This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs License which permits noncommercial use and distribution provided the original author(s) and source are credited. (See https://creativecommons.org/licenses/by-nc-nd/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Structured Abstract
Background:
Socioeconomically disadvantaged patients, who bear a disproportionate burden from chronic obstructive pulmonary disease (COPD), often lack resources to optimize management of their disease, including limited access to pulmonary specialists. We conducted a randomized controlled trial (RCT) to assess the efficacy of a health coaching model to improve COPD-related quality of life (QOL) and self-management for patients with COPD.
Objectives:
The specific aims of the study were to compare patients receiving health coaching with patients receiving usual care with respect to COPD-related QOL, including degree of dyspnea, number of COPD exacerbations, exercise capacity, and patient self-efficacy of managing COPD.
Methods:
We conducted an RCT of 9 months of health coaching vs usual care for low-income English- or Spanish-speaking patients at least 40 years of age with moderate to severe COPD from 7 primary care practices serving low-income, urban adults. Patients randomized to the intervention arm were assigned a health coach who supported them in working toward personal health goals and in self-management skills such as correct inhaler use. Coaches accompanied patients to their primary care and specialty visits and met with them between visits. Coaches facilitated review of patient care plans by a pulmonary nurse practitioner. Patients in the usual care arm received any resources their provider and their clinic offered as part of standard care, including access to COPD educators, respiratory therapists, COPD education classes, pulmonary rehabilitation, or smoking cessation classes. The primary outcomes were COPD-related QOL and the dyspnea subscale of the Chronic Respiratory Disease Questionnaire (CRQ). Secondary outcomes were self-efficacy for COPD self-management, exercise capacity (6-Minute Walk Test [6MWT]), and number of COPD exacerbations. Additional outcomes included the Patient Assessment of Chronic Illness Care (PACIC) mean item score (range 1-5); COPD symptoms measured by the COPD Assessment Test; forced expiratory volume at 1 second percentage predicted, measured by spirometry; smoking status by patient self-report; number of bed days due to COPD in past 4 weeks; adequate inhaler use (observed using checklist of steps); and COPD knowledge (4 questions). We assessed outpatient visits related to COPD, emergency department (ED) visits, and hospitalizations, both COPD related and not COPD related, by review of medical records. Additional outcomes not prespecified were concordance with international Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines for COPD management and symptoms of depression measured with the Patient Health Questionnaire (PHQ). We used generalized linear mixed modeling to adjust for differences in baseline values and to account for clustering by clinic.
Results:
We enrolled 192 of 282 patients determined to be eligible (68%), of whom 158 (82%) completed 9-month follow-up. Patients enrolled were representative of the target population with similar characteristics by study arm. Mean age was 61 years; 66% were male; 32% had less than a high school education; 44% reported an income of <$10 000/year; 15% were homeless or marginally housed; and 51% had a diagnostic code for tobacco use, 17% for alcohol abuse, and 29% for other substance use. Most (92%) reported a high level of COPD symptoms (GOLD classification B or D). There was no significant difference at 9 months between health coached and usual care arms for the primary outcome of improvement in QOL, either by total CRQ score (4.58 vs 4.43; adjusted difference = 0.14; 95% CI, −0.15 to 0.43) or CRQ dyspnea domain score (4.98 vs 4.78; adjusted difference = 0.26; 95% CI, −0.13 to 0.65). There were also no significant differences in the secondary outcomes of number of exacerbations, exercise capacity, or self-efficacy. Among other prespecified outcomes, we saw significant differences in favor of the health coached arm for quality of care and adequate inhaler use. At 9 months, patients in the health coached arm reported higher quality of care on the PACIC (adjusted difference in mean item score = 0.38; 95% CI, 0.07-0.68; P = .02) and were more likely to demonstrate adequate inhaler use (adjusted difference = 39.7%; 95% CI, 19.6-59.8; P < .001). None of the differences for the remaining prespecified outcomes were significantly different. Patients in the health coached arm were more likely to receive guideline-concordant treatment (adjusted difference = 14.6%; 95% CI, 3.3-25.9; P = .01) and were less likely to report symptoms of moderate to severe depression (adjusted difference = −18.9%; 95% CI, −33.1 to −4.8; P = .01) (both post hoc outcomes).
Conclusions:
Using unlicensed health coaches to work with patients, primary care providers, and pulmonary specialists did not improve of QOL or exercise capacity or reduce the number of COPD exacerbations, the primary and secondary outcomes of the study. We did find evidence for improvement in quality of care, both as reported by patients and as seen in adherence to guidelines.
Limitations:
Limitations of the study were that patients, rather than primary care providers or clinics, were randomized and the intervention was not blinded, which may have caused a halo effect whereby patients in the usual care arm may have benefited from the presence of health coaching, as clinicians were aware of coaching activities. These results should help inform expectations regarding the limitations and benefits of health coaching for patients with COPD. Results may be useful to health policy experts in assessing the potential value of reimbursement and incentives for health coaching-type activities for patients with chronic disease. Future studies could explore targeted versions of a model focusing on the positive outcomes noted in the current study.
Background
Chronic obstructive pulmonary disease (COPD) affects more than 14 million US adults and is the third leading cause of death in the United States.1 COPD costs the US economy $38.8 billion annually in direct health care costs and lost work productivity. COPD impacts quality of life (QOL) for patients in many ways, including anxiety associated with difficulty breathing, worry about disease progression, and social isolation.2-5 While COPD is chronic and incurable, evidence-based care for COPD can substantially reduce disease burden, improve QOL, and prevent emergency visits and hospitalizations for exacerbations of disease.6
Evidence-based care for patients with COPD recommended by the Global Initiative for Chronic Obstructive Lung Disease (GOLD),6 including immunizations, smoking cessation, inhaled medications, and pulmonary rehabilitation, have been shown to affect both symptoms and overall health status of patients and reduce exacerbation rates.7,8 Despite the widespread availability of evidence-based guidelines for care of patients with COPD, such care is underutilized in the outpatient management of COPD by primary care clinicians.9-13 In studies, fewer than half of primary care physicians reported knowing that COPD guidelines exist14 and only 25% of physicians reported using guidelines for COPD care.15 It has been estimated that adults with obstructive lung disease, including COPD and asthma, in the United States receive only 55% of recommended care.16 These care gaps are even more pronounced for vulnerable low-income and minority patients14,17 and likely contribute to disparities in disease severity. In the urban underserved and among those with lower educational attainment and household income, COPD is more severe, and it is associated with worse lung function, greater exercise limitation, more emergency visits and hospitalizations, a lower health-related QOL, and a higher risk of dying from COPD.18,19
Barriers to providing evidence-based care for patients with COPD seen in primary care include primary care clinicians' lack of knowledge of guidelines, lack of experience with interpreting spirometric data or assessing response to pharmacotherapy, competing clinical demands from managing comorbid conditions,20-22 complexity of COPD care, and lack of structural and team support for care.14,23,24 For patients, management of COPD is often complex, requiring frequent medical appointments, multiple medications, and behavioral changes.25 These challenges are generally recognized as even greater for vulnerable populations, which often face competing demands with fewer personal resources.26
Multiple models to improve the delivery of guideline-based care have been proposed. A Cochrane review in 2014 found that integrated disease management programs, defined as programs that include 2 or more health disciplines using 2 or more treatment modalities, were effective in improving multiple outcomes of patients with COPD.27 Clinic-based case management, usually by a highly trained nurse, has been shown to improve outcomes.28-30 However, integrated disease management and dedicated professional case managers are generally not available in resource-limited federally qualified health centers (FQHCs) and similar organizations. While patient education is necessary, by itself it does not appear to be sufficient; patient engagement and activation are thought to also be important.31 It has been suggested that providing individualized, patient-centered support integrated into primary care is an effective model for improving COPD care.32 Such a patient self-management support model would be feasible in an FQHC setting and could potentially be delivered primarily by unlicensed health workers such as community health workers or medical assistants.
The health coaching model developed at our institution trains unlicensed health workers to support patient self-management using commonly recognized patient-centered techniques such as motivational interviewing and action planning. Health coaching recognizes that people living with chronic disease are the primary decision makers in their care; it is a tailored approach that builds on the strengths and expertise of patients and helps ensure that they have the knowledge and skills to be active participants within the medical encounter. Health coaches help patients identify their goals and create action plans in conjunction with their primary care provider (PCP). Health coaching is thus distinct from patient navigation (which focuses on helping patients access medical resources), patient education (which provides information but is not focused on patient-centered goals and motivation), or case management (which generally involves a medical professional tracking adherence to guideline-based best practices and patient health status). Health coaches in our model may, however, engage in some activities also provided by care managers, patient navigators, and educators. For example, coaches may track care targets and conduct gap analyses to identify suboptimal areas. Coaches also help patients get the support they need by facilitating access to community, clinic, and specialist support, and improving communication between patients and providers.33,34 Our research group and others around the country have shown that health coaching can improve management for patients with poorly controlled diabetes35-38 and hypertension.39,40
The health coaching model is based in part on the Chronic Care Model (CCM) (Figure 1) proposed by Wagner et al,41 which is used as a basis for American Thoracic Society (ATS) recommended care.42,43
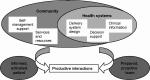
Figure 1
Chronic Care Model.
By coordinating with the patient, the PCP, and a pulmonary specialist (Figure 2), the health coach provides several functions key to the CCM. Coaches work within the health systems sphere to provide patient decision support and to improve care delivery. Coaches also provide patient-centered management support and help connect patients to clinical and community services and resources. Health coaching also addresses multiple barriers to the primary care of patients with COPD, as shown in Table 1.
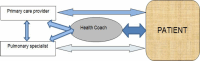
Figure 2
Role of Health Coaches.
Table 1
Barriers to Evidence-Based COPD Care Addressed by Health Coaching.
While health coaching as part of primary care is a promising model for patients with COPD, more evidence is needed to determine its effectiveness. We designed the current research study, the Aides in Respiration (AIR) health coaching study, to address this gap by evaluating the effectiveness of a health coach-based model for vulnerable and low-income patients with COPD.
The goal of our study was to evaluate the effectiveness of a health coach model for improving outcomes for low-income urban patients with COPD. We conducted a randomized trial, AIR, comparing 9 months of health coaching plus usual care (health coached arm) with usual care alone (usual care arm) for patients with moderate to severe COPD cared for at 7 public health clinics in San Francisco. The 7 clinics have characteristics of FQHCs and are designated FQHC look-alikes by the Health Services Resources and Services Administration (https://bphc.hrsa.gov/programopportunities/lookalike/index.html).109 For purposes of this report, we refer to them as FQHCs.
The specific aims of the study were the following:
- Specific aim 1 (primary outcome). To compare disease-specific QOL for patients randomized to receive 9 months of health coaching plus usual care with patients randomized to usual care alone. Our hypothesis was that mean COPD-related QOL total and the dyspnea domain of the overall QOL at 9 months would be greater in patients in the health coached arm than in the usual care arm.
- Specific aim 2 (secondary outcome). To compare the number of exacerbations of COPD experienced by patients in the health coached arm with those in the usual care arm during the 9-month period starting at enrollment. We defined COPD exacerbation as a COPD-related ED visit or hospitalization, or the outpatient prescription of oral steroids and/or antibiotics for COPD-related diagnosis. Our hypothesis was that patients in the health coached arm would experience fewer COPD exacerbations compared with the usual care patients.
- Specific aim 3 (secondary outcome). To compare exercise capacity at 9 months for patients in the health coached arm with those in the usual care arm. Our hypothesis was that patients in the health coached arm would have greater exercise capacity than patients in usual care.
- Specific aim 4 (secondary outcome). To compare self-efficacy for management of COPD for health coached vs usual care patients at 9 months. Our hypothesis was that mean self-efficacy would be greater in patients in the health coached arm than in the usual care patients.
