This work was produced by Guthrie et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Guthrie B, Rogers G, Livingstone S, et al. The implications of competing risks and direct treatment disutility in cardiovascular disease and osteoporotic fracture: risk prediction and cost effectiveness analysis. Southampton (UK): National Institute for Health and Care Research; 2024 Feb. (Health and Social Care Delivery Research, No. 12.04.)

The implications of competing risks and direct treatment disutility in cardiovascular disease and osteoporotic fracture: risk prediction and cost effectiveness analysis.
Show detailsBackground
Preventative treatments are different from interventions for acute conditions. Preventative treatments may have both immediate and persistent harms, but their benefits are deferred. It follows that shared decision-making between the patient and clinician about whether or not to start a preventative treatment should focus on whether or not the (potential) long-term health benefits are sufficient to justify the (certain) immediate burden and (possible) immediate or delayed harms. Clinical practice guidelines may provide recommendations, indicating whether or not this trade-off is positive for an average person. In England, at least, the developers of such guidelines are often informed by a quantitative analysis exploring the benefits, harms and costs of treatments compared with each other or with no treatment. This usually takes the form of a decision-analytic model that quantifies the net effects of competing approaches in terms of QALYs, encompassing expected benefits and harms, and compares these with net costs. Interventions will typically receive positive recommendations only if the guideline developers are confident that offering the treatment in question will result in population-level QALY gain (without imposing opportunity costs that imply even greater QALY loss in the wider health system). Therefore, prescribers may assume that, all things being equal, the preventative treatments that are recommended in trustworthy guidelines provide net benefit, and this seems like helpful information until one acknowledges that, in practice, all things never are equal.
Two particular dimensions in which any given person will diverge from population average are their clinical characteristics – especially with regard to long-term conditions other than the one of interest – and their preferences – in terms of whether or not they perceive a treatment is worth taking. Clinical guidelines often neglect to consider these factors, except in nebulous terms (e.g. encouraging prescribers to consider individualised risks and benefits, without providing any objective basis on which to introduce them to an options talk).
In the cost-effectiveness sections of this project, reported in this chapter and in Chapter 7, we aim to address this gap. We explore how accounting for clinical characteristics and preferences (accounted for using DTD) could alter the balance of benefits, harms and costs of preventative interventions. By adapting current methods used to populate model-based CEA of preventative medicines, we can better reflect the potential for subgroups of the patient populations to accrue different degrees of net benefit.
Our first objective is to tailor estimates according to competing mortality risk. As explored in Chapters 2–4, all people at risk of a given health condition are also at risk of other events that preclude the incidence of the condition of interest (unrelated death is an obvious example in almost all cases). We will see that, unlike epidemiological risk prediction models, decision-analytic economic models usually have some structural way of accounting for such competing events. However, decision modellers seldom pay close attention to how the likelihood of competing events is parameterised. Often, for example, decision modellers use an unadjusted estimate of general population mortality to reflect deaths that are not related to the condition of primary interest. We wanted to assess how much a failure to handle competing risks robustly might bias model outputs. This chapter explores an example using a cohort-level state-transition model (i.e. statins for the primary prevention of CVD) and Chapter 7 looks at a patient-level discrete-event simulation (i.e. bisphosphonates for the primary prevention of osteoporotic fragility fracture). In both cases, we build on the models that were used to underpin national guidance from NICE. In the case of statins, we use the model developed for Clinical Guideline 181 (CG181).10 For bisphosphonates, we start from the model for Technology Appraisal 464 (TA464).12 We do not necessarily assert that these are the best possible models in each decision space, and our choice to adopt and adapt the models arises from our interest in the extent to which existing population-level guidance might be affected by the issues under consideration.
Our second objective is to incorporate the evidence regarding DTD generated in Chapter 5, using the TTO method into modelled estimates of the cost-effectiveness of statins and bisphosphonates. Having found that a majority of people would be prepared to trade some of their life expectancy to avoid the inconvenience of taking statins or bisphosphonates, it is an obvious next step to ask whether or not the benefits of the interventions are sufficient to counterbalance this level of disutility. By integrating existing evidence with our new findings, we can derive QALY estimates for preventative medicines, compared with no intervention, that help us weigh up the overall balance of options for people with different levels of preference to avoid medicines.
Modifying existing methods in these ways allows us to define cost-effective subgroups of people who reflect different levels of competing risk (defined by specified levels of competing risk at different levels of baseline risk) and different degrees of DTD (defined by specified levels/durations of DTD).
Methods
We used a cohort-based decision-analytic model to address the stated decision problem (Table 14).
TABLE 14
Key design criteria for decision-analytic model for statins for primary prevention of CVD
Selection of the statins case study
The decision-analytic model we adopted and developed for this decision problem takes a cohort-level state-transition (Markov) approach to predicting events of interest and estimating costs and effects.10
Decision-analytic models of this type account for competing risks by design. For example, consider a Markov model with the three states of (1) well, (2) myocardial infarction (MI) and (3) dead (for our purposes, we will define the latter two as absorbing states from which further transitions are not of interest) and let per-cycle transition probabilities pwell→MI and pwell→dead both be 0.1. Assuming the whole cohort starts in the well state, after one cycle of the model 10% of people will be in the dead state and 10% will be in the MI state, leaving 80% in the well state. Note that, via the transition to death, 10% of the people who were initially at risk of a MI have been taken out of the at-risk state before cycle 2 without experiencing a MI. After the second cycle, we will have 18% of the cohort in the MI state (1 × 0.1 + 0.8 × 0.1); however, if we had a two-state well MI model with no competing risk of death, then it would have been 19% (1 × 0.1 + 0.9 × 0.1). If we run the model on for 10 cycles, and we find that 44.6% of people have had a MI, then without the competing transition to death the number would be 65.1%.
This simple example demonstrates that a competing risk structure is hardwired into the kind of state-transition models that are very commonly used to assess the cost-effectiveness of healthcare interventions. The example also shows that, to parameterise any given transition within such a structure, it is appropriate to rely on evidence that censors for competing events. Were we to base our inputs on time-to-event models that, themselves, adjust for competing risks (e.g. models explored in Chapters 2 and 3), our model would underestimate the probability of events of interest, as we would be effectively double counting the competing risks.
Clearly, however, it is important that the time-to-event evidence on which a decision model relies should be valid for the population to which the decision problem pertains. It should be clear from the above that if we have an inappropriate estimate of any one of our transitions, then it is not only with respect to that outcome that our model will be biased, as it will also compromise our estimate of any events with which it competes.
