Introduction
The need for a randomised controlled trial of community treatment orders
As the Introduction to this study made clear, CTOs were highly controversial when they were introduced and have remained so (see Chapter 5). The key issues of contention in the period before their implementation included:
- ethics – whether or not CTOs ought to be enacted
- legality – whether or not CTOs would comply with constitutional and/or human rights
- empirical issues – whether or not established CTO schemes had the intended beneficial outcomes.
The only methodology which could convincingly address concerns about the effectiveness of CTOs was an RCT. We therefore designed the OCTET Trial to:
- provide rigorous evidence to inform the debate about CTOs
- demonstrate whether or not adding CTOs to community care reduced readmission rates and affected a range of other outcomes
- identify patient characteristics and care patterns associated with positive and negative outcomes
- inform an economic evaluation.
This chapter draws substantially on papers by members of the OCTET Coercion Programme Group: the trial protocol summarised in The Lancet (Burns et al.,93 with permission from Elsevier); its primary and secondary outcome findings as published in The Lancet (Burns et al.,16 with permission from Elsevier) and the tertiary outcome and subgroup findings (Rugkåsa et al.,94 with permission from John Wiley & Sons).
Development of the Trial design: consultation exercise
The aim of the OCTET Trial was to compare CTO use to voluntary outpatient treatment. A number of ethical, legal and practical issues rendered this a complex undertaking.
In view of concerns being raised about the ethical, legal and practical issues of methodologies to test the impact of CTOs, we conducted a consultation exercise with clinicians and legal, policy and ethical groups in the spring and summer of 2008, before finalising the protocol for the OCTET Trial. As is reported below, the exploratory work on the legal constraints involved led to our seeking a detailed legal opinion; we report this separately (see Chapter 11).
This detailed consultation exercise involved numerous opportunistic discussions and a series of more structured meetings to explore the ethical, legal, clinical and practical aspects to the proposed study methodology and to obtain agreement about, and support for, the strongest test of the intervention, in order to produce a detailed study protocol. The consultation exercise involved > 50 people from the full range of stakeholders from the following groups:
- patients and carer organisations and mental health voluntary organisations
- MHA practitioners
- mental health lawyers
- academic legal experts
- the MHRT
- approved social workers
- clinicians in inpatient and outpatient services
- ethicists.
There were some differences between the different groups of stakeholders, in particular between those working with patients in a clinical capacity and those who practised mental health law as solicitors or as representatives of the MHRT. Here we summarise the views expressed by each group, in turn. The summaries represent the breadth of views in each group and the main points, from across the groups, which were of relevance to the trial’s feasibility and design. It should be noted that this consultation was conducted after the legislation had been passed by Parliament in 2007 but prior to the actual introduction of CTOs on 3 November 2008, so none of the consultees had any practical experience of the new regime.
Clinicians and approved social workers
The long and heated debate preceding the introduction of CTOs nationally was reflected in the views raised during our consultations. Some were concerned that CTOs constituted ‘incarceration in the community’ and that CTOs might lead to a lowering of the threshold for compulsion so that a larger proportion of people at relatively low risk might be subject to compulsion. Others commented that, in practice, the new provision did not represent much change and that ‘we are doing it anyway with Section 17 as a long leash: at least a CTO is honest’.
Clinicians noted that attitudes to CTO use had changed since the new legislation had been passed by Parliament. Much of the opposition had dissolved and CTOs were rapidly becoming accepted as part of the provision that was soon to be implemented. This was expressed in concerns or even fear associated with not complying with the new legislation: ‘As a clinician, I will opt for a CTO because of the new law [when it takes effect]. We would be frightened what will happen if we do not.’ They were also concerned about public harm and liabilities in the event that tribunals would discharge patients in the non-CTO group who subsequently committed crimes. As such, CTOs were considered the more restrictive option, with more control remaining with the clinician.
At the time of the consultation, few mental health professionals had received training on the amended MHA and how it would impact on services. There was considerable uncertainty about how CTOs would work in practice and whether or how Section 17 Leave would change. Prolonging Section 17 Leave was seen as being likely to be challenged by tribunals, as the new Code of Practice indicated that this would not constitute ‘good practice’. Psychiatrists were concerned that mental health lawyers might be eager to test the amended MHA, and worries were expressed about how to explain to tribunals why an RC had repeated Section 17 Leave instead of placing the person on a CTO. Several psychiatrists stated ‘I don’t want to be involved in the first judicial review’.
Mental health lawyers
Lawyers representing patients in tribunals emphasised the right of patients to make decisions about their treatment, including taking part in trials; they suggested that legal representatives would be obliged to take a client’s participation in any study into account when representing them at a hearing. They strongly emphasised that patients must be fully informed about the trial and that the different mechanisms for treatment following from randomisation should be made clear to patients prior to enrolment in the study.
The legal representatives we spoke to expressed surprise at the degree of fear of the MHRT among clinicians. Their view was that a tribunal only recommends treatment and that it is a matter for clinicians to ‘stick to their guns’ regarding what they consider an appropriate course of action.
Academic legal experts
Unlike many clinicians, who believed that the clinical effectiveness of CTOs needed to be tested, some of the academic legal experts we consulted saw the overall research question as being of limited interest to the law, since the amended MHA had already been passed by Parliament and CTOs were soon to become part of the MHA.
Some legal experts expressed uncertainty whether or not the new Act presented the option of using Section 17 Leave as the control arm of the trial, given the directions in the Code of Practice; they anticipated that MHRT hearings would insist on the Code being followed. They thus saw the MHRT, despite its inability to go beyond recommending treatment, as potentially exerting influence over the trial.
The legal experts also identified a worry about a shifting threshold for detention, and they described the new Act as ‘vague’ on this point. Some believed that the introduction of CTOs might lead to an increasingly ‘defensive practice’ in which clinicians might leave it to tribunals to discharge patients from CTOs. Moreover, would some such ‘defensive’ clinicians, if a patient were randomly allocated to be managed without CTO, end up using Section 17 Leave for longer periods than they would have done otherwise and thus threatening the naturalistic design of the trial? Some experts were also sceptical as to whether or not clinicians would be willing to put patients forward given that the randomisation would lead to some patients who were deemed appropriate for CTOs not being put on to CTOs.
The legal experts raised two related issues regarding clinical equipoise. First, would there be genuine equipoise when the clinical judgement was that CTO would be appropriate? Second, would clinicians who were willing to put patients on to a CTO be in equipoise? In other words, once a clinician deemed that a CTO was appropriate for a particular patient, would they then deem CTOs to have benefits and by definition not be in equipoise?
Mental Health Review Tribunal
Those representing the MHRT expressed a different view of the Code of Practice compared with some academic lawyers. Although, from the point of view of Government, there would be little point in issuing a new Act with provision for CTOs without a steer as to their use, they argued that case law illustrates that the Code does not constitute a statute; making decisions contrary to the Code might therefore be in adherence with the MHA.95 Moreover, they pointed out that the Code is directed towards clinicians rather than tribunals.
Regarding the appropriate treatment arms for a study, the MHRT stakeholders raised the issue of what would constitute the least restrictive option. Established case law and European law require a tribunal to apply the least restrictive option. As a matter of law, the MHRT stakeholders considered that if a patient were subject to Section 17 Leave, this would mean that Section 3 was still active and the patient was still detained, whereas a CTO would represent a form of discharge from Section 3 (unless the patient was recalled), so that the patient would not be considered to be detained despite the restrictions to their personal freedom. Contrary to the impression of most clinicians, they thus saw CTOs as the least restrictive option, based on Article 5 of the European Convention on Human Rights (albeit not yet tested in a court of law).96 Following from this, they considered that it would be difficult for a tribunal to believe simultaneously both that Section 17 Leave was appropriate and that a CTO was appropriate for a particular patient. They expressed a degree of surprise at what they saw as ‘liberal’ views and uses of Section 17 Leave among clinicians, as, from their point of view, it constituted part of Section 3.
This position on the relative restrictedness of CTO and Section 17 Leave was seen as relevant to the equipoise of any clinician participating in a trial. The MHRT stakeholders explained that when a tribunal looked at a patient’s circumstances (e.g. treatment, potential risks) and decided whether or not, on balance, continued detention or a CTO was justified, only clinical arguments would be considered. If the clinician were in genuine equipoise, it was suggested, they might be unable to provide such an argument. They advised us to seek separate legal advice to address this issue.
