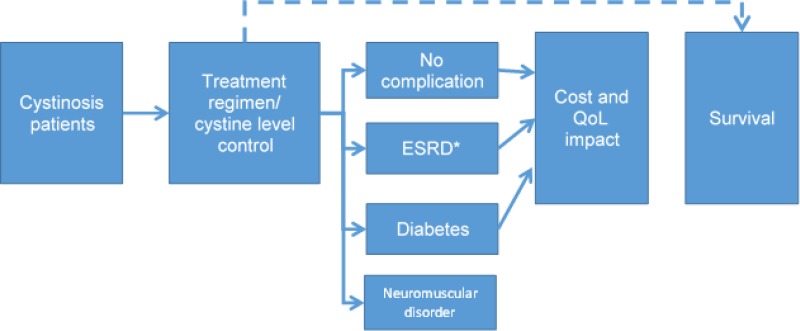
Manufacturer’s Model Structure
The manufacturer submitted a partitioned survival model comparing delayed-release cysteamine to no treatment. Cysteamine is the only specific treatment existing for nephropathic cystinosis in Canada. The manufacturer noted that a short-acting formulation (immediate-release cysteamine) was available through a federal special access program, although this was to be stopped upon the entry of delayed-release cysteamine to the Canadian market.
The manufacturer considered a lifetime horizon (i.e., 100 years in 1-year cycles with half-cycle correction) in a two-year-old child with cystinosis. The analysis adopted the Canadian public health care payer perspective with an annual discount rate of 1.5% on health benefits and costs. In addition to death, three different complications are followed throughout the model: end-stage renal disease (ESRD), diabetes, and neuromuscular disorder, with the possibility for patients to have no complication, only one complication, any combination of two complications, or three complications. The model structure as presented by the manufacturer can be seen in .
The manufacturer used a retrospective study in a cohort of 86 nephropathic cystinosis patients diagnosed in France in the years 1961 to 1995 as the basis for the time to events.9 In this retrospective cohort, 75 of the 86 patients (87%) received the short-acting formulation of cysteamine (40 patients started treatment before age five years; eight patients started treatment after age five years, but prior to any complications; and 27 patients only started treatment once ESRD was established, i.e., 22.6 ± 5.7 years). Eleven (11) patients never received cysteamine. Therefore, this retrospective study provides estimation of the time to events (death, ESRD, diabetes, neuromuscular disorder) in untreated patients as well as those starting cysteamine short-acting before age five years and after age five years.
The median age of onset for the three modelled complications, and death, as derived from the Brodin-Sartorius study9 for immediate-release cysteamine are provided in .
Empirical data on the long-term impact of delayed-release cysteamine are not available. The manufacturer consulted a clinical expert (Dr. Brodin-Sartorius) who noted that as compliance was only reported as good for 35% of patients and quite good for 41% of patients, there may be additional patient benefits associated with delayed-release cysteamine due to the side effect profile, compliance, early diagnosis, and monitoring. The revised estimates based on feedback from the clinical expert are also provided in . The manufacturer stated that monitoring and subsequent control of ½ cystine levels (and general health) have improved over the intervening years.
Table 9Median Patient Age at Complication Onset Used in the Model
View in own window
| Event | Untreated9 | Immediate-Release Cysteamine (Starting Age < 5)9 (Yrs) | Assumption For Delayed-Release Cysteamine (Starting Before Age 5) (Yrs) | Difference Between Delayed-Release Cysteamine And Untreated (Yrs) | Difference Between Delayed-Release Cysteamine And Immediate-Release Cysteamine (Yrs) |
|---|
| ESRD | 9 | 15 | 20 | 11 | 5 |
| Diabetes | 15 | 38 | 40 | 25 | 2 |
| Neuromuscular disorder | 25 | 35 | 45 | 20 | 10 |
| Death | 23 | 37 | 50 | 27 | 13 |
ESRD = end-stage renal disease; yrs = years.
Source: Manufacturer’s Pharmacoeconomic Submission8
In the model, these data were considered through hazard ratios using a Weibull distribution, which was modelled to allow the manufacturer to transform these to different probabilities that patients would be in each Markov health state.
Table 10Data Sources
View in own window
| Data Input | Description of Data Source | Comment |
|---|
| Efficacy | Risks for complications and mortality in the no-treatment group were taken from a retrospective cohort study with immediate-release cysteamine.9 Assumptions on the superiority of delayed-release cysteamine compared with immediate-release cysteamine were based on expert opinion. | The assumption of incremental effectiveness of delayed-release cysteamine compared with immediate-release cysteamine is based on the opinion of one expert, and is not supported by published evidence. The expert based his opinion on the potential for better adherence to treatment with cysteamine delayed-release. Some of the reasons given by the expert (e.g., early diagnosis and monitoring) are unlikely to have an impact as these changes in practice have occurred independently of effects from cysteamine therapy. Expert opinion on this important parameter was not obtained through state-ofthe-art methodology.11,12 Feedback from the clinical expert consulted by CADTH indicated that the biologic plausibility of the assumption of an incremental benefit of delayed-release cysteamine compared with immediate-release cysteamine is rational; greater adherence will hopefully lead to delayed morbidity. However the magnitude of the incremental benefit, if any, is not known. Therefore, the assumption of equivalent effectiveness in the model is acceptable. |
| Natural history | Risk of complications and mortality based on a retrospective cohort study9 | The efficacy of cysteamine is based on a retrospective study where immediate-release cysteamine was used in 86 adults. Only 11 individuals were in the untreated cohort for the survival analysis. For diabetes and neuromuscular disorders, the cohorts were more or less equal. It is uncertain whether the small number of patients in the untreated group and each subgroup were sufficient for the statistical comparisons. As most of the comparisons were based on hypothesis tests with P values; standard errors and confidence intervals were not reported. Thus, it is difficult to evaluate the precision and the clinical relevance of the statistical findings. |
| Utilities | Disutilities of adverse events, and complications from the medical literature. | In general, it is unclear which values have been used from the cited sources and how disutilities were calculated from the utilities reported in the sources. More specifically:
The diabetes value reported in Dale’s systematic review 30 comes from a time-trade-off study in 17 Canadian health care workers. Utilities in Canadian diabetes patients can be found in the literature. The manufacturer’s used MS as a proxy for neuromuscular disorders. According to the clinical expert consulted by CADTH, PD would be more appropriate; while MS may come and go with different severity, PD is cumulative in terms of deterioration, which more closely resembles this complication.
Furthermore, utilities were rarely tested in the PSA. |
| Baseline utility for a child with cystinosis from mapping of PedsQL 4.0 collected at month 1 in Langman’s study (40 children; average age 11.5 years).31 | The PedsQL 4.0 values from the RP103-03 study31 transformed into utilities via an algorithm were inflated by the manufacturer, i.e., from 0.873 to 0.95 on the basis that study patients were rather sick, and this would not be representative of a 2-year-old child starting on treatment. No norm is available yet from the EuroQol group on the EuroQol5-Dimension-Y (EQ-5D-Y).32 However, a Canadian study in (likely healthy) 3,421 Grade 5 students (i.e., aged 11-12 years old, as per Langman’s study) reported an average EQ-5D-Y index score of 0.86.33
Utilities mapped from quality of life questionnaires are not recommended for Health Technology Assessment.22 Even the author of the PedsQL mapping algorithm noted the variance in prediction accuracy across the range of fitted EQ-5D-Y utility scores.34 Furthermore, the UK tariffs were used for the development of the algorithm. Using the Canadian tariff set might give different results.
PedsQL 4.0 values not published in Langman’s article31 and the manufacturer has not provided a report of their analysis. |
| Resource use | Treatment information from RP103-03 trial (assuming no dropout) | Dose in RP103-03 trial is lower than recommended dose from product monograph |
ESRD: dialysis modalities and waiting time to transplant from CORR20,21
Time to graft failure from medical literature | The manufacturer used data from adults to populate dialysis modality usage and waiting time for kidney transplant. However, as the age at which ESRD is established is estimated to be 9 years in the no-treatment group, it would have been more appropriate to also use the information from children. There are differences between adults and children in the usage of peritoneal dialysis (54% in children vs. 22.7% in adults age 20 to 44 years) and waiting time for a kidney transplant (18 months in children vs. 47.3 months in adults).20,21 This could impact the costs and QALYs. Van Stralen reports 17.9% hemodialysis, 39.6% peritoneal dialysis, 35.1% transplant, 7.5% unknown in starting the renal replacement therapy modality in patients with cystinosis.35 |
| Adverse events | Gastric acid production in 10.4% of patients (source: RP103-03 study) managed by proton pump inhibitors | Feedback from the clinical expert consulted by CADTH suggested clinicians are moving away from proton pump inhibitors due to their potential impact on kidney function. H2 blockers are the alternative. |
| Mortality | From medical literature9 | Data are limited (86 patients) relatively old (diagnosis between 1961 and 1995) and from France only, hence questionable generalizability to Canadian setting. |
| Costs | | |
|---|
| Drug | Cost of delayed-release cysteamine provided by the manufacturer | Appropriate |
| Administration | No administration costs | Oral formulation. Note that recommended dosage represents 10 to 26 capsules per day |
| Event | ESRD: base case uses an aggregated value from a paper published in 2007 but reporting in 2000 $CAD36 This paper obtained its estimated from various sources, including the medical literature and Ontario medical fee schedule.
The manufacturer uses a so-called ‘micro-costing’ approach in the sensitivity analyses where a cost for hemodialysis, peritoneal dialysis, kidney transplant first year, and kidney transplant subsequent year are used. | The ESRD cost estimates for the base case are old and would have benefited from more recent values and better adaptation to the children population. For example, the 2000 CORR data used for this estimate reports 22% on peritoneal dialysis while in children the proportion was 54% in the 2016 report.20,21
The ESRD ‘micro-costing’ is not operational in the notreatment group in the version of the model provided to CADTH. |
| Diabetes costs from medical literature (study in Ontario)37 | It is difficult to understand which value of the publication has been used for the model as the publication reports different values for each year after diagnosis for men and women. |
| Cost for neuromuscular disorders from medical literature38 | Although the source uses Canadian data (Ontario and BC), the costs are for MS patients.38 Feedback from the clinical expert consulted by CADTH suggested that PD would be a better proxy than MS for neuromuscular disorders due to the progressive nature of the disease. |
| Routine care: MD visit: $38.05 (Ontario); hemicystine test: $342 (US$ value converted to C$ using 1.35 rate) | US dollar to Canadian dollar conversion rate is higher than the current exchange rate. The ½ cystine test can be done in Canada; however, CADTH was not able to find a public price. |
| The manufacturer reports inflation-adjusted values in the submitted pharmacoeconomic report.8 Adjustments for inflation are not included in the model, aggregate values are presented. Therefore, it is difficult to validate which value has been used from the source and if inflation adjustment has been done properly. Greater transparency should have been provided.
Note that manufacturer estimates standard error for PSA as 0.10 or 0.25 of average cost depending on cost rather than using 95% confidence interval. |
| Adverse events | Proton pump inhibitor $11.54 per month (ODB) | According to the clinical expert consulted by CADTH, clinicians are moving away from proton pump inhibitors due to their potential impact on kidney function. H2 blockers are the alternative. However, this value has very limited impact on the ICUR. |
| Health state | No complication
ESRD
Diabetes
Neuromuscular disorder
Any combination of 2 of these 3 complications
Death | Feedback from the clinical expert consulted by CADTH confirmed that these are the most important complications in this patient population. |
CORR = Canadian Organ Replacement Registry; EQ-5D-Y (Youth) = EuroQol5-Dimension-Y; ICUR = incremental cost-utility ratio; ESRD = end-stage renal disease; MS = multiple sclerosis; ODB = Ontario Drug Benefit; PD = Parkinson’s disease; PedsQL 4.0 = Pediatric Quality of Life Inventory version 4.0; PSA = probabilistic sensitivity analysis; QALY = quality-adjusted life-year.
Table 11Manufacturer’s Key Assumptions
View in own window
| Assumption | Comment |
|---|
| Patients were assumed to enter the model at 2 years of age. | This is appropriate based on the product monograph which notes that the safety and efficacy of delayed-release cysteamine in patients under 2 years of age have not been established.
However, for the model information was provided as to whether the patients entering at 2 years of age had existing disease or whether these were newly presenting patients. |
| Survival partition approach assumes the risks of complications and mortality are independent of each other. | This is a simplification of the reality as the impact of diabetes or kidney failure on survival is well documented. Therefore, the chosen modelling approach limits the possibility of assessing the impact of delayed-release cysteamine on survival if the product was to have a greater impact on one complication over another. For example, if the impact of cysteamine is greater on kidney failure than on diabetes, the model might underestimate the impact on survival.
Furthermore, when survival equations are independent of each other, care must be taken in sensitivity analyses to avoid logical fallacy (i.e., time to a complication is longer than time to death).39
Feedback from the clinical expert consulted by CADTH highlighted the interdependent nature of the complications of cystinosis. |
| Risks of complications and death are conditional on the age when cysteamine therapy started (i.e., Brodin-Sartorius shows that treatment before the age of 5 years leads to improved outcomes compared with treatment starting after the age of 5 years). | In the Brodin-Sartorius study,9 the size of the 3 cohorts varies according to the complication. For example, for ESRD, the cohort starting after age 5 consists of only 8 patients. For death, there are only 11 untreated patients. Therefore, some of these subgroups are very small and even if a statistically significant difference were observed, the statistical power might be low. For example, no difference is seen for ESRD incidence between starting after age 5 and not starting before reaching ESRD, however, only 8 patients started after age 5. For diabetes, neuromuscular disorders, and death, starting after age 5 was much closer to the ‘starting before age 5’ curves. The ‘after age 5’ group was bigger in all cases (n = 17 for diabetes; n = 28 for neuromuscular disorders; n = 35 for death) |
| Model projections over a lifetime are beyond the observed follow-up time. | Acceptable |
| The risk estimates are predicted based on assumptions for the shape of the long-term hazard function for each event (i.e., Weibull or Gompertz). | The shape of the distribution for a survival function is often Weibull or Gompertz, the ones used by the manufacturer; however, a better approach would have been to test various functions and choose the most appropriate.40 |
| Quality of life values were based on an analysis of trial outcomes, then mapped using a published algorithm. | Utilities mapped from quality of life questionnaires are not recommended for Health Technology Assessment.22 |
| The impact of delayed-release cysteamine on median time to complications is from the opinion of one single expert. | Although the expert consulted by the manufacturer is the author of the natural history paper and has experience using immediate-release cysteamine, his opinion was based on assumptions regarding the side effect profile, patient compliance, and monitoring of delayed-release cysteamine. This assumption highly increases the uncertainty around the estimates. |
| The routine care of cystinosis and management of complications are assumed to have no overlap with each other. | Acceptable |
| The micro-costing and micro-tariff approach for mean ESRD cost and tariff is based on the assumption that post-ESRD survival of all patient categories would follow an exponential distribution. | Reasonable. However, the micro-costing and micro-tariff approach was not used by the manufacturer in their base case, and was not programmed in the notreatment group in the version of the model provided to CADTH. |
| It is appropriate to use time to transplant as a marker of disease activity. | This is complicated by the fact that when a patient is deemed ready for transplant, there may not be a donor. Feedback from the clinical expert consulted by CADTH indicated that the wait time is influenced by a variety of factors, including donor availability, health of the recipient, HLA matching and sensitization (i.e., previous exposure to blood antigens affecting the ease of finding an HLA-matched donor). It was also indicated that children may preferentially receive peritoneal dialysis more than adults due to factors limiting hemodialysis use in a small body. |
| The curves of age of complication onset and age of death for different patient categories are assumed to share the same curve shape. | Acceptable |
| The Kaplan–Meier curves in the Brodin-Sartorius et al. study (Figure 2) represent complication free with death considered as an event. | This is unlikely the case. The survival curve referenced by the manufacturer likely represents the overall survival in all 86 patients, some of them with 1 or more complications. |
| Maximum of 2 kidney transplants over the lifetime. | Reasonable. A US study showed that 42 out of 100 patients received a second transplant.28 |
ESRD = end-stage renal disease; HLA = human leukocyte antigen; n = number of patients in subgroup.
Manufacturer’s Results
The manufacturer’s base case estimates the incremental expenses to using delayed-release cysteamine at $8,770,005 over the lifetime of a patient with an incremental quality-adjusted life-year (QALY) gain of 12.98 and an incremental life-year gain (LYG) of 18.11 years. The resulting incremental cost-utility ratio (ICUR) is $675,605 per QALY or $536,168 per LYG (deterministic analysis). below reproduces the manufacturer’s results.
Table 12Manufacturer’s Base Case Results (Deterministic Analysis)
View in own window
| Delayed-Release Cysteamine | No Treatment | Incremental |
|---|
| Total average cost | $9,531,676 | $761,671 | $8,770,005 |
|---|
| Cost of drug treatment for cystinosis | $8,144,642 | $0 | |
|---|
| Cost of routine management | $55,018 | $4,134 | |
|---|
| ESRD costs | $1,239,937 | $733,000 | |
|---|
| Diabetes costs | $11,346 | $7,275 | |
|---|
| Neuromuscular disorder costs | $80,692 | $17,262 | |
|---|
| Adverse event costs | $41 | $0 | |
|---|
| LYs | 34.47 | 18.11 | 16.36 |
|---|
| QALYs | 27.46 | 14.48 | 12.98 |
|---|
| Incremental cost per LY gained | | | $536,168 |
|---|
| Incremental cost per QALY gained | | | $675,605 |
|---|
ESRD = end-stage renal disease; LY = life-year; QALY = quality-adjusted life-year.
Source: Manufacturer’s pharmacoeconomic submission8
The ICUR from the probabilistic sensitivity analysis (PSA) has been recalculated from of the manufacturer’s pharmacoeconomic submission by dividing the average incremental costs by the average incremental QALY (i.e., $8,784,414/12.93). This gives an ICUR of $679,382. Also to be noted, only 2,000 iterations have been included in the PSA. According to the manufacturer’s analysis, 95% of the iterations are found between $562,277 and $860,714 per QALY.
The manufacturer produced a series of scenario analyses (0% and 3% discounting; 75% and 50% of children initiating treatment below age of five years; time horizon at 60 years; exclusion of diabetes and neuromuscular disorder as complications; using 0.80 as baseline utility value rather than 0.95; using a ‘micro-tariff’ for ESRD utility). Only results of the deterministic analyses were presented in the manufacturer’s report. The lowest ICUR ($612,497 per QALY) was seen with the scenario where diabetes and neuromuscular complications were excluded. This is a little counterintuitive. While costs were only slightly lower ($8,702,504 versus $8,770,005 in the base case), QALY gain was higher (14.21 versus 12.98 in the base case). The highest ICUR ($821,927 per QALY) was seen in the scenario where only 50% of the children initiated treatment below the age of five years. Costs were lower ($7,012,105 versus $8,770,005 in the base case), and so was the QALY gain as well (8.53 versus 12.98 in the base case).
CADTH identified the following limitations with the manufacturer’s model in addition to those listed in the main body of the report:
CADTH Common Drug Review Reanalyses
Two publications estimating the cost-effectiveness of immediate-release cysteamine were found in the literature.41,42 One, published as an abstract only, reported the cost-effectiveness of cysteamine in Poland.41 The other, published as a full paper, reported a cost-consequence analysis in the US setting.42 Both models were decision trees focusing on renal complications only. Cysteamine was assessed as being cost-effective in the Polish analysis, but the publication format (i.e., abstract only), does not allow full appraisal of the analysis. In the US analysis, incremental costs were estimated at $4,000 over the patient lifetime for an incremental survival of 5.5 years. This was based on annual immediate-release cysteamine costs of $1,600 per year (likely 1996/1997 costs). In comparison, delayed-release cysteamine annual costs are estimated to be $136,109 and $321,711 based on recommended dose and body surface area. Therefore, these analyses do not provide a lot of insight to the current submission.
In view of the limitations of the manufacturer’s base case noted above, CADTH undertook a series of reanalyses to determine the CADTH base case:
Increase
PSA iterations to 5,000 to be consistent with CADTH guidelines
Adjust cysteamine delayed-release dose standard deviation to 31.5% of dose (using the relationship between standard deviation of the daily dose and daily dose in the RP103-03 study as a proxy) to account for variability in dose
Adjust cysteamine delayed-release dose to the product monograph recommended dose, i.e., 1,300 mg/m2 (rather than 1,083 mg/m2)
Reduce the efficacy of delayed-release cysteamine to that of immediate-release cysteamine
Use a different set of utilities and costs for neuromuscular disorders and the baseline ().
The literature was reviewed to identify more appropriate inputs in particular for utilities and costs. Preference was given to recent Canadian values. CADTH inputs are listed in .
Table 13CADTH Base Case Inputs Compared With Manufacturer’s Inputs
View in own window
| Parameter | Manufacturer’s Value [SE] | Manufacturer’s Source | CADTH Value [SE] | CADTH Source |
|---|
| Clinicala |
|---|
| Time to ESRD | HR vs. no treatment: 0.83 [0.05]
Untreated: 9 yrs
Cysteamine: 20 yrs | Brodin-Sartorius9 and expert elicitation | HR: 1.00 [0.05]
Untreated: 9 yrs
Cysteamine: 15 yrs | Brodin-Sartorius9 |
| Time to diabetes | HR: 0.93 [0.05]
Untreated: 15 yrs
Cysteamine: 40 yrs | Brodin-Sartorius9 and expert elicitation | HR: 1.00 [0.05]
Untreated: 15 yrs
Cysteamine: 38 yrs | Brodin-Sartorius9 |
| Time to neuromuscular disorder | HR: 0.89 [0.05]
Untreated: 25 yrs
Cysteamine: 45 yrs | Brodin-Sartorius9 and expert elicitation | HR: 1.00 [0.05]
Untreated: 25 yrs
Cysteamine: 35 yrs | Brodin-Sartorius9 |
| Death | HR: 0.11 [0.05]
Untreated: 23 yrs
Cysteamine: 50 yrs | Brodin-Sartorius9 and expert elicitation | HR: 1.00 [0.05]
Untreated: 23 yrs
Cysteamine: 37 yrs | Brodin-Sartorius9 |
| Utilities or disutility |
|---|
| Neuromuscular disorderb | −0.32 [0.05] | Karampampa43 | −0.22 [0.04] | Pohar44 |
| Baseline utility | 0.95 [0.05] | Study RP103-0310 | 0.860 [0.0025] | Wu33 |
| Costs and health care resources |
|---|
| Neuromuscular disorders | $17,993 per year [mfr value divided by 4] | Amankwah38 | $3,104
Sampling performed on number of hospitalizations in PD (2.30 [0.10]) and control patients (2.10 [0.09]). Inpatient PD costs: $15,521 [$1,285]) per case | Hobson45 for hospitalizations. OCCI for inpatient costs |
| Cysteamine daily dose | 1,083 mg/m2 [50] | Study RP103-0310 | 1,300 mg/m2 [410] | Manufacturer submission24 |
- a
The manufacturer is using a ‘Hazard Ratio’ as a multiplier used to adjust the survival curves of the short-acting formulation from Brodin-Sartorius’ publication to reflect the assumptions on the delayed-release formulation
- b
Disutility: negative value
ESRD = end-stage renal disease; HR = hazard ratio; mfr = manufacturer; m2 = metre squared; mg = milligram; OCCI = Ontario Case Costing Initiative; PD = Parkinson’s disease; SE = standard error; yrs = years.
Table 14CADTH Reanalysis: Base Case Details (Probabilistic Analysis)
View in own window
| Scenario | Element | | Total Cost | Total QALY | ICUR |
|---|
| CADTH base case: | 5,000 iterations | Delayed-release cysteamine | $9,558,609 | 27.5043 | |
| No treatment | $759,460 | 14.4902 | |
| Incremental analysis | $8,799,149 | 13.0141 | $676,126 |
| Dose standard error changed to 31.5% of dose | Delayed-release cysteamine | $9,559,847 | 22,4317 | |
| No treatment | $760,541 | 14.4658 | |
| Incremental analysis | $8,799,306 | 12.9658 | $678,653 |
| Dose changed to product monograph recommended dose, i.e., 1,300 mg/m2/day | Delayed-release cysteamine | $11,205,210 | 27.4147 | |
| No treatment | $761,318 | 14.4675 | |
| Incremental analysis | $10,443,892 | 12.9472 | $806,655 |
| Efficacy similar to short-acting formulation | Delayed-release cysteamine | $8,724,302 | 22.1341 | |
| No treatment | $761,861 | 14.4575 | |
| Incremental analysis | $7,962,441 | 7.6766 | $1,037,235 |
| Utility for neuromuscular disorder changed to −0.22 [0.04] | Delayed-release cysteamine | $8,710,132 | 22.4375 | |
| No treatment | $759,846 | 14.5715 | |
| Incremental analysis | $7,950,285 | 7.8661 | $1,010,705 |
| Baseline utility changed to 0.860 [0.0025] | Delayed-release cysteamine | $8,706,370 | 20.2849 | |
| No treatment | $760,177 | 13.1620 | |
| Incremental analysis | $7,946,193 | 7.1230 | $1,115,576 |
| Neuromuscular disorder costs | Delayed-release cysteamine | $8,732,065 | 20.2680 | |
| No treatment | $749,283 | 13.1679 | |
| Incremental analysis | $7,982,782 | 7.1000 | $1,124,329 |
ICUR = incremental cost-utility ratio; m2 = metres squared; mg = milligram; QALY = quality-adjusted life-year.
Sensitivity on the price of delayed-release cysteamine was performed (Table 4 and ). A price reduction of more than 95% was necessary to bring the ICUR below $100,000 per QALY. An analysis of the impact on various cost items showed that a large part of the costs associated with delayed-release cysteamine treatment is related to the management of ESRD. Although delayed-release cysteamine delays the incidence of ESRD, by increasing the survival, it also increases the risk of patients developing ESRD or other complications. In the CADTH base case, ESRD costs totalled $733,000 (98.1% of the total costs) in the untreated cohort. In the delayed-release cysteamine group, ESRD costs increased to $1,063,297. Similar increases were seen for neuromuscular disorders, while diabetes costs were stable. Even when the price of delayed-release cysteamine were reduced by 95%, the total costs were twice as high in the delayed-release cysteamine group as in the no-treatment group, with increased ESRD costs being responsible for 50% of the increase in total costs.
Table 15CADTH Reanalyses: Additional Price Reduction Analysis
View in own window
| Scenario | Element | | Total Costs | Total QALY | ICUR |
|---|
| Scenario 1: | Base case | Delayed-release cysteamine | $8,732,065 | 20.2680 | |
| No treatment | $749,283 | 13.1679 | |
| Incremental analysis | $7,982,782 | 7.1000 | $1,124,329 |
| 25% price reduction | Delayed-release cysteamine | $6,786,525 | 20.2685 | |
| No treatment | $745,439 | 13.1625 | |
| Incremental analysis | $6,041,087 | 7.1060 | $850,139 |
| 50% price reduction | Delayed-release cysteamine | $4,893,737 | 20.2621 | |
| No treatment | $744,824 | 13.1539 | |
| Incremental analysis | $4,148,913 | 7.1081 | $583,685 |
| 75% price reduction | Delayed-release cysteamine | $2,997,932 | 20.2937 | |
| No treatment | $740,366 | 13.1687 | |
| Incremental analysis | $2,257,565 | 7.1251 | $316,847 |
| 95% price reduction | Delayed-release cysteamine | $1,501,616 | 20.2748 | |
| No treatment | $744,718 | 13.1704 | |
| Incremental analysis | $756,898 | 7.1044 | $106,540 |
ICUR = incremental cost-utility ratio; QALY = quality-adjusted life-year.