In an epidemiological risk prediction model, failing to account for competing risks will result in an overestimate of event rates for the whole cohort. In a state-transition decision model, the danger is that we will fail to account accurately for competing risks, which will lead to overprediction of events in people with higher-than-average competing risk and underprediction of events in people with lower-than-average competing risks.
Model overview
Table 14 summarises the decision problem and scope of the analysis for the model-based CEA. The model from which we started directly replicates the model developed to inform decision-making about primary prevention of CVD in CG181.10 The published guideline provides a detailed description of methods.10
In summary, the model takes a cohort-level state-transition (Markov) approach. The model has a 1-year cycle length and a 40-year time horizon. Figure 18 illustrates the model structure. As the decision problem relates to primary prevention, the whole cohort starts in the ‘no known CVD’ state. As time progresses in the model, the model simulates a proportion of people experiencing one or more of seven distinct non-fatal cardiovascular events [i.e. stable angina (SA), unstable angina (UA), MI, stroke, TIA, peripheral arterial disease (PAD) and heart failure]. The model also accounts for cardiovascular death and other-cause mortality. For each of the non-fatal cardiovascular events, an initial 1-year cycle reflects an acute event or diagnosis and a post-event state reflects people living with a history of the event in subsequent years. Repeat acute events are possible for MI and stroke. Where evidence was available to the CG181 modellers, other secondary events occur from most acute and post-event states. However, in the base case, no further events are allowed from PAD and heart failure (as these events are associated with the highest probability of cardiovascular death, meaning that additional events would unrealistically confer an improvement in prognosis).

FIGURE 18
Structure of state-transition model assessing statins for primary prevention of CVD. HF, heart failure.
However, in the base case, no further events are allowed from PAD and heart failure (as these events are associated with the highest probability of cardiovascular death, meaning that additional events would unrealistically confer an improvement in prognosis).
The model simulates a cohort comprising men and/or women of a specified age and a baseline 10-year risk of a first cardiovascular event (i.e. events counted by QRISK3: SA, UA, MI, stroke, TIA and cardiovascular death). (Note that the model handles heart failure and PAD separately on the assumption that their incidence is proportional to that of the QRISK3 events.) Calculations are based on true baseline risk, that is, absent competing events, 20% of people with a 20% 10-year risk will experience a first cardiovascular event in the first 10 years of the model.
The ways in which we have adapted the model fall into two broad categories. First, there were ‘general model updates’ that reflect steps we took to bring the CG181 model up to date and improve the parameterisation and/or implementation of existing functionality. Second, we introduced new features to explore the issues of interest for this project (i.e. accounting for competing risk and the incorporating DTD).
General model updates
Except where otherwise described, our version of the model retains the inputs used for the CG181 analysis (see guideline documentation for full details10).
Natural history: mortality statistics
The CG181 model relied on ONS lifetables for England for 2010–2. We configured the model to simulate present-day life expectancy using ONS lifetables for 2017–9.126 While we were in the latter stages of preparing this report, ONS lifetables for 2018–20 became available; however, we did not update the model to use these data, as the data include substantial excess mortality owing to the COVID-19 pandemic that began in 2020.
The model also needs an estimate of the proportion of deaths that are caused by CVD. For this estimate, the CG181 model used ONS death registration statistics for England and Wales from 2012, selecting all deaths recorded under ICD-10 codes I00–I99. For this study we did exactly the same, using data for 2019 that are now available through an ONS Application Programming Interface.127
Type of first cardiovascular event
In the CG181 model,10 as preserved in our update, a cohort’s cardiovascular risk is defined by its specified 10-year QRISK3 predicted risk. The model treats this value as deterministically exact, that is, absent competing risks, 20% of a cohort with a 20% 10-year cardiovascular risk will experience a first qualifying event in the first 10 years of the model.
The model subdivides the proportion of people experiencing a first event into eight event-specific states. Six events were covered by QRISK3 (i.e. SA, UA, MI, stroke, TIA and cardiovascular death) plus two additional events (i.e. heart failure and PAD) (see Figure 18). In CG181, the evidence used to define the split came from the original NICE technology appraisal on statins,128 which took data from a variety of sources.129–131 This evidence is 25–40 years old and it does not account for underlying level of risk (i.e. it is plausible that people at low risk of cardiovascular events experience a different distribution of first events from people at high risk). Moreover, it is suboptimal to derive a subdivision like this using disparate sources of evidence. Therefore, we used the CPRD data set generated for the cardiovascular element of this project (see Chapter 2) to develop a new estimate of type of first cardiovascular event, conditional on some event having occurred. We fitted a multinomial logistic regression model to estimate the relative probabilities of a cardiovascular event being of each type, according to sex, age at event and baseline QRISK3 predicted risk. Appendix 6 provides full methods, including a worked example calculation and resulting inputs for the decision-analytic model.
Increasing cardiovascular risk over time
The CG181 model incorporates a year-on-year linear increase in cardiovascular risk (0.3% per year for men and 0.08% per year for women), based on a regression undertaken for the original NICE technology appraisal on statins.128 The model applies this yearly increment in the initial 10-year period, over which the user specifies baseline risk (centring the risk around the middle of the period so that the cumulative risk over the 10 years is very close to the specified level) and then extends the same linear increase beyond to the model’s time horizon.
We retained the assumption that the increase is linear over the initial 10 years. However, beyond the period of fixed risk, we made use of evidence from the QRISK3 derivation study,25 that is increase in cardiovascular risk accelerates as people get older. We configured the model to use the age coefficients from QRISK3 to estimate HRs for people as they age. For men, QRISK3 estimates age-related risk in terms of (age/10)−1 and (age/10)3, for which the log-HRs are −17.84 and 0.0023, respectively. For women, the parameters are (age/10)−2 and (age/10) and the log-HRs are −8.14 and 0.797, respectively. We verified that our implementation of these data exactly matches the ‘HRs by age’ depicted in supplementary material for the QRISK3 derivation paper.25 Using this approach, our model calculates HRs for people as they age, compared with their risk at the end of the initial 10-year period, and applies the HRs to calculate yearly risk increases.
When performing this update, our study also identified an error in the CG181 model in how increasing baseline risk interacts with treatment effect,10 as this leads to anomalous results where statins – despite being notionally subject to a constant relative risk indicating benefit – are associated with raised risk of some events (i.e. stroke and cardiovascular death) in later cycles of the model. A negative effect of statins also develops for heart failure, even though, in its base case, the CG181 model10 and ours assume a relative risk of 1 (i.e. no benefit) for this outcome. We corrected this error in our implementation of the model.
