future research.
Conclusions and Implications for Decision or Policy Making
This report identified safety evidence regarding the use of PIO in patients with pre-diabetes or type 2 diabetes. Five SRs28–32 (four with MAs28,30–32 and one with NMA29), two RCTs,33,34 and six NRSs35–40 were identified and included in this review.
The identified literature were heterogenous and revealed mixed conclusions regarding the safety of PIO in patients with pre-diabetes. No statistically significant difference was reported regarding PIO’s potential effects on mortality40 and major adverse cardiovascular events.30 While lumbar spine BMD was worse for pre-diabetes PIO users,32 no clear direction emerged regarding odds of fractures, with some studies finding no effect32 and others favouring placebo.40 However, an identified NRS in patients with pre-diabetes suggest that PIO was associated with an increased likelihood of weight gain and edema when compared to placebo.40 Whether these increases would be considered clinically meaningful changes, particularly with respect to weight gain, was not discussed in the study. The same NRS reported no significant between group differences in hospitalization or incident cancer.40
With regards to the safety of PIO in patients with type 2 diabetes, the identified literature were heterogenous and revealed mixed conclusions. No clear direction emerged regarding the drug’s potential effects on mortality, with one SR finding no effect30 and one NRS favouring PIO in specific comparisons.35 Results were mixed (some statistically significant and non-significant findings) regarding major adverse cardiovascular events (i.e., non-fatal MI, non-fatal stroke, or cardiovascular death),30,35,39 blood pressure,31,34 weight change,28,33,34 edema,28,31,33,34 renal function,28,33,37 or incidence of HF among PIO users.30,35,37,38 However, an NMA found that PIO was worse than LIR for decreasing left ventricular diastolic function, but found no difference when PIO was compared with placebo, EXE, SIT, or LIN.29 There were no differences between PIO and GLIM in the incidence of fractures,33 or between PIO and various comparators (e.g., ALE, MET, SUs, DPP-4 inhibitors, ACAR, MEG, insulin) for the incidence of cancer.28,31,37 Two analyses of PIO found it to be less likely to cause hypoglycemia;31,33 however, in a third analysis, the odds depended on the comparator used.28 As such, there remains some uncertainty around the overall safety profile of PIO.
The limitations of the included studies, especially heterogeneity (e.g., baseline characteristics, follow-up period, and lack of standard definition of pre-diabetes), should be considered when interpreting the results. The findings highlighted in this review come with a high degree of uncertainty. The lack of consensus in the identified literature suggests that more comparative studies are required in patients with pre-diabetes and type 2 diabetes.
References
- 1.
Triplitt
CL, Reasner
CA, Isley
WL. Chapter 77: Diabetes Mellitus. In: DiPiro
JT, Talbert
RL, Yee
GC, Matzke
GR, Wells
BG, Posey
LM, eds. Pharmacotherapy: a pathophysiologic approach. 7th
ed. New York (NY): McGraw-Hill; 2008.
- 2.
Arnason
T, Mansell
K. Diabetes mellitus. In:
Compendium of therapeutic choices. Ottawa (ON): Canadian Pharmacists Association; 2019.
- 3.
- 4.
Institute for Health Metrics and Evaluation. The Global Burden of Disease study data visualization hub : 2017 estimated incidence of diabetes mellitus type 2, for both sexes and all ages in Canada. Seattle (WA): University of Washington; 2020:
http://ihmeuw.org/55bv. Accessed 2020 Jun 8.
- 5.
Institute for Health Metrics and Evaluation. The Global Burden of Disease study data visualization hub : 2017 estimated prevalence of diabetes mellitus type 2, for both sexes and all ages in Canada. Seattle (WA): University of Washington; 2020:
http://ihmeuw.org/55bw. Accessed 2020 Jun 8.
- 6.
Institute for Health Metrics and Evaluation. The Global Burden of Disease study data visualization hub : 2017 estimated deaths due to diabetes mellitus type 2, for both sexes and all ages in Canada. Seattle (WA): University of Washington; 2020:
http://ihmeuw.org/55bt. Accessed 2020 Jun 8.