Participation of Patients and Other Stakeholders
Choice of Stakeholders, Including Patient Partners
At the point of conception of the study, we identified stakeholder groups to include in the study process. The primary group was vulnerable patients with COPD cared for at the study sites, which consisted of FQHCs in the San Francisco Health Network. Because we designed the health coaching intervention to be delivered as part of patient care, we identified clinic leadership, staff, and clinicians as additional stakeholders. A major purpose of the health coaching intervention was to improve evidence-based patient care by facilitating communications between the consulting pulmonary specialist and the PCP. We thus included pulmonary specialists (ie, pulmonologists, pulmonary nurse specialists, and respiratory therapists) as stakeholder groups. Implementation and dissemination of the coaching intervention required support from the San Francisco Department of Public Health or similar networks. We therefore included system leadership as a stakeholder group. We included experts in patient education both as content experts and to help with dissemination activities.
Identification and Recruitment of Stakeholders
We recruited leaders at each of the community clinic study sites as community partners both before application for study funding (to establish support and feasibility) and after funding was received to operationalize the implementation of the study. We based recruitment at the clinic level on past experience conducting studies at the community clinic sites and networking existing contacts throughout the San Francisco Health Network. We made initial contacts with the clinic medical director, the clinic manager, or the nursing supervisor. We based choice of initial contact on preexisting relationships and availability. We included some members of the community clinics on the study advisory board, as described below.
We recruited stakeholders as members of the study advisory board, as indicated in Table 2. Members of the study advisory board were expected to help ensure that the health coaching intervention met the needs of the patients enrolled in the study, that study protocols were optimized for recruitment and retention, and that measures of outcome were appropriate. Members also advised on the dissemination of the study findings. Additional description of the activities of the study advisory board is provided in the following section.
Table 2
Stakeholder Groups for the Study Advisory Board.
Description of Engagement With Stakeholders
Community Clinic Partners
In designing the study, we worked with community-based clinics, initially contacting and meeting with clinic leadership (ie, medical director and staff and nursing managers) to identify benefits of the study to the clinic, address concerns, and establish protocols to minimize disruption to clinic function. During these meetings we identified and discussed ways in which the proposed study could benefit patient care at the clinic. For example, we provided results from spirometry and exercise capacity to clinicians for all patients enrolled in the study by entering the information into the patients' electronic medical record (EMR) and notifying the clinician. Patients randomized to the health coached arm also received education including instructions regarding inhaler use and other support, and their clinicians received expert recommendations about medication management from a pulmonary nurse practitioner specialist (PNPS). We extended the latter benefit to include patients in the usual care arm after they completed the study. At the end of the study, we provided in-service education to clinic staff. Members of our research team also participated in a health fair for 1 clinic (Southeast Health Center), providing instructions on inhaler use and spirometric assessment for patients. At the end of the study, our team met with clinic staff and providers to report back results of the study.
AIR Study Advisory Board
The advisory board served several functions over the course of the study. The study co-primary investigators (co-PIs) met with members of the advisory board to solicit their initial reaction to the idea of health coaching for patients with COPD and to discuss how coaching might be implemented. Feedback from members provided the basis for applying to PCORI for study funding. Once funding was obtained, the research team met with the advisory board to discuss health coaches' engagement with patients. For example, we discussed the appropriate protocol for health coaches conducting home visits. Feedback from our patient advisors included the importance of ensuring that patients felt in control of the process, providing a menu of options for how to use the home visits (eg, including review of the home for allergy triggers or review of medications), using tools such as a home assessment of potential allergy triggers with transparency and in collaboration with patients (to avoid the perception of judgment), and treading carefully in discussions of pets and how to mitigate their adverse effects on lung health. This discussion resulted in significant modifications to our protocol and tools. During the conduct of the study, we met with the advisory board to discuss recruitment and retention strategies and to brainstorm ways to make the study most useful to patients and clinicians. We also presented and discussed study data at baseline and at 9 months. Toward the end of the study, we focused on how to disseminate results to patients, clinicians, and investigators. The advisory board met 7 times over the course of the preparation for the execution of the study. In addition, some advisory board members took part in a health coach curriculum planning meeting, in 1 or more of the training sessions for health coaches, and in the end-of-project celebration for patients and clinic staff. Patient advisors in particular helped pilot and provided feedback on instruments and took part in training the health coaches.
Patient Partner Included in the Study Team
Our research team included a patient partner with COPD, Mr Devon Low, who has had COPD for >10 years. Mr Low was also a member of the study advisory board. He attended monthly research team meetings (30 in total) to review study progress, respond to challenges that arose during the course of the study, and review data collected. Mr Low named the study the Aides in Respiration (AIR) study. He was a member of the hiring team that selected our health coaches. Mr Low also helped train our health coaches and met with the coaches over the course of the study to discuss their experience and provide ongoing mentorship (15 meetings in total). For example, when confronted with patient resistance to using oxygen, the health coaches discussed with Mr Low the reasons for resistance and various strategies to address it. Mr Low's role was expanded to include participation in other health coaching projects conducted within the University of California, San Francisco (UCSF) Center for Excellence in Primary Care, and he was invited by the study team to participate as a speaker in several conferences.
Poststudy Presentation and Celebration
All study participants and stakeholders were invited to a poststudy presentation and celebration, held on July 21, 2017, where the main study results were presented and discussed with more than 2 dozen patients and stakeholders.
Impacts of Stakeholder Engagement
Choice of Research Question and Study Design
The decision to study health coaching for patients with COPD arose from our experience with health coaching for patients with diabetes and the need of patients with COPD for ongoing support for disease management that could be delivered where they lived and received their primary care. While study investigators chose the randomized controlled trial (RCT) design, patients and other stakeholders shaped the specifics of the design, including the choice of study measures and the content of the intervention. For example, based on their feedback, we modified the initial questionnaire assessing environmental triggers for asthma (for patients with asthma/COPD overlap syndrome) to be more sensitive and respectful. Based on their feedback, we also modified or dropped other measures to reduce respondent burden.
Health Coaching Intervention
Advice received from the study advisory board and from our patient research partner, Mr Low, shaped the content of the intervention. Mr Low helped with health coach training and met periodically with the coaches over the course of the study to address challenges and provide feedback. For example, he designed a training session to help coaches understand the impact of COPD (eg, walking and climbing stairs while breathing through a straw), role played using his own life experiences, provided coaches with insights into the barriers and competing demands for patients with COPD, and advised when to reassess an approach that was not working. Other examples of expertise contributed by stakeholders include training of research assistants (RAs) in spirometry by respiratory therapist Eula Lewis and incorporation of patient educational resources and support activities by patient advocates (Chris Garvey and DorAnn Donesky).
Participant Recruitment/Retention
Patient recruitment and retention was discussed both at our study advisory board meetings and at our monthly study team meetings. We implemented suggestions regarding how to best advertise the study (eg, study flyers in clinic, enlisting front desk staff) and how to best reach out to patients who were difficult to contact. When, for example, the health coaches experienced difficulty in convincing patients with signs of exacerbations to seek medical care, the study advisory team and our study team discussed strategies for proactively discussing red flags and barriers to seeking care before symptoms.
Data Analysis or Results Interpretation
Members of the study advisory board provided suggestions for data analysis and interpretation. For example, 1 member (Chris Garvey) was struck by the inclusiveness of our recruitment strategy, noting that most clinical studies avoid recruitment of patients with comorbid conditions or psychosocial challenges. With her encouragement, we have written and submitted a paper that reports our experience with recruitment and retention of groups underrepresented in research (eg, racial/ethnic minorities, patients with substance use or mental health issues or who are homeless).
Dissemination
Our patient research partner, Mr Low, suggested and helped us stage a photo shoot to secure action health coaching shots for presentations, publications, and materials. He was our artistic director and photographer for the shoot. Mr Low has also helped disseminate the AIR study results by becoming involved as a patient advocate and representative with other groups. For example, in 2015, he gave a presentation at Stanford Medicine on the patient's perspective in living with COPD and the need for more patient-centered support such as health coaching. The presentation and his video story can be viewed online.51,52
Methods
Study Overview
The goal of our study was to evaluate the effectiveness of a health coach model for improving outcomes for low-income urban patients with COPD. We conducted an RCT comparing 9 months of health coaching plus usual care vs usual care alone, with the specific aims of measuring differences in COPD-related QOL, number of exacerbations, exercise capacity, and patient self-efficacy of managing COPD. Additional outcomes of interest included patient-reported quality of care, concordance of prescribed medications to international guidelines, correct use of inhalers, level of COPD symptoms, lung function, knowledge of COPD, and smoking status.
Study Design
The AIR health coaching study was a 9-month single-blinded parallel RCT comparing health coaching plus usual care vs usual care alone for patients with moderate to severe COPD. We chose an RCT design to provide a high degree of internal validity. Blinding of patients and clinical teams was not feasible due to the nature of the intervention. However, data analysis was blinded during the course of the study and analyses for investigators and members of the data safety monitoring board.
Participants
The study enrolled low-income and vulnerable patients receiving care at FQHCs because these patients experience disparities in quality of care and disease burden and have been underrepresented in clinical research studies of COPD. Multiple clinic sites were necessary to obtain an adequate sample size. Spanish-speaking patients were recruited by a bilingual RA. To maximize participation from underrepresented groups, we minimized exclusion criteria.
Specifically, we did not exclude patients who were homeless or had substance abuse, mental illness, or other conditions as long as they were able to receive health coaching and participate in the study.
Eligibility for the study required a diagnosis of COPD confirmed by the study pulmonologist, Dr George Su. The diagnosis required that a patient met the common criteria of a postbronchodilator force expiratory volume at 1 second (FEV1) of <0.7, although in some cases in which the FEV1/FVC (forced vital capacity) was between 0.7 and 0.75 the diagnosis was made based on the overall clinical data. In addition to having a diagnosis of COPD, patients had to have COPD that was considered moderate to severe based on meeting at least 1 of the criteria listed in Table 3.
Table 3
Criteria for Moderate to Severe COPD.
Patients believed to be potentially eligible for the study were identified primarily through review of the electronic record for patients of the San Francisco Department of Health system with an ED, inpatient, or outpatient diagnostic code suggestive of COPD during a 24-month period. The ICD-9 codes of interest were 491, 492, 496, 490 + 305.1, 493 + 305.1, and 786 + 305.1. During the course of the study the ICD-10 coding system was introduced. The approximately corresponding ICD-10 codes were J42, J43, J44, J40 + Z72, J45 + Z72, and R06 + Z72. In addition, we obtained a list of patients referred to the COPD and asthma clinics. Potentially eligible patients could also be identified by providers or staff at study clinics.
Five trained members of the study team reviewed medical records for patients identified as described above to ascertain the diagnosis of COPD considered to be at least moderate (Table 3) and to confirm the patient did not meet 1 or more of the exclusion criteria shown in Table The criterion for at least moderate COPD reflects common clinical criteria for moderate or severe COPD6: meeting spirometric criteria of FEV1 <80% predicted (criterion 1); being on home oxygen or meeting criteria for being on home oxygen (criteria 2, 3, and 4); exacerbations of COPD in the past 12 months (criteria 5, 6, and 7); or being prescribed medication used for moderate COPD (criteria 8 and 9). Of note, exacerbations are used in GOLD guidelines to define patients at high risk, although for our study we required only 1 or more exacerbations vs the 2 or more in GOLD guidelines. Names of patients considered potentially eligible following medical record review were then given to their PCPs, who could exclude patients not appropriate for participation in the study due to medical or mental health problems, or who met exclusion criteria not ascertained by medical record review (eg, patient had moved, or patient did not speak English or Spanish). Patients not excluded by their provider were entered into a recruitment tracking database and RAs attempted to contact them by phone, in person at the time of a clinic visit, or by letter. RAs made at least 5 attempts to reach patients. When we were unable to reach patients by phone or letter, we approached them at the time of a medical visit. When the RA contacted the patient, he or she confirmed eligibility (age, language, continuing care at one of the study FQHCs, ability to travel to clinic) and determined if the patient was interested in participating in the study. If the patient was eligible and interested in participating in the study, the RA arranged to meet the patient at the patient's FQHC. There were no differences in how we formed the health coached and usual care arms.
Table 4
Exclusion Criteria.
