Copyright © 2024 - Canadian Agency for Drugs and Technologies in Health. Except where otherwise noted, this work is distributed under the terms of a Creative Commons Attribution-NonCommercial- NoDerivatives 4.0 International licence (CC BY-NC-ND).
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Andexanet Alfa (Ondexxya): CADTH Reimbursement Review: Therapeutic area: Reversal of FXa inhibitor anticoagulant effects [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2024 Mar.

Andexanet Alfa (Ondexxya): CADTH Reimbursement Review: Therapeutic area: Reversal of FXa inhibitor anticoagulant effects [Internet].
Show detailsExecutive Summary
The executive summary comprises 2 tables (Table 1 and Table 2) and a conclusion.
Table 1
Submitted for Review.
Table 2
Summary of Economic Evaluation.
Conclusions
The CADTH Clinical Review of the 3 weighted comparative analyses (ANNEXA-4 trial versus the ORANGE study; ANNEXA-4 trial versus the Hartford Health care study; and the ANNEXA-4 trial versus the RETRACE-II study) found several limitations that prevented definitive conclusions regarding the comparative efficacy of andexanet alfa and prothrombin complex concentrate (PCC). CADTH’s appraisal of the indirect treatment comparisons (ITCs) found evidence of heterogeneity with the inclusion and exclusion criteria between studies (e.g., definition of major bleeding, recent history of blood product, intracranial hemorrhage [ICH] severity, recent history of thromboembolism, expected survival). Additional residual confounding due to inadequate adjustment for prognostic factors or treatment effect modifiers was also listed as a major limitation. As such, the clinical effectiveness of andexanet alfa compared with PCC is uncertain, both in the short-term (30 days) and long-term.
CADTH identified limitations in the sponsor’s economic analysis: overestimation of long-term survival for ICH patients prescribed andexanet alfa, inappropriate utilities reported for ICH patients who used andexanet alfa, underestimated relative risk (RR) of 30-day mortality, and lack of publicly available sources for the unit cost of PCC in Canada. To derive the CADTH base case, the following changes were made: applying equal modified Rankin Scale (mRS) distributions for both the andexanet alfa and PCC treatment groups and changing ICH long-term utilities for andexanet alfa to align with the PCC value (0.62). In the CADTH’s base case, a price reduction of at least 27% would be required to make andexanet alfa an optimal treatment option at a s (WTP) threshold of $50,000 per quality-adjusted life-year (QALY) gained. A scenario analysis conducted by CADTH demonstrated that the estimated incremental cost-effectiveness ratio (ICER) is highly sensitive to changes in the RR of 30-day mortality. Feedback obtained by CADTH from the clinical experts highlights that the 30-day mortality RR is likely higher (i.e., the difference between treatments is smaller) than what is reported in the sponsor submission. The true value of the ICER may be higher than the CADTH base case; as such, a further price reduction may therefore be warranted.
Stakeholder Input Relevant to the Economic Review
This section is a summary of the feedback received from the patient groups, registered clinicians, and drug plans that participated in the CADTH review process.
CADTH received input from 2 patient groups, the Canadian Venous Thromboembolism Research Network (CanVECTOR) and the HeartLife Foundation. The CanVECTOR input was collected through interviews (n = 33) with patients and caregivers in 7 countries with lived experience with venous thromboembolism (VTE). Of these 33 interviews, 8 were completed with patients in Canada. The HeartLife Foundation noted that the use of FXa inhibitor can increase the risk of bleeding and patients with heart failure may require invasive surgeries, which put them at risk of surgical bleeds. The group expressed a need for a treatment that can rapidly and effectively reverse the anticoagulant effect of FXa inhibitor to prevent further bleeding and ensure the best possible outcome in patients who require urgent surgery or are experiencing life-threatening bleeds. According to the CanVECTOR input, participants all had VTE and varied treatment experience; this included experience with warfarin, direct oral anticoagulants (DOACs), and low-molecular-weight heparin. Participants had been on treatment anywhere from 1 to 6 months to long-term (more than 3 years). A diverse group of participants was selected that included a mix of ages, education, and racial and gender identities. Several treatment options are available for VTE, and personal factors affect a patient’s treatment preferences. Generally, participants described the burden of treatment with warfarin and low-molecular-weight heparin to be higher than DOACs. Some patients started on warfarin or low-molecular-weight heparin and later switched to a DOAC. While treatment with DOACs was preferred by patients, a lack of reversal drugs available for DOACs was named as a concern and that the absence of reversal drugs could prevent patients from undergoing a surgery in the case of an emergency. No participants within the patient group had experience with andexanet alfa.
The clinician group input was obtained from 5 clinician groups: Thrombosis Canada (represented by 2 clinicians), members of the thrombosis and Anticoagulation Team at Dalhousie University (represented by 3 clinicians), faculty members at McMaster University in hematology and/or thromboembolism (represented by 4 clinicians), Canadian Stroke Consortium (represented by 5 clinicians), and a journal club comprising local emergency department physicians in the Peel Region (represented by 5 clinicians). The clinician groups note that PCCs have no effect on eliminating the active inhibitory action of oral factor Xa (FXa) inhibitors and while they are currently the treatment of choice, their use is considered off-label. One clinician group highlighted that there would be a clear division between the patients who would receive PCC versus andexanet alfa. The group noted that PCCs would likely be used for average cases requiring DOAC reversal and andexanet alfa would be used only in select patients (life-threatening bleeding that does not respond to supportive management, critical site bleeding, a need for emergent surgery). The clinicians noted that as most centres do not have FXa inhibitor drug assays available, treatment is considered based on the timing of the last dose or other factors, including examination findings, patient liver or kidney function, and clinician judgment. The groups supported the use of andexanet alfa by specialists and in the hospital setting. These settings included critical care units, operating rooms, trauma centres, stroke or neurosurgical referral centres, emergency departments.