Treatment effectiveness
We have not updated any estimates of the effectiveness of statins in preventing CVD and, therefore, these estimates remain as in CG181.10 For high-intensity regimens, this means relative risks of 0.46 (95% CI 0.37 to 0.59) for MI, 0.80 (95% CI 0.70 to 0.91) for stroke and 0.73 (95% CI 0.61 to 0.88) for cardiovascular death. As in CG181,10 we assume that statins’ effect in reducing MI also applies to SA, UA and PAD, and that the effect for stroke applies to TIA. We do not model a benefit for statins in reducing incidence of heart failure.
The base-case model presented in CG18110 included a benefit for statins in reducing non-cardiovascular mortality. The evidence supporting this assumption was weak in 2014, and we are not aware of any stronger data since the guideline was published. Therefore, we have removed this assumption from our base case (i.e. we do not model any non-cardiovascular benefit for statins).
Health-related quality of life: underlying
The developers of the model that informed the original NICE technology appraisal for statins128 undertook a linear regression on patient-level data from the study reported by Kind et al.132 to estimate baseline quality of life. This analysis, which the CG181 model subsequently adopted, uses data from the general population and defined utility as a function of age, without accounting for sex or any non-linear effects. We considered that it could be important to account for these factors in a more flexible way and so we undertook a new regression, using a pooled sample of respondents from the Health Survey for England (HSE)133 (see Appendix 6 for full details).
Health-related quality of life: cardiovascular events
To quality-adjust expected survival for cost–utility analysis of a state-transition model, we require estimates of the quality of life associated with each of the model states. We performed a systematic literature review to update the health state utility values from the CG181 model.10 To identify estimates for each model state, we adopted a pragmatic approach, following recommendations in the NICE Decision Support Unit’s technical support document for utility values.134 Appendix 6 provides details of the values we selected for the updated model and the process by which we identified and selected them. Appendix 6, Table 51, provides full details of the final inputs.
Adverse drug events
The CG181 model accounted for one adverse effect of statins, based on evidence appearing to show hastened onset of type 2 diabetes at the time.10 However, a recent systematic review found no association between randomisation to statins and incidence of diabetes [odds ratio (OR) 1.01, 95% CI 0.88 to 1.16].135 Therefore, we removed this feature from our version of the model.
The adverse event with which people most commonly believe statins are associated is muscle pain. However, it is hard to find objective evidence confirming a harm of meaningful magnitude in this domain. A recent comprehensive systematic review summarising the experience of over 65,000 trial participants found that statins are significantly associated with an increase in muscle symptoms, but the effect is extremely small, with 15 (95% CI 1 to 29) extra events per 10,000 patient-years.135 Two UK-based randomised crossover trials that postdate the review found no difference in muscle symptoms between the phases when participants took statins and phases when participants received placebo.43,136,137
The meta-analysis also found very small increased risks of liver, kidney and eye problems with statins compared with controls, with increased incidences of 8, 12 and 14 events per 10,000 patient-years, respectively.135 These events are all mild and, in the case of liver and kidney reports, largely based on laboratory findings rather than clinically overt symptoms.
Even if we were to assume that all these events were serious, their incidence is increased so little that including the events in a cost–utility analysis would make no material difference to results. For example, if we ascribe the substantial disutilities that have been reported for people living with chronic complaints affecting the same body parts [−0.212 for ‘Other problems of bones/joints/muscles’, −0.102 for ‘Other digestive complaints’ (including liver), −0.176 for ‘Kidney complaints’ and −0.114 for ‘Cataract/poor eye sight/blindness’138] and assume each adverse event lasts for 1 month (except for eye problems, which we extend to a mean of 5 years to reflect the possibility of long-term harm), the net expected health loss across all these categories would total 0.0008 QALYs, which is equivalent to less than one-third of a day in perfect health. As this is a negligible amount, and almost certainly a substantial overestimate (as reported events are nowhere near as serious as those from which we obtained the utility decrements), we concluded that there would be no benefit in configuring our model to simulate adverse events.
Nevertheless, the fact that many people believe that statins cause harms is, in itself, a harm associated with statins. A recent crossover trial by Howard et al.43 also included no-treatment periods along with statin and placebo phases. Although there was no difference between statin and placebo periods, the level of self-reported muscle symptoms was significantly higher when participants took either pill than it was for the no-treatment phases, and this strongly suggests that statins have ‘nocebo’ effects. Howard et al.43 conclude that ‘It is clear that this cohort were indeed intolerant of statin tablets, but also that the source of the intolerance was primarily the tablet, not the statin’. We will argue below that our exploration of DTD provides a useful paradigm in which to conceptualise nocebo effects as an authentic harm of treatment.
Resource use and costs: acquisition of statins
We updated the acquisition costs of statins to present-day levels, using data from the NHS Drug Tariff (November 2021).139 The new prices (per pack of 28 tablets) were £0.92 for simvastatin 10 mg (i.e. low intensity), £0.96 for simvastatin 20 mg (i.e. medium intensity), £1.10 for atorvastatin 20 mg (i.e. high intensity) and £1.68 for atorvastatin 80 mg (i.e. high intensity and secondary prevention).
Resource use and costs: cardiovascular events
In addition to the costs of the interventions modelled, the model requires annual per-person costs of NHS and Personal Social Services care associated with each state (in GBP and for the relevant price year) to calculate lifetime costs of CVD in each cohort. We updated all model inputs in this area on the basis of a rapid review we undertook using a pearl-growing approach based on two previously known sources (one for stroke/TIA140 and one for cardiovascular events141). Appendix 6 details our methods and shows the resulting model inputs.
New model features specific to this project
Accounting for competing risk of non-cardiovascular death
The CG181 model10 accounts for background non-cardiovascular mortality using general population lifetables. A key motivation of this project (see Background) was to improve on this by ensuring that, for any modelled cohort, the competing risk of non-cardiovascular death reflects the life expectancy of people with the specified level of cardiovascular risk. Official lifetables stratify by age and sex; however, the lifetables do not tell us how all-cause mortality might be associated with cause-specific factors, such as cardiovascular risk. Therefore, we needed a method that will enable us, in effect, to estimate cardiovascular risk-specific non-cardiovascular lifetables.
To do this, we fitted a relative survival model to the cardiovascular CPRD data set used in the cardiovascular element of this project (see Chapter 2), using the multiplicative method of Andersen et al.142 This approach is a variant of a Cox proportional hazards model that uses the life expectancy of a reference group (in this case, non-cardiovascular survival in the general population) as a time-varying covariate. For a formal definition of the model, see Appendix 6.