An RA met with patients interested in participating in the study at the patient's primary care clinic to obtain informed consent and administer baseline measures, which included the patient survey, 6-Minute Walk Test (6MWT) (if patient was physically capable and did not have a contraindication to completing the test), and spirometry if indicated. Spirometry measures the amount and speed of air flow during a deep inspiration and forced expiration. Patients breathe into a mouthpiece connected to a portable spirometer. Data on airflow volume over time are recorded and converted to commonly used measures such as FVC (total volume of air expired during forced expiration) and FEV1 (volume of air expired during the first second of forced expiration), and represented graphically. Conducting spirometry before and after having the patient use a bronchodilator inhaler determines the amount of nonreversible outflow obstruction. Typically, a ratio of FEV1FVC of <0.70 after use of a bronchodilator, in the absence of other underlying lung conditions (eg, chronic bronchiectasis), is considered to indicate COPD. Spirometry, including postbronchodilator measures of FEV1/FEV, was considered indicated to determine eligibility if the patient did not already meet criteria for COPD. Spirometry was also indicated for patients who met criteria for COPD but who had not had a spirometry in the previous 3 months in order to obtain a baseline measure of lung function.
After baseline measures were completed, the patients were given the next randomly ordered envelope, which assigned them to the coached or to the usual care arm. Participant contact information, including phone numbers, addresses, and emails, were obtained at enrollment and the best way(s) to contact the participant was established.
Participants in both study arms were contacted every 3 months by the RA and asked to complete a brief interval survey, which asked about recent exacerbations and current smoking status. Participants were paid $10 for each interval survey, $10 if they completed the 6MWT, and $10 if they completed spirometry, for a total of up to $30 for the baseline measures. The window for administering the 3-month survey was between 2 to 4 months (61-122 days) after enrollment; the window for the 6-month survey was from 5 to 7 months (152-213 days) after enrollment; the window for the 9-month survey was from 8 to 12 months (243-365 days) after enrollment. RAs made a minimum of 3 attempts to contact patients for the 3- and 6-month surveys and 9 attempts to contact patients for the 9-month measures. To the extent possible, the same RA followed up with a participant each time to build trust and improve response rates. For the 9-month measures, which required an in-person visit, RAs met with the patient at the patient's primary care clinic.
Intervention (Health Coaching) and Control (Usual Care)
Health Coaching Intervention
Two health coaches delivered the study intervention. One was full time, the other 80% time. Because their time was paid for using study funds, they were UCSF employees. Health coaches were supervised clinically by the PNPS member of the study team, with backup from the coprimary investigators, a pulmonologist (Dr Su), and a family physician (Dr Thom). Coaches could also consult with the patient's PCP as needed. Both coaches were 4-year college graduates bilingual in Spanish and English. One had worked as a health coach for 4 years, primarily with patients with diabetes. The second had worked as an RA on a previous study of health coaching but had no direct experience as a health coach. Both received approximately 100 hours of training over 3 months using a COPD health coaching curriculum specific to the study. The curriculum comprised 2 primary domains: health coaching techniques and COPD-specific knowledge. The health coaching curriculum (available at http://cepc.ucsf.edu/content/health-coaching-curriculum) covers active listening and nonjudgmental communication, harm reduction, navigating health care systems, gathering information on medication adherence, creating self-management goals, and closing the loop (checking for comprehension by asking patients to describe the key messages in their own words). COPD-specific training was delivered by 2 pulmonary specialists and covered the physiology of COPD, related comorbidities, GOLD guidelines (which are international guidelines for the management of COPD), prevention and management of exacerbations, and lifestyle management. Upon completion of training, coaches were required to score at least 90% on 3 examinations assessing content knowledge and to demonstrate mastery of coaching skills through simulated role-plays and observed health coaching sessions.
Patients received health coaching for 9 months. Each health coach worked with a total of 50 patients over the 2-year duration of the study, with a maximum caseload of 30 patients at any given time. Standardized care included a minimum number of contacts and completion of specific objectives, such as reviewing medications, assessing and reinforcing patient knowledge about warning signs of COPD exacerbations, and facilitating an assessment of the care plan by a PNPS. Health coaches could use their discretion to increase the number or frequency of contacts and address issues of interest to the patient. Minimum frequency of contact based on study protocol was once every 3 weeks, for a total of 13 contacts over the course of the study. Health coaches were expected to complete an initial visit within 2 to 3 weeks of enrollment; to meet with the patient at least 4 additional times over the course of the study; and to have a phone check-in call at least every 3 weeks, including within 2 weeks after each medical visit. Coaches were also expected to conduct at least 1 in-depth consultation with the study PNPS and to attend at least 3 medical visits. The purpose and intended content for each of these activities are described below. All interactions between health coaches and patients were documented in a database created for the study that included date, mode of contact, duration of contact, topics discussed, and any relevant notes.
Coaches met with patients in person at the clinic or at the patient's home when possible, or by phone if an in-person meeting was not feasible. The purpose of the initial meeting was to build rapport and understand the patients' motivations, strengths, and barriers to self-management. Subsequent meetings, which generally lasted 15 to 90 minutes, were used to assess patient knowledge, provide education about COPD, review patient medications, assess patients' ability to correctly use their inhalers and provide instruction in correct inhaler use, and help patients develop an action plan regarding COPD exacerbations. More generally, health coaches worked with patients to identify their health-related goals, to address barriers to reaching their goals, and to create action plans to achieve their goals. Health coaches also assisted with navigation of health and community resources (including making and keeping appointments, accessing classes and smoking cessation resources, and meeting with a member of the behavioral health team). Home visits were used most frequently by patients who had difficulties with public transportation or general mobility. Home visits were also used to identify COPD/asthma triggers within the home, to acquire accurate knowledge of what medications a patient had in the home (including any duplicate or expired medications), to identify barriers to medication adherence, and to ensure patients on oxygen had the necessary equipment. Phone check-ins were conducted to provide ongoing support for patient self-management, check for barriers to self-management, and provide reminders for upcoming appointments. In contrast to patient support modes that focus on 1 or 2 areas such as increasing exercise, improving medication adherence, or following a specific action plan for COPD exacerbations, the health coaches for the AIR study had a broad range of activities, as shown in Table 5.
Table 5
Health Coaching Activities.
Consultations between the health coaches and PNPS involved several steps. Health coaches recorded patient medical history and comorbidities, smoking history, risk and symptom assessment, COPD and asthma medications and treatment history, environmental triggers, and screens for symptoms of sleep apnea, from review of the EMR and information supplied by the patient (the health coaches created a tool in consultation with the PNPS that can be found at http://cepc.ucsf.edu/health-coaching-chronic-lung-conditions). The health coach then presented the patient's information to the PNPS, who could gather additional information from the medial record if needed. The PNPS created a set of recommendations for changes in care using GOLD guidelines, generally without the need to see the patient. Recommendations could include changes to inhaler therapy; further diagnostic testing; and referrals to pulmonology, pulmonary rehabilitation, physical therapy, and other appropriate programs. The PNPS communicated recommendations to the patient's PCP via the EMR and/or through secure email, and the health coach followed up with the PCP to support desired changes.
Recommendations were considered implemented if the PCP acted on the recommendation (eg, prescribed a new medication, made a referral, ordered a test). The health coach followed up with the patient to see if the recommendations had been implemented by the PCP and to provide education and support to the patient for implementing the recommendations.
Health coaches accompanied patients to medical visits with their PCP whenever possible. Coaches met with the patient immediately before the medical visit to conduct medication reconciliation and to help the patient establish priorities and goals for the visit. With the patient's permission, the health coach was present during the medical visit and could offer clarifications and support as needed. After the visit, the health coach reviewed the PCP's recommendations with the patient to ensure patient understanding, and helped patients choose attainable goals and create an action plan for making changes to achieve those goals. The health coach called the patient approximately 2 weeks later to follow up on action plans.
We used several methods to assess the fidelity of the intervention. The project manager conducted several observations of the health coaches, both immediately after training and periodically throughout the project. Using a standardized checklist, the project manager provided immediate feedback to the coaches regarding skills. In addition, the health coaches used the skills checklist to observe and provide feedback to each other, thus reinforcing the key components of the intervention. The health coaches met regularly with the project manager to review progress, and they used several metrics to assess success in engaging patients, such as the proportion of patients with whom they had a substantive interaction in the past 3 weeks and the proportion of patients with at least 1 in-person visit. They also categorized patients using a “stoplight” worksheet as having high (green), medium (orange), or poor (red) engagement, and they discussed strategies for better engaging those categorized in orange or red groups. The coaches met weekly with the pulmonary nurse practitioner specialist to review patient cases and keep up to date on relevant changes in care guidelines or formulary. For example, Advair was taken off formulary by a major MediCal managed care provider during the course of the study, resulting in confusion among patients, clinicians, and pharmacists. The PNPS and the health coaches discussed the inhalers being prescribed in its stead and the common questions emerging for patients and clinicians. Finally, health coaches took part in monthly study team meetings, in which they could raise concerns and troubleshoot issues with implementation of study protocols.
Usual Care Arm
Patients randomized to usual care continued to have visits with their PCP over the course of the 9-month period. They received any resources their provider and their clinic offered as part of standard care, including, but not limited to, access to COPD educators, respiratory therapists, COPD education classes, pulmonary rehabilitation, or smoking cessation classes. During the study period, relatively few usual care patients took advantage of COPD education classes (n = 1) or smoking cessation classes (n = 0), according to rosters from these classes.
Usual care included referral by the PCP to pulmonary specialists at the county hospital (San Francisco General Hospital [SFGH]) via an electronic referral system operated by the San Francisco Department of Public Health. During the study period, 23 patients in the usual care arm (25%) saw a pulmonary specialist. Specialists working at the SFGH had access to the same EMR as used in the study FQHCs. All clinics provided access to mental health services. Three of the clinical sites had complex care management programs for their patients at heightened risk for hospitalizations, which could include people who had COPD. Medication reconciliation, an activity performed by health coaches, was not routinely available to patients in the usual care arm.
Study Outcomes
All study participants met with an RA at their primary care clinic. The RA administered the baseline survey in either in English or Spanish, based on the patient preference. The RA also administered spirometry using the VMAX Vyntus SPIRO with SentrySuite and the 6MWT, and recorded weight, height, blood pressure, pulse, and transcutaneous oxygen saturation.
Study outcome variables are shown in Table 6. The primary outcomes for the study were COPD-related QOL and dyspnea at 9 months (specific aim 1 in the study proposal), which we measured using the short form of the Chronic Respiratory Disease Questionnaire (CRQ).53-55 The CRQ generates scores in 4 domains (dyspnea, fatigue, physical function, and mastery) and an overall score, and has been validated in multiple studies.57-59 The CRQ has the advantage of having a near-normal total score distribution,60,61 being sensitive to change,58-60 and having established minimum clinically important differences (MCIDs) for total score and for each of its 4 domain scores.61,62
Table 6
Outcome Variables.
At 9 months we also measured secondary outcomes for the study, corresponding to the additional specific aims in the study proposal: COPD exacerbations (specific aim 2), exercise capacity (specific aim 3), and patient self-efficacy for self-management of COPD (specific aim 4). Reducing COPD exacerbations is an important goal for patients as exacerbations restrict daily activities,56 decrease QOL,63 and can have lasting negative psychological impact.64 We defined an exacerbation as worsening of respiratory symptoms resulting in prescription of a course of oral steroid medication, an unscheduled or emergency visit, or a hospitalization related to COPD. Similar utilization-based definitions have been used in previous studies for moderate to severe exacerbations63 and are consistent with guideline definition.6 We measured exercise capacity using the standardized 6MWT, which measures how far a patient can walk in 6 minutes. An RA administered the test using an established protocol recommended by the ATS.65 The distance a patient is able to walk in 6 minutes is a well-recognized and widely used measure of exercise capacity.66 The 6MWT has been described as “a simple test to perform, does not require special training of personnel, and is easy to administer, well tolerated, and reflects the functional exercise.”67 The MCID for the 6MWT is generally considered to be 25 to 50 meters.71 Previous studies have documented the 6MWT as sensitive to measuring change over time.60,69-71 Self-efficacy has been shown to predict functional capacity and QOL for patients with COPD.72 We chose to measure self-efficacy using the 6-item scale developed by Dr Kate Lorig and others at Stanford.73
Additional outcomes prespecified in ClinicalTrials.gov are the following:
- COPD symptoms, measured with the COPD Assessment Test (CAT), a validated and widely used scale76-78
- Lung function, measured by spirometry as the forced expiratory volume at 1 second (FEV1) percentage predicted
- Smoking status by patient self-report
- Observed use of prescribed inhalers, assessed by an RA using a checklist of steps specific for each type of inhaler (eg, MDI [metered dose inhaler], Spiriva HandiHaler, Advair DISKUS, Respimat Soft Mist), patterned after previously published measures,81,85 and consistent with manufacturers' directions for inhaler use.