- 7.
Institute for Health Metrics and Evaluation. The Global Burden of Disease study data visualization hub : 2017 estimated years of life lost due to diabetes mellitus type 2, for both sexes and all ages in Canada. Seattle (WA): University of Washington; 2020:
http://ihmeuw.org/55bu. Accessed 2020 Jun 8.
- 8.
- 9.
Inzucchi
SE, Lupsa
B. Thiazolidinediones in the treatment of type 2 diabetesmellitus. In: Post
TW, ed.
UpToDate. Waltham (MA): UpToDate; 2020:
www.uptodate.com. Accessed 2020 Jun 8.
- 10.
Dormandy
JA, Charbonnel
B, Eckland
DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial in macroVascular Events): a randomised controlled trial.
Lancet. 2005;366(9493):1279–1289. [
PubMed: 16214598]
- 11.
- 12.
- 13.
Neumann
A, Weill
A, Ricordeau
P, Fagot
JP, Alla
F, Allemand
H. Pioglitazone and risk of bladder cancer among diabetic patients in France: a population-based cohort study.
Diabetologia. 2012;55(7):1953–1962. [
PMC free article: PMC3369136] [
PubMed: 22460763]
- 14.
Lewis
JD, Ferrara
A, Peng
T, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study.
Diabetes Care. 2011;34(4):916–922. [
PMC free article: PMC3064051] [
PubMed: 21447663]
- 15.
Lewis
JD, Habel
L, Quesenberry
C, et al. Proteinuria testing among patients with diabetes mellitus is associated with bladder cancer diagnosis: potential for unmeasured confounding in studies of pioglitazone and bladder cancer.
Pharmacoepidemiol Drug Saf. 2014;23(6):636–645. [
PubMed: 24764283]
- 16.
Lewis
JD, Habel
LA, Quesenberry
CP, et al. Pioglitazone use and risk of bladder cancer and other common cancers in persons with diabetes.
JAMA. 2015;314(3):265–277. [
PubMed: 26197187]
- 17.
Home
PD, Pocock
SJ, Beck-Nielsen
H, et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial.
Lancet. 2009;373(9681):2125–2135. [
PubMed: 19501900]
- 18.
- 19.
Idris
I, Warren
G, Donnelly
R. Association between thiazolidinedione treatment and risk of macular edema among patients with type 2 diabetes.
Arch Intern Med. 2012;172(13):1005–1011. [
PubMed: 22688528]
- 20.
Ambrosius
WT, Danis
RP, Goff
DC
Jr, et al. Lack of association between thiazolidinediones and macular edema in type 2 diabetes: the ACCORD eye substudy.
Arch Ophthalmol. 2010;128(3):312–318. [
PMC free article: PMC3010554] [
PubMed: 20212201]
- 21.
Viscoli
CM, Inzucchi
SE, Young
LH, et al. Pioglitazone and risk for bone fracture: safety data from a randomized Clinical Trial.
J Clin Endocrinol Metab. 2017;102(3):914–922. [
PMC free article: PMC5460686] [
PubMed: 27935736]
- 22.
Ota
H, Goto
T, Yoshioka
T, Ohyama
N. Successful pregnancies treated with pioglitazone in infertile patients with polycystic ovary syndrome.
Fertil Steril. 2008;90(3):709–713. [
PubMed: 18423625]
- 23.
- 24.
Shea
BJ, Reeves
BC, Wells
G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both.
BMJ. 2017;358:j4008. [
PMC free article: PMC5833365] [
PubMed: 28935701]
- 25.
Jansen
JP, Trikalinos
T, Cappelleri
JC, et al. Indirect treatment comparison/network meta-analysis study questionnaire to assess relevance and credibility to inform health care decision making: an ISPOR-AMCP-NPC Good Practice Task Force report.
Value Health. 2014;17(2):157–173. [
PubMed: 24636374]
- 26.