Our rationale for using a relative survival model is threefold. First, we needed some way of not only characterising observed survival in the CPRD data set (which has a median follow-up of 5 years), but also extrapolating to the lifetime horizon of the decision model. Decision-analytic economic models commonly use parametric models to accomplish this type of task; however, such models invariably characterise a discrete cohort of which we can plausibly assume that the members share a survival distribution (e.g. people with newly diagnosed cancer of a given type). In this case, we want to simulate heterogeneous groups of people (e.g. 40-year-old women with a 10-year cardiovascular risk of 2%; 80-year-old men with a 10-year cardiovascular risk of 40%) and it is implausible to assume that we can describe the expected survival of all such groups with a common parametric foundation. With a relative survival model, we do not need to define a functional form, we simply have to estimate a model that we can apply to a baseline drawn from empirical lifetable data, as this will naturally produce survival curves that vary in shape and scale, even though the relative difference between the general population and the modelled cohort is a constant function of the covariates. Second, we wanted an approach we could use to estimate the life expectancy of cohorts for whom the decision to initiate statins takes place in the present day. Characterising the relative relationship between observed survival and expected population survival from contemporaneous lifetables provides us with a model we can then apply to present-day lifetables. In doing this, we make the assumption that, although absolute life expectancy may have changed over time, the extent to which factors of interest affect it generalise across time with minimal bias. Third, relative survival models provide a parsimonious way of summarising complex data. If, as discussed above, we relied on parametric models to extrapolate to lifetime expectation, then we would inevitably need to fit multiple models to discrete subsets of the data; however, if we had chosen too few, then we would have inappropriately lumped people with heterogeneous expectations together, and if we had chosen too many, then we would have generated high-variance estimates with a tendency to overfit to artefacts in uncertain data. In contrast, the relative survival approach enables us to make use of all available data while adjusting for the things that might define groups with different expectations. Another reasonable approach would have been to fit a multistate model to the CPRD data; however, this would not solve the problem of extrapolation beyond the observed evidence and may have given us less flexibility in simulating cohorts with different baseline characteristics and risk profiles.
The output of the relative survival model is the multiplicative difference in time to event (in our case, non-cardiovascular death) between a person in a specific population and someone of the same age and sex in the general population. The coefficients show how this difference varies with each covariate.
For our models, the critical covariate is ΔQ, which we define as the difference between an individual’s predicted 10-year QRISK3 score and the average score for a person of the same age and sex. Because QRISK3 predicted risk is a probability it is bounded between 0 and 1 and, therefore, it becomes mathematically convenient to transform the estimates to a log-odds scale and so we work with Δlogit(Q). Because population lifetables do not provide information on QRISK3 scores we estimated average 10-year risks by age and sex using a regression on the CPRD data set (see Appendix 6, Table 54 and Figure 39). Owing to computational constraints, we were unable to fit a single relative survival model to the whole CPRD extract and, therefore, we developed separate models for men and women.
To begin with, we fitted relative survival models with a single coefficient [i.e. Δlogit(Q)].
As lifetables are already stratified by age, it is theoretically not necessary to include a term for it in a relative survival model. However, it is plausible that the effect of other covariates – in this case, Δlogit(Q) – varies with age. Therefore, we also fitted a more complicated model including age and its interaction with Δlogit(Q). At the same time, we explored introducing polynomial terms to account for non-linearity of effect. We found that using quadratic terms for both Δlogit(Q) and age improved the fit of the model (reducing AIC by several hundred points); however, the inclusion of higher-order polynomials provided diminishing returns. Therefore, the linear component of our more complex model takes the form:
Table 15 shows the results of simple and polynomial models fitted for men and women separately.
TABLE 15
Relative survival models: non-cardiovascular mortality as a function of predicted cardiovascular risk and age in men and women
To give a worked example, consider a 60-year-old man with a 10-year QRISK3 predicted CVD risk of 20%. First, using Appendix 6, Table 54, we calculate the average logit(QRISK3) for a person with those characteristics:
On a probability scale, this is a 10-year risk of 14% (i.e. our hypothetical man is subject to higher-than-average risk). Next, we calculate Δlogit(Q), that is the difference between observed and expected risk, and in this case it is logit(0.2) minus −1.815 = 0.429. Plugging this value into our polynomial model gives:
(Note that for summation we use the natural logarithm of the coefficients given in Table 15 and age is centred around the population mean of 45.43. Therefore, in this case, 60 – 45.43 = 14.57.)
When exponentiated, this value provides a HR of 1.72. Therefore, we estimate that a 60-year-old man with a 10-year QRISK3-predicted CVD risk of 20% is, alongside his raised cardiovascular risk, subject to a hazard of non-cardiovascular death that is more than two-thirds higher than that of an average person of that age and sex. This is the value that we apply to general population lifetables (adjusted to remove cardiovascular deaths) when we simulate the non-cardiovascular life expectancy of a cohort with those characteristics.
Figure 19 shows estimates of non-cardiovascular mortality generated in the way described above, compared with empirical data across different categories of cardiovascular risk and age. The empirical Kaplan–Meier curves represent observed non-cardiovascular mortality (censored for cardiovascular death) in the CPRD extract for people of the stated age, with baseline QRISK3 predictions in the specified brackets. For our modelled estimates, we start from ONS 3-year lifetables for England and Wales (for this illustration we use 2009–11 tables, as this is in the middle of the period covered by the CPRD data). We adjust these data to remove cardiovascular deaths, estimated using proportions recorded under ICD-10 codes I00–I99 in ONS’s ‘Deaths registered in England and Wales’ series. For each combination of risk and age bracket, we calculate HRs, as described above (for comparability, we fit at the mean QRISK3 prediction and age observed within that category in the CPRD data), and apply the HRs to the ONS curves, and this produces the adjusted curves shown in Figure 19.

FIGURE 19
Fitted relative survival models for non-cardiovascular mortality compared with empirical data. (a) Men; and (b) women.
This exercise shows that the approach produces an excellent fit to the observed data. Even though the simple models (i.e. short dash) depend on a single variable, the simple models capture most of the observed variability in non-cardiovascular death. The polynomial models with age interactions (i.e. long dash) improve this fit somewhat further, at least falling within the 95% confidence limits of the observed survival functions in almost all cases. (One exception is 45- to 54-year-old women with a 10-year QRISK3 CVD prediction of 25% ± 2.5%, as these women appear to have better survival than women of the same age with lower cardiovascular risk; however, we consider this an implausible artefact of the relatively small sample of women in the category.)
Note that within each age bracket the unadjusted general population estimate (see Figure 19) remains the same across cardiovascular risk categories. Estimating competing-cause mortality on the basis of age and sex alone, without accounting for the way in which it correlates with the risk of interest, is the approach the CG181 model takes,10 but this analysis shows that such an approach can misestimate competing mortality risk to a potentially substantial degree.