From the viewpoint of the MHRT stakeholders, it was mental health lawyers who might provide a challenge to the trial: they might be eager to test the new provision via judicial review should they believe that a tribunal had been influenced in any way by any factors other than Statute. They also noted, however, that a robust clinical argument would in most cases suffice for the use of either option; while the tribunal might recommend a CTO, clinicians did not have to follow their recommendation. They commented that perhaps some clinicians ‘fear the tribunals too much’.
Service user representatives
There was less enthusiasm for taking part in the consultations among service user representatives and mental health organisations than among the other groups of stakeholders and thus fewer people took part or expressed their views. Overall, there was strong support for testing the effectiveness of CTOs, but they expressed concern over the lawfulness of randomisation and whether or not clinicians would find it ethical to randomise. They also reiterated the importance of fully informing patients about what taking part would entail and of their right to withdraw, and recommended using a wide range of outcome measures.
Key issues raised in the consultation exercise
The consultation exercise thus elicited a range of views as to the various legal, clinical and practical aspects of the proposed protocol. In particular, although many clinicians considered CTO to represent a more coercive alternative than Section 17 Leave, many lawyers considered it to be less restrictive. Three issues emerged overall:
- what would constitute appropriate treatment arms of the study
- issues of equipoise
- whether randomisation between different legal statuses would be lawful.
These are summarised here along with the conclusions we reached about our trial design based on the consultation.
Treatment arms It was clear that to randomise between a CTO and voluntary status, as the two US RCTs had been able to do, would be unacceptable to clinicians and ethicists, and might be considered unlawful should it be tried in the courts. The consultation exercise confirmed that patients in the control arm of our trial should instead leave hospital via Section 17 Leave. Many consultees were concerned about the lawfulness of prolonged use of Section 17 Leave or how it would impact on the research. To preclude the threat of prolonged use of Section 17 Leave during the trial, which would serve as a de facto CTO and contaminate the results, we decided that we would recruit only from clinicians who used Section 17 Leave as intended by the Code of Practice that accompanies the amended MHA. This states that if extending Section 17 Leave beyond seven consecutive days, the RC should consider a CTO instead. This gives a clear indication that Section 17 Leave should be treated as a short-term measure, whereas prolonged community compulsion should be managed by a CTO.
As the legal analysis indicated, for the trial to be lawful, patients needed to be randomised to two equally restrictive positions. Therefore, this confirmed that it would not be lawful to randomise a patient either to be put on a CTO or to be discharged to voluntary status. The solution, which our experts deemed to be both ethically and legally valid, was to randomise the patients to leave hospital either on a CTO or via Section 17 Leave. Those in the control arm (henceforth referred to as the ‘non-CTO arm’) would then proceed to voluntary care.
Equipoise The issue of equipoise is central to all RCTs, as no clinician should put forward a patient to a trial if he or she considers treatment in one arm to be inferior to that in the other. Given the fact that there was no robust evidence for CTO effectiveness at the time and that English psychiatrists did not have any clinical experience on which to base an opinion, by definition a state of equipoise existed. We decided to recruit only from psychiatrists who accepted this. This was in order to address the concern that a psychiatrist might be willing to refer one patient but not another (cherry-picking the RCT patients). Given the state of the evidence, if one were in equipoise in one case, one must be in equipoise on all cases. We did accept, however, that there might be exceptional circumstances (such as insistence from family) when an eligible patient was not put forward. Moreover, there were psychiatrists who, perhaps as a result of the heated debate preceding the introduction of CTOs, were convinced that CTOs would be the best option, and there were also psychiatrists who were equally convinced that CTOs were not effective or should not be used for ethical reasons. It was considered unlikely that either of these groups of psychiatrists would participate in the trial. This is a limitation that applies to most RCTs. (For further details about the state of equipoise, see Chapter 11.)
Lawfulness of randomisation The legal experts involved in our consultation exercise identified a need for further work on understanding the legal issues arising from our proposed trial design and this issue was also raised by the Research Ethics Committee. Following their initial review, and the stakeholder consultation, we therefore commissioned a formal legal opinion from John Dawson, Professor of Law, and Marion Rickman, mental health lawyer (and subsequently tribunal judge). This concluded that our protocol was lawful. To arrive at this decision, they examined three features of the law in detail to answer the questions:
- Do the legal criteria for the two chosen treatment arms overlap? Is there therefore a group of patients who may simultaneously meet the legal criteria for both treatments?
- Are both the treatment arms equally restrictive in terms of the patient’s liberty?
- Is there a situation in which RCs can be genuinely uncertain as to which of the two treatments is the more appropriate option for an individual patient?
They concluded, respectively, that:
- there would be a number of patients fulfilling the criteria for detention under both regimes
- it was difficult to ascertain which of the two treatment arms would constitute a more or less restrictive option
- genuine equipoise existed, making it both ethical and lawful for RCs to allow a specific group of patients to be randomised between a CTO and Section 17 Leave.
Thus, within particular constraints, their legal opinion was that an RCT of CTOs would be feasible. (The legal analysis is reported in detail in Chapter 11.)
The legal analysis also concluded that the period soon after the change in the MHA would provide a window of opportunity to study the outcomes for patients of the varying clinical practices that were likely to emerge from the position of equipoise in which clinicians would find themselves at that point, because of genuine uncertainty about both treatment efficacy and the proper application of the law. The legal analysis also advised on the correct procedure for randomisation and advised that clinical decision-making should not be influenced by the patient’s participation in the trial or randomised allocation (see Overview of the OCTET Trial).
The exploration of legal, ethical and feasibility issues during the consultation period shaped the final research protocol. In addition to clarifying the randomisation process, it also helped shape the design of eligibility criteria, outcome measurement and strategies for recruitment and data collection, all of which we describe next.
Overview of the OCTET Trial
Design
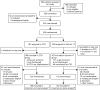
The OCTET Trial was a single-outcome, parallel-arm, non-blinded randomised trial. Its primary objective was ‘to conduct the most rigorous trial possible of CTOs with prolonged, high-quality care incorporating a broad range of outcomes, and identify patient and service predictors of response’. Our aim was to compare CTO use to voluntary outpatient treatment. This aim was modified on the basis of the legal analysis, as explained above. Our trial therefore randomised the patients to leave hospital either on a CTO or via Section 17 Leave. There was an understanding that for patients in the non-CTO arm, Section 17 Leave was to be used according to the MHA Code of Practice48 and be restricted to a short period of days, or at most weeks, before discharge to voluntary care took place. The trial thus randomly assigned patients to receive one of two forms of mandatory outpatient care at the point of discharge from inpatient care: the two main options for clinicians regarding patients who needed ongoing supervision in the community from November 2008 onwards. Patients were assessed at baseline, and at 6 and 12 months, and the primary outcome measure was readmission to hospital, as detailed below and represented in the OCTET flow chart (Figure 3).
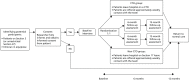
FIGURE 3
The OCTET Trial procedure.
Taking into account the findings from the North Carolina RCT,64 which suggested a correlation between outcomes and the combination of duration of CTO and intensity of services, we designed the OCTET Trial so that patients in both arms would receive approximately weekly contact with services. This was in line with common practice for this group of patients in English services.
Eligibility
Patients were deemed eligible for the study when the RC and AMHP agreed that they needed ongoing supervision in the community, but (after having considered the relevant legal standards, including the guidelines in the Code of Practice) when clinicians recognised the uncertainty as to which option was more appropriate (CTO or non-CTO). Potential referring clinicians who held strong views in favour of certain patients being likely to be more or less well on a CTO were actively discouraged from referring any patients to the trial, so that the referring clinicians would be those, reflecting the evidence base, with a neutral view (accepting the state of equipoise), thus avoiding cherry-picking. Clinicians who used Section 17 Leave as a de facto CTO were excluded from recruiting to the trial.
During the preparations for the amendment of the MHA, discussions around CTOs focused almost exclusively on the situation of so-called revolving-door patients, that is, patients experiencing frequent relapse and readmission to hospital. Both expert views and the evidence from other jurisdictions suggested that CTOs were likely to be mostly used for patients with psychosis in adult mental health services. The amendment did, however, pave the way for the use of CTOs for a much wider group of patients, including those with learning disabilities and in forensic detention. Given the potentially different clinical uses of CTOs for different patient groups, we decided to design the trial to focus on a relatively homogeneous group of patients. We therefore decided to exclude those on restricted forensic sections and those without a primary diagnosis of psychosis, as well as those in adolescent or older adult services. Including the other groups would require a significantly larger sample size, which could prove unachievable given restrictions of time and resources. The group included matched those studied in the majority of CTO studies internationally, to facilitate comparisons.