Downs
SH, Black
N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions.
J Epidemiol Community Health. 1998;52(6):377–384. [
PMC free article: PMC1756728] [
PubMed: 9764259]
- 27.
Liberati
A, Altman
DG, Tetzlaff
J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration.
J Clin Epidemiol. 2009;62(10):e1–e34. [
PubMed: 19631507]
- 28.
Han
CL, Qu
CZ. Cardiovascular risk and safety evaluation of a dual peroxisome proliferator-activated receptor-alpha/gamma agonist, aleglitazar, in patients with type 2 diabetes: a meta-analysis.
J Cardiovasc Pharmacol. 2020;75(4):351–357. [
PubMed: 31929323]
- 29.
Ida
S, Kaneko
R, Imataka
K, et al. Effects of oral antidiabetic drugs and glucagon-like peptide-1 receptor agonists on left ventricular diastolic function in patients with type 2 diabetes mellitus: a systematic review and network meta-analysis.
Heart Fail Rev. 2020. [
PubMed: 32080782]
- 30.
Zhou
Y, Huang
Y, Ji
X, Wang
X, Shen
L, Wang
Y. Pioglitazone for the primary and secondary prevention of cardiovascular and renal outcomes in patients with or at high risk of type 2 diabetes mellitus: a meta-analysis.
J Clin Endocrinol Metab. 2020;105(5). [
PubMed: 31822895]
- 31.
Alam
F, Islam
MA, Mohamed
M, et al. Efficacy and safety of pioglitazone monotherapy in type 2 diabetes mellitus: a systematic review and meta-analysis of randomised controlled trials.
Sci Rep. 2019;9(1):5389. [
PMC free article: PMC6441028] [
PubMed: 30926892]
- 32.
Zuo
L, Wang
J, Zhang
N, Wang
J. Pioglitazone therapy decreases bone mass density and increases fat mass: a meta-analysis.
Curr Pharm Des. 2019;25(33):3590–3596. [
PubMed: 31538886]
- 33.
Kim
JM, Kim
SS, Kim
JH, et al. Efficacy and safety of pioglitazone versus glimepiride after metformin and alogliptin combination therapy: a randomized, open-label, multicenter, parallel-controlled study.
Diabetes Metab J. 2020;44(1):67–77. [
PMC free article: PMC7043969] [
PubMed: 31339011]
- 34.
Khaloo
P, Asadi Komeleh
S, Alemi
H, et al. Sitagliptin vs. pioglitazone as add-on treatments in patients with uncontrolled type 2 diabetes on the maximal dose of metformin plus sulfonylurea.
J Endocrinol Invest. 2019;42(7):851–857. [
PubMed: 30535871]
- 35.
Yen
FS, Wang
HC, Pan
CW, Wei
JC, Hsu
CC, Hwu
CM. Pioglitazone exposure reduced the risk of all-cause mortality in insulin-treated patients with type 2 diabetes mellitus.
J Clin Endocrinol Metab. 2020;105(3). [
PubMed: 31544207]
- 36.
- 37.
Cid Ruzafa
J, Ulrichsen
SP, Bennett
D, Ehrenstein
V. Post-authorisation safety study of pioglitazone use and safety endpoints of interest in Denmark after direct healthcare professional communication.
Drugs Real World Outcomes. 2019;6(3):133–140. [
PMC free article: PMC6702533] [
PubMed: 31376066]
- 38.
Miao
S, Dong
X, Zhang
X, et al. Detecting pioglitazone use and risk of cardiovascular events using electronic health record data in a large cohort of Chinese patients with type 2 diabetes.
J Diabetes. 2019;11(8):684–689. [
PMC free article: PMC6850402] [
PubMed: 30597747]
- 39.
Patorno
E, Gopalakrishnan
C, Brodovicz
KG, et al. Cardiovascular safety of linagliptin compared with other oral glucose-lowering agents in patients with type 2 diabetes: a sequential monitoring programme in routine care.