The drug plan input received by CADTH noted concerns regarding the anticipated budget impact and sustainability. The drug plans requested input on the proportion of major bleeds that would need to be reversed with a reversal drug and the overall market size that was estimated based on the ITC of the ANNEXA-4 trial versus the ORANGE study. Additional concerns were noted on the use of claims data from IQVIA to determine the number of patients on apixaban and rivaroxaban. Lastly, the drug plans highlighted additional concerns with the implementation of andexanet alfa due to the costly unit price in comparison with PCC. The drug plans noted that a prioritization scheme that could be implemented in hospitals would be required.
Several of these concerns were addressed in the sponsor’s analysis and the CADTH reanalysis:
- The feedback obtained by CADTH from the clinical experts suggests that the proportion of patients eligible for treatment with a reversal drug is reasonable; no additional findings in the literature have suggested otherwise.
- The sponsor noted that an epidemiologically based approach was considered; however, due to the limited data on the prevalence of patients receiving treatment with apixaban and rivaroxaban in Canada, the number of patients using FXa inhibitors in Canada was estimated indirectly using IQVIA claims data.
Economic Review
The current review is for andexanet alfa (Ondexxya) for adult patients treated with FXa inhibitors (rivaroxaban or apixaban) when rapid reversal of anticoagulation is needed due to life-threatening or uncontrolled bleeding.
Economic Evaluation
Summary of Sponsor’s Economic Evaluation
Overview
The sponsor submitted a cost-utility analysis assessing andexanet alfa compared with PCC for the treatment of adult patients treated with FXa inhibitors (rivaroxaban or apixaban) when rapid reversal of anticoagulation is needed due to life-threatening or uncontrolled bleeding.1 The modelled population aligned with the patients from the ANNEXA-4 trial on which the Health Canada indication was based. The reimbursement request is aligned with the Heath Canada–indicated population.2
Andexanet alfa is administered as an IV bolus with a target rate of 30 mg/minute, followed by continuous infusion with a target rate of 4 mg/minute to 8 mg/minute for up to 120 minutes. The recommended dose for andexanet alfa varies between 5 (low dose) to 9 (high dose) 200 mg vials, depending on the rivaroxaban or apixaban dose.3 The low dose of andexanet alfa comprises an initial IV bolus of 400 mg at a rate of 30 mg/minute, followed by a continuous IV infusion of 4 mg/minute for 120 minutes.3 The high dose of andexanet included an initial IV bolus of 800 mg at a rate of 30 mg/minute followed by a continuous IV infusion of 8 mg/minute for 120 minutes.3 To determine the average cost of andexanet alfa in the base case, the sponsor derived the proportion of patients receiving a low or high dose from the ANNEXA-4 pivotal study.2 The submitted price of andexanet alfa is $4,590 per 200 mg vial.1 Based on a mean patient weight of 77.8 kg, in alignment with the ANNEXA-4 trial, the average total treatment cost per patient is $26,787 (weighted cost for low dose = $18,153 and high dose = $8,634).2 Administration costs for andexanet alfa were sourced from the Ontario Schedule of Benefits, where andexanet alfa was considered a complex single-drug therapy with prolonged administration.4 Acute care costs were assumed to be equivalent to hospitalization costs associated with bleeding events and were sourced from the Canadian Institute for Health Information Patient Cost Estimator.5 Long-term costs for managing the health of bleed survivors were sourced from Canadian literature that assessed costs associated with each bleed.6-9 The associated cost of administration was $75.
At the time of the analysis, no specific reversal drugs were approved in Canada for the reversal of direct FXa inhibitors in adults with severe or life-threatening bleeding events.1 As such, the comparator used in the analysis was PCC and was used as a proxy for usual care. As human prothrombin complexes Beriplex and Octaplex are the only PCC products approved in Canada, PCC costing was based on the cost of these 2 products.1 The cost of PCC was calculated by weighting the cost of Beriplex and Octaplex by the percentage of patients who received each drug in the ORANGE study.10 As no Canadian list prices are published for PCC, unit costs were sourced from the median of available published list prices for PCC as specified by the Patented Medicine Prices Review Board (PMPRB) for assessing foreign prices of medications.11 The sponsor calculated an average weighted treatment cost of |||||| for PCC by using the percentage of patients who received each drug in the ORANGE study.12 The cost of administration was sourced from the Ontario Schedule of Benefits, where PCC is considered to be an agent with minor toxicity that may require monitoring.4 The associated cost of administration was $54.25 for PCC. Wastage was included in all cost calculations for both andexanet alfa and PCC.
The economic analysis was performed from the perspective of the Canadian publicly funded health care system. Costs and clinical outcomes (life-years and QALYs) were simulated over a model horizon of 22 years and discounted at an annual rate of 1.5%.