We measured use of outpatient services (ie, primary care visits, specialty care visits, urgent care visits, ED visits, and hospitalizations) by reviewing EMRs of the participating clinics and the pulmonary clinics at Zuckerberg San Francisco General Hospital (ZSFGH). We ascertained ED visits and hospitalizations by reviewing records from the county hospital and from all outside hospitals where patients indicated having received emergency care or being hospitalized for the period from 1 year before enrollment to the end of the study. We measured knowledge of COPD using 4 questions developed for the AIR study.
Other outcomes, which were not prespecified in ClinicalTrials.gov before the start of data collections, were concordance between medications prescribed and those recommended by COPD category I GOLD guidelines, documented by chart review; and symptoms of depression defined as a score of 15 or greater on the Patient Health Questionnaire-8 (PHQ-8).82 Concordance of medications with guideline recommendations is a measure of quality of clinical care (which we listed as an outcome in the study proposal but did not further define); we included symptoms of depression as an outcome in the study proposal but did not register it as a prespecified outcome on ClinicalTrials.gov. We decided to include these measures in our analyses after the data had been collected.
Study Setting
We conducted this study at 7 urban county-operated primary care clinics designated as FQHCs that primarily serve a low-income, publicly insured patient population. Two of these sites are large academic residency teaching practices based at the public hospital that is part of the county-owned system. Pulmonary specialty care is available through the public hospital that is part of the health network and can be accessed via a referral system. In the year before study enrollment, 31.3% of participants had at least 1 visit with a pulmonary specialist. Clinic sites have integrated behavioral health services. All sites have had prior exposure to health coaching for diabetes or hypertension, and/or as part of complex care management programs. As part of the San Francisco Health Network, FQHC sites and pulmonary consultation clinics, which were at the county hospital (ZSFGH), shared a common EMR system (e-ClinicalWorks), which is separate from the UCSF EMR system (EPIC). Health coaches had access to the EMRs for the patients they coached.
Time Frame for the Study
We chose an intervention period of 9 months based on the expected time needed to show an impact of health coaching and based on the funding available. In a previous RCT of peer health coaching for patients with diabetes, we found a significant improvement in glucose control after 6 months. We expected coaching for COPD to be more challenging and expected it potentially could take longer to show an effect.
Analytical and Statistical Approaches
Sample Size
The original target sample size for enrollment was 250 patients allocated in a 1:1 ratio between study arms. Sample size and power estimates for comparison of the health coached and usual care arms assumed a target power of 0.80 or higher and significance to be defined by a 2-sided α = .05. We derived expected effect sizes and MCIDs for each outcome from previous studies for the primary measures for the first 3 aims: COPD-related QOL,54 number of exacerbations,86 and the 6MWT.87 Expected differences and minimal clinically important differences were not available for the fourth specific aim, patient self-efficacy of COPD management. Based on our previous experience in conducting RCTs in this population,36 we assumed an attrition rate of 20%, resulting in 200 patients available for analysis at the end of the study. We further assumed an intraclass correlation coefficient of 1% between clinic sites based on our previous experience. The target sample size gave us sufficient power to detect the anticipated differences between arms for each outcome variable, which are at least as large as the MCIDs previously established, as shown in Table 7.
Table 7
Study Power Based on Original Planned Enrollment of 250 Patients.
Data Analysis
We compared outcomes by arm assignment (intention to treat). Hypothesis tests were 2-sided, with P < .05 considered statistically significant. We compared baseline participant characteristics between study arms and tested for significance using chi-square for categorical variables, t tests for normally distributed continuous variables, and appropriate nonparametric tests for non-normally distributed continuous variables. Outcomes are reported as means with standard deviations for continuous variables, as proportions, or as counts per person-year for exacerbations, outpatient visits, ED visits and hospitalizations, with adjusted P values and 95% CIs. We compared outcomes by arm assignment (intention to treat) using generalized linear models with a normal distribution with identity link for continuous outcomes (eg, scale scores and proportions), Poisson distribution with log link for count outcomes (eg, exacerbations and hospitalizations), and binomial distribution with logit link for binary outcomes (eg, smoking status). We used a robust standard error in all analyses to account for clustering, and it accommodates missing data under the assumption that the outcomes are missing at random.88-92 We examined the assumption that data were missing at random by comparing characteristics of patients lost to follow-up by study arms to look for evidence of differential attrition. In all models, we entered baseline levels of the outcome as a predictor and follow-up levels as the dependent variable, with use of a robust SE. Event rates (exacerbations, outpatient visits, ED visits, and hospitalizations) are reported as events per person-year and adjusted for baseline rate and for clustering by participant, as patients could have more than 1 event. We used goodness of fit diagnostics to assess influential points, outliers, overdispersion, and heteroscedasticity. We dichotomized symptoms of depression as a PHQ-8 score of <15 and ≥15 (indicating moderately severe to severe depression).82
We conducted the following planned sensitivity analyses: (1) repeating primary analyses with multiple imputation procedures; (2) limiting intervention participants to those who received a prespecified minimal amount of the intervention (per-protocol analyses); (3) adjusting for patient characteristics that differed between study arms if the P value for the difference was <.10; and (4) adjusting for season of enrollment, patient age, race, and gender. We examined heterogeneity of effects for 3 prespecified subgroups: English vs Spanish as primary language; current smokers vs other; and COPD classification GOLD category D (high symptoms and high risk) vs other.6 We also conducted post analyses to look for evidence of effect modification by clinic site.
Changes to the Original Study Protocol
The most notable changes to the original study protocol were the reduction in target enrollment sample size from 250 to 190 and the dropping of the 15-month measures (6 months after the end of the intervention period). We made these changes (and PCORI approved them) due to unforeseen delays in starting the study and the need to perform more spirometries than anticipated. Of the 1881 people with a diagnostic billing code for chronic bronchitis, emphysema, or COPD (ICD-9 codes 491, 493, 496), 1471 (78%) did not have spirometric data documenting obstruction (FEV1/FVC <0.7) in their medical record. Thus, the study took on significantly more burden than expected to secure needed diagnostic spirometry before enrollment. The power for the revised sample size is presented in Table 8.
Table 8
Revised Study Power Based on 76 Participants in Each Arm at End of Study.
We changed the measure of self-efficacy using the Stanford scale rather than the 15-item Pulmonary Rehabilitation Adapted Index of Self-efficacy93 both to reduce patient response burden and because the Stanford scale appeared to be widely used and accepted. We dropped the Consumer Assessment of Healthcare Providers and Systems (CAHPS) measures for access to care and coordination of care to reduce the survey response burden and because these measures overlapped with the PACIC measure of quality of care. We dropped the pulmonary function test because we considered it to be nonspecific (as it includes multiple measures of lung function, of which the most clinically relevant are captured by spirometry). We dropped the use of home oxygen because of small numbers of patients on home oxygen. We also dropped the measure of self-reported physical activity before starting the study because patients found it difficult to answer in pilot testing. We added 2 outcomes not initially listed with ClinicalTrials.gov to the site as post hoc measures. The first was symptoms of moderately severe to severe depression, defined as a PHQ-8 score of 15 or greater. We included symptoms of depression, as measured by the PHQ, as an outcome measure in the study proposal. We based the decision to dichotomize the measure at 15 or greater on 15 being a commonly used clinical threshold. The second outcome measure was the proportion of patients who received prescription medication concordant with GOLD recommendations. While we listed quality of COPD care as an outcome in the proposal, we did not operationalize this definition. We chose medication concordant with GOLD recommendations as a straightforward measure of quality of COPD care.
Results
Recruitment and Enrollment
Recruitment and enrollment flow is shown in the CONSORT diagram in Figure 3. Of 2504 patients identified as potentially having COPD, we excluded 1478 (59%), more than half because they did not meet at least 1 of the criteria for at least moderate severity. We excluded an additional 235 patients after chart review based on their not being an active patient at one of the study clinics. An additional 147 patients were identified by their PCP as no longer being at the clinic site or being deceased (12). The RAs were able to contact 661 (64%) of the 1026 patients not excluded. Of these 661 patients, 177 (27%) were found to be ineligible. We could not determine eligibility for 202 patients (31%), either because they declined to be screened or because the RA was able to only partially screen them. Of the 282 patients determined to be eligible, 90 either explicitly declined enrollment or missed their enrollment appointment(s) and could not be successfully rescheduled. The study enrolled 192 patients (slightly above the revised goal of 190 patients), or 68% of the 282 patients determined to be eligible. The first patient enrolled on November 12, 2014, and the last 9-month survey was completed on May 6, 2017.
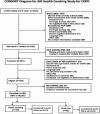
Figure 3
Patient Flow.
Compared with enrolled patients, patients who did not enroll were significantly older, but were similar in gender and in the proportion prescribed COPD controller medications (Table 9).
Table 9
Characteristics of Enrolled and Eligible but Not Enrolled.
We assessed follow-up at 3, 6, and 9 months, as shown in Table 10. The lower rates of completion at 3 months reflect competing demands of patient recruitment and enrollment at that time.
Table 10
Response Rates at Point of Follow-up by Study Arm.
Of the 34 patients lost to follow-up at 9 months, 6 had died, 3 had moved out of the area, and 25 could not be contacted despite multiple attempts.
We compared participants who completed the 9-month follow-up with those who did not, and we found no significant differences by age, gender, disease severity, smoking status, substance use, social support, housing, or symptoms of depression (Table 11).
Table 11
Study Completers vs Noncompleters at 9 Months.
There were no significant differences in the characteristics of patients lost to follow-up between study arms, which supported our assumption that data were missing at random.
Most participants (93.2% [n = 179]) in the study had obstruction confirmed by postbronchodilator spirometry (FEV1/FVC <0.7) (Table 12). As per protocol, a study pulmonologist reviewed results of patients who had postbronchodilator FEV1/FVC ≥0.7 and ≤0.75, and based on case history and other diagnostic imaging determined that 6 other individuals (3.1%) were eligible for enrollment. An additional 7 individuals (3.6% of the study sample) could not complete the spirometry maneuver but were determined by the pulmonologist to have obstruction and to be eligible for the study.
Table 12
Documentation of Pulmonary Obstruction.
Baseline Characteristics
Baseline characteristics were similar between the study arms (Table 13). A greater proportion of the health coached arm identified as white (29% vs 13%) and a lower proportion reported speaking Spanish as their primary language (7% vs 12%). Finding 2 variables that differ significantly between study arms from more than 40 variables examined is within the range of what would be expected by chance and does not indicate a failure in randomization.
Table 13
Baseline Characteristics by Study Arm.
Baseline outcome measures also did not vary significantly between study arms, with the exception of rates of hospitalizations, which were higher in the usual care arm (Table 14).
Table 14
Baseline Outcome Measures.
As expected, there were significant differences in patient demographic characteristics of race, ethnicity, national origin, education, and income among the 7 clinics, reflecting the characteristics of the populations served in their geographic neighborhood. We did not find significant differences across clinics in age, gender, GOLD classification of COPD, or any of the primary or secondary outcomes, with the exception of rates of COPD exacerbations.