Clinical decision-making
Once we had recruited a patient and conducted the baseline interview, legal advice indicated that the correct procedure was for the independent statistician to advise the RC on the random allocation, who would then allocate the patient to either the CTO group or the non-CTO group. In practice, RCs referring to the trial undertook to allocate the patient according to the statistician’s advice; this is no different from the usual randomisation procedure in any RCT. This procedure is referred to simply as ‘randomisation’ below.
After patients had been recruited and randomised, it was stipulated that when RCs made decisions about them, they should strictly adhere to the statutory process and criteria in every case, as the law required, and should fully consider every option open to them within the legal regime and the relevant factors listed in the statute or the Code of Practice. We thus did not exercise control over clinical decisions subsequent to randomisation. Patients in both groups retained all their usual legal rights, including their right of access to the MHRT, which might discharge them from compulsory treatment. No attempt was made to influence how clinicians subsequently managed these patients, including whether the clinicians decided to renew a patient’s treatment on Section 17 Leave or renew their CTO. When a patient was discharged from involuntary treatment by the RC or the MHRT, or transferred from Section 17 Leave to a CTO, for instance, as required by law, this would simply be counted as one outcome of the process for that patient.
Patients in the CTO arm of the study might therefore, during the follow-up period, be recalled to hospital, have their CTO revoked or be discharged from it. Patients in the non-CTO arm might be discharged to voluntary status after the initial period of leave, or might have it renewed, or they might, in exceptional cases, be put on a CTO. (Details of which of these scenarios we handled as protocol violations are given below, under Methods, while actual protocol violations appear under Results.) Given the Code of Practice, there was a reasonable expectation that the majority of patients randomised to the non-CTO arm of the trial might proceed fairly swiftly to voluntary status. We collected data on changes to legal status systematically as part of the trial.
Recruitment of trusts, teams and patients
Procedures and NHS permissions
Permission to carry out the trial needed to be obtained for each trust, and each research assistant needed to obtain personal permission to access professionals, patients and medical records in each trust. We sought permission to conduct the trial locally from R&D offices in around 60 NHS trusts that provided mental health services in England. All trusts within a reasonable travelling distance were approached.
Having secured R&D approval, we carefully mapped that trust’s services before embarking on extensive information sharing and a series of meetings with the mental health teams to secure their collaboration.
We then identified potentially eligible patients from a network of CMHTs and AOTs agreeing to participate in the trial. In NHS trusts with a split between inpatient and outpatient services, patients were recruited via the inpatient service. This happened, however, in agreement with the outpatient service to which the patient would be transferred when leaving hospital. All community teams had to be able to provide approximately weekly contact with the patient.
Following identification of an eligible patient, a member of the care team then approached the patient and secured agreement that one of the study researchers could make contact. This researcher then assessed and recorded the patient’s capacity to participate in research and sought written informed consent for inclusion in the study. Once the consent form had been signed and the baseline assessment completed, the patient was randomised.
For the follow-up interviews, the researcher contacted the mental health team to confirm that the patient was still in their care or, if not, where they were then based; and whether they believed that it was appropriate to contact the patient.
Obtaining access in NHS trusts
Obtaining permission in the NHS trusts we approached proved much more time-consuming than expected. The time required to deal with our applications varied considerably. Although one trust was able to process the necessary paperwork in a matter of days, for others it took nearly a year; in some cases, it took longer and we were eventually obliged to give up. The extent of documentation required varied and, although this was usually easily dealt with, it caused some delays, in particular in the cases when a trust required specific contracts drawn up between themselves and the host trust. One trust refused collaboration because the service leads did not give their support to the study. Two others refused as a result of their own internal peer reviews of the protocol: one review concluded that there was no immediate benefit to that trust; the other deemed the protocol unethical. It took us between 2 days and, in one case, 11 months, to gain R&D permissions in the 44 trusts. Of these 44 trusts, 32 trusts – predominantly in the Midlands and southern England – recruited to the trial (see Results, below).
Each of our research assistants (14 in all, over the 28 months of recruitment and data collection) was required to obtain individual permissions in each of the collaborating trusts in which they were working. We found that a wide range of procedures for obtaining such letters of access was required. Despite NHS guidelines, a number of trusts required new Criminal Records Bureau (CRB) checks to be conducted, which caused several months delay in each case. In total, 607 letters of access, 56 honorary contracts, 17 research passports and seven clinical licences were processed and issued to our researchers: on average, 21 contracts per trust. In addition to this, 51 CRB checks were also performed (on top of those issued on behalf of the employing trust).
Getting mental health teams and their patients on board
We initially focused on recruiting from AOTs, so began by mapping the location and contact details of these teams in the participating trusts. It proved more difficult than we had expected to find out the number and location of AOTs, as it was hard to find people within each trust with an overview of how services were organised and divided both geographically and functionally. This process resulted in a largely complete map of AOTs across our chosen trust areas, of which there were approximately 200.
We had initially envisaged that the vast majority of the patients placed on CTOs in England would come from AOTs, as both AOTs and CTOs were designed to be employed for revolving-door patients. We quickly realised, however, that a considerable proportion of patients from regular adult CMHTs were also being placed on CTOs, and we therefore made the decision to recruit from these CMHTs as well as AOTs. This was a huge undertaking. Not only were there on average three times the number of CMHTs as AOTs in each trust, but also the functional split between inpatient and outpatient care meant that all of the inpatient services into which CMHTs fed had to be contacted separately. This meant that we had a total of approximately 1000 potential teams, including CMHTs and AOTs, as well as approximately 500 inpatient units.
We contacted by e-mail and telephone the team leaders, the consultant psychiatrist for each team and the medical directors for each trust. It was at this point that it became apparent to us that services were increasingly being split into those providing care for inpatients and those providing care for outpatients. This situation differed from the pre-existing arrangement in which a consultant psychiatrist was attached to a particular geographical area, within which he or she had responsibility for the care of patients regardless of whether they were currently in hospital or in the community. This increased the number of consultant psychiatrists we were to contact considerably.
Once contact had been established with the mental health teams and information about the OCTET Trial had been sent to them, we used a combination of large meetings, forums, academic meetings and individual team meetings to inform teams and doctors about the study and agree a plan for the recruitment of their patients to the study by their own referral. We also met with ward managers and inpatient consultants to secure their agreement for us to monitor the inpatient wards for eligible patients. We then e-mailed or telephoned the clinicians responsible for potentially eligible candidates to seek their permission to meet the patient and proceed with the consent process. Our recruitment therefore proceeded along two routes: both through the direct referral of patients by the consultant and team and through the identification of eligible patients from wards.
In getting teams on board, holding large meetings in which many teams and doctors were present was most successful. In these meetings, the issues surrounding the OCTET Trial were thoroughly discussed. In each case, we then held a series of smaller meetings with individual interested teams and consultants, to discuss practicalities and specifically to ascertain whether or not there were any suitable patients known to the team. This ensured that research assistants mainly travelled to individual teams that had already expressed interest in the study and that were aware of the broad outline of the study. Even so, many teams were unable to find eligible candidates once they heard the inclusion criteria in more detail, even when they were happy to do so in principle. In these cases, the inpatient ward monitoring process was designed to ensure that should any eligible candidates exist in the future, they would be identified.
Ultimately, recruitment was most successful with teams and doctors who had met a member of the team in person and had the opportunity to discuss any issues and concerns in detail. Owing to the number of teams involved, this initial setting-up phase of the study was both resource heavy and time-consuming. Once the teams and doctors were familiar with the study team, however, they were often happy to receive further e-mails prompting them to refer or asking permission to see a patient identified on the ward. This system made recruitment reliable and efficient, despite requiring a considerable input of effort initially. Because of the complexity of the study, the recruitment of patients by a less personal process, for example solely by e-mail, often resulted either in a refusal to recruit, or in confusions and misunderstandings about the study process, and ultimately wasted time. As teams were located in such disparate locations as Cornwall, Lincolnshire, Greater Manchester and Birmingham, one of the biggest challenges in the OCTET Trial was to use this personal and hands-on approach with a team of six researchers, all of whom were based in Oxford.