Model Structure
The sponsor submitted a combined decision tree and Markov model with a cycle length of 1 month to capture short- and long-term costs and health consequences associated with the use of andexanet alfa or PCC.1 The decision tree represented short-term survival (30 days) and was structured to represent the expected cohort of patients in Canada requiring rapid reversal of anticoagulation due to major bleeding, including: ICH, gastrointestinal (GI) bleeding, and intraspinal, intraocular, pericardial, and retroperitoneal bleeding. These bleeding sites requiring rapid reversal aligned with the inclusion criteria of the ANNEXA-4 study.2 Bleed survivors entered the Markov model in health states corresponding to the site of the bleeding. All survivors remained in their health state or transitioned into the death health state. Rebleeding was not included in the model, and it was assumed that all bleed survivors resumed anticoagulation therapy. A schematic of the sponsor’s model structure is available in Figure 1.
Model Inputs
The modelled population had baseline characteristics that were similar to the population enrolled in the ANNEXA-4 pivotal study.2 Patients had a mean body weight of 77.8 kg, and 53.1% of patients were male. The starting age in the model was 77.70 years, based on the mean age of trial participants.
In the sponsor’s model, as no head-to-head efficacy data were available for andexanet alfa compared with PCC, the comparative effectiveness in the base case was informed by an ITC. The ITC used patient-level data from the single-arm ANNEXA-4 pivotal trial and the ORANGE observational study to compare 30-day mortality rates.10 Functional outcomes were informed by an ITC by Huttner et al. (2022) that compared the distribution of functional outcomes following FXa inhibitor–associated ICH at 30 days or discharge.13 As adults treated with apixaban or rivaroxaban may experience major bleeding in several sites, the starting distribution of major bleed sites in the decision tree was informed by the ORANGE study and the proportions of patients experiencing a major bleed was matched with the ANNEXA-4 study.10 The 30-day mortality rates in patients who received andexanet alfa in the decision tree were derived using the propensity score–matched RR of all-cause 30-day mortality for andexanet alfa versus PCC.10 To estimate long-term mortality for ICH survivors in the Markov model, the sponsor used a study by Huybrechts et al. (2008), which provided long-term mortality estimates for 1,276 stroke survivors, measured with the mRS.14 The mRS distributions were redistributed to exclude death, as it was already captured in the 30-day decision tree. Long-term mortality estimates for severe GI bleed and other major bleeds in the Markov model were sourced from Friberg et al. (2007), which assessed the risk of death in a cohort of 3,824 patients with atrial fibrillation compared with a matched general population.15 The standardized mortality ratio (SMR) reported by the study was recalculated to a monthly SMR to determine the long-term survival of non-ICH patients with an uncontrolled bleeding event.15 Adverse events were not included in the economic analysis due to the low incidence of treatment-emergent adverse events in the ANNEXA-4 trial.
The decision tree component of the model incorporated utilities associated with acute, life-threatening, and uncontrollable bleeding events in the 30-day period following the major bleeding event. ICH utility scores were based on an EQ-5D health questionnaire sourced from the National Institute for Health and Care Excellence (NICE).16 A disutility was applied for non-ICH bleeds and was based on results from the Canadian Community Health Survey.17 Long-term utilities were incorporated into the Markov model component. For ICH survivors, long-term utility values were adjusted by mRS score and were derived from a published decision analytic model defining health state by mRS-defined ICH severity. For GI bleed survivors and pericardial bleed survivors, long-term utility was assumed to be the same as the utility for the general population in Canada. Disutilities for all other bleeds were derived from additional literature.18-20
Summary of Sponsor’s Economic Evaluation Results
The sponsor submitted a probabilistic analysis based on 2,500 model iterations. The deterministic and probabilistic results were similar. The deterministic findings are presented subsequently.
Base-Case Results
In the sponsor-submitted base case, andexanet alfa was associated with incremental costs of $43,634, incremental life-years of 1.249, and incremental QALYs of 0.994 in comparison with PCC, resulting in an ICER of $43,891 per QALY (Table 3). The probability of being cost-effective at a $50,000 per QALY WTP threshold was 76.9%.
Table 3
Summary of the Sponsor’s Economic Evaluation Results.
Sensitivity and Scenario Analysis Results
The sponsor conducted several sensitivity analyses involving short- and long-term utilities, 30-day mortality, and long-term care costs. When a value of 0.32 was used for long-term utility estimates for ICH bleeds, the resulting ICER was $57,723 per QALY gained.
CADTH Appraisal of the Sponsor’s Economic Evaluation
CADTH identified several key limitations to the sponsor’s analysis that have notable implications on the economic analysis:
- Long-term survival for ICH survivors using andexanet alfa is overestimated: In its economic analysis, the sponsor used the results from the ANNEXA-4 trial versus the RETRACE-II study to inform the distribution of mRS scores in the ICH survivor clinical population. In this ITC, the 1-month baseline mRS scores for andexanet alfa and PCC were derived through propensity-score adjustment and resulted in differing mRS scores, with a higher proportion of the patients prescribed andexanet alfa remaining in the less severe mRS categories than patients prescribed PCC. Feedback obtained from the clinical experts consulted by CADTH highlighted that they did not expect a difference in survival or mRS distribution for patients prescribed andexanet alfa in comparison with PCC. The CADTH Clinical Review notes that the mean difference for mRS at discharge from the ANNEXA-4 trial versus the RETRACE-II study was not statistically significant (P value = 0.261 for unadjusted analysis and 0.110 for inverse probability of treatment weighting adjusted analysis), and that there is insufficient evidence to justify different mRS distributions between the 2 treatment groups. Additionally, CADTH’s clinical appraisal suggests a risk of bias due to unmeasured confounding, as demographic data such as age, sex, and weight were not included in the adjustment model.