For example, for women aged 65–74 years the unadjusted general population estimate suggests that women of this age have around an 84% chance of surviving 10 years without experiencing non-cardiovascular death. However, if a woman in this category had a predicted cardiovascular risk between 7.5% and 12.4%, then she would be uncommonly healthy and so her true 10-year non-cardiovascular survival would be around 91%. Conversely, if a woman had a high QRISK3-predicted risk of the order 27.5%–32.4%, then she would have below-average health and her 10-year non-cardiovascular survival probability would be around only 70%. In the former case, a model relying on unadjusted competing-cause mortality estimates would underestimate likely survival by a non-trivial amount. In the latter case, the bias is reversed. Note that in both cases the unadjusted population expectation is not within the confidence limits of the observed Kaplan–Meier estimate, whereas our adjusted estimates pass closely through them.
Incorporating direct treatment disutility
The TTO exercise we conducted as part of this project (see Chapter 5) established that most respondents would be prepared to sacrifice an amount of life expectancy to avoid the inconvenience of taking statins to prevent CVD. Across all participants, the average estimated utility was 0.966 (95% CI 0.961 to 0.971). Note that, as explained in Chapter 5, we exclude participants who would trade 50% of their life expectancy to avoid taking statins, as we take the view that people with such marked preferences are not relevant to the decision problem of whether or not to offer statins for primary prevention of CVD because they would always decline.
We explore the effect of including DTD at this level on the outputs of our model in four scenarios:
- Permanent DTD (i.e. disutility of the specified level throughout the time simulated patients are taking statins for primary prevention of CVD).
- Diminishing DTD (i.e. a linear decline in disutility from the specified level to zero over a given number of cycles and, again, we start from 10 years and explore further).
In our base-case analyses, we apply DTD multiplicatively (e.g. a multiplier of 0.966) and we test the model’s sensitivity to this assumption in additional scenarios in which we assume an absolute effect (e.g. a decrement of 0.034).
We do not apply DTD to states representing cardiovascular events and post-event life. Although the model assumes that people will take statins in these states and includes a cost for them, our elicitation exercise was explicitly focused on the use of statins for primary prevention in people with no cardiovascular history, and it is not clear that people would make similar trade-offs when it comes to statins for secondary prevention. It would also be difficult to apply any time-dependent DTD for secondary prevention in our model, as it has a ‘memoryless’ Markov structure, with a proportion of people experiencing first cardiovascular events at every cycle.
Model verification
Before making any updates or amendments, we verified that our version of the model exactly reproduced reported results from CG181.10 We also performed technical verification to confirm that the model results changed in the expected direction when extreme values were used for specific model inputs.
Base-case analysis
Clinical Guideline 18110 presented cost–utility results for no preventative treatment and for statins at three levels of intensity (i.e. low, medium and high). All of the statin arms assumed class-level effects and used the acquisition costs of a single representative drug (i.e. simvastatin 10 mg/day, simvastatin 20 mg/day and atorvastatin 20 mg/day for low-, medium- and high-intensity therapy, respectively). An additional arm used identical effects to the high-intensity arm, but adopted the costs of atorvastatin 80 mg/day. Therefore, there were five arms in total.
Our model retains these five arms, and full incremental results are presented in Appendices 7 and 8. However, to simplify interpretation, the main analyses presented below focus on a pairwise comparison between high-intensity therapy with atorvastatin 20 mg/day and no treatment, as this is the approach the guideline ultimately recommended.
Sensitivity analyses
We use three types of sensitivity analyses (i.e. probabilistic sensitivity analysis based on 1000 iterations per output set, deterministic one-way sensitivity analysis and scenario analyses) to look at the impact of different assumptions about DTD.
Results
General model updates
Before accounting for competing risks or DTD, in Table 16 we break down the independent and cumulative impact of each of the steps detailed in General model updates (above). The updates with the largest impact serve to improve the estimated cost-effectiveness of statins. Correcting the miscalculation by which statins were erroneously associated with increased incidence of some cardiovascular effects has the largest effect and, unsurprisingly, this increases the incremental QALYs conferred by statins and reduces their net costs. Updating costs relating to cardiovascular events also has a large effect. Because we now use higher estimates of cost across most categories (substantially so in some cases), the value of statins in preventing events increases. For both men and women, the net effect of the updates is to increase incremental QALYs and decrease incremental costs, although this does not lead to large changes in estimated cost-effectiveness.
TABLE 16
Independent and cumulative impact of general updates to CG181 model: incremental costs and effects of high-intensity statins (atorvastatin 20 mg/day) compared with no treatment
Appendix 7 provides full results for all five simulated strategies at varying levels of cardiovascular risks, as well as a range of deterministic and probabilistic sensitivity analyses.
Accounting for competing risk
Before examining the impact that introducing adjustment for competing risks has on the cost-effectiveness of statins, it is useful to understand how it affects the modelled natural history of people at risk of CVD without accounting for any treatment effect. Appendix 8, Figure 47, provides model state occupancy graphs in two untreated cohorts with and without adjustment for competing risk of non-cardiovascular mortality. From these examples (see Appendix 8, Figure 47), we can see that the impact of adjusting for competing risk depends on a person’s cardiovascular risk compared with an average person of their sex and age:
- A 60-year-old woman with a 10-year QRISK3-predicted risk of 10% has above-average risk. In Figure 19, we can see that people in this category have slightly shorter non-cardiovascular life expectancy than people of the same age and sex in the general population. Consequently, when we include this adjustment in the cost-effectiveness model, the cohort experiences somewhat diminished life expectancy. The unadjusted lifetables tell us that a woman of this age has around a 35% chance of surviving until her 90th birthday. However, once we adjust for the raised risk of non-cardiovascular death that is associated with a 10-year cardiovascular risk of 10%, that figure drops to around 25%. A direct corollary of this is that the average woman with these characteristics will spend somewhat less time at risk of cardiovascular events, and we can see that the areas reflecting fatal and non-fatal cardiovascular history in the state occupancy graph (see Appendix 8, Figure 47) shrink between the unadjusted and adjusted versions.
- Conversely, a 60-year-old man with a 10-year QRISK3-predicted risk of 10% has below-average risk (note that in Figure 19 empirical and modelled data show a slightly longer non-cardiovascular life expectancy than would be expected for an average person of that age and sex). Now we have the opposite result, that is the man spends longer alive in the model than the unadjusted lifetable would suggest. The man’s chance of reaching 90 years of age rises from around 28% to around 38%. In addition, the man spends longer at risk of cardiovascular events and so the relevant areas grow instead of shrinking.