Diabetes Obes Metab. 2019;21(8):1824–1836. [
PMC free article: PMC6785989] [
PubMed: 30941884]
- 40.
Spence
JD, Viscoli
CM, Inzucchi
SE, et al. Pioglitazone therapy in patients with stroke and prediabetes: a post hoc analysis of the IRIS randomized clinical trial.
JAMA Neurol. 2019;76(5):526–535. [
PMC free article: PMC6515584] [
PubMed: 30734043]
- 41.
Naka
KK, Pappas
K, Papathanassiou
K, et al. Lack of effects of pioglitazone on cardiac function in patients with type 2 diabetes and evidence of left ventricular diastolic dysfunction: a tissue doppler imaging study.
Cardiovasc Diabetol. 2010;9:57. [
PMC free article: PMC2955641] [
PubMed: 20863381]
- 42.
Pala
S, Esen
O, Akcakoyun
M, et al. Rosiglitazone, but not pioglitazone, improves myocardial systolic function in type 2 diabetic patients: a tissue Doppler study.
Echocardiography. 2010;27(5):512–518. [
PubMed: 20412274]
- 43.
Oe
H, Nakamura
K, Kihara
H, et al. Comparison of effects of sitagliptin and voglibose on left ventricular diastolic dysfunction in patients with type 2 diabetes: results of the 3D trial.
Cardiovasc Diabetol. 2015;14(1):83. [
PMC free article: PMC4473835] [
PubMed: 26084668]
- 44.
Scalzo
RL, Moreau
KL, Ozemek
C, et al. Exenatide improves diastolic function and attenuates arterial stiffness but does not alter exercise capacity in individuals with type 2 diabetes.
J Diabetes Complications. 2017;31(2):449–455. [
PMC free article: PMC5787373] [
PubMed: 27884660]
- 45.
Yamada
H, Tanaka
A, Kusunose
K, et al. Effect of sitagliptin on the echocardiographic parameters of left ventricular diastolic function in patients with type 2 diabetes: a subgroup analysis of the PROLOGUE study.
Cardiovasc Diabetol. 2017;16(1):63. [
PMC free article: PMC5426055] [
PubMed: 28490337]
- 46.
- 47.
Bizino
MB, Jazet
IM, Westenberg
JJM, et al. Effect of liraglutide on cardiac function in patients with type 2 diabetes mellitus: randomized placebo-controlled trial.
Cardiovasc Diabetol. 2019;18(1):55. [
PMC free article: PMC6492440] [
PubMed: 31039778]
- 48.
Scalzo
RL, Rafferty
D, Schauer
I, et al. Sitagliptin improves diastolic cardiac function but not cardiorespiratory fitness in adults with type 2 diabetes.
J Diabetes Complications. 2019;33(8):561–566. [
PMC free article: PMC7278036] [
PubMed: 31182338]
- 49.
Diabetes Canada Clinical Practice Guidelines Expert Committee. Diabetes Canada 2018 clinical practice guidelines for the prevention and management of diabetes in Canada.
Can J Diabetes. 2018;42(Suppl 1):S1–S325. [
PubMed: 29650079]
- 50.
American Diabetes Association. 2. Classification and diagnosis of diabetes.
Diabetes Care. 2016;39(Suppl 1):S13–22. [
PubMed: 26696675]
- 51.
Bone
HG, Lindsay
R, McClung
MR, Perez
AT, Raanan
MG, Spanheimer
RG. Effects of pioglitazone on bone in postmenopausal women with impaired fasting glucose or impaired glucose tolerance: a randomized, double-blind, placebo-controlled study.
J Clin Endocrinol Metab. 2013;98(12):4691–4701. [
PubMed: 24057294]
- 52.
Ruilope
L, Hanefeld
M, Lincoff
AM, et al. Effects of the dual peroxisome proliferator-activated receptor-alpha/gamma agonist aleglitazar on renal function in patients with stage 3 chronic kidney disease and type 2 diabetes: a phase IIb, randomized study.