- Within the economic analysis, the sponsor also used differing utility values for ICH survivor health states between the andexanet alfa and PCC treatment groups, driven by the mRS distributions. As noted by the clinical experts and highlighted in the CADTH Clinical Review appraisal of the ANNEXA-4 trial versus the RETRACE-II study ITC, there is insufficient evidence to suggest mRS utilities will differ between treatments.
- As part of the base case, in consultation with clinical experts, CADTH set mRS distributions as equal between andexanet alfa and PCC to inform ICH survival and long-term utilities.
- Comparative clinical effectiveness of andexanet alfa is uncertain: In the absence of direct comparative evidence, the sponsor submitted an ITC using patient-level data from the single-arm ANNEXA-4 pivotal trial and the prospective ORANGE observational study to compare 30-day mortality rates. Based on this indirect evidence, the sponsor assumed a 30-day mortality RR of 0.43 (95% confidence interval [CI], 0.29 to 0.63) for andexanet alfa compared with PCC. To derive the RR, patients from the ORANGE study were matched based on baseline characteristics including age, bleed site, and medical history. Additional characteristics including sex, weight, and creatinine clearance were excluded from the adjustment, as the authors considered the characteristics to have no significant effect on 30-day mortality. However, the clinical experts consulted by CADTH highlighted that these characteristics are influential factors and were relevant in the other studies. The lack of adjustment for these treatment effect modifiers may result in confounding of the study results and may have led to an overestimation of comparative effectiveness, resulting in a bias that favours andexanet alfa.The CADTH Clinical Review appraisal of the sponsor-submitted ITC notes that several limitations preclude definitive conclusions regarding the comparative efficacy of andexanet alfa and PCC. The Clinical Review highlighted a risk of selection bias for the studies that were included in the analysis, given the absence of a systematic literature review. Additionally, heterogeneity was found within the study population, with patients in the ANNEXA-4 trial generally presenting as healthier than those in the ORANGE study. In the pharmacoeconomic model, the RR for 30-day mortality was an important parameter in determining the cost-effectiveness of andexanet alfa. The estimate of the RR for 30-day mortality is highly uncertain.
- CADTH could not address the potential bias in 30-day mortality in the reanalysis, as no other appropriate estimates were available. CADTH conducted a scenario analysis to explore the impact that this uncertainty has on cost-effectiveness. In consultation with the clinical experts, CADTH used the upper bound of the 95% CI (0.63) around the RR for 30-day mortality from the ANNEXA-4 trial versus the ORANGE study ITC.
- Comparator pricing based on publicly available prices: The sponsor’s analysis estimated the cost of PCC using published list prices for Beriplex and Octaplex in the PMPRB basket of 11 comparator countries. Unit prices for Beriplex and Octaplex were available in only 2 and 3 of the 11 countries, respectively. Therefore, the price of PCC (Beriplex and Octaplex) does not reflect any confidential pricing that may have been negotiated by Canadian Blood Services (CBS). As such, the estimated drug acquisition costs for PCC are uncertain.
- Due to confidentiality surrounding the negotiated price of PCC in Canada, CADTH was unable to address this limitation. To explore the potential impact of this uncertainty on the ICER, CADTH conducted a scenario analysis in which the drug price for PCC was reduced by an arbitrary 10%.
Additionally, the following key assumptions were made by the sponsor and have been appraised by CADTH (Table 5).
Table 4
Key Assumptions of the Submitted Economic Evaluation (Not Noted as Limitations to the Submission).
CADTH Reanalyses of the Economic Evaluation
Base-Case Results
CADTH corrected the sponsor’s model by updating the acute care hospitalization costs for the reported bleed sites using the Canadian Institute for Health Information Patient Cost Estimator.5 CADTH used the same Case Mix Group (CMG) codes used in the sponsor submission to determine the acute care costs, including CMG 025 for ICH bleeds, CMG 254 for GI bleeds, and CMG 782 for all other bleeds. The difference in cost is highlighted in Table 6. Additionally, CADTH corrected the unit cost for apixaban to that reported in the Ontario Drug Benefit Formulary.21
The CADTH base case was derived by making changes in the model parameter values and assumptions, in consultation with the clinical experts. These changes include applying equal mRS distributions for both the andexanet alfa and PCC treatment groups and changing the ICH long-term utilities for andexanet alfa to be equal to the PCC value (0.62). Table 6 details the changes made to derive the CADTH reanalysis; the summary results of that reanalysis are presented in Table 7. The CADTH base-case results were broadly similar to the sponsor’s submitted base case. Treatment with andexanet alfa was more costly than PCC (incremental cost = $36,604) while improving overall survival (incremental life-year = 0.854) and quality-adjusted survival (incremental QALY = 0.592). The probability of being cost-effective at a $50,000 per QALY WTP threshold was 14.4%.
Table 5
CADTH Revisions to the Submitted Economic Evaluation.
Table 6
Summary of the Stepped Analysis of the CADTH Reanalysis Results.