When we layer treatments effects, costs and QALYs on these dynamics, we derive cost-effectiveness results with similar characteristics (Table 17). When estimating the cost-effectiveness of statins, the impact of adjusting for competing risk depends on the cohort’s cardiovascular risk compared with an average person of their sex and age:
TABLE 17
Effect of adjusting for competing risk of non-cardiovascular death on estimated cost-effectiveness of statins: 60-year-olds with a 10-year cardiovascular risk of 10%
- For the 60-year-old women with a 10-year QRISK3-predicted risk of 10%, expected QALYs in both intervention and control arms decrease by around 0.65, reflecting the cohort’s below-average health. This means that a proportion of the cohort will die before they accrue all of the benefits of statins previously predicted for them. Consequently, health gains associated with high-intensity therapy (i.e. atorvastatin 20 mg/day), compared with no treatment, reduce by around 0.04 QALYs compared with the unadjusted model.
- Our 60-year-old men with a 10-year QRISK3-predicted risk of 10% were more healthy than their average contemporaries and so our cohort accrues around 0.6 QALYs per person more in both arms when adjusting for competing risk. This additional life expectancy potentially increases each man’s capacity to benefit from statins. Therefore, we see the incremental benefits of high-intensity therapy (i.e. atorvastatin 20 mg/day), compared with no treatment, rise by a little under 0.04 QALYs per person compared with the unadjusted model.
However, these adjustments affect treatment and non-treatment arms to a fairly similar degree. As a result, the cost-effectiveness of statins is not materially changed. The incremental cost-effectiveness ratio (ICER) for 60-year-old men with a 10-year QRISK3-predicted risk of 10% goes down by around £500 per QALY. For women with the same age and risk, the ICER goes up by a similar amount. In all cases, ICERs remain far below conventional thresholds representing an effective use of NHS resources.
Appendix 8, Figure 54, illustrates the same comparisons when analysed probabilistically. The same features are evident as in the deterministic results. For 60-year-olds with a 10% 10-year cardiovascular risk, adjusting for competing risk of non-cardiovascular death increases QALYs for men and decreases QALYs for women, although resulting cost-effectiveness estimates are not materially altered.
In Appendix 8, Figure 48, we depict the cost-effectiveness of statins for people of different ages and cardiovascular risks, and how adjusting for competing risk of non-cardiovascular death affects these results. Even before adopting this adjustment, the model suggests that statins represent a good use of resources for almost everyone. It is only for people aged ≥ 60 years with the lowest cardiovascular risk that statins represent poor value for money. However, adjusting for competing risk of non-cardiovascular death removes even this small subgroup. In practice, the distinction is moot if QRISK3 is used to predict cardiovascular risk, as it is essentially impossible for people in those age brackets to have 10-year risks low enough to enter the cost-ineffective zone. If such people did exist, then they would have extraordinary life expectancy, which is why the adjusted model concludes that it would still be good value to offer them statins, as there is every chance that even the oldest people would live to realise their benefit.
Appendix 8 provides full results from the updated model, adjusted for competing risk for all five simulated strategies at varying levels of cardiovascular risks, as well as a range of deterministic and probabilistic sensitivity analyses.
Incorporating direct treatment disutility
Introducing DTD to the analysis has a substantial impact on the results. Table 18 shows cost-effectiveness results for 60-year-old men and women under our four DTD scenarios. For the permanent scenario (with a utility multiplier of 0.966), DTD throughout the period people are taking statins for primary prevention is enough to render the statins net harmful. Although the intervention provides around 0.3 QALYs, the small day-to-day disutility of taking the tablets amounts to more than 0.4 discounted QALYs over a lifetime, and so the tablets end up doing more harm than good. Assuming that DTD lasts for no more than 10 years (with either constant or diminishing detriment over that period) attenuates but does not eradicate the benefits of statins in these cohorts, and so the statins remain reasonable value for money, assuming that QALYs are valued at conventional levels.
TABLE 18
Effect of incorporating DTD on the estimated cost-effectiveness of statins
We see the effect of differing DTD assumptions on the cost-effectiveness of statins across a range of ages and baseline cardiovascular event risks in Appendix 8, Figure 50.
Even though a lifetime’s unremitting DTD represents a substantial decrement to expected quality of life, statins remain an effective use of resources for younger people with higher levels of risk. This is not only because people with high levels of risk stand to gain more from taking preventative statins (as they are more likely to have an event to prevent), but also because they spend less long in the primary prevention state (as they move to the event states more swiftly and they are also, now we adjust for competing risk of non-cardiovascular mortality, more likely to die of other causes). In contrast, people with lower levels of risk may need to take statins for a long time before realising benefits, and this becomes a poor trade-off when DTD is permanent. Older people, too, experience net harm from statins if they cannot get used to taking them, and this is because the lower life expectancy of older people gives them limited time in which to experience potential benefits of the intervention, while its harms are unavoidable.
For time-limited (10-year) DTD, the age–risk threshold at which statins become cost-effective is very close to the mean QRISK3-predicted risk observed in the population. Under this scenario, anyone with above-average risk stands to gain from treatment, whereas the model suggests that anyone with below-average risk will experience net harm.
If we can assume that DTD declines over time, then statins remain good value for everyone except people with a theoretically possible – although practically unlikely – profile reflecting extraordinarily good health for their age.
We show probabilistic versions of these calculations for a representative range of age–risk profiles in Appendix 8, Figure 58. In Appendix 8, Figure 58, the ‘clouds’ converge as age and risk rises, showing that DTD has less of an absolute effect on model outputs (owing to shorter time on preventative treatment with lesser life expectancy and higher event rates). However, net value for money remains relatively unaffected. In the cost-effectiveness acceptability curves, statins are always associated with less than 20% chance of cost-effectiveness when we assume permanent DTD (assuming we value QALYs at ≥ £20,000). Conversely, without DTD, statins are certain to be cost-effective as long as we value QALYs at ≥ £6000. The temporary DTD scenarios also result in a high probability of cost-effectiveness, with the exception of 10-year DTD in 80-year-olds with a 30% risk of cardiovascular event. Under this scenario, there is around a 50 : 50 chance that statins are worth paying for, and this is fairly invariant to the value we place on QALYs.
The interaction between life expectancy, level of risk and duration of DTD discussed above begins to suggest another way of conceptualising benefit–harm trade-offs. We have previously argued that the ‘pay-off time’, that is the minimum time until people can expect net benefit from a course of action with up-front harms and long-term benefits, may provide a useful heuristic for thinking about these issues in shared decision-making.41 Appendix 8, Figure 59, shows cumulative incremental QALYs over time for four example profiles across our four DTD scenarios (we do not discount outcomes in this instance, as we take the view that it would be impossible for a patient to arrive at their own conception of the interaction between benefit and time while mentally adjusting for the fact that an interaction of that type is hardwired into the calculation).