The amount of missing data at baseline was low (<3%) for most variables, with the exception of exercise capacity (6MWT) and lung function (FEV1 measured by spirometry). Both these measures require significant patient effort and therefore have contraindications, as explained in the methods section. Fifty-six patients (29%) were unable to complete the 6MWT—44 because of a medical contraindication and 12 who refused. In addition, the RA terminated the test early for 2 patients due to signs of distress. We included results for these 2 individuals as the distance completed at the time the test was terminated, per testing protocol. Noncompleters of the 6MWT were more likely to be classified as GOLD category D (57.4% vs 41.9%; P = .05). Forty patients (21%) did not have FEV1 (% predicted) measured at baseline. Spirometry was contraindicated for 15 of these patients, it was not possible to get adequate spirometry for 22 patients despite repeated attempts, and 3 patients could not be scheduled for spirometry within the baseline window.
Outcomes
Outcomes at 9 months are presented in Table 15 and Figures 4 to 8. There were no significant differences between study arms for any of the outcomes specified in the study-specific aims: disease-related QOL, number of exacerbations, exercise capacity, or self-efficacy for management of COPD. Of the additional prespecified outcomes, we saw significant differences for improved inhaler use and patient-reported quality of care and for the proportion of patients demonstrating adequate inhaler use, but not for COPD symptoms, lung function, bed days, or COPD knowledge. The proportion of current smokers declined in both study arms but did not significantly differ between arms at 9 months.
Table 15
Outcomes at 9 Months by Study Arm.
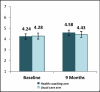
Figure 4
CRQ Mean Scores by Arm.
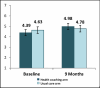
Figure 5
CRQ Dyspnea Scores by Arm.
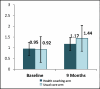
Figure 6
COPD Exacerbations Per Person-Year by Arm.
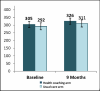
Figure 7
Exercise Capacity (Meters) by Arm.
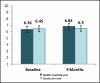
Figure 8
Self-efficacy for Managing COPD by Arm.
Patients had a total of 251 ED visits (176 at the county and 75 at outside hospitals) and 80 hospitalizations (56 from county and 24 from outside hospitals) over the course of the study. Outside hospitals responded to 91.5% of our requests for medical records (88.2% of requests in the health coached arm and 95.3% in the usual care arm; P = .14). Over the course of the 9-month study period, the rate of ED visits, both related and not related to COPD, was similar in the 2 study arms. The rate of hospitalization for COPD exacerbation was approximately half as high in the health coached arm as in the usual care arm (0.27 vs 0.51 per 100 person-years, but this difference was not significant in adjusted analyses). In a post hoc analysis, we found that patients in the health coached arm seen in the ED primarily for a COPD exacerbation were less likely to be hospitalized than those in the usual care arm (adjusted difference in proportion −30.6%; 95% CI, −53.7 to −7.6; P = .01).
We also found significant differences in 2 outcomes that were not prespecified: a reduction in the proportion of patients with symptoms of moderate to severe depression and an increase in the proportion of patients who received guideline-concordant medications in the health coached arm. The proportion of patients with clinically important symptoms of depression (PHQ score of >15) dropped from 13% to 6% in the health coached arm but did not change in the usual care arm, a significant difference. While the proportion of patients prescribed guideline-concordant medications increased from baseline to 9 months in both the health coached and usual care arms, this increase was significantly greater for patients in the health coached arm. This difference was due to greater concordance of medications for patients classified as having a high degree of symptoms but being at low risk for ED visits or hospitalizations (GOLD category B). Specifically, >90% of patients in the health coached arm with GOLD category B COPD were receiving guideline-recommended medications at 9 months, compared with 75% of patients in the usual care arm.
Table 16 shows outcomes for the CRQ total QOL scores, the CRQ dyspnea domain scores, number of bed days, and days of reduced activities due to COPD in the previous 4 weeks, and proportion of patients who reported having smoked in the past 30 days. There were no significant differences by study arm at any time point, and no significant differences in linear trends over time by study arm.
Table 16
Outcomes at Baseline, 3, 6, and 9 Months by Study Arm.
Sensitivity Analyses
Repeating analyses using multiple imputation for missing data had minimal effect and did not materially change the results of the analyses. Using a per-protocol analysis, excluding 15 participants in the health coached arm who did not receive at least half the intended amount of contact with the health coach (ie, 7 interactions, of which 3 were in-person meetings) did not materially affect the results. Including additional baseline variables, which differed between study arms at P < .10 (FEV1/FEV, FEV1 % predicted, primary language other than English) or patient demographic characteristics (age, gender, race) did not change the results.
Analyses for Heterogeneity of Treatment Effect
There were no significant differences in treatment effect between the prespecified subgroups defined by language, smoking status, and GOLD classification. There was no evidence for heterogeneity of effect by clinic site, either using an omnibus test for heterogeneity or using pair-wise comparisons.
Implementation of Recommendations From the PNPS by PCPs
We examined recommendations for guideline-concordant care made by the PNPS for patients presented by the health coach for consultation (patients did not need to be seen by the PNPS in most cases). A summary of recommendations made and their implementation by the PCP, as documented in the electronic medical record, is provided in Table 17. The most common types of recommendations were for changes in COPD medications (70%) or assessment for other conditions (43%). There was a high rate of referrals, with the exception of referral to sleep clinic (which is a more complicated referral in the health system under study), and of further testing. There was a somewhat lower rate of implementation of recommendations for changes to medications for COPD (77%) and tobacco cessation (71%).
Table 17
PNPS Recommendations for Guideline-Based Care (n = 87).
Fidelity and Intensity of the Intervention
Per study protocol, we defined delivery of the intervention as meeting all of the following requirements:
- Meeting in person with the patient at least every 2 months (5 visits over 9 months)
- Attending clinic primary care visits with the patient at least 3 times during the study
- Contacting the patient at least once every 3 weeks (13 contacts over 9 months)
- Consulting 1 or more times with the COPD nurse to determine recommendations based on GOLD criteria
Of the 100 patients assigned to the health coaching intervention, 47% met all components of the study protocol (Table 18).
Table 18
Adherence to Study Protocol (n = 100).
The criterion least often met was that the health coach take part in at least 3 medical visits with the patient and his or her PCP or pulmonary specialist, with only half (49%) meeting this criterion. Of those who did not meet the criteria, the median number of interactions was 24, with 9 in-person interactions. Most patients (59% of those who did not have at least 3 medical visits) had at least 13 interactions with their health coach and 68% had at least 5 in-person meetings, suggesting that failure to meet this criterion was not a symptom of lack of engagement with the health coach. Of the 51 patients who failed the meet the criterion for 3 medical visits with the health coach, 27 (53%) had 2 or fewer PCP and chest clinic visits, suggesting that for this arm, there was a lack of engagement with the broader health care system. An additional 18 patients assigned to the health coached arm (35% of those who failed to meet the visit criteria) had only 3 to 4 total medical visits during the time period, and scheduling challenges may have played into the failure to meet the criterion. A similar portion of patients seen by each health coach met all the criteria (50.0% vs 44.0%; P = .55).
The study protocol did not place a cap on the number of visits, and some patients received a more intense intervention, with an interquartile range of 6 to 14 in-person visits and 15 to 41 contacts reported during the study period. Per the health coaches, this intensity most often reflected patient-initiated contacts, but the health coach would also reach out more often if concerned about the patient's well-being.
Safety Data
The AIR study included a Data and Safety Monitoring Board to which the study team reported the number of ED visits, hospitalizations, and deaths. We reported deaths at the time we became aware of them. As shown in Table 19, there were no significant differences in hospitalizations, ED visits, or deaths between study arms over the course of the study.
Table 19
Deaths, Hospitalizations, and ED Visits by Study Arm.
Discussion
Context for Study Results
The AIR study compared health coaching plus usual care with usual care for patients with moderate to severe COPD receiving care at 7 FQHCs. At 9 months, there were no significant differences between study arms for any of the primary or secondary outcomes specified in the study-specific aims: disease-related QOL, number of exacerbations, exercise capacity, or self-efficacy for management of COPD. Of the additional prespecified outcomes, we saw significant differences in the proportion of patients demonstrating adequate inhaler use and in patient-reported quality of care. We also found significant differences in 3 other outcomes that were not prespecified: a reduction in proportion of patients with symptoms of moderate to severe depression, an increase in the proportion of patients receiving guideline-concordant medications in the health coached arm, and a reduction in the proportion of patients with a COPD-related ED visit who were hospitalized. The proportion of current smokers declined in both study arms but was not significantly different between arms at 9 months. While patients in the health coached arm had nearly 50% fewer hospitalizations for COPD during the study period, this difference was not statistically significant in adjusted analyses.
We identified 7 studies that examined the impact of individual coaching for patient self-management support based on 1 or more models for patient-centered behavioral change (eg, transtheoretical mode with motivational interviewing94,95 or self-regulation theory96 for patients with COPD). The outcomes for these studies are summarized in Table 20. An 18-month study by Coultas et al in 2016, which used an RCT design and a coaching-based intervention similar to ours but which was directed primarily at increasing physical activity, also found no significant differences in exercise capacity measured by the 6MWT or in COPD-related QOL or dyspnea using the CRQ measures.97,98 A study by Bucknall et al, which used a registered nurse as a health coach for patients recently discharged from the hospital following a COPD admission, similarly found no difference in the primary outcome of COPD-related deaths or hospitalizations and no difference in COPD-related QOL with the exception of a single subscale on the St. George's Respiratory Questionnaire.63 A study by Wood-Baker et al used an RCT of community health nurse mentors (trained in the transtheoretical model of change and motivational interviewing) over 12 months and found no difference in general QOL except for the physical functioning subscale of the SF-36 (36-Item Short Form Health Survey), no differences in dyspnea, and no differences in hospital admissions.99 In another RCT, a clinical pharmacist delivered an intervention aimed at improving medication adherence and reducing smoking, based on motivational interviewing and action planning.100 At 6-month follow-up, the investigators reported that the health coached arm had significantly fewer hospital admissions, ED visits, and unscheduled primary care appointments for COPD. Intervention patients also reported significantly better disease-specific QOL, adherence to COPD medication, and COPD knowledge. There was no significant difference in FEV1 or smoking abstinence. An RCT in Sweden that used a patient-centered intervention found significant improvement in patient COPD-related QOL and in patient knowledge about COPD.101 In this Swedish study, the intervention was delivered by a team comprising a COPD nurse and physician, plus, as needed, a dietician, medical social worker, physical therapist, and occupational therapist. A prospective, controlled (but not randomized) study in the Netherlands also used a multidisciplinary team (pulmonologist and respiratory therapist) to deliver a combination of group education and 2 to 3 sessions of individual health coaching.102 This study found significant improvement in COPD-related QOL at 1 year and a reduction in moderate to severe exacerbations over 2 years. Finally, a recent RCT by Benzo et al, which employed health coaching over 8 weekly sessions by a registered nurse or respiratory therapist for patients following hospitalization for COPD exacerbation, found that patients in the health coached arm had significantly fewer hospital admissions for COPD at 1 and 6 months.103,104 Coached patients also reported significantly better COPD-related QOL (measured by the CRQ) at 6 and 12 months and fewer COPD exacerbations over 12 months.
Table 20
Summary of Previous Studies Using Similar, Patient-Centered Health Coaching Type of Intervention.
Several factors could explain differences and similarities between outcomes from the above studies and our study. Of the 7 studies described above, 4 found no differences in QOL or a difference for only a single subscale that was not prespecified, which is consistent with our results. Two of the 3 studies that reported a significant benefit to QOL for patients in the health coached arm were conducted outside the United States and used teams rather than a single coach. The study by Benzo et al used a nurse practitioner or respiratory therapist. In contrast, our study used a single unlicensed health worker as a health coach. While not conclusive, these results suggest that health care professionals, particularly working as teams, may be more effective at improving QOL than coaching by a single unlicensed health worker.