Scenario Analysis Results
CADTH undertook a price reduction analysis based on the sponsor’s base case and the CADTH reanalysis (Table 9). The results show that a price reduction of 27% is required for andexanet alfa to be considered cost-effective at a WTP threshold of $50,000 per QALY gained.
Table 7
CADTH Price Reduction Analyses.
CADTH conducted a scenario analysis to explore the impact of changing the 30-day mortality RR for andexanet alfa. In this analysis, the mean estimate of 30-day survival was set to the upper bound of the 95% CI around the 0.63 RR reported in the ITC for the ANNEXA-4 trial versus the ORANGE study. In this scenario, the ICER increased to $85,235 per QALY gained (incremental costs = $32,687; incremental QALYs = 0.384) (results in Table 12). In the sponsor’s base case, the QALYs generated in the decision tree were 0.035 for andexanet alfa and 0.028 for PCC (incremental QALYs = 0.007). In the CADTH scenario analysis, the QALYs generated in the decision tree were 0.033 for andexanet alfa and 0.028 for PCC (incremental QALYs = 0.004).
Issues for Consideration
- Andexanet alfa may be used outside of its indication: The feedback obtained from the clinical experts consulted by CADTH suggests that andexanet alfa may be used outside of its indication in clinical practice. The clinical experts explained that while the indication is limited to use on major bleeds due to the FXa inhibitors apixaban and rivaroxaban, major bleeds often occur in emergent care and patients may be prescribed andexanet alfa outside of a need for DOAC-related bleed reversals. This may lead to an increase in the incremental budgetary impact, as it was not accounted for in the budget impact analysis (BIA).
- Division in treatment population for andexanet alfa in comparison with PCC: The feedback obtained from the clinical experts consulted by CADTH suggests that in clinical practice, andexanet alfa is expected to be prescribed for more emergent and severe cases, and PCC will continue to be used for other major bleeds. In the sponsor’s submission, it was assumed PCC and andexanet alfa could be used interchangeably. However, additional clinical input also highlighted that there would be a clear division between the patients, with PCC prescribed for average DOAC reversals and andexanet alfa prescribed for more severe cases. As the division between these patient groups was not reported in the trial, the incremental budgetary impact may be uncertain.
Overall Conclusions
The CADTH Clinical Review of the 3 weighted comparative analyses (ANNEXA-4 trial versus the ORANGE study, ANNEXA-4 versus the Hartford Health care study, and ANNEXA-4 versus the RETRACE-II study) found several limitations that prevented definitive conclusions regarding the comparative efficacy of andexanet alfa and PCC. CADTH’s appraisal of the ITCs found evidence of heterogeneity with the inclusion and exclusion criteria between studies (e.g., definition of major bleeding, recent history of blood product, ICH severity, recent history of thromboembolism, expected survival). Additional residual confounding due to inadequate adjustment for prognostic factors or treatment effect modifiers was also listed as a major limitation. As such, the clinical effectiveness of andexanet alfa compared with PCC is uncertain, both in the short-term (30 days) and long-term.
CADTH identified limitations in the sponsor’s economic analysis: overestimation of long-term survival for ICH patients prescribed andexanet alfa, inappropriate utilities reported for the ICH patients who used andexanet alfa, underestimated RR for 30-day mortality, and lack of publicly available sources for the unit cost of PCC in Canada. To derive the CADTH base case, the following changes were made: applying equal mRS distributions to both the andexanet alfa and PCC treatment groups and changing the ICH long-term utilities for andexanet alfa to align with the PCC value (0.62). In CADTH’s base case, a price reduction of at least 27% would be required to make andexanet alfa an optimal treatment option at a WTP threshold of $50,000 per QALY gained. A scenario analysis conducted by CADTH demonstrated that the estimated ICER is highly sensitive to changes in the RR for 30-day mortality. The feedback obtained by CADTH from the clinical experts highlights that the 30-day mortality RR is likely higher (i.e., the difference between treatments is smaller) than what is reported in the sponsor submission. The true value of the ICER may be higher than the CADTH base case; as such, a further price reduction may therefore be warranted.
Abbreviations
- BIA
budget impact analysis
- CI
confidence interval
- DOAC
direct oral anticoagulant
- FXa
factor Xa
- GI
gastrointestinal
- ICER
incremental cost-effectiveness ratio
- ICH
intracranial hemorrhage
- ITC
indirect treatment comparison
- mRS
modified Rankin Scale
- PMPRB
Patented Medicine Prices Review Board
- PCC
prothrombin complex concentrate
- QALY
quality-adjusted life-year
- RR
relative risk
- WTP
willingness to pay
Appendix 1. Cost Comparison Table
Note this appendix has not been copy-edited.
The comparators presented in the following table have been deemed to be appropriate based on feedback from the clinical experts and drug plans. Comparators may be recommended (appropriate) practice or actual practice. Existing Product Listing Agreements are not reflected in the table and as such, the table may not represent the actual costs to public drug plans.
Table 8
CADTH Cost Comparison Table for Adults Treated With FXa Inhibitors Resulting in Life-Threatening or Uncontrolled Bleeding.
Appendix 2. Submission Quality
Note this appendix has not been copy-edited.
Table 9
Submission Quality.
Appendix 3. Additional Information on the Submitted Economic Evaluation
Note this appendix has not been copy-edited.
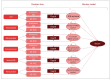
Figure 1
Model Structure.
Detailed Results of the Sponsor’s Base Case
Table 10
Disaggregated Results of the Sponsor's Base Case.