Potential obstacles to referral were many and varied. First, some team members or consultants were not in equipoise, so were opposed in principle to allocating their patients randomly to one of two different legal statuses. Both the multidisciplinary nature of mental health teams and the functional inpatient–outpatient split increased the number of professionals involved in, and responsible for, the care of individual patients, which ultimately increased the likelihood of at least one of those professionals objecting to the patient’s involvement in the trial. This proved to be the biggest problem: when the parties in disagreement were the inpatient and outpatient clinicians for one patient, one of whom would implement the CTO, or not, depending on the outcome of randomisation, and the other of whom would manage the patient in the community. If these parties could not agree on the acceptability of randomisation then recruitment could not go ahead. Once a patient had been identified as eligible and permission had been gained from both the inpatient and outpatient consultants, and the relevant team members where necessary, we would be able to approach the patient.
This final stage of recruitment also posed several problems. First, access to wards in trusts that were remote from our own proved at times unreasonably difficult, given that we had official researcher access to all relevant sites. Second, a lack of communication between doctors and ward staff, or within the ward, resulted in a few cases in patients being sent on leave or discharged before we could see them. This was despite the rapidity with which the research team responded to patient referrals, often seeing patients no more than a few hours after they had been referred. A third issue was premature referral, which resulted in some patients being too unwell at the time we saw them to give informed consent. This did not often prevent the patient being recruited, as the researchers would return to the ward when they were well enough, but it did waste time and resources. Last, patients themselves sometimes refused to take part or were judged by the research team to lack the capacity to consent. We stayed in contact with the recruiting teams regularly via e-mails or phone calls, and we produced a monthly newsletter in which we provided information about the progress of the trial and relevant information about CTOs. We stayed in touch with patients via postcards at Christmas and made contact again some weeks before each 6-monthly interview.
In these extensive recruitment activities, we got considerable support from the Mental Health Research Network, the clinical support officers of which often provided considerable practical assistance in local NHS trusts, such as making contact with hospital wards and local clinicians, and in some cases helping with obtaining R&D access and accessing medical records.
In total, the study team assessed 442 patients, of whom 336 were randomised, as we detail in Results.
Methods
Sample
Recruitment took place from 10 November 2008 to 22 February 2011.
Eligible patients were those:
- aged 18–65 years (in line with local administrative procedures for adult mental health services)
- diagnosed with psychosis
- currently admitted under Section 3 or Section 37 (without restrictions) of the MHA
- not currently subject to any other legal restrictions
- judged by their clinicians (RC and AMHP) to need ongoing community treatment under supervision
- able to consent to take part in research and give written and informed consent
- not participating in the study already (i.e. patients with multiple admissions throughout the recruitment period should participate in the study only once).
We did not exclude patients with insufficient English language to complete the interview. As the research instruments were not validated in different languages, these patients could not be interviewed, but we collected data from their medical records and invited them to take part in the Qualitative Study in their own language.
Patients were not eligible if they were:
- unable to give informed consent (e.g. advanced dementia or mental disorder too severe)
- subject to incompatible legal restrictions on treatment
- considered to be clear candidates for immediate discharge to voluntary treatment
- already on Section 17 Leave for > 30 days.
We invited mental health professionals to complete questionnaires about their therapeutic relationships with the patient and the patient’s quality of life if they were:
- currently the care co-ordinators of the recruited patient and employed by the NHS at baseline and/or 12 months
- providing care for the recruited patient (for at least the past 3 months).
We originally intended to recruit family carers of the sampled patients to undertake a questionnaire survey at the 6-month follow-up point. We later decided to omit this, however (see Changes to protocol from original proposal).
Objectives and hypotheses
The primary objective of the OCTET Trial was to test the hypothesis that patients with psychosis and a history of compulsory admissions in the CTO arm of the trial would experience a reduction in readmissions to hospital compared with those not placed on a CTO. The secondary and tertiary objectives were to investigate whether or not such patients would experience a greater delay to readmission, shorter admissions or improvements in a range of clinical and social outcomes.
The exploratory objectives of the trial were to identify the baseline characteristics of patients associated with differential treatment effects; to examine the factors (other than treatment group) associated with readmission; and to explore the effect on readmission of process variables such as rate of contact.
Cost-effectiveness was also evaluated and this is reported separately (see Chapter 7).
Sample size and power calculation
The North Carolina trial64 demonstrated a difference of 16% in the proportions readmitted to a psychiatric hospital within 12 months (32% in the group under outpatient commitment vs. 48% in the control arm). We calculated that to detect a similar difference with a significance level of 5% and power of 80%, assuming that rates of readmission would be comparable in our control group, would require a sample size of 288 patients. We predicted that attrition would be negligible, as primary outcome data would be available from medical records. With this number of patients, we calculated that we would also be able to detect either of the following differences as statistically significant at the 5% level with 80% power:
- a 14-day difference in the mean number of days spent in hospital over 12 months (e.g. a reduction of 28 days to 14 days)
- a difference of 0.43 in the mean number of readmissions over 12 months.
Data collection
We collected data from medical notes and structured patient interviews with independent, trained researchers at baseline and at 6 and 12 months. Detailed assessments included demographics, clinical history, prior MHA use and criminal justice history. We assessed current status using well-validated and widely used structured questionnaires, as detailed in Instruments. The researcher read out the questions and recorded the patient’s replies. The full assessment battery took between 45 and 90 minutes to complete. We pursued notes from other teams or hospitals or from MHA offices when applicable. In all cases, we collected all primary outcome data from Trust information systems. We collected data on diagnosis and current medication from case notes. We also confirmed data on sociodemographics and psychiatric history, collected in the Socio-demographic Schedule (see Instruments), from case notes.
We collected 6-month data for use in the analyses along with the 12-month data, but did not intend to report them independently. We used the three data points in order to have sufficient power for the modelling of the within-patient variability in the multilevel analysis. Collecting 6-month data also ensured that the research team was able to keep in contact with the patients and as a safety net in case there proved to be significant difficulties in follow-up at 12 months.
Outcomes
We chose outcome measures for the trial based on the evidence in the literature about the most appropriate hospitalisation outcomes to capture the outcome and process of CTOs, as well as relevant clinical and social functioning outcomes (see Chapter 5, Background). We chose the rate of readmission as our primary outcome because CTOs had been legislated explicitly for this outcome. It was the primary outcome in most major studies,3,4,61 including the only two published randomised trials.63,64 It is also accepted as the best measure for relapse prevention85 and therefore a proxy for community stability.
The primary outcome measure was therefore psychiatric readmission in the 12-month follow-up period, as a binary outcome. We defined a readmission as the period between the patient’s admission date and the date on which the patient left hospital, which should include at least one overnight stay. Readmissions could be either voluntary or involuntary. We did not classify recall to hospital of a patient on a CTO as a readmission, as it was understood as being part of the CTO process rather than an outcome. If a recall ended in the CTO being revoked, we counted this as a readmission, calculated from the first day of the recall. The follow-up point was defined as 365 days after randomisation (referred to below as ‘follow-up’).
Secondary outcomes related to readmission followed the same readmission definition and constraints. We mainly obtained these from medical records. They represented patterns of readmission, as follows:
- number of nights in psychiatric hospital from randomisation (index) to follow-up
- days in community until first readmission
- number of readmissions from first discharge to follow-up, per patient
- days under legal compulsion (measured by time being subject to the MHA 2007 under Sections 2, 3, 4, 37, 48, 49, 135, 136 or on a CTO) from randomisation (index) to follow-up
- number of patients with multiple readmissions.
Tertiary outcomes were mainly self-reported patient outcomes. These were:
- clinical and social outcomes
- medication
- experiences of and satisfaction with services
- experiences of leverage and perceived coercion
- employment.
Safety outcomes were covered by self-reported items in the clinical research forms. Additional safety outcomes included death and cause of death.
Instruments
We utilised the following instruments with patients to measure the outcomes detailed above. Unless otherwise specified, they were patient rated at baseline and at 6- and 12-month follow-up, and relate to the 6-month period prior to interview.
Demographics and psychiatric history
- The OCTET Socio-demographic Schedule This collected data on three areas:
- ‘Self and Home’ (basic information including age, sex, ethnicity and educational achievement, employment, family, including marital status; living situation).
- ‘Clinical History’ (diagnosis, psychiatric history, current psychiatric medication), which was corroborated from medical records.
- ‘Legal History’ (criminal convictions, imprisonments).