Of the 4 studies that reported rates of hospitalization for COPD, 2 found significantly fewer hospitalizations for COPD in the health coached arm.100,104 The magnitude of the differences found in these 2 studies was similar to the approximately 50% reduction found in our study. In an unplanned, exploratory analysis for our study, we found that coached patients seen in the ED for a COPD exacerbation were significantly less likely to be hospitalized for that exacerbation. This outcome was not reported in the above studies. Because it was a post hoc outcome for our study, it should be viewed with caution.
Exercise capacity, smoking cessation, and forced expiratory volume were each measured in just 1 of the 7 previous studies, with no differences found, which is consistent with our results. In contrast to our study, COPD knowledge significantly improved in the 1 study that reported this outcome,101 although knowledge was measured with a single question asking patients to rate their knowledge of COPD.
Integrated disease management, defined as management by 2 or more categories of health care providers and 2 or more areas of intervention (education/self-management, exercise, psychosocial, smoking cessation, medication use, nutrition, financial incentives, or structural changes)10 has many of the same goals as health coaching, but requires substantially more resources. A Cochrane review of 27 RCTs found a significantly greater improvement on quality-of-life scores for patients who received integrated disease management for COPD, although most of the difference was seen in RCTs focused on exercise improvement.105 The review also found that patients who received integrated disease management were less likely to be hospitalized for a respiratory-related condition and had shorter hospital stays. The review did not find significant differences in the outcomes of COPD exacerbations, lung function, smoking status, or depression.
We found a significantly higher level of patient-reported quality of chronic disease care received in the health coached arm of the AIR study compared with the usual care arm. None of the 7 health coaching studies reported quality of chronic disease care as an outcome. Of note, an RCT of integrated disease management failed to find a difference in care quality as assessed by the same measure (PACIC).27 The improvement in patient-reported quality of chronic illness care seen in our study may be due to the emphasis of health coaching on several aspects of care quality measured by the PACIC, specifically goal setting, shared decision-making, care planning, and follow-up between visits.
The AIR study did find a highly significant and likely clinically important greater increase (26%) in the proportion of coached patients who demonstrated adequate inhaler use compared with usual care patients (6%). Inhaler use was not reported in any of the 7 studies of health coaching but was evaluated in a 3-month RCT conducted in 170 community pharmacies in Belgium.106 The Belgium study compared a pharmacist-based education intervention against usual care for patients with COPD provided in two 1-hour educational sessions that covered use of COPD medications, lifestyle advice, and smoking cessation support. Patients randomized to the health coached arm had significantly improved observed inhaler use compared with those in usual care. Several additional studies have compared the efficacy of didactic instruction vs teach-back techniques for improving inhaler use. In teach-back, or closing-the-loop, techniques, which were the basis for inhaler instruction in the AIR study, patients are asked to demonstrate correct techniques and receive additional instruction until they can conduct the steps correctly. These studies found significantly more improvement when teach-back techniques were used compared with didactic education.107,108
The AIR study also found a significant decrease in the proportion of patients who reported symptoms of moderate to severe depression in the health coached arm. The 1 study that measured depression symptoms reported no difference63; however, this study used a different measure (Hospital Anxiety and Depression scale) than our study did (PHQ-8).
Another outcome of note in the AIR study was the receipt of guideline-concordant medications based on a patients' GOLD classification category. While the proportion of patients prescribed guideline-concordant medications increased from baseline to 9 months in both the health coached and usual care arms, this increase was significantly greater for patients in the health coached arm. This result was consistent with additional analyses demonstrating a high level of implementation by the PCP of recommendations made by the PNPS working with the health coach. We could not locate other RCTs for health coaching that used guideline-concordant care as an outcome.
Viewing the results from the AIR study in the context of previous studies of health coaching or similar interventions for patients with COPD suggests several implications. While the impact on disease-specific QOL and dyspnea trended in the correct direction, these differences were not significant despite a fairly intense amount of coaching. These results suggest that any benefit of health coaching in our model was likely to be small and not clinically important. Similarly, we found no impact on exercise capacity, a finding that was not unexpected given that interventions that affect this outcome have generally been much more intensive and focused pulmonary rehabilitation programs. The lack of effect on all exacerbations of COPD was disappointing but finding that coached patients seen in the ED for a COPD exacerbation were significantly less likely to be hospitalized suggests that health coaching may be effective in changing when a patient seeks care for an exacerbation.
Health coaching in the AIR study also appeared to improve inhaler use and patient-reported quality of care and reduce the proportion of patients with symptoms of moderate to severe depression. The first finding is quite robust, and likely reflects the effectiveness of the health coaching and the low rate of appropriate inhaler use seen at baseline. The improvement in patient-reported quality of care probably is due to the concordance of many of the health coaching activities with the quality items, as both our health coaching model and the PACIC were informed by the CCM.41 The impact on symptoms of depression is encouraging, but it is not clear if this is a nonspecific result from increased attention or is related to specific health coaching support activities or referral to behavioral health.
An intriguing finding from the AIR study that we have not seen previously reported from RCTs of health coaching or similar interventions is the impact on the prescription of guideline-concordant medications. There is evidence that guideline-based medication based on a patient's COPD classification by GOLD criteria can reduce the burden of COPD, and that lack of concordance with recommended care in general is more common for low-income, vulnerable patients such as those enrolled in the AIR study. It seems likely that improvement in guideline-based medications reflects the success of the pulmonary nurse practitioner-health coach consultation model employed in the AIR study in which the health coaches gather information from the medical record but also from the patients. This information includes patients' experience with and preferences for treatment and identification of barriers to receiving recommended care. This model allows specialist recommendations for guideline-based care to be made without the patient having to be seen in person in most cases. The model thus has the potential to increase access to specialty consultation and recommendations, particularly for patients who find it difficult to travel to see the specialist.
Generalizability of the Findings
The AIR study purposively enrolled patients seen at FQHCs using minimal exclusion criteria. We did not find evidence for differential effects by clinic sites or by patient subgroups, suggesting the results should be generalizable to other FQHCs that have shared EMRs and access to a pulmonary specialist interested in working with health coaches. The FQHCs for the AIR study had previous experience with health coaching, primarily for patients with diabetes, which may have facilitated the acceptance of health coaching for patients with COPD. The health coach model used in the AIR study involved a substantial amount of training and multiple contacts with patients, including home visits and accompanying patients to their medical visits, an intensive model that not all FQHCs will be able to support. A less intensive intervention may be able to achieve aims such as ensuring guideline-concordant prescribing or improving inhaler use, but it is not clear from this study which aspects of our program are necessary to achieve those ends.
Implementation of Study Results
Our experience with the AIR study indicates that using unlicensed health workers to improve care for underserved patients with COPD was acceptable to patients, clinicians, and staff and resulted in significant improvement of the outcomes of appropriate inhaler use, guideline-concordant care, patient-reported quality of care, and symptoms of depression. Health coaches were able to work with the PCP and the pulmonary specialists, as intended in our health coaching model. Based on our experience, we would advise implementing health coaching in a more targeted way, focusing on areas where we saw the most evidence of benefit, including inhaler use and guideline-based prescriptions. Health coaching appears unlikely to reduce the number of COPD exacerbations, but it does show some promise for reducing COPD-related hospitalizations, suggesting a potential for more cost-effective care provision. The model of consultation with a pulmonary specialist used in the AIR study, in which the patient is coached without having to be physically present, could potentially increase access of care to patients who cannot or do not want to travel to a central specialty clinic for consultation or could improve efficiency and reduce wait times in settings where the supply of specialist time is limited.
Based on our experience, we feel that the commitment of the pulmonary specialist to work with the health coach is very important. Gaining buy-in from PCPs and clinic leaders is crucial. The groundwork laid from previous studies of health coaching and from meetings with leaders, clinicians, and nonclinical staff at each clinic was undoubtedly important to and effective for our study. Choosing health coaches with excellent interpersonal skills and commitment to coaching and experience working with patients seen at FQHCs also helped. It would have been very difficult for the health coach to work effectively with the patient, the pulmonary specialist, and the PCP without a shared EMR record that allowed for relatively easy written communication. Any program should include 1 or more patient partners with COPD as co-trainers and as ongoing advisors who periodically meet with health coaches to review coaching activities and provide advice and support. Online training for health coaches using the AIR study model has been developed (https://cepc.ucsf.edu/get-trained), and resources are available at http://cepc.ucsf.edu/health-coaching-chronic-lung-conditions.
Subpopulation Considerations
We did not find any evidence of differential impact in subpopulations defined by language, disease severity, or smoking status.
Study Limitations
A potentially important limitation of our study design was that patients, rather than PCPs or clinics, were randomized, resulting in many PCPs having patients in both study arms. This may have caused a halo effect whereby patients in the usual care arm may have benefited from the presence of health coaching, as clinicians were aware of coaching activities. For example, PCPs received recommendations to improve medication regimens in accordance with international guidelines, and this likely caused them to apply similar principles to care of other patients. Another limitation is that the study was not well powered to look for differences in effect between subgroups. The reduction in the recruitment goal may have limited the power to detect differences. Due to time and budget constraints we were not able to assess the persistence of the differences between groups that we did find.
Future Research
Future research is needed to evaluate the implementation and effectiveness of health coaching for patients with COPD in other FQHC environments. The use of health coach consultation with a pulmonary specialist, usually without the need for the patient to visit the specialist, should be further evaluated for effectiveness and acceptability.
Conclusions
We conclude that the benefits from health coaching suggested by the AIR study probably do not justify a 9-month intensive health coaching program. This is consistent with previous studies, which have also found it difficult to change outcomes such as QOL and COPD exacerbations, even when using health professionals as coaches. Our study found that significant improvement in patient-reported quality of care, guideline-concordant care, and appropriate inhaler use did not translate into better hard outcomes of improved QOL, better exercise capacity, or fewer total exacerbations. This is likely because many other factors contribute to these hard outcomes. The results of the AIR study also suggest ways in which the health coaching model might be more effectively focused.
Our results should be helpful to FQHCs that already use health coaching, or that are interested in implementing a health coaching program at their clinic. Materials and training resources to support such a program are available through the UCSF Center for Excellence in Primary Care (http://cepc.ucsf.edu/content/health-coaching-curriculum). Patients who have a choice to work with a health coach should find our results helpful in weighing the benefits of working with a health coach against the commitment required. The results will also be useful to health policy experts in assessing the potential value of reimbursement and incentives for health coaching-type activities for patients with chronic disease.
References
- 1.
- National Institutes of Health: NHLBI. Morbidity and Mortality: 2012 Chart Book on Cardiovascular, Lung, and Blood Diseases. National Institutes of Health; 2012.
- 2.
- Caress A, Luker K, Chalmers K. Promoting the health of people with chronic obstructive pulmonary disease: patients' and carers' views. J Clin Nurs. 2010;19(3-4):564-573. [PubMed: 20500291]
- 3.
- Willgoss TG, Yohannes AM, Goldbart J, Fatoye F. “Everything was spiraling out of control”: experiences of anxiety in people with chronic obstructive pulmonary disease. Heart Lung. 2012;41(6):562-571. [PubMed: 22939112]
- 4.
- Boeckxstaens P, Deregt M, Vandesype P, Willems S, Brusselle G, De Sutter A. Chronic obstructive pulmonary disease and comorbidities through the eyes of the patient. Chron Respir Dis. 2012;9(3):183-191. [PubMed: 22848068]
- 5.
- Miravitlles M, Anzueto A, Legnani D, Forstmeier L, Fargel M. Patient's perception of exacerbations of COPD—the PERCEIVE study. Respir Med. 2007;101(3):453-460. [PubMed: 16938447]
- 6.
- Vogelmeier CF, Criner GJ, Martinez, FJ, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2017 Report). Am J Respir Crit Care Med. 2017;195(5):557-582. [PubMed: 28128970]
- 7.
- Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532-555. [PubMed: 17507545]
- 8.
- Omachi TA, Sarkar U, Yelin EH, Blanc PD, Katz PP. Lower health literacy is associated with poorer health status and outcomes in chronic obstructive pulmonary disease. J Gen Intern Med. 2013;28(1):74-81. [PMC free article: PMC3539035] [PubMed: 22890622]
- 9.