Appendix 4. Additional Details on the CADTH Reanalyses and Sensitivity Analyses of the Economic Evaluation
Note this appendix has not been copy-edited.
Detailed Results of CADTH Base Case
Table 11
Disaggregated Summary of CADTH’s Economic Evaluation Results.
Scenario Analyses
Table 12
Summary of CADTH Scenario Analysis.
Appendix 5. Submitted BIA and CADTH Appraisal
Note this appendix has not been copy-edited.
Table 13
Summary of Key Takeaways.
Summary of Sponsor’s BIA
The sponsor-submitted BIA assessed the budgetary impact resulting from reimbursing andexanet alfa for the treatment of adult patients treated with FXa inhibitors (rivaroxaban or apixaban) when rapid reversal of anticoagulation is needed due to life-threatening or uncontrolled bleeding.22 The BIA was conducted from the perspective of CBS over a 3-year horizon (2024 to 2027) with 2023 as the base year using a combination of a claims-based and epidemiological approach. The population size was derived using a claims-based approach by gathering IQVIA PharmaStat claims data for apixaban or rivaroxaban and was further narrowed epidemiologically to those experiencing a major bleed that would be eligible for reversal.23 Key inputs to the BIA are documented in Table 15.
The following key assumptions were made by the sponsor:
- Historical claims data for apixaban and rivaroxaban were extracted from the IQVIA PharmaStat database for all CADTH-participating regions, regardless of payer (public versus private). Claims were standardized to 30 days using public and private historical days data for each jurisdiction, as available. Private days data were used as a proxy for the whole jurisdiction when no public days data were available. No claims data were available for Prince Edward Island; as such, Nova Scotia claims data were used as a proxy, adjusted for the population difference between Nova Scotia and Prince Edward Island.
- The sponsor assumed 23.6% of all major bleeds are eligible to be treated with a reversal agent.
- The sponsor assumed andexanet alfa would capture up to ||| of the market if reimbursed.
Table 14
Summary of Key Model Parameters.
Summary of the Sponsor’s BIA Results
The sponsor’s base case reported that the reimbursement of andexanet alfa for the treatment of adult patients treated with FXa inhibitors (rivaroxaban or apixaban) when rapid reversal of anticoagulation is needed due to life-threatening or uncontrolled bleeding would result in an incremental budget impact of $15,952,349 in year 1, $34,389,366 in year 2, and $56,656,154 in year 3. The total 3-year incremental cost of $106,997,869. Sensitivity analyses were completed to (i) include an alternative proportion of patients eligible for reversal, (ii) adjust for pessimistic (−25%) and optimistic ( + 25%) market shares, and (ii) include an alternative low-dose/high-dose split. These sensitivity analyses impacted the 3-year incremental budget impact, which varied from $80,248,402 to $133,747,337.
CADTH Appraisal of the Sponsor’s BIA
CADTH identified several key limitations to the sponsor’s analysis that have notable implications on the results of the BIA:
- Use of a claims-based approach to estimate market size introduces uncertainty with the anticipated budget impact of andexanet alfa: The sponsor estimated market size based on public claims data for patients using apixaban or rivaroxaban. While the sponsor did translate claims into the number of users, the sponsor assumes that all public claims for apixaban and rivaroxaban are at risk for major bleeding. Given the claims database does not specify the indication and the proportion of claims pertaining to use for other indications is unknown, using a claims-based approach to estimate market size introduces significant uncertainty in the estimated market size.
- CADTH is unable to address this limitation due to the lack of literature on the pan-Canadian use of apixaban or rivaroxaban.
- Proportion of patients treated with a reversal agent is uncertain: The sponsor’s submitted BIA assumed that 100% of patients with an ICH would require a reversal. However, the clinical expert feedback obtained by CADTH suggests that a low (< 7) Glasgow Coma Scale score and a large (> 60 cc) hematoma size are indicative of severe ICH bleeds and would not require reversal, as any means of treatment would be considered futile. Therefore, the proportion of patients with an ICH bleed eligible for reversal is overestimated. Additionally, the Little et al. (2001) study was used to determine the bleed type distribution.26 This study, however, focused on a population-based cohort of adults aged 66 years or older and thus more susceptible to bleeding, and results were based on 4 oral anticoagulants (apixaban, rivaroxaban, dabigatran, and warfarin). Given that apixaban and rivaroxaban have lower GI bleeding rates in comparison with warfarin, the bleed type distribution is uncertain.
- To address this limitation, CADTH undertook a scenario analysis to explore the budgetary impact of reducing the proportion of patients treated with a reversal agent. Given that no alternative estimates were available, the CADTH scenario analysis used an arbitrary value of 18.6% (i.e., a 5% reduction in the size of the eligible population).
- The price of drugs paid by CBS is uncertain: Due to the confidentiality of list prices for CBS products, both the sponsor’s and CADTH’s analyses are based on an estimation of the cost of PCC using published list prices for Beriplex and Octaplex in PMPRB basket of 11 comparator countries. Unit prices for Beriplex and Octaplex were only available in 2 and 3 of the 11 countries, respectively. In the sponsor’s base case, a median price was used of the published list prices.
- To address this limitation, CADTH undertook a scenario analysis by using the lowest and highest list prices available in the PMPRB for Beriplex and Octaplex.