Clinical and social outcomes
- Symptom severity The Brief Psychiatric Rating Scale (BPRS),97 a researcher-rated instrument, was used to assess symptom severity on a scale from ‘1’ (not present) to ‘7’ (extremely severe) over the last 2 weeks. The total score (range 24–168) is a sum of ratings across 24 symptom domains.
- Insight The Insight and Treatment Attitudes Questionnaire (ITAQ)98 is an 11-item questionnaire assessing patient awareness of illness and need for treatment on a three-point Likert scale (no = 0, possibly = 1, yes = 2). Total scores range from ‘0’ (no insight) to ‘22’ (full insight). Its two subscales are the Awareness of Illness Scale and the Attitude to Treatment Scales (range 0–10 and 0–12, respectively).
- Substance misuse The CAGE99,100 is a screening questionnaire for alcohol and drug misuse. Four items are rated for each area, covering the last 30 days, with yes/no responses. Two or more positive responses indicate a drink/drug problem. Its four questions focus on ‘Cutting down’, ‘Annoyance by criticism’, ‘Guilty feeling’ and ‘Eye-openers’, providing the acronym for the scale.
- Social functioning The Global Assessment of Functioning (GAF),101 a researcher-rated, single-item scale, was used to assess impairment in functioning over the previous 2 weeks. Scores range from ‘1’ (severe, life-threatening impairment) to ‘100’ (superior functioning). The rating is based on the information collected during the course of the interview.
- Overall social outcomes The Objective Social Outcomes Index (SIX)102 summarises objective indicators of social outcomes (employment, housing, living status and social contacts) in one overall score (range 0–6), with higher scores indicating better social outcome.
- Health-related quality of life The EuroQol-5 Dimensions (EQ-5D),103 a non-disease-specific five-item scale, is commonly applied in health economics research and was used to ascertain the patient’s self-description and valuation of their health status. Data from this measure are reported under OCTET Economic Evaluation (see Chapter 7).
Medication
- Type of medication We collected data on prescribed psychotropic medication from medical records.
- Attitudes and adherence to medication The Drug Attitude Inventory, 10-item version (DAI-10)104,105 assesses experiences of, and attitudes towards, medication. Patients rate 10 statements as true or false (variously scored as ‘–1’ or ‘1’). The total score ranges from ‘–10’ (negative subjective response/adherence) to ‘10’ (positive subjective response/adherence).
Experiences of services
- Therapeutic relationships The Scale To Assess Therapeutic Relationship–Patient Version (STAR-P)106 assesses community patients’ relationships with their care co-ordinators. Patients rate, on a five-point Likert scale (0–4), the frequency with which communication, consultation and trust is present in interactions across 12 items, which constitute three subscales: Positive Collaboration (range 0–24), Positive Clinician Input (range 0–12) and Non-Supportive Clinician Input (range 0–12). The total STAR-P score ranges from ‘0’ to ‘48’. Higher scores indicate better relationships on all scales (with Non-Supportive Clinician Input being reverse scored).
- Satisfaction with services The Client Satisfaction Questionnaire (CSQ-8)107 assesses satisfaction with health services on eight items using a four-point Likert scale (1–4). It yields a total score ranging from ‘8’ (low satisfaction) to ‘32’ (high satisfaction).
- Preference for joint decision-making and information-seeking The Autonomy Preference Index (API),108 adjusted to the mental health setting,109 is a 23-item questionnaire on preferred autonomy (14 items were utilised for the purposes of our analysis). Its two subscales are the Decision Making Preference Scale, which measures patients’ desire for their own rather than their psychiatrists’ involvement in clinical decision-making, and the Information Seeking Preference Scale, which measures patients’ desire to be informed about their illness and treatment. Both use five-point Likert scales (strongly agree = 1, strongly disagree = 5). Adjusted scores for each subscale range from ‘0’ to ‘100’, where ‘0’ is a lack of desire to be involved/informed, ‘100’ the strongest possible desire and ‘50’ indicates a neutral stance.
Experience of leverage and informal coercion
- Experience of coercion The MacArthur Leverage Interview7 ascertains patients’ experience of leverage. Leverage was defined as making support to obtain housing, money and child custody conditional on treatment adherence or reducing or dropping criminal charges if patients adhered. It is a semistructured interview designed to ascertain experience of leverage during the 6 months prior to interview and across the patient’s lifetime. Here we report experience over only the previous 6 months. We adapted it for use in the English setting for the ULTIMA Study, including adding a section asking about child access. Questions test for both access to, and potential withdrawal of, benefits. Any positive response within a specific coercion area counts as ‘reported’ (Box 2).
- Perceived coercion The MacArthur Admission Experience Survey (AES),111 adapted for outpatient use,112 contains 14 statements rated on a five-point Likert scale (1 = strongly agree, 5 = strongly disagree). There are three subscales:113 the Perceived Coercion Scale assesses perception of influence and control in treatment decisions (range 5–25, with a high score indicating a high level of perceived coercion); the Negative Pressures Scale assesses experienced threats and force (range 6–30, with a high score indicating higher negative pressure after reverse scoring); and the Procedural Justice Scale assesses experience of having a say in one’s care (range 3–15, with a high score indicating feeling less involved in one’s care). No total score for the AES was utilised.
- Fairness and effectiveness of pressure The Index of Fairness assesses patient agreement with statements on the fairness of any treatment pressure they have experienced in the last 6 months, and the Index of Effectiveness assesses their views of how effective this pressure has been in making them stay in treatment and gain control.114,115 It is rated on a five-point Likert scale (1 = strongly agree, 5 = strongly disagree) with a total score for each scale (range 4–20), with higher scores indicating that the pressure is viewed as more fair/effective.
BOX 2
Questions used to identify experienced leverage
Service usage
- A modified version of the Client Service Receipt Inventory (CSRI),116 which measures the number and duration of contacts the patient has had with a range of health and social care professionals during the 6 months prior to interview and the location of these meetings (24 items). (Some data from this measure are reported below but it was most extensively used for the OCTET Economic Evaluation reported in Chapter 7.)
(The full Schedule of Procedures and the instruments listed here are available from the authors on request.)
Outcome data from medical records
We collected outcome data on readmissions and MHA use from patients’ medical records. This included questions on legal status (whether under section or not), CTO recalls and conditions (both over 12 months), tribunals and managers’ hearings. We also pursued notes from other hospitals and from the criminal justice system where applicable.
Blinding
It would, of course, be impossible to blind clinicians or patients in a trial for which knowledge of the intervention is part of the intervention itself. Moreover, it would be unlawful not to inform each party of legal status. It was not possible to blind researchers to randomisation. Research assistants were blind to randomised status during the baseline assessment.
Randomisation and masking
Randomisation was a stratified block design with a 1 : 1 allocation ratio. Eligible patients had an equal probability of assignment to each arm of the trial. Patients were randomised individually to either the CTO or the non-CTO arm by an independent statistician using stratified block randomisation for gender (male/female), schizophrenic status (yes/no) and duration of illness (< 2 years, ≥ 2 years). We developed the randomisation code using a computer random number generator to select random permuted blocks. The block lengths were two, four and six, varied randomly.
A researcher independent to the trial team enclosed assignments in sequentially numbered, opaque, sealed envelopes and stored them. The details of the sequence remained unknown to all members of the trial team until recruitment, data collection and analyses had been completed. The sealed envelope was labelled with the stratum number, gender, schizophrenic status, duration of illness and an envelope number. A matching label inside, also numbered, specified the intervention arm. Randomisation took place after consent had been obtained and the baseline interview had been performed. The envelope was opened on the day of the interview by the independent researcher after recording the patient’s trial identification number on the envelope. She then communicated the randomised allocation to the recruiting researcher by telephone. As randomisation involved allocation to different legal statuses, it was both impossible and unlawful to mask research assistants, treating clinicians or patients.
Protocol violations
Protocol violations were predefined as arising when patients were:
- withdrawn
- ineligible
- discharged to the wrong treatment arm
- never discharged from hospital.
Patients who were protocol violators of any of these predefined types were included in our intention-to-treat analysis. We counted those patients who never left hospital during the follow-up period as having had one readmission of 365 days.
Statistical methods
All analyses were performed using Stata/SE version 12 (StataCorp LP, College Station, TX, USA).
The trial team wrote and signed off a detailed statistical analytical plan before any data were analysed (see Appendix 2). All analyses were done according to the analysis plan. Blinding of the statistical analysis, methods for dealing with outliers, missing data, computation and methods are detailed there.