- Jochmann A, Neubauer F, Miedinger D, Schafroth S, Tamm M, Leuppi JD. General practitioner's adherence to the COPD GOLD guidelines: baseline data of the Swiss COPD Cohort Study. Swiss Med Wkly. 2010;140. doi:10.4414/smw.2010.13053 [PubMed: 20407960] [CrossRef]
- 10.
- Kaufmann C, Markun S, Hasler S, et al. Performance measures in the management of chronic obstructive pulmonary disease in primary care—a retrospective analysis. Praxis (Bern 1994). 2015;104(17):897-907. [PubMed: 26286494]
- 11.
- Ta M, George J. Management of chronic obstructive pulmonary disease in Australia after the publication of national guidelines. Intern Med J. 2011;41(3):263-270. [PubMed: 20002857]
- 12.
- Barrecheguren M, Monteagudo M, Ferrer J, et al. Treatment patterns in COPD patients newly diagnosed in primary care. A population-based study. Respir Med. 2016;111:47-53. [PubMed: 26758585]
- 13.
- Price D, West D, Brusselle G, et al. Management of COPD in the UK primary-care setting: an analysis of real-life prescribing patterns. Int J Chron Obstruct Pulmon Dis. 2014;9:889-904. [PMC free article: PMC4154894] [PubMed: 25210450]
- 14.
- Barr RG, Celli BR, Martinez FJ, et al. Physician and patient perceptions in COPD: the COPD Resource Network Needs Assessment Survey. Am J Med. 2005;118(12):1415. [PubMed: 16378794]
- 15.
- Foster JA, Yawn BP, Maziar A, Jenkins T, Rennard SI, Casebeer L. Enhancing COPD management in primary care settings. MedGenMed. 2007;9(3):24. [PMC free article: PMC2100091] [PubMed: 18092030]
- 16.
- Agency for Healthcare Research and Quality (AHRQ). National Healthcare Disparities Report 2011. US Department of Health & Human Services; 2011. Updated October 2014. https://archive
.ahrq .gov/research/findings /nhqrdr/nhqr11/index.html - 17.
- Gershon AS, Dolmage TE, Stephenson A, Jackson B. Chronic obstructive pulmonary disease and socioeconomic status: a systematic review. COPD. 2012;9(3):216-226. [PubMed: 22497534]
- 18.
- Eisner MD, Blanc PD, Omachi TA, et al. Socioeconomic status, race and COPD health outcomes. J Epidemiol Community Health. 2011;65(1):26-34. [PMC free article: PMC3017471] [PubMed: 19854747]
- 19.
- Wong AW, Gan WQ, Burns J, Sin DD, van Eeden SF. Acute exacerbation of chronic obstructive pulmonary disease: influence of social factors in determining length of hospital stay and readmission rates. Can Respir J. 2008;15(7):361-364. [PMC free article: PMC2679571] [PubMed: 18949105]
- 20.
- Salinas GD, Williamson JC, Kalhan R, et al. Barriers to adherence to chronic obstructive pulmonary disease guidelines by primary care physicians. Int J Chron Obstruct Pulmon Dis. 2011;6:171-179. [PMC free article: PMC3064423] [PubMed: 21468169]
- 21.
- Perez X, Wisnivesky JP, Lurslurchachai L, Kleinman LC, Kronish IM. Barriers to adherence to COPD guidelines among primary care providers. Respir Med. 2012;106(3):374-381. [PMC free article: PMC3377746] [PubMed: 22000501]
- 22.
- Sandelowsky H, Hylander I, Krakau I, Modin S, Stallberg B, Nager A. Time pressured deprioritization of COPD in primary care: a qualitative study. Scand J Prim Health Care. 2016;34(1):55-65. [PMC free article: PMC4911027] [PubMed: 26849465]
- 23.
- Lundell S, Tistad M, Rehn B, Wiklund M, Holmner A, Wadell K. Building COPD care on shaky ground: a mixed methods study from Swedish primary care professional perspective. BMC Health Serv Res. 2017;17(1):467. [PMC free article: PMC5504776] [PubMed: 28693473]
- 24.
- Craig BM, Kraus CK, Chewning BA, Davis JE. Quality of care for older adults with chronic obstructive pulmonary disease and asthma based on comparisons to practice guidelines and smoking status. BMC Health Serv Res. 2008;8:144. [PMC free article: PMC2500012] [PubMed: 18611245]
- 25.
- George J, Kong DC, Thoman R, Stewart K. Factors associated with medication nonadherence in patients with COPD. Chest. 2005;128(5):3198-3204. [PubMed: 16304262]
- 26.
- King TE, Wheeler MB. Medical Management of Vulnerable and Underserved Patients: Principles, Practice, and Populations. 2nd ed. McGraw Hill Education Medical; 2016.
- 27.
- Kruis AL, Boland MR, Assendelft WJ, et al. Effectiveness of integrated disease management for primary care chronic obstructive pulmonary disease patients: results of cluster randomised trial. BMJ. 2014;349:g5392. doi:10.1136/bmj.g5392 [PMC free article: PMC4160285] [PubMed: 25209620] [CrossRef]
- 28.
- Bischoff EW, Akkermans R, Bourbeau J, van Weel C, Vercoulen JH, Schermer TR. Comprehensive self management and routine monitoring in chronic obstructive pulmonary disease patients in general practice: randomised controlled trial. BMJ. 2012;345:e7642. doi:10.1136/bmj.e7642 [PMC free article: PMC3514071] [PubMed: 23190905] [CrossRef]
- 29.
- Bourbeau J, Casan P, Tognella S, Haidl P, Texereau JB, Kessler R. An international randomized study of a home-based self-management program for severe COPD: the COMET. Int J Chron Obstruct Pulmon Dis. 2016;11:1447-1451. [PMC free article: PMC4934557] [PubMed: 27418817]
- 30.
- Chuang C, Levine SH, Rich J. Enhancing cost-effective care with a patient-centric chronic obstructive pulmonary disease program. Popul Health Manag. 2011;14(3):133-136. [PubMed: 21214417]
- 31.
- Benzo R. Collaborative self-management in chronic obstructive pulmonary disease: learning ways to promote patient motivation and behavioral change. Chron Respir Dis. 2012;9(4):257-258. [PubMed: 23129803]
- 32.
- Bourbeau J, Saad N. Integrated care model with self-management in chronic obstructive pulmonary disease: from family physicians to specialists. Chron Respir Dis. 2013;10(2):99-105. [PubMed: 23382555]
- 33.
- Bennett HD, Coleman EA, Parry C, Bodenheimer T, Chen EH. Health coaching for patients with chronic illness. Fam Pract Manag. 2010;17(5):24-29. [PubMed: 21121566]
- 34.
- Thom DH, Wolf J, Gardner H, et al. A qualitative study of how health coaches support patients in making health-related decisions and behavioral changes. Ann Fam Med. 2016;14(6):509-516. [PMC free article: PMC5389392] [PubMed: 28376437]
- 35.
- Thom DH, Ghorob A, Hessler D, De Vore D, Chen E, Bodenheimer TA. Impact of peer health coaching on glycemic control in low-income patients with diabetes: a randomized controlled trial. Ann Fam Med. 2013;11(2):137-144. [PMC free article: PMC3601392] [PubMed: 23508600]
- 36.
- Willard-Grace R, Chen EH, Hessler D, et al. Health coaching by medical assistants to improve control of diabetes, hypertension, and hyperlipidemia in low-income patients: a randomized controlled trial. Ann Fam Med. 2015;13(2):130-138. [PMC free article: PMC4369595] [PubMed: 25755034]
- 37.
- Ruggiero L, Moadsiri A, Butler P, et al. Supporting diabetes self-care in underserved populations: a randomized pilot study using medical assistant coaches. Diabetes Educ. 2010;36(1):127-131. [PMC free article: PMC3758241] [PubMed: 20185612]
- 38.
- Ivey SL, Tseng W, Kurtovich E, et al. Evaluating a culturally competent health coach intervention for Chinese American patients with diabetes. Diabetes Spectr. 2012;25(2):93-102. [PMC free article: PMC3849128] [PubMed: 24311938]
- 39.
- Turner BJ, Hollenbeak CS, Liang Y, Pandit K, Joseph S, Weiner MG. A randomized trial of peer coach and office staff support to reduce coronary heart disease risk in African-Americans with uncontrolled hypertension. J Gen Intern Med. 2012;27(10):1258-1264. [PMC free article: PMC3445668] [PubMed: 22570108]
- 40.
- Chen EH, Thom DH, Hessler DM, et al. Using the Teamlet Model to improve chronic care in an academic primary care practice. J Gen Intern Med. 2010;25(Suppl 4):S610-S614. [PMC free article: PMC2940441] [PubMed: 20737236]
- 41.
- Wagner EH. Chronic disease management: what will it take to improve care for chronic illness? Eff Clin Pract. 1998;1(1):2-4. [PubMed: 10345255]
- 42.
- Adams SG, Smith PK, Allan PF, Anzueto A, Pugh JA, Cornell JE. Systematic review of the chronic care model in chronic obstructive pulmonary disease prevention and management. Arch Intern Med. 2007;167(6):551-561. [PubMed: 17389286]
- 43.
- Nici L, ZuWallack R; American Thoracic Society Subcommittee on Integrated Care of the CP. An official American Thoracic Society workshop report: the integrated care of the COPD patient. Proc Am Thorac Soc. 2012;9(1):9-18. [PubMed: 22421582]
- 44.
- Yawn BP, Wollan PC. Knowledge and attitudes of family physicians coming to COPD continuing medical education. Int J Chron Obstruct Pulmon Dis. 2008;3(2):311-317. [PMC free article: PMC2629969] [PubMed: 18686740]
- 45.
- Dolce JJ, Crisp C, Manzella B, Richards JM, Hardin JM, Bailey WC. Medication adherence patterns in chronic obstructive pulmonary disease. Chest. 1991;99(4):837-841. [PubMed: 2009784]
- 46.
- van Grunsven PM, van Schayck CP, van Deuveren M, van Herwaarden CL, Akkermans RP, van Weel C. Compliance during long-term treatment with fluticasone propionate in subjects with early signs of asthma or chronic obstructive pulmonary disease (COPD): results of the Detection, Intervention, and Monitoring Program of COPD and Asthma (DIMCA) study. J Asthma. 2000;37(3):225-234. [PubMed: 10831147]
- 47.
- Melani AS, Canessa P, Coloretti I, et al. Inhaler mishandling is very common in patients with chronic airflow obstruction and long-term home nebuliser use. Respir Med. 2012;106(5):668-676. [PubMed: 22277996]
- 48.
- Melzer AC, Ghassemieh BJ, Gillespie SE, et al. Patient characteristics associated with poor inhaler technique among a cohort of patients with COPD. Respir Med. 2017;123:124-130. [PMC free article: PMC5310832] [PubMed: 28137488]
- 49.
- Souza ML, Meneghini AC, Ferraz E, Vianna EO, Borges MC. Knowledge of and technique for using inhalation devices among asthma patients and COPD patients. J Bras Pneumol. 2009;35(9):824-831. [PubMed: 19820807]
- 50.
- Twyman L, Bonevski B, Paul C, Bryant J. Perceived barriers to smoking cessation in selected vulnerable groups: a systematic review of the qualitative and quantitative literature. BMJ Open. 2014;4(12):e006414. https://bmjopen
.bmj.com /content/4/12/e006414 [PMC free article: PMC4275698] [PubMed: 25534212] - 51.
- Stanford Medicine X ePatient Scholar: Devon Low. Vimeo. https://vimeo.com/143775926. October 27, 2015.
- 52.
- Low D. Medicine X ePatient Scholar: Devon Low. YouTube. https://www
.youtube.com /watch?v=Z4zRJFQhDCo. September 30, 2015. - 53.
- Schunemann HJ, Griffith L, Jaeschke R, et al. A comparison of the original chronic respiratory questionnaire with a standardized version. Chest. 2003;124(4):1421-1429. [PubMed: 14555575]
- 54.