BMC Nephrol. 2014;15(1):180. [
PMC free article: PMC4364102] [
PubMed: 25407798]
- 53.
Henry
RR, Lincoff
AM, Mudaliar
S, Rabbia
M, Chognot
C, Herz
M. Effect of the dual peroxisome proliferator-activated receptor-α/γ agonist aleglitazar on risk of cardiovascular disease in patients with type 2 diabetes (SYNCHRONY): a phase II, randomised, dose-ranging study.
Lancet. 2009;374(9684):126–135. [
PubMed: 19515415]
- 54.
Herz
M, Gaspari
F, Perico
N, et al. Effects of high dose aleglitazar on renal function in patients with type 2 diabetes.
Int J Cardiol. 2011;151(2):136–142. [
PubMed: 20837369]
- 55.
Vaccaro
O, Masulli
M, Nicolucci
A, et al. Effects on the incidence of cardiovascular events of the addition of pioglitazone versus sulfonylureas in patients with type 2 diabetes inadequately controlled with metformin (TOSCA.IT): a randomised, multicentre trial.
Lancet Diabetes Endocrinol. 2017;5(11):887–897. [
PubMed: 28917544]
- 56.
Yoshii
H, Onuma
T, Yamazaki
T, et al. Effects of pioglitazone on macrovascular events in patients with type 2 diabetes mellitus at high risk of stroke: the PROFIT-J study.
J Atheroscler Thromb. 2014;21(6):563–573. [
PubMed: 24477028]
- 57.
DeFronzo
RA, Tripathy
D, Schwenke
DC, et al. Pioglitazone for diabetes prevention in impaired glucose tolerance.
N Engl J Med. 2011;364(12):1104–1115. [
PubMed: 21428766]
- 58.
Kaku
K, Daida
H, Kashiwagi
A, et al. Long-term effects of pioglitazone in Japanese patients with type 2 diabetes without a recent history of macrovascular morbidity.
Curr Med Res Opin. 2009;25(12):2925–2932. [
PubMed: 19835463]
- 59.
Mazzone
T, Meyer
PM, Feinstein
SB, et al. Effect of pioglitazone compared with glimepiride on carotid intima-media thickness in type 2 diabetes: a randomized trial.
JAMA. 2006;296(21):2572–2581. [
PubMed: 17101640]
- 60.
Jain
R, Osei
K, Kupfer
S, Perez
AT, Zhang
J, Lannon
MM. Long-term safety of pioglitazone versus glyburide in patients with recently diagnosed type 2 diabetes mellitus.
Pharmacotherapy. 2006;26(10):1388–1395. [
PubMed: 16999648]
- 61.
Morikawa
A, Ishizeki
K, Iwashima
Y, et al. Pioglitazone reduces urinary albumin excretion in renin-angiotensin system inhibitor-treated type 2 diabetic patients with hypertension and microalbuminuria: the APRIME study.
Clin Exp Nephrol. 2011;15(6):848–853. [
PubMed: 21823043]
- 62.
Schernthaner
G, Matthews
DR, Charbonnel
B, Hanefeld
M, Brunetti
P, Quartet Study G. Efficacy and safety of pioglitazone versus metformin in patients with type 2 diabetes mellitus: a double-blind, randomized trial.
J Clin Endocrinol Metab. 2004;89(12):6068–6076. [
PubMed: 15579760]
- 63.
Tanaka
R, Yamashiro
K, Okuma
Y, et al. Effects of pioglitazone for secondary stroke prevention in patients with impaired glucose tolerance and newly diagnosed diabetes: the J-SPIRIT study.
J Atheroscler Thromb. 2015;22(12):1305–1316. [
PubMed: 26269002]
- 64.
Goke
B, German Pioglitazone Study Group. Improved glycemic control and lipid profile in a randomized study of pioglitazone compared with acarbose in patients with type 2 diabetes mellitus.