Intention-to-treat population
Analysis was carried out on an intention-to-treat basis (n = 336), except for the tertiary analyses for which the sample was 333. The intention-to-treat population included all randomised patients; we thus analysed data from dropouts or protocol violators according to their randomised group.
Baseline characteristics, interview refusers and loss to follow-up
We assessed the baseline comparability of the two randomised groups by tabulating patient characteristics and treatment experiences; we did not perform any statistical tests on baseline data. We compared the baseline characteristics of any patients who refused to participate in follow-up interviews (or who had inadequate English language for this) to those of patients completing the follow-up interviews.
Primary analysis
The primary analysis was a test of the difference in the proportion of patients readmitted to a psychiatric hospital during the 12-month follow-up period between the CTO arm and the non-CTO arm. We analysed the primary outcome using a log-binomial regression model adjusted for the stratification factors (sex, schizophrenic status and duration of illness). Results are presented as the relative risk of readmission for the CTO group compared with the non-CTO group, with appropriate 95% confidence intervals (CIs) and two-sided p-values.
Secondary analyses
We conducted secondary analyses using the intention-to-treat population. There were no missing data for secondary outcomes. We analysed secondary outcomes in the same way as primary outcomes, using multiple regression models with adjustment for stratification factors. The type of regression model depended on the data distribution. All model assumptions were assessed.
Number of readmissions and number of nights in psychiatric hospitalisation are count outcomes, and we analysed these using adjusted zero-inflated Poisson and negative-binomial regression models, respectively. Results are presented as adjusted incidence–density ratios (IDRs) with 95% CIs, and interpreted in the same way as relative risks.
The number of nights from first discharge to first readmission and the time spent under compulsion are time-to-event outcomes. We therefore performed these analyses using adjusted proportional hazards regression, and present the results as hazard ratios (HRs) with 95% CIs. Kaplan–Meier plots are also presented and the median time to readmission is calculated with 95% CIs. We used the log-rank test to compare the median time under compulsion between the two arms, whereas we used the Wilcoxon rank-sum test for comparison of time to first readmission, as this variable violated the log-rank test assumptions.
Tertiary analyses
We conducted tertiary analyses using the population of 333 patients (omitting the three exclusions). Thus we included all available data from all of the patients, and imputed missing values intrinsically within the model rather than requiring multiple imputations.
Sensitivity analyses
We conducted a repeated measures sensitivity analysis for end points measured at multiple time points using multivariable mixed-effects regression models. We used the Stata command xtmixed for continuous end points, xtlogit for binary end points and xtmelogit for multiple category end points. We included all available data from all patients, with missing values intrinsically imputed within the model rather than requiring multiple imputations. We entered treatment, stratification factors and time point (time since randomisation) into the model as fixed effects, and the model contained a patient-specific random intercept. We treated an interaction between time point and treatment group as a fixed effect to allow estimation of treatment effect at each time point. We also used likelihood ratio tests to assess whether or not time should be included in the model as a random effect. We also explored different covariance structures.
As a sensitivity analysis, we imputed missing data in self-reported outcomes using multiple imputations117 in the mi routine in Stata. Subsequently, we used regression modelling to estimate the association between change from baseline to 12 months and study arm, adjusting for baseline end point and randomisation factors. We chose the regression models according to the distribution of the end points (logistic models for CAGE, leverage and employment; linear models for CSQ-8, STAR-P, BPRS, GAF, ITAQ, DAI-10, API, SIX, AES subscales, Index of Fairness and Index of Effectiveness).
We performed safety analyses according to the statistical analysis plan (see Appendix 2).
Subgroup analysis
Subgroup analyses are designed to explore whether or not any treatment effect tested in an RCT varies across subgroups defined by baseline patient characteristics.118
We defined binary subgroups a priori, as identified in the literature to be related to outcomes. We performed subgroup analysis for the primary outcome, all of the secondary outcomes apart from time under compulsion and for the main clinical outcomes, BPRS and GAF. Potential errors (i.e. increased type 1 error and decreased type 2 error) introduced by a subgroup analysis were controlled by selecting the subgroups and stabilising the hypothesis to test (i.e. equal direction of effect as for the overall sample) prior to accessing the data and thus performing the analysis. With 13 subgroups there is a 12% chance of at least one subgroup resulting significant at the 5% level. All conclusions were written paying due consideration to this fact.
We evaluated 13 groups in this analysis, the first three of which were used as stratification factors during randomisation:
- diagnosis: schizophrenia spectrum versus other psychoses
- duration of illness: < 2 years versus ≥ 2 years
- gender: male versus female
- age: ≤ 40 years versus > 40 years
- ethnicity: white, black, Asian, mixed race and ‘other’
- immigration history: UK versus other
- marital status: married/co-habiting versus single/separated/divorced
- living status: living alone (including homelessness) versus living with others
- accommodation status: independent versus supported/homeless
- years of education: ≤ 12 years versus > 12 years (as 12 years of education is compulsory in England)
- tertiary education: ‘yes’ versus ‘no’
- BPRS: ≤ 33 versus > 33
- GAF: ≤ 49 versus > 49.
We performed the subgroup analysis by fitting the same models as for primary and secondary outcome measures plus an additional interaction effect for interactions between study arm and the relevant subgroup variable. The p-value of interest is that for the interaction test.
Data management
The researchers recorded data in the clinical research forms by hand and two different researchers double-entered them into IBM SPSS version 20 (IBM Corporation, Armonk, NY, USA). Double-entered data were compared against each other, and discrepancies were discussed and corrected by the research assistants under supervision. The statistician responsible for the analysis conducted additional data quality evaluations. These included range checks and logical and consistency checks that might not be picked up by checks at the individual patient level by the research staff who collected and entered the data. In the case of variables that were a function of other variables (e.g. length of a particular readmission), these were checked by automatic calculation of the variable’s values, except for total scoring of the individual instruments, which was performed automatically using a validated code. We froze the final cleaned data before analysis commenced.
Changes to protocol from original proposal
Family carers questionnaire
According to the original protocol, we were to ask the patients to identify family carers and ask their consent to contact the carer. These carers were then to be sent a carer questionnaire by post, enclosing a letter with full information about the study. We initially undertook this part of the trial but it had a poor response rate. In July 2010, of the 135 patients who had reached the 6-month follow-up point, 38 had agreed to a carer being contacted and only two of those carers had returned a questionnaire. It was anticipated that many of the patients who were eligible for the trial had limited contact with their family, so this result was not surprising. With a response rate of 3%, we decided not to pursue this part of the study, as the results would not be representative or robust. Some of the items covered in the questionnaire, such as carer strain and perceptions of patient well-being, were included in the topic guide for the qualitative interviews with family carers.
Clinical staff questionnaire
According to the original protocol, care co-ordinators were to be asked to complete two validated instruments covering demographics, the therapeutic relationship with the patient, and their assessment of the patients’ health-related quality of life, at both baseline and 12 months. The questionnaires were handed to the relevant staff, or were posted to them, or both. A poor response rate at baseline, however, was compounded by multiple changes of staff, which made identifying the appropriate staff group at follow-up almost impossible. The information sought was designed to be analysed in conjunction with patients’ views of their own health, quality of life and therapeutic relationships, and was thus time sensitive and dependent on a response from a particular individual. Given the difficulties in obtaining this, we therefore decided not to pursue this part of the study.
Statistical analysis
We conducted an additional sensitivity analysis for the primary outcome. This analysis excluded all protocol violators. It was not a prespecified analysis but was conducted to address concerns raised about the number of protocol violators, particularly those who had been discharged to the wrong arm of the trial.
The analyses undertaken were all in line with the spirit of the analyses detailed in the original proposal, but we made some changes to the original proposal when writing the statistical analysis plan: these were changes to the types of statistical models or tests performed to allow for more sophisticated adjusted regression models to be used as the primary comparisons, with the simpler unadjusted tests used for secondary sensitivity analyses. Adjusted regression models have more statistical power (i.e. are more precise) than unadjusted tests such as t-tests, and thus make better use of the data collected.
The original protocol makes reference to minimisation factors. No minimisation procedure was carried out during treatment assignment. Instead, a stratified block design was used. Tertiary outcomes were specified. In particular, we decided to treat satisfaction with service as a tertiary outcome instead of a secondary one. We did not analyse the effect of the discipline of the clinical supervisor in readmissions, as originally planned, as information on this outcome was not available.