- Schunemann HJ, Puhan M, Goldstein R, Jaeschke R, Guyatt GH. Measurement properties and interpretability of the chronic respiratory disease questionnaire (CRQ). COPD. 2005;2(1):81-89. [PubMed: 17136967]
- 55.
- Tsai CL, Hodder RV, Page JH, Cydulka RK, Rowe BH, Camargo CA Jr. The short-form chronic respiratory disease questionnaire was a valid, reliable, and responsive quality-of-life instrument in acute exacerbations of chronic obstructive pulmonary disease. J Clin Epidemiol. 2008;61(5):489-497. [PubMed: 18394543]
- 56.
- Weldam SW, Schuurmans MJ, Liu R, Lammers JW. Evaluation of quality of life instruments for use in COPD care and research: a systematic review. Int J Nurs Stud. 2013;50(5):688-707. [PubMed: 22921317]
- 57.
- Davies N, Gibbons E, Fitzpatrick R. A Structured Review of Patient-reported Outcome Measures for COPD: An Update 2009. Patient-Reported Outcome Measurement Group: University of Oxford; 2009.
- 58.
- Harper R, Brazier JE, Waterhouse JC, Walters SJ, Jones NM, Howard P. Comparison of outcome measures for patients with chronic obstructive pulmonary disease (COPD) in an outpatient setting. Thorax. 1997;52(10):879-887. [PMC free article: PMC1758425] [PubMed: 9404375]
- 59.
- Hajiro T, Nishimura K, Tsukino M, Ikeda A, Koyama H, Izumi T. Comparison of discriminative properties among disease-specific questionnaires for measuring health-related quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(3 Pt 1):785-790. [PubMed: 9517591]
- 60.
- de Torres JP, Pinto-Plata V, Ingenito E, et al. Power of outcome measurements to detect clinically significant changes in pulmonary rehabilitation of patients with COPD. Chest. 2002;121(4):1092-1098. [PubMed: 11948037]
- 61.
- Schunemann HJ, Goldstein R, Mador MJ, et al. A randomised trial to evaluate the self-administered standardised chronic respiratory questionnaire. Eur Respir J. 2005;25(1):31-40. [PubMed: 15640320]
- 62.
- Griffiths TL, Burr ML, Campbell IA, et al. Results at 1 year of outpatient multidisciplinary pulmonary rehabilitation: a randomised controlled trial. Lancet. 2000;355(9201):362-368. [PubMed: 10665556]
- 63.
- Bucknall CE, Miller G, Lloyd SM, et al. Glasgow supported self-management trial (GSuST) for patients with moderate to severe COPD: randomised controlled trial. BMJ. 2012;344:e1060. doi:https://www
.bmj.com/content/344/bmj .e1060 [PMC free article: PMC3295724] [PubMed: 22395923] - 64.
- Kessler R, Stahl E, Vogelmeier C, et al. Patient understanding, detection, and experience of COPD exacerbations: an observational, interview-based study. Chest. 2006;130(1):133-142. [PubMed: 16840393]
- 65.
- Laboratories ATSCoPSfCPF. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111-117. [PubMed: 12091180]
- 66.
- Puhan MA, Mador MJ, Held U, Goldstein R, Guyatt GH, Schunemann HJ. Interpretation of treatment changes in 6-minute walk distance in patients with COPD. Eur Respir J. 2008;32(3):637-643. [PubMed: 18550610]
- 67.
- Divo M, Pinto-Plata V. Role of exercise in testing and in therapy of COPD. Med Clin North Am. 2012;96(4):753-766. [PubMed: 22793943]
- 68.
- Redelmeier DA, Bayoumi AM, Goldstein RS, Guyatt GH. Interpreting small differences in functional status: the Six Minute Walk test in chronic lung disease patients. Am J Respir Crit Care Med. 1997;155(4):1278-1282. [PubMed: 9105067]
- 69.
- Lacasse Y, Goldstein R, Lasserson TJ, Martin S. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;(4):CD003793. doi:10.1002/14651858.CD003793.pub2 [PubMed: 17054186] [CrossRef]
- 70.
- Rejbi IB, Trabelsi Y, Chouchene A, et al. Changes in six-minute walking distance during pulmonary rehabilitation in patients with COPD and in healthy subjects. Int J Chron Obstruct Pulmon Dis. 2010;5:209-215. [PMC free article: PMC2921688] [PubMed: 20714374]
- 71.
- von Leupoldt A, Hahn E, Taube K, Schubert-Heukeshoven S, Magnussen H, Dahme B. Effects of 3-week outpatient pulmonary rehabilitation on exercise capacity, dyspnea, and quality of life in COPD. Lung. 2008;186(6):387-391. [PubMed: 18408968]
- 72.
- Jackson BE, Coultas DB, Ashmore J, et al. Domain-specific self-efficacy is associated with measures of functional capacity and quality of life among patients with moderate to severe chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11(3):310-315. [PMC free article: PMC4028735] [PubMed: 24447029]
- 73.
- Lorig KR, Ritter PL, Laurent DD, Plant K. Internet-based chronic disease self-management: a randomized trial. Med Care. 2006;44(11):964-971. [PubMed: 17063127]
- 74.
- Glasgow RE, Wagner EH, Schaefer J, Mahoney LD, Reid RJ, Greene SM. Development and validation of the Patient Assessment of Chronic Illness Care (PACIC). Med Care. 2005;43(5):436-444. [PubMed: 15838407]
- 75.
- Gugiu PC, Coryn C, Clark R, Kuehn A. Development and evaluation of the short version of the Patient Assessment of Chronic Illness Care instrument. Chronic Illn. 2009;5(4):268-276. [PubMed: 19933249]
- 76.
- Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648-654. [PubMed: 19720809]
- 77.
- Jones PW, Harding G, Wiklund I, et al. Tests of the responsiveness of the COPD assessment test following acute exacerbation and pulmonary rehabilitation. Chest. 2012;142(1):134-140. [PubMed: 22281796]
- 78.
- Tsiligianni IG, van der Molen T, Moraitaki D, et al. Assessing health status in COPD. A head-to-head comparison between the COPD assessment test (CAT) and the clinical COPD questionnaire (CCQ). BMC Pulm Med. 2012;12:20. [PMC free article: PMC3431277] [PubMed: 22607459]
- 79.
- Schillinger D, Handley M, Wang F, Hammer H. Effects of self-management support on structure, process, and outcomes among vulnerable patients with diabetes: a three-arm practical clinical trial. Diabetes Care. 2009;32(4):559-566. [PMC free article: PMC2660485] [PubMed: 19131469]
- 80.
- Gill TM, Allore H, Guo Z. Restricted activity and functional decline among community-living older persons. Arch Intern Med. 2003;163(11):1317-1322. [PubMed: 12796067]
- 81.
- Pothirat C, Chaiwong W, Phetsuk N, Pisalthanapuna S, Chetsadaphan N, Choomuang W. Evaluating inhaler use technique in COPD patients. Int J Chron Obstruct Pulmon Dis. 2015;10:1291-1298. [PMC free article: PMC4501446] [PubMed: 26185435]
- 82.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. [PMC free article: PMC1495268] [PubMed: 11556941]
- 83.
- Guyatt GH, Sullivan MJ, Thompson PJ, et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132(8):919-923. [PMC free article: PMC1345899] [PubMed: 3978515]
- 84.
- Ingle L, Shelton RJ, Rigby AS, Nabb S, Clark AL, Cleland JG. The reproducibility and sensitivity of the 6-min walk test in elderly patients with chronic heart failure. Eur Heart J. 2005;26(17):1742-1751. [PubMed: 15831556]
- 85.
- Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105(6):930-938. [PubMed: 21367593]
- 86.
- Calverley PM. Minimal clinically important difference—exacerbations of COPD. COPD. 2005;2(1):143-148. [PubMed: 17136975]
- 87.
- Holland AE, Hill CJ, Rasekaba T, Lee A, Naughton MT, McDonald CF. Updating the minimal important difference for six-minute walk distance in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2010;91(2):221-225. [PubMed: 20159125]
- 88.
- Fairclough DL. Design and Analysis of Quality of Life Studies in Clinical Trials: Interdisciplinary Statistics. Chapman & Hall/CRC; 2002.
- 89.
- Little RJA, Rubin DB. Statistical Analysis With Missing Data. Wiley; 1987.
- 90.
- Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatry. 2002;59(10):877-883. [PubMed: 12365874]
- 91.
- Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods. 2nd ed. Sage Publications; 2002.
- 92.
- Hedeker DR, Gibbons RD. Longitudinal Data Analysis. Wiley-Interscience; 2006.
- 93.
- Vincent E, Sewell L, Wagg K, Deacon S, Williams J, Singh S. Measuring a change in self-efficacy following pulmonary rehabilitation: an evaluation of the PRAISE tool. Chest. 2011;140(6):1534-1539. [PubMed: 21737490]
- 94.
- Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am J Health Promot. 1997;12(1):38-48. [PubMed: 10170434]
- 95.
- Miller WR, Rose GS. Toward a theory of motivational interviewing. Am Psychol. 2009;64(6):527-537. [PMC free article: PMC2759607] [PubMed: 19739882]
- 96.
- Clark NM, Gong M, Kaciroti N. A model of self-regulation for control of chronic disease. Health Educ Behav. 2001;28(6):769-782. [PubMed: 11720277]
- 97.
- Coultas DB, Jackson BE, Russo R, et al. A lifestyle physical activity intervention for patients with chronic obstructive pulmonary disease. A randomized controlled trial. Ann Am Thorac Soc. 2016;13(5):617-626. [PMC free article: PMC5018892] [PubMed: 26785249]
- 98.
- Ashmore J, Russo R, Peoples J, et al. Chronic obstructive pulmonary disease self-management activation research trial (COPD-SMART): design and methods. Contemp Clin Trials. 2013;35(2):77-86. [PMC free article: PMC3703772] [PubMed: 23680985]
- 99.
- Wood-Baker R, Reid D, Robinson A, Walters EH. Clinical trial of community nurse mentoring to improve self-management in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2012;7:407-413. [PMC free article: PMC3402057] [PubMed: 22848153]
- 100.
- Khdour MR, Kidney JC, Smyth BM, McElnay JC. Clinical pharmacy-led disease and medicine management programme for patients with COPD. Br J Clin Pharmacol. 2009;68(4):588-598. [PMC free article: PMC2780284] [PubMed: 19843062]
- 101.
- Efraimsson EO, Hillervik C, Ehrenberg A. Effects of COPD self-care management education at a nurse-led primary health care clinic. Scand J Caring Sci. 2008;22(2):178-185. [PubMed: 18489687]
- 102.
- Steurer-Stey C, Dalla Lana K, Braun J, Ter Riet G, Puhan MA. Effects of the “Living well with COPD” intervention in primary care: a comparative study. Eur Respir J. 2018;51(1):1701375. doi:10.1183/13993003.01375-2017 [PubMed: 29301921] [CrossRef]
- 103.
- Benzo R, Vickers K, Ernst D, Tucker S, McEvoy C, Lorig K. Development and feasibility of a self-management intervention for chronic obstructive pulmonary disease delivered with motivational interviewing strategies. J Cardiopulm Rehabil Prev. 2013;33(2):113-123. [PMC free article: PMC3586298] [PubMed: 23434613]
- 104.
- Benzo R, Vickers K, Novotny PJ, et al. Health coaching and chronic obstructive pulmonary disease rehospitalization. A randomized study. Am J Respir Crit Care Med. 2016;194(6):672-680. [PMC free article: PMC5027231] [PubMed: 26953637]
- 105.
- Kruis AL, Smidt N, Assendelft WJ, et al. Integrated disease management interventions for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;(10):CD009437. doi:10.1002/14651858.CD009437.pub2 [PubMed: 24108523] [CrossRef]
- 106.
- Tommelein E, Mehuys E, Van Hees T, et al. Effectiveness of pharmaceutical care for patients with chronic obstructive pulmonary disease (PHARMACOP): a randomized controlled trial. Br J Clin Pharmacol. 2014;77(5):756-766. [PMC free article: PMC4004396] [PubMed: 