Treat Endocrinol. 2002;1(5):329–336. [
PubMed: 15832486]
- 65.
Hu
YY, Ye
SD, Zhao
LL, Zheng
M, Wu
FZ, Chen
Y. Hydrochloride pioglitazone decreases urinary cytokines excretion in type 2 diabetes.
Clin Endocrinol (Oxf). 2010;73(6):739–743. [
PubMed: 20874769]
- 66.
Erdem
G, Dogru
T, Tasci
I, et al. The effects of pioglitazone and metformin on plasma visfatin levels in patients with treatment naive type 2 diabetes mellitus.
Diabetes Res Clin Pract. 2008;82(2):214–218. [
PubMed: 18778865]
- 67.
Alba
M, Ahren
B, Inzucchi
SE, et al. Sitagliptin and pioglitazone provide complementary effects on postprandial glucose and pancreatic islet cell function.
Diabetes Obes Metab. 2013;15(12):1101–1110. [
PubMed: 23782502]
- 68.
Rosenstock
J, Kim
SW, Baron
MA, et al. Efficacy and tolerability of initial combination therapy with vildagliptin and pioglitazone compared with component monotherapy in patients with type 2 diabetes.
Diabetes Obes Metab. 2007;9(2):175–185. [
PubMed: 17300593]
- 69.
Tan
M, Johns
D, Gonzalez Galvez
G, et al. Effects of pioglitazone and glimepiride on glycemic control and insulin sensitivity in Mexican patients with type 2 diabetes mellitus: a multicenter, randomized, double-blind, parallel-group trial.
Clin Ther. 2004;26(5):680–693. [
PubMed: 15220012]
- 70.
Perriello
G, Pampanelli
S, Di Pietro
C, Brunetti
P, Italian Pioglitazone Study G. Comparison of glycaemic control over 1 year with pioglitazone or gliclazide in patients with Type 2 diabetes.
Diabet Med. 2006;23(3):246–252. [
PubMed: 16492206]
- 71.
Jovanovic
L, Hassman
DR, Gooch
B, et al. Treatment of type 2 diabetes with a combination regimen of repaglinide plus pioglitazone.
Diabetes Res Clin Pract. 2004;63(2):127–134. [
PubMed: 14739053]
- 72.
Pérez-Monteverde
A, Seck
T, Xu
L, et al. Efficacy and safety of sitagliptin and the fixed-dose combination of sitagliptin and metformin vs. pioglitazone in drug-naive patients with type 2 diabetes.
Int J Clin Pract. 2011;65(9):930–938. [
PubMed: 21849007]
- 73.
Rosenstock
J, Inzucchi
SE, Seufert
J, Fleck
PR, Wilson
CA, Mekki
Q. Initial combination therapy with alogliptin and pioglitazone in drug-naive patients with type 2 diabetes.
Diabetes Care. 2010;33(11):2406–2408. [
PMC free article: PMC2963503] [
PubMed: 20724648]
- 74.
Ramachandran
A, Snehalatha
C, Salini
J, Vijay
V. Use of glimepiride and insulin sensitizers in the treatment of type 2 diabetes - a study in Indians.
J Assoc Physicians India. 2004;52(JUN):459–463. [
PubMed: 15645955]
- 75.
Bray
GA, Smith
SR, Banerji
MA, et al. Effect of pioglitazone on body composition and bone density in subjects with prediabetes in the ACT NOW trial.
Diabetes Obes Metab. 2013;15(10):931–937. [
PubMed: 23551856]
- 76.
Grey
A, Bolland
M, Fenwick
S, et al. The skeletal effects of pioglitazone in type 2 diabetes or impaired glucose tolerance: a randomized controlled trial.