- The market uptake of andexanet alfa is uncertain: The sponsor’s submitted BIA indicated that andexanet alfa would result in a market uptake of ||| in year 1, ||| in year 2, and ||| in year 3 based on internal sponsor market shares. However, CADTH obtained clinical expert feedback indicating that the market uptake of ||| in year 3 does not align with clinical expectations and indicated the sponsor likely overestimated andexanet alfa uptake. The clinical experts highlighted that while PCC is not indicated for the rapid reversal of anticoagulation due to life-threatening or uncontrolled bleeding in patients using FXa inhibitors (apixaban and rivaroxaban), clinicians are comfortable using PCC due to the robust published data and experience with the comparator. Additionally, the clinical experts reported uncertainty on the clinical efficacy of andexanet alfa in comparison with PCC and that the overestimation of the market share could be a result of the sponsor’s overestimation of the difference in benefit between PCC and andexanet alfa. CADTH clinical expert feedback deemed the sponsor’s estimate in year 1 and year 2 to be reasonable, but they indicated that the andexanet alfa market share would likely |||||| |||| by year 3.
- To address this limitation, CADTH undertook a scenario analysis by revising the market share for andexanet alfa in the new-drug scenario to 25% in year 3.
CADTH Reanalyses of the BIA
Table 15
CADTH Revisions to the Submitted Budget Impact Analysis.
CADTH did not undertake a base case reanalysis. Instead, CADTH explored the potential impact of several scenario analyses which included:
- Using the lowest and highest PCC (Beriplex and Octaplex) list prices from the available PMPRB published prices.
- ||||||| |||||||| || || ||||||||| |||||||| || || ||||||||| |||||||| |||| || ||||||||| |||||||| || || ||||||||| |||||||| |||| || ||||||||||||||||| |||||||| || || |||||||| |||||||| || || ||||||||| |||||||| |||| ||||||| |||||||| || || ||||||||| |||||||| |||| || |||||||||. Assuming 25% market uptake of andexanet alfa in year 3, due to feedback obtained from clinical experts consulted by CADTH.
- Assuming a lower proportion (18.6%) of patients treated with a reversal agent.
- Exploring 100% of patients on low-dose andexanet alfa or high-dose andexanet alfa.
- 27% reduction in the price of andexanet alfa.
The results are presented in Table 18. The reimbursement of andexanet alfa was associated with a 3-year incremental budget impact of $106,997,869 in the base case. A price reduction of 27% reduces the budget impact, for a 3-year incremental budget impact of $76,452,748. In the first scenario analysis, using the lowest available pricing for PCC resulted in an increase of the budget impact, with a 3-year incremental budget impact of $108,452,021. Alternatively, the revised market uptake of andexanet alfa using feedback obtained from clinical experts consulted by CADTH resulted in a decrease, with a 3-year budget impact of $85,751,811. Similarly, reducing the proportion of patients treated with a reversal agent by 5% to a total of 18.6% resulted in a decrease in the 3-year budgetary impact, with a total of $84,366,966. When exploring the impact of the weight of low- or high-dose andexanet alfa, the fifth scenario highlights that 100% of patients on a low dose would result in a reduction in the 3-year budget impact for a total of $91,326,507. Alternatively, in the sixth scenario, altering the proportion of patients on a high dose to 100% results in a marked increase in budgetary impact, with a 3-year total of $169,293,482.
Table 16
Detailed Breakdown of the CADTH Reanalyses of the BIA.
References
- 1.
- Pharmacoeconomic evaluation [internal sponsor's report]. In: Drug Reimbursement Review sponsor submission: Ondexxya (andexanet alfa) 200 mg powder for solution for intravenous infusion. Mississauga (ON): AstraZeneca Canada, Inc.; 2023 Mar 2.
- 2.
- Clinical Study Report 14-505 (ANNEXA-4). Prospective, open-label study of andexanet alfa in patients receiving a factor Xa inhibitor who have acute major bleeding (ANNEXA-4) [internal sponsor's report]. San Francisco (CA): Portola Pharmaceuticals, Inc.; 2020.
- 3.
- PrOndexxya® (andexanet alfa): 200 mg powder for solution for intravenous infusion [product monograph]. Mississauaga (ON): AstraZeneca Canada, Inc; 2023 Jun 16.
- 4.
- Ontario schedule of benefits: physician services under the health insurance act (October 5, 2022 (effective December 1, 2022)). Toronto (ON): Ontario Ministry of Health; 2022: https://www
.health.gov .on.ca/en/pro/programs /ohip/sob/physserv /sob_master_20221201.pdf. Accessed 2023 Mar 1. - 5.
- Patient Cost Estimator. 2023; https://www
.cihi.ca/en /patient-cost-estimator). Accessed 2023 Mar 1. - 6.
- Mittmann N, Seung SJ, Hill MD, et al. Impact of disability status on ischemic stroke costs in Canada in the first year. Can J Neurol Sci. 2012;39(6):793-800. [PubMed: 23041400]
- 7.
- Chan BC-F, Cadarette SM, Wodchis WP, Krahn MD, Mittmann N. The lifetime cost of spinal cord injury in Ontario, Canada: a population-based study from the perspective of the public health care payer. J Spinal Cord Med. 2019;42(2):184-193. [PMC free article: PMC6419658] [PubMed: 29923798]
- 8.