We added type of medication as a tertiary outcome, as we considered this to be clinically relevant, given a reported association between CTOs and the use of depot injections.4 We included this before any of the data analyses were conducted. Patient-rated adherence to medication could not be adequately measured at baseline (patients were recruited while detained in hospital for treatment) and data collected on this during follow-up were of insufficient quality to be included as an outcome. We therefore used the DAI-10, which correlates with clinician-rated adherence,104 as the reported measure for adherence. Missing data made it impossible to analyse different types of leverage separately or divide leverage experience into types of pressure. We originally planned to analyse tertiary outcomes by type of service (e.g. ACT, early intervention, crisis teams), but ongoing service reconfigurations rendered this analysis redundant.
Results
Recruitment
We recruited patients from 32 trusts to the trial, predominantly in the Midlands and southern England (Figures 4 and 5).
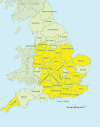
FIGURE 4
Recruiting counties.
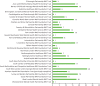
FIGURE 5
Recruitment rates in participating NHS mental health trusts in England.
The study team assessed 442 patients in total, of whom we recruited 336. The 106 patients who were not recruited after being approached either refused (n = 91), were found not to be eligible (n = 6) or lacked capacity (n = 9) (Figure 6). The Ethics Committee did not grant us permission to collect any data on those who refused to enter the study or were considered for referral to the study by their clinicians but then not referred.
Participant flow
Participant flow is presented in our CONSORT (Consolidated Standards of Reporting Trials) diagram (see Figure 6). The 336 recruited patients were randomly assigned to either the CTO arm (167 patients) or the non-CTO (169 patients) arm of the trial. One patient withdrew and two patients were identified as ineligible directly after randomisation.
Primary and secondary outcome data
We based all of our analyses (apart from the tertiary analyses) on all 336 patients, as we conducted them on an intention-to-treat basis. We had data on the primary and secondary measures for all 333 patients at baseline and 12 months. We included the three patients who either withdrew or were ineligible, with their data missing for all variables, apart from data on the inclusion criteria and the randomisation factors.
Interview data
Of the 336 patients in the trial, 14 were not interviewed at baseline (10 because they wanted to take part but did not want to be interviewed and four because of inadequate English). The remaining 322 patients completed baseline interviews. We compared the baseline characteristics of the 14 patients not interviewed to those of the interviewed patients; there were no obvious differences that might have skewed the subsequent analyses.
At 12 months, we interviewed 241 patients [72%: 125 (75%) of the CTO group and 116 (69%) of the non-CTO group], of whom 189 (56%) completed all three interviews [98 (59%) of the CTO group and 91 (54%) of the non-CTO group]. Those not interviewed at 12 months either refused or did not attend [61 (18%) overall: 27 (16%) of the CTO and 34 (20%) of the non-CTO group], were non-contactable (20 (6%) overall: 8 (5%) of the CTO and 12 (7%) of the non-CTO group), had inadequate English language [4 (1%) overall: 2 (1%) of each group], were deceased [5 (1%) overall: 3 (2%) of the CTO and 2 (1%) of the non-CTO group] or were not interviewed because the clinical team advised against it (one patient in the CTO group) or for other reasons (one patient in the non-CTO group).
Sample: baseline data
Baseline sociodemographic data and data on psychiatric and legal history are shown in Table 2. Of the 336 patients included in the final data analyses, 225 (67%) were males. The vast majority (n = 316, 98%) were not employed. The majority of the patients (n = 206, 61%) were white, with 78 (23%) being black. The majority (n = 259, 77%) had been born in the UK. They had received 12 years of education on average. The majority (n = 241, 72%) were living in independent accommodation and were living alone or homeless (n = 239, 75%). Only a minority (n = 112, 37%) had an identified carer. They had been ill for 12 years on average, with only 14 patients (4%) having been ill for < 2 years. They had experienced an average of four involuntary psychiatric hospital admissions in the past and reported that they had spent a total of 15 months in psychiatric hospital in their lifetime. A substantial minority (n = 133, 40%) had criminal convictions and just over one-quarter (n = 86, 26%) had previously been in prison (see Table 2).
TABLE 2
Patient characteristics at baseline: sociodemographics, psychiatric history and legal history
Table 3 shows baseline values of clinical characteristics, experiences of services variables and experiences of leverage and perceived coercion. The majority (n = 286, 85%) had a primary clinical diagnosis of schizophrenia. The sample was only mildly to moderately symptomatic (BPRS, median: 38), as would be expected for patients being discharged to the community following successful inpatient treatment. They were a significantly impaired group, however, in terms of their functioning (GAF, mean: 39). Five patients (2%) screened as positive for alcohol or drugs. The sample reported a fairly low level of overall social outcome (SIX, mean: 2.5). They had fairly high levels of insight (ITAQ, median: 13.5), including more positive attitudes to treatment but less awareness of illness (medians: 9 and 6, respectively). The majority (n = 265, 79%) were on oral medication, a small minority were prescribed clozapine (Clozaril, Novartis, Basel, Switzerland) (n = 43, 13%) and a substantial proportion (n = 188, 56%) were on depot medication. They reported somewhat negative attitudes and adherence to medication (DAI-10, median: –2), which was mainly first generation antipsychotic medication.
TABLE 3
Patient characteristics at baseline: clinical characteristics and experiences of services, leverage and perceived coercion
Patients reported their therapeutic relationships to be fairly positive (STAR-P, mean: 30.3), with similar levels across the three subscales. They reported fairly neutral attitudes to being involved in clinical decision-making (API, mean: 56.6) but a greater desire to be informed about their illness and treatment (API, mean: 76.5). They were fairly satisfied with services (CSQ-8, mean: 21.3).
Seventy (23%) of the patients reported having had experience of leverage in the previous 6 months. They rated their care as fairly coercive (Perceived Coercion Scale, mean: 14.8) and reported that they had experienced a fairly high number of negative pressures (Negative Pressures Scale, mean: 14.5). They reported not having much of a say in their own care (Procedural Justice Scale, mean: 8.4). They regarded the treatment pressure they had experienced as only moderately fair (Index of Fairness, mean: 12.8) and effective (Index of Effectiveness, mean: 12.3) (see Table 3).
Baseline characteristics did not differ between those who were interviewed at 12 months and those who were not (Table 4).
TABLE 4
Patient characteristics at baseline for those interviewed or otherwise at 12 months
Protocol violations
It was impossible to control for protocol violations, as clinical decision-making needed to be unconstrained by the study design for the trial to be lawful (see Introduction/Overview of the OCTET Trial). The treatment of 35 patients in the CTO arm and 40 in the non-CTO arm (22.5% of the whole sample) did not follow the randomised status: these 35 patients from the CTO arm of the trial were never put on a CTO, whereas the 40 patients from the non-CTO arm were put on a CTO directly after leaving hospital. Seven further patients from the CTO arm of the trial and six from the non-CTO arm were never discharged from hospital, making a total of 42 protocol violations in the CTO group and 46 in the non-CTO group: 88 in total. Combined with the three patients who were withdrawn or ineligible, this made a total of 91 protocol violations (Table 5).
TABLE 5
Protocol violations by treatment group
Primary outcome
There was no difference in the primary outcome of psychiatric readmission in the 12-month follow-up period between the two groups, with 59 and 60 patients readmitted in the CTO and non-CTO groups, respectively (see Table 6).
TABLE 6
Outcomes at 12-month follow-up
Secondary and major clinical outcomes
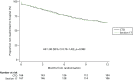
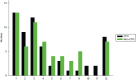
There were no differences in any of the secondary hospitalisation outcomes between the two groups or in the major clinical outcomes of symptoms and functioning. At 12 months, neither the total duration of all psychiatric hospitalisations, nor the number of readmissions per patient, the number of patients experiencing multiple readmissions and the number of days spent in the community until the first readmission differed between the two groups (Table 6). (Time to first readmission is also presented in Figure 7.) The pattern of duration of individual readmissions was similar for each group (Figure 8). Neither severity of symptoms nor social functioning differed between the groups at 12 months (see Table 6).
We conducted a per-protocol sensitivity analysis for the primary and secondary outcomes, removing the 91 protocol violations (i.e. excluding those patients who were discharged to the wrong arm of the trial or were not discharged, and the three ineligible or withdrawn patients, for whom we had no data on the primary outcome). This analysis was based on 245 cases with no protocol violations. It did not alter the findings (Table 7).