Eur J Endocrinol. 2014;170(2):255–262. [
PubMed: 24217934]
Abbreviations
- ACAR
acarbose
- ADA
American Diabetes Association
- AE
adverse event
- ALE
aleglitazar
- ALO
alogliptin
- AMSTAR 2
A Measurement Tool to Assess Systematic Reviews 2
- BMD
bone mineral density
- BMI
body mass index
- CAD
coronary artery disease
- CI
confidence interval
- CRD
University of York Centre for Reviews and Dissemination
- CV
cardiovascular
- CVD
cardiovascular disease
- DPP-4
dipeptidyl peptidase-4
- eGFR
estimated glomerular filtration rate
- EXE
exenatide
- FDA
United States Food and Drug Administration
- GLIC
gliclazide
- GLIM
glimepiride
- GLP-1
glucagon-like peptide-1
- HbA1c
glycated hemoglobin A1c
- HF
heart failure
- HOMA-IR
homeostatic model assessment of insulin resistant
- HR
hazard ratio
- ITT
intention-to-treat
- LIN
linagliptin
- LIR
liraglutide
- MA
meta-analysis
- MEDLINE
Medical Literature Analysis and Retrieval System Online
- MEG
meglitinide
- MeSH
medical subject headings
- MET
metformin
- MI
myocardial infarction
- NMA
network meta-analysis
- NNH
number needed to harm
- NRS
non-randomized study
- OAD
oral antidiabetic drugs
- PIO
pioglitazone
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RCT
randomized controlled trial
- REP
repaglinide
- ROS
rosiglitazone
- RR
relative risk
- SBP
systolic blood pressure
- SD
standard deviation
- SIT
sitagliptin
- SMD
standardised mean difference
- SR
systematic review
- SU
sulphonylurea
- TIA
transient ischemic attack
- TZD
thiazolidinediones
- VIL
vildagliptin
- WHO
World Health Organization
Appendix 1. Selection of Included Studies
Appendix 5. Further Information
Additional References
Case reports
Xue
J, Liu
W, Shi
F, Zheng
J, Ma
J. Pleural effusion due to use of pioglitazone: a case report.
Metab Syndr Relat Disord. 2020
Apr;18(3):168–171.
PubMed: P32250209 [
PubMed: 32250209]
Karakurt
F, Kargili
A, Kasapoglu
B. Pioglitazone induced reversible pancytopenia.
Exp Clin Endocrinol Diabetes. 2010
Feb;118(2):96–97.
PubMed: PM19834871 [
PubMed: 19834871]
Alternative Intervention – Thiazolidinedione Drug Class in General
Alternative Population – Mixed Type of Diabetes
Garry
EM, Buse
JB, Gokhale
M, et al. Study design choices for evaluating the comparative safety of diabetes medications: an evaluation of pioglitazone use and risk of bladder cancer in older US adults with type-2 diabetes.
Diabetes Obes Metab. 2019
Sep;21(9):2096–2106.
PubMed: PM31087620 [
PMC free article: PMC7025290] [
PubMed: 31087620]
Relevant Systematic Reviews Published Before 2019
Adil
M, Khan
RA, Ghosh
P, et al. Pioglitazone and risk of bladder cancer in type 2 diabetes mellitus patients: a systematic literature review and meta-analysis of observational studies using real-world data.
Clin Epidemiol Glob Health. 2018
Jun;6(2):61–68. 10.1016/j.cegh.2017.08.002 [
CrossRef]
Davidson
MB and Pan
D. An updated meta-analysis of pioglitazone exposure and bladder cancer and comparison to the drug’s effect on cardiovascular disease and non-alcoholic steatohepatitis.
Diabetes Res Clin Pract.
PubMed: PM29146119 [
PubMed: 29146119]
Note: erratum - PubMed: PM29935912
Pavlova
V, Filipova
E, Uzunova
K, Kalinov
K, Vekov
T. Pioglitazone therapy and fractures: systematic review and meta- analysis.
Endocr Metab Immune Disord Drug Targets. 2018;18(5):502–507.
PubMed: PM29683100 [
PubMed: 29683100]
Bundhun
PK, Janoo
G, Teeluck
AR, Huang
F. Adverse drug effects observed with vildagliptin versus pioglitazone or rosiglitazone in the treatment of patients with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials.