- Persson J, Aronsson M, Holmegaard L, et al. Long-term QALY-weights among spouses of dependent and independent midlife stroke survivors. Qual Life Res. 2017;26:3059-3068. [PMC free article: PMC5655581] [PubMed: 28664459]
- 9.
- Hogan M-E, Taddio A, Katz J, Shah V, Krahn M. Incremental health care costs for chronic pain in Ontario, Canada: a population-based matched cohort study of adolescents and adults using administrative data. Pain. 2016;157(8):1626-1633. [PubMed: 26989805]
- 10.
- Cohen AT, Lewis M, Connor A, et al. Thirty‐day mortality with andexanet alfa compared with prothrombin complex concentrate therapy for life‐threatening direct oral anticoagulant‐related bleeding. J Am Coll Emerg Physicians Open. 2022;3(2):e12655. [PMC free article: PMC8898077] [PubMed: 35280921]
- 11.
- Potential sources for foreign prices: PMPRB11. Ottawa (ON): Government of Canada; 2023: https://www
.canada.ca /en/patented-medicine-prices-review /services /are-you-patentee /potential-sources-foreign-prices.html. Accessed 2023 Mar 1. - 12.
- Green L, Tan J, Morris JK, et al. A three-year prospective study of the presentation and clinical outcomes of major bleeding episodes associated with oral anticoagulant use in the UK (ORANGE study). Haematologica. 2018;103(4):738. [PMC free article: PMC5865412] [PubMed: 29371325]
- 13.
- Huttner HB, Gerner ST, Kuramatsu JB, et al. Hematoma expansion and clinical outcomes in patients with factor-Xa inhibitor–related atraumatic intracerebral hemorrhage treated within the ANNEXA-4 trial versus real-world usual care. Stroke. 2022;53(2):532-543. [PubMed: 34645283]
- 14.
- Huybrechts KF, Caro JJ, Xenakis JJ, Vemmos KN. The prognostic value of the modified Rankin Scale score for long-term survival after first-ever stroke. Cerebrovasc Dis. 2008;26(4):381-387. [PubMed: 18753743]
- 15.
- Friberg L, Hammar N, Pettersson H, Rosenqvist M. Increased mortality in paroxysmal atrial fibrillation: report from the Stockholm Cohort-Study of Atrial Fibrillation (SCAF). Eur Heart J. 2007;28(19):2346-2353. [PubMed: 17670754]
- 16.
- Apixaban for the treatment and secondary prevention of deep vein thrombosis and/or pulmonary embolism. NICE technology appraisal guidance (TA341). London (UK): National Institute for Health and Care Excellence; 2023: https://www
.nice.org.uk/guidance/ta341. Accessed 2023 Mar 1. - 17.
- Guertin JR, Feeny D, Tarride J-E. Age-and sex-specific Canadian utility norms, based on the 2013–2014 Canadian Community Health Survey. CMAJ. 2018;190(6):E155-E161. [PMC free article: PMC5809215] [PubMed: 29440335]
- 18.
- Wittenborn JS, Clemons T, Regillo C, Rayess N, Kruger DL, Rein D. Economic evaluation of a home-based age-related macular degeneration monitoring system. JAMA Ophthalmol. 2017;135(5):452-459. [PMC free article: PMC5470421] [PubMed: 28358948]
- 19.
- Matza LS, Chung K, Van Brunt K, et al. Health state utilities for skeletal-related events secondary to bone metastases. Eur J Health Econ. 2014;15:7-18. [PMC free article: PMC3889679] [PubMed: 23355121]
- 20.
- Hogan M-E, Taddio A, Katz J, Shah V, Krahn M. Health utilities in people with chronic pain using a population-level survey and linked health care administrative data. Pain. 2017;158(3):408-416. [PubMed: 27902568]
- 21.
- Ontario Ministry of Health, Ontario Ministry of Long-Term Care. Ontario drug benefit formulary/comparative drug index. 2022; https://www
.formulary .health.gov.on.ca/formulary/. Accessed 2023 Aug 1. - 22.
- Budget Impact Analysis [internal sponsor's report]. In: Drug Reimbursement Review sponsor submission: Ondexxya (andexanet alfa) 200 mg powder for solution for intravenous infusion. Mississauga (ON): AstraZeneca Canada, Inc.; 2023 Mar 2.
- 23.
- PharmaStat. [Ottawa (ON)]: IQVIA; 2022: https://www
.iqvia.com/. Accessed 2023 Aug 1. - 24.
- Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981-992. [PubMed: 21870978]
- 25.
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883-891. [PubMed: 21830957]
- 26.
- Little DH, Sutradhar R, Cerasuolo JO, et al. Rates of rebleeding, thrombosis and mortality associated with resumption of anticoagulant therapy after anticoagulant-related bleeding. CMAJ. 2021;193(9):E304-E309. [PMC free article: PMC8034308] [PubMed: 33649169]
- Executive Summary
- Stakeholder Input Relevant to the Economic Review
- Economic Review
- Abbreviations
- Cost Comparison Table
- Submission Quality
- Additional Information on the Submitted Economic Evaluation
- Additional Details on the CADTH Reanalyses and Sensitivity Analyses of the Economic Evaluation
- Submitted BIA and CADTH Appraisal
- References
- Pharmacoeconomic Review - Andexanet Alfa (Ondexxya)Pharmacoeconomic Review - Andexanet Alfa (Ondexxya)
Your browsing activity is empty.
Activity recording is turned off.
See more...