TABLE 7
Patient characteristics at baseline for patients with no protocol violations, N = 245
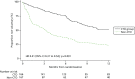
Table 8 shows details of the treatment process (days in the community under the randomised legal compulsion, days under any legal compulsion during the follow-up period, and the number of contacts with the service per month). The number of days spent in the community after hospital discharge under the randomised compulsory outpatient supervision (i.e. randomised compulsion) was substantially longer in the CTO group (median: 183 days compared with 8 days for the non-CTO group) and this was highly statistically significant. The time to first voluntary status was also substantially longer for the CTO group (Figure 9). Overall, the total number of days under any compulsion during the 12-month follow-up period was significantly greater in the CTO group (median: 255 days) than in the non-CTO group (102 days), which was again highly statistically significant (p < 0.0001). This total number of days included compulsion under the randomised regime (either CTO or non-CTO), any time on CTOs for crossover patients in the non-CTO group and any time as a compulsory inpatient or on a subsequent CTO. The self-reported number of contacts with the service per month during the time outside hospital did not differ between the two groups (with a median of two contacts reported in each group) (see Table 8).
TABLE 8
Treatment process
Table 9 shows the number of nights spent in psychiatric hospital during the 12-month follow-up period for the whole sample and for those patients who were readmitted. For the whole sample, the number of nights in hospital from randomisation (index) to 12 months did not differ significantly between the two groups [incident rate for CTO group 0.9 times the incident rate for the non-CTO group (95% CI 0.65 to 1.26; p = 0.550; not tabulated]. When patients who were never discharged were excluded from this analysis, the difference remained non-significant. The time from randomisation to discharge from hospital was similar between groups [median 8 days in the CTO group vs. 16 days in the non-CTO group, HR 1.08 (95% CI 0.86 to 1.34); not tabulated; Figure 10]. For those 119 patients who were readmitted only, the number of nights in hospital from randomisation to 12 months did not differ between the CTO and non-CTO groups and nor did the number of nights from randomisation to first discharge from hospital or the number of nights from the first readmission to the end of the follow-up period (whether or not excluding those who were never discharged) (see Table 9).
TABLE 9
Number of nights of psychiatric hospitalisation
Tertiary outcomes
Table 10 presents the clinical and social factors for the whole sample at baseline and 12 months, along with the estimated average change over time. Table 11 presents the same clinical and social factors at 12 months for the CTO and non-CTO groups, along with the test statistics for the difference in change over time between the two groups.
TABLE 10
Clinical and social factors at 12 months: total sample compared to baseline, N = 333
TABLE 11
Clinical and social factors at 12 months: comparison of change over time between the CTO and non-CTO groups
There were some statistically significant changes over the 12 months in the total sample of 333 (see Table 10). Patients reported higher effectiveness of treatment pressure (Index of Effectiveness, average change 2.10 points, 95% CI 1.32 to 2.89 points; p < 0.001) and more positive views of the fairness of the pressure applied (Index of Fairness, average change 1.31 points, 95% CI 0.54 to 2.08 points; p = 0.001). They also reported more positive relationships with clinicians at 12 months compared with baseline (STAR-P, average change 2.97 points, 95% CI 0.97 to 4.97 points; p = 0.004). This included more positive collaboration (average change: 1.81 points, 95% CI 0.71 to 2.93 points; p = 0.001) and less non-supportive clinician input (average change: 0.66 points, 95% CI 0.06 to 1.25 points; p = 0.031). Although the sample expressed a desire for gaining control in decision-making and to be given information about their illness, the scores were lower at 12 months than at baseline (Decision Making Preference, average change –4.83 points, 95% CI –8.24 to –1.41 points; p = 0.006 – Information Seeking Preference, average change –3.94 points, 95% CI –6.98 to –0.90; p = 0.011). There was an increase over time in patients scoring positively for potential problem drinking [odds ratio (OR) 5.01, 95% CI 1.31 to 19.19; p = 0.019]. Attitudes to medication were more positive across the sample (DAI-10, average change 1.74 points, 95% CI 0.57 to 2.91 points; p = 0.004) and more patients were in employment at 12 months (2.74 points, 95% CI 0.99 to 4.48 points; p = 0.002). Significantly fewer patients were prescribed clozapine (OR 0.13, 95% CI 0.03 to 0.63; p = 0.011) and depot medication (OR 0.09, 95% CI 0.03 to 0.24; p < 0.001) at 12 months compared with baseline.
There were no significant differences between the two arms in the change from baseline to 12 months in any of the reported outcomes except for the Index of Effectiveness (see Table 11). Those in the CTO group showed a smaller increase over time than those in the non-CTO group in their agreement that the treatment pressure they had experienced had been helpful. The difference was small (–1.22 points, 95% CI –2.32 to –0.14; p = 0.028).
The sensitivity analysis using multiple imputation did not alter the outcome results.
Subgroup analyses
The observed values for the primary outcome (psychiatric hospital readmission) and main secondary outcomes (nights in hospital and days to first readmission) in the subgroup analysis are shown in Table 12, and for the main clinical outcomes (BPRS and GAF) in Table 13. Adjusted values for the statistically significant interactions (adjusted for the randomisation factors) are presented in the text.
TABLE 12
Subgroup outcomes at 12 months: hospitalisation outcomes, N = 333
TABLE 13
Subgroup outcomes at 12 months: GAF and BPRS, N = 333
There were no statistically significant interactions between any of the subgroups and the primary outcome measure of readmission or for time to first readmission, duration of readmissions or social functioning.
Severity of symptoms (BPRS) demonstrated crossover interactions with age in the two arms (interaction coefficient –4.49; p = 0.043). In the CTO group, those aged < 40 years had more severe symptoms than those aged > 40 years [39.54 (95% CI 37.55 to 41.52) vs. 37.96 (95% CI 35.58 to 40.34)]. Among the non-CTO group, those aged < 40 years had less severe symptoms than the older group [38.64 (95% CI 36.53 to 40.75) vs. 41.56 (95% CI 39.35 to 43.78)].
There were also crossover interactions between symptoms and subgroups with and without some tertiary education (interaction coefficient = –5.62; p = 0.024). In the CTO group, those without tertiary education had more severe symptoms than those with tertiary education [40.05 (95% CI 38.35 to 41.76) vs. 34.92 (95% CI 31.78 to 38.07)]. Among the non-CTO group, those without tertiary education had less severe symptoms than those with tertiary education [39.93 (95% CI 38.13 to 41.73) vs. 40.42 (95% CI 37.62 to 43.22)].
The proportion of patients on depot medication at baseline was not statistically different between the two groups [85/166 (51%) in the CTO arm vs. 103/167 (62%) in the non-CTO arm; p = 0.054). The statistically significant reduction in the number of patients prescribed depot medication over time was unexpected. Our exploratory analyses showed that the reduction was significant in both groups (OR 0.04, 95% CI 0.01 to 0.11; p < 0.001) and that it was significantly larger in the CTO group than among the non-CTO group (OR 6.08, 95% CI 1.48 to 24.97; p = 0.012). There was no statistically significant difference in the primary outcome measure of readmission between those on depot and those not. There was also no interaction effect of the CTO and non-CTO groups with being prescribed depot (interaction coefficient 0.85; p = 0.586).
Deaths
Five patients died during follow-up: two deaths by suicide and one by accidental death from a drug overdose were recorded in the CTO group, and one death by suicide and one death from natural causes were recorded in the non-CTO group. The DMC did not regard these deaths as necessitating the ending of the trial.
Conclusions
- CTO use did not reduce the rate of readmission to hospital.
- The length of the initial compulsory outpatient treatment was hugely greater for the CTO group than the non-CTO group (median 183 days vs. 8 days).
- CTO use had no impact on:
- time to readmission
- number and duration of hospital admissions
- any measured clinical and social outcomes.
- There were no differences between CTO and non-CTO outcomes for any of the prespecified subgroups.
Publication Details
Copyright
Included under terms of UK Non-commercial Government License.
Publisher
NIHR Journals Library, Southampton (UK)
NLM Citation
Burns T, Rugkåsa J, Yeeles K, et al. Coercion in mental health: a trial of the effectiveness of community treatment orders and an investigation of informal coercion in community mental health care. Southampton (UK): NIHR Journals Library; 2016 Dec. (Programme Grants for Applied Research, No. 4.21.) Chapter 6, OCTET Trial.