BMC Pharmacol Toxicol. 2017
Oct
23;18(1):66.
PubMed: PM29058622 [
PMC free article: PMC5651605] [
PubMed: 29058622]
Lee
M, Saver
JL, Liao
HW, Lin
CH, Ovbiagele
B. Pioglitazone for secondary stroke prevention: a systematic review and meta-analysis.
Stroke. 2017
Feb;48(2):388–393.
PubMed: PM27999139 [
PubMed: 27999139]
Bolen
S, Tseng
E, Hutfless
S, et al. AHRQ Comparative Effectiveness Reviews. Diabetes Medications for Adults With Type 2 Diabetes: An Update. Rockville (MD): Agency for Healthcare Research and Quality (US); 2016.
PubMed: PM27227214 [
PubMed: 27227214]
Vos
RC, van Avendonk
MJ, Jansen
H, et al. Insulin monotherapy compared with the addition of oral glucose-lowering agents to insulin for people with type 2 diabetes already on insulin therapy and inadequate glycaemic control.
Cochrane Database Syst Rev. 2016
Sep
18;9:CD006992.
PubMed: PM27640062 [
PMC free article: PMC6457595] [
PubMed: 27640062]
Billington
EO, Grey
A, Bolland
MJ. The effect of thiazolidinediones on bone mineral density and bone turnover: systematic review and meta-analysis.
Diabetologia. 2015
Oct;58(10):2238–2246.
PubMed: PM26109213 [
PubMed: 26109213]
Gray
LJ, Dales
J, Brady
EM, Khunti
K, Hanif
W, Davies
MJ. Safety and effectiveness of non-insulin glucose-lowering agents in the treatment of people with type 2 diabetes who observe Ramadan: a systematic review and meta-analysis.
Diabetes Obes Metab. 2015
Jul;17(7):639–648.
PubMed: PM25777247 [
PubMed: 25777247]
Mearns
ES, Saulsberry
WJ, White
CM, et al. Efficacy and safety of antihyperglycaemic drug regimens added to metformin and sulphonylurea therapy in type 2 diabetes: a network meta-analysis.
Diabet Med. 2015
Dec;32(12):1530–1540.
PubMed: PM26104021 [
PubMed: 26104021]
About the Series
CADTH Rapid Response Report: Summary with Critical Appraisal
Funding: CADTH receives funding from Canada’s federal, provincial, and territorial governments, with the exception of Quebec.
Suggested citation:
Pioglitazone for type 2 diabetes mellitus and pre-diabetes: a review of safety. Ottawa: CADTH; 2020 Jun. (CADTH rapid response report: summary with critical appraisal).
Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services.
While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up-to-date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. The views and opinions of third parties published in this document do not necessarily state or reflect those of CADTH.
CADTH is not responsible for any errors, omissions, injury, loss, or damage arising from or relating to the use (or misuse) of any information, statements, or conclusions contained in or implied by the contents of this document or any of the source materials.
This document may contain links to third-party websites. CADTH does not have control over the content of such sites. Use of third-party sites is governed by the third-party website owners’ own terms and conditions set out for such sites. CADTH does not make any guarantee with respect to any information contained on such third-party sites and CADTH is not responsible for any injury, loss, or damage suffered as a result of using such third-party sites. CADTH has no responsibility for the collection, use, and disclosure of personal information by third-party sites.
Subject to the aforementioned limitations, the views expressed herein are those of CADTH and do not necessarily represent the views of Canada’s federal, provincial, or territorial governments or any third party supplier of information.
This document is prepared and intended for use in the context of the Canadian health care system. The use of this document outside of Canada is done so at the user’s own risk.
This disclaimer and any questions or matters of any nature arising from or relating to the content or use (or misuse) of this document will be governed by and interpreted in accordance with the laws of the Province of Ontario and the laws of Canada applicable therein, and all proceedings shall be subject to the exclusive jurisdiction of the courts of the Province of Ontario, Canada.