Under the permanent DTD scenario, for a 50-year-old with a 10-year CVD risk of 5%, a decision to take statins would confer net QALY gains only after 48 years of treatment. For 60-year-olds at 10% risk, 70-year-olds at 20% risk and 80-year-olds at 30% risk, the expected pay-off times are 36 years, 25 years and 22 years, respectively. When we assume temporary DTD of one form or another, the expected pay-off times are typically in the range of 10–20 years.
Interaction of competing risk and direct treatment disutility
Bringing the analyses above together, we can look at the combined effect of competing risk of non-cardiovascular death and DTD on the effectiveness and cost-effectiveness of statins for the primary prevention of CVD.
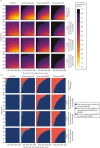
To do this, we present a 4 × 4 cross-categorisation of scenarios, within each of which we derive results for people at different ages and different levels of baseline risk. For competing risk calculations, we present two additional scenarios in which we halve and double the cohort’s hazard of non-cardiovascular death. These scenarios are representative of a decision problem in which people are atypically healthy or atypically well to a degree over and above what we would expect by adjusting for their cardiovascular risk alone.
Figure 20a visualises the expected incremental QALY gains associated with high-intensity statins compared with none in each of these scenarios. Without DTD, statins are associated with very little disutility in the model and, therefore, statins generate some degree of QALY benefit for people at any age and any level of cardiovascular and competing risk (see first column Figure 20a). However, when we introduce DTD, the threshold at which treatment confers net QALY gains begins to depend on age. For someone who is persistently averse to pill-taking (implying lifelong DTD; see last column of Figure 20a), many combinations of age and risk result in statins doing more harm than good (see black area in Figure 20a). For example, people with such preferences in their mid-70s and older would need a 10-year QRISK3-predicted risk of over 30% before statins would be net beneficial. If people had additional long-term conditions leading to a doubled risk of non-cardiovascular death, then that threshold would rise to 40%. Even if we assume taking pills is something people get used to (i.e. time-limited DTD), it is easy to find combinations of age and risk where statins’ DTD outweighs their cardiovascular benefits.
Figure 20b provides a similar analysis but introduces expected costs to the equation to establish the circumstances in which statins would be judged to provide an effective use of NHS resources (when QALYs are valued at NICE-recommended levels of £20,000–30,000). The combinations of characteristics for which statins do not provide good value for money are similar to characteristics for which they generate QALY loss (except under unusual circumstances, a technology will not be cost-effective if it is not associated with QALY gains). However, there are a small number of combinations where, although the model predicts positive QALY gains for statins, the positive QALY gains are so small that the cost of the drugs outweighs the gains (e.g. under the permanent DTD scenario, 50-year-olds with a 10% risk of cardiovascular event and an adjusted but unaltered hazard of non-cardiovascular death, as such people expect a tiny lifetime benefit of 0.006 QALYs, but at a cost that equates to an ICER of around £44,000 per QALY).
As a scenario analysis, we also explored applying DTD as an absolute decrement rather than a relative multiplier, and this results in a slightly greater impact for DTD. For example, for 80-year-olds under time-limited and permanent DTD scenarios, statins do more harm than good at all levels of risk we analyse (see Appendix 8, Figure 60).
Discussion
Main findings
When it comes to state-transition decision models, the question is not whether or not to account for competing risks, but how to do so. Our analysis shows that, where competing risks are handled in a naive manner (e.g. assuming population lifetables for all-cause death), analyses overestimate the clinical effectiveness and cost-effectiveness of preventative interventions in people with above-average risk and underestimate the clinical effectiveness and cost-effectiveness in people with below-average risk. In the case of statins for the primary prevention of CVD, the intervention appears to be almost universally cost-effective in a way that renders these biases of limited impact. However, we have shown that the potential for meaningful differences exists. Indeed, one only has to imagine high-intensity statins cost £18 per pack instead of £1.10, and appropriate adjustment for competing risk of non-cardiovascular death would make the difference between the intervention meeting and not meeting conventional thresholds of cost-effectiveness for some people, for example 75-year-old men with a 10-year cardiovascular risk of 15% (unadjusted ICER compared with no treatment, £25,700/QALY; adjusted ICER, £18,100/QALY).
Incorporating DTD has a more immediately obvious effect on the cost-effectiveness outputs of the model. However, although we see no reason for decision models not to include the most robust estimates of competing risks possible in their base cases, we would hesitate to recommend that modellers should include population-average DTD in their base-case cost–utility results, as this would result in statins being recommended for only people who appear in the dark blue areas in the bottom-right of Figure 20b for whichever DTD scenario decision-makers prefer. In Chapter 5, we argued that, rather than assuming blanket disutility, we should provide decision-makers with evidence of circumstances under which, and people for whom, DTD might tip the balance of benefits and harms (if any). In turn, decision-makers should use this information to provide guidance to prescribers that can inform shared decision-making. This case study illustrates the point well.
To return to our example of a 75-year-old man with a 10-year cardiovascular risk of 15%, without accounting for DTD, we would expect the man to achieve net QALY gains from statins (i.e. an expected value of 0.19 QALYs, which is equivalent to over 2 months in perfect health). However, once we introduce DTD, that expectation reduces. Under the diminishing DTD scenario, the man can expect fewer than 0.06 QALYs, whereas the time-limited and permanent DTD scenarios predict net QALY loss.
Nevertheless, even if we think the permanent DTD scenario is the best way to capture population-level average disutility, we would not argue in favour of adjusting everyone’s expectation to account for it (which would have the effect of rendering statins poor value for money in people with these characteristics). This is because – as clearly shown in Chapter 5 – some people anticipate negligible disutility from taking statins and, indeed, some people may even anticipate benefit over and above the directly clinical effects (i.e. ‘peace of mind’). To deny such people access to an effective treatment would clearly not maximise societal welfare, even if the average person would not feel the same. Therefore, we suggest that the appropriate way to make use of this information is as an objective basis on which decision-makers can identify what NICE refers to as ‘preference-sensitive decision-points’.125 In this paradigm, guidelines should help prescribers to summarise benefits and harms of possible options for people for whom they are indicated, and this should include the process characteristics underlying DTD.
Precisely how such information makes it into shared decision-making is an unanswered question. It is theoretically possible to envisage ex ante preference elicitation, along similar lines as our experiment, which could be integrated into a personalised QALY-maximising analysis to provide recommendations for individual patients, and, unquestionably, this would be practically onerous, but, arguably, it would be unnecessarily prescriptive. However, we think it is useful to augment prescribers’ understanding of the strength of preference for avoiding a technology that would be necessary to outweigh its expected benefits, and our findings give an example of how this might be possible.
