| Patient characteristics | Baseline characteristics were informed by the GiACTA trial (phase III, 52-week, randomized, double-blind, placebo-controlled; n = 251).4 | Appropriate |
| Efficacy | Efficacy on time to first flare and transition to subsequent flares were taken from the GiACTA trial.4 | Likely appropriate for year 1, but uncertainty exists regarding extrapolation in year 2. Further, prednisone dosing is protocolized and may differ from real-world administration (which may impact relative efficacy between the 2 treatment groups).
It is inappropriate to assume continued benefit of tocilizumab after year 2 (when no further tocilizumab is administered). |
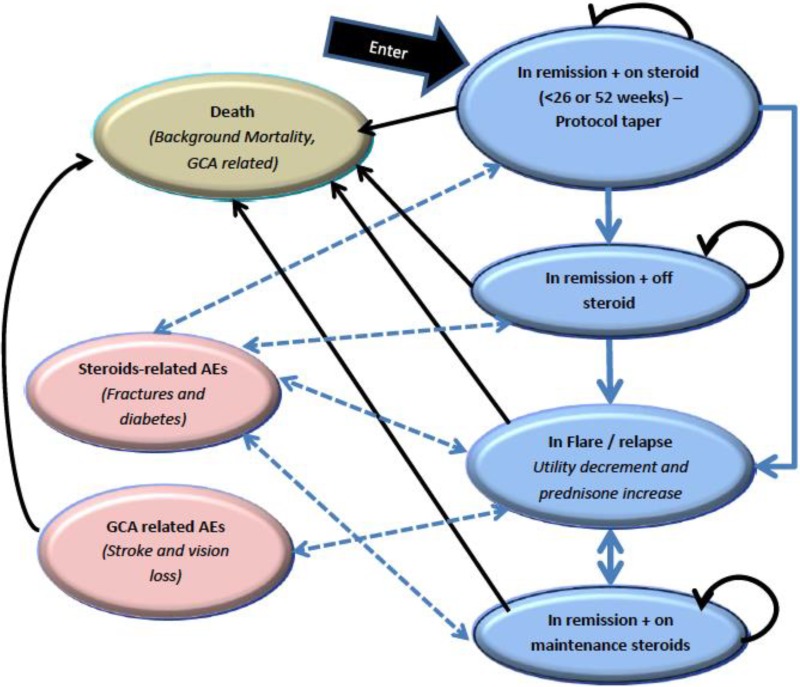
| Natural history | Model structure was conceptualized after considering the natural history of the disease and the insights from the manufacturer’s clinical team regarding the GiACTA data.4 | Appropriate; disease is not common. |
| Utilities | Health-state utilities were obtained from the GiACTA trial data.4
Decrements for GCA or prednisone-related adverse events were obtained from the literature. | While appropriate, the specific details of modelling from EQ-5D data collected from the trial were not provided.
All studies used to inform were non-Canadian, as studies on Canadian populations were unavailable; this approach is reasonable. |
| Resource use | See costs section. | |
| Dose of prednisone | The prednisone dose for the treatment period was derived from the GiACTA trial.4 Until the first flare (primary remission), the cumulative dose was 2,632 mg for TCZ and 3,945 mg for prednisone alone.
During flare, a predictive equation of the prednisone dose increase was estimated from trial data, based on the last effective dose (1.6472 for TCZ and 1.6493 for prednisone alone).
After the flare, patients would switch to the “escape” prednisone tapering regimen, a logistic growth regression was applied derived from the GiACTA trial, and ▬ ▬ ▬ ▬ ▬.6 | Appropriate. However, prednisone dosing is driven by the protocol. Actual dosing may differ in clinical practice and there is noted variability in treatment. |
| Adverse events (indicate which specific adverse events were considered in the model) | Adverse events related to TCZ were not included as isolation of these events from AEs caused by prednisone or GCA was not possible, and GiACTA trial showed that the rates of AEs between the treatment groups were very similar.4
GCA-related AEs included both vision loss and stroke (minor or major), as they were the most costly and debilitating AEs. Rate estimated from the NHS HTA report.5
Prednisone-related AEs included fractures and diabetes mellitus where considered most relevant from literature review and ▬ ▬ ▬.6 Algorithms were developed from ▬ ▬ ▬ ▬ ▬. | Uncertain, but the GiACTA trial showed the rates of AEs and SAEs between the treatment groups were similar.
Appropriate although uncertain; uncommon events not captured in trial and estimated from observational data.
Appropriate although uncertain; these events were not obtained from the trial but estimated using observation data linking cumulative dose of prednisone and development of fractures and diabetes. |
| Mortality | The model considered background mortality for all patients based on Canadian life tables. Mortality due to GCA was indirectly incorporated via the occurrence of death with major stroke (in 50%). | Mortality due to stroke is higher than the Canadian data.9 |
| Costs |
|---|
Drug
(tocilizumab, TCZ) | The cost of TCZ was based on the mode prices reported by the provincial formularies. A cost of $358.905 was used for each 162 mg syringe of TCZ. Patients were assumed to remain on weekly TCZ until the end of the second year. | Appropriate |
Drug
(prednisone) | A costing algorithm was used to calculate the minimum cost for each prednisone dose required by patients. The costs used for prednisone tablets were based on the mode prices reported by the provincial formularies. The weekly cost of prednisone for the first year was $4.59; after one year, the weekly cost was $0.75 for TCZ + prednisone and $0.28 for prednisone only. | Appropriate |
| Flare management | The total cost for a flare was the sum of the cost of a visit to the rheumatologist (code A480 from the Ontario Schedule of Benefits) and the cost of additional prednisone for one cycle. | Appropriate |
| GCA-related AEs | Annual costs of $3,152 for vision loss, $25,655 for non-fatal stroke, and $9,295 for fatal stroke were obtained from a Canadian study.8 | Appropriate |
| Prednisone-related AEs | The cost of diabetes was derived from a Canadian costing study in which incident diabetes cases in Ontario were matched with subjects without diabetes to determine the attributable costs.7
For factures, a tariff cost per event was obtained from the Ontario case costing and a weighted average cost of $10,971 was used in the model assuming all fractures were treated in-patient. | Appropriate.
According to the CDR clinical expert, most vertebral fractures (31.4% of fractures) would be treated in an outpatient setting; the approach used likely overestimates costs. |