Since CG181, there have been two CEAs143,144 of statins for the primary prevention of CVD that predicted cardiovascular events and non-cardiovascular death based on a common set of risk factors. To do this, the analyses used either separate models144 or cause-specific survival models.143 Like our relative survival model, these approaches should accurately predict time spent living with CVD and, therefore, QALYs because they capture the crucial correlation between cardiovascular risk and competing risk of non-cardiovascular death. However, neither analysis tested whether or not its results were sensitive to this competing risk adjustment, that is neither analysis modelled scenarios where risk of non-cardiovascular death was assumed to be independent of cardiovascular risk factors for comparison. In addition, one of these analyses found that cost-effectiveness was sensitive to DTD in the form of an additive decrement ascribed to ‘pill burden’, which was applied to each QALY.144 Another primary prevention model124 has also reported that the cost-effectiveness of statins is sensitive to an arbitrary disutility for DTD.
There is recent, compelling evidence that many self-reported muscle symptoms in people taking statins can be explained as ‘nocebo’ effects.43 Although this finding may represent a fascinating psychological insight, it is of limited practical value to prescribers, as few people experiencing pain when taking a statin will easily accept that their symptoms are ‘all in the mind’. However, we argue that DTD provides a helpful way to conceptualise and quantify the authentic and predictable harm that such people experience. Authors usually cite process and inconvenience factors to explain why people ascribe disutility to taking a preventative medicine they have been assured is benign.40 However, it is also likely that part of the internal calculus reflects (1) a degree of mistrust that the substance truly has no adverse effects and (2) a reluctance to become ‘a patient’ who – among other inconveniences – will henceforth be on-guard for unpleasant symptoms.145 Although few people would describe it in such terms, no one wants to volunteer for a nocebo effect. Therefore, by quantifying the things that people ex ante want to avoid from preventative treatment, we believe that we capture some or all of the subtler harms that people taking them report ex post. As explored in this chapter, this enables us to balance these harms against the expected benefits and costs of treatment to estimate whether or not we expect net benefit overall.
Limitations
First, we have not been able, as originally intended, to take account of the extent to which specific co-existing long-term conditions might complicate the issues explored in this chapter. In particular, we wanted to look at type 1 diabetes and CKD, as NICE identified these conditions as areas of interest in the surveillance proposal in which it announced its intention to update CG181.13 However, we found that including additional binary covariates for one or both of these factors did not improve the fit of our relative survival models. Therefore, this implies that the impact of type 1 diabetes and CKD on cardiovascular risk is well captured by the QRISK3 algorithm, and the factors do not differentially confound the relationship between cardiovascular risk and non-cardiovascular mortality. We also found that type 1 diabetes and CKD had no meaningful effect on the type of first cardiovascular event people experience. Having found no clear point of differentiation, it would not have been meaningful to stratify our analyses. Of course, it would also be possible to tailor other model inputs (e.g. baseline utility, event disutility, cost of events) to represent people with particular long-term conditions, and this might affect outputs in non-trivial ways.
Second, our relative survival models use predicted cardiovascular risk (i.e. QRISK3 10-year predicted risk) as an overarching indicator of non-cardiovascular life expectancy. We believe that we achieved a very good fit to observed data in this way (see Figure 19). Nevertheless, it would be possible to construct a more sophisticated model that, instead of using a summary risk prediction measure, estimates relative survival as a function of each of the individual covariates on which the prediction itself relies. For example, an 80-year-old non-smoking man with type 2 diabetes and SBP of 140 mmHg has an identical 10-year QRISK3-predicted CVD risk to an 80-year-old moderate-smoking man with no diabetes and SBP of 166 mmHg. If the factors that distinguish these two individuals affect their non-cardiovascular life expectancy differentially, then our relative survival model will fail to capture this. By accounting for these factors, it might be possible to achieve an even more accurate prediction of life expectancy. However, as noted above, we found that including terms for type 1 diabetes and/or CKD did not improve the fit of our relative survival models. Nevertheless, we cannot rule out the possibility that other variables would explain some residual heterogeneity between observed and modelled survival expectation.
Third, because secondary prevention of CVD is beyond the scope of our project, we did not review any evidence about the natural and treated history of people who have had a cardiovascular event. For all secondary transitions, the model relies on the same inputs that the developers identified for CG181.10 Although this part of the pathway is outside our decision problem, it has an important impact on our estimates of lifetime costs and effects, as it defines some of the value that an intervention provides by preventing first events. We suggest that any future updates to the model should review evidence in this area, even if secondary prevention is not the analysts’ focus.
Fourth, we do not make any adjustments to the model’s effectiveness inputs to account for imperfect patient adherence to statins, and this means we effectively assume adherence is identical to that observed in the intention-to-treat trials that underpin the effect estimates. If adherence in practice is worse than in trials, then the model is likely to overestimate, to some degree, the clinical effectiveness and cost-effectiveness of statins.
Finally, the model compares all-or-nothing strategies. In reality, the counterfactual to offering statins to a given cohort is not never offering them statins, it is not offering statins for the time being, with the option of revisiting the decision later. To simulate this decision problem, we would require detailed longitudinal data with which to project the development of risk factors over time. It would also probably necessitate moving away from a cohort model to a stochastically evaluated individual patient simulation. However, we would argue that this analysis highlights some of the major advantages of analytically evaluated cohort models. The kind of many-way scenario analyses we have been able to generate would be computationally intractable and muddied by Monte Carlo error in a patient-level simulation, and we feel sure that the kind of analytical flexibility from which our analysis benefits outweighs the flexibility in representing the pathway that would come with a patient-level simulation.
Conclusion
This analysis has shown that updating the CG181 model with newer model inputs did not materially change the conclusion that statins are an effective use of healthcare resources. In the case of statins for the primary prevention of CVD, the intervention appeared to be almost universally cost-effective in a way that renders the impact of including competing risk inconsequential. However, we argue that failure to account for competing risk in CEAs of primary prevention strategies will produce biased results and the impact of this omission is likely to become more apparent for interventions with relatively higher costs compared with their health benefits. Incorporating DTD had a more immediately obvious effect on the cost-effectiveness of statins. We advise that the impact of including DTD in sensitivity analyses on the outputs of a decision-analytic model should be an integral component of CEAs of primary prevention strategies.
- Cost-effectiveness analysis accounting for competing risks and direct treatment ...Cost-effectiveness analysis accounting for competing risks and direct treatment disutility: statins for the primary prevention of cardiovascular disease - The implications of competing risks and direct treatment disutility in cardiovascular disease and osteoporotic fracture: risk prediction and cost effectiveness analysis
Your browsing activity is empty.
Activity recording is turned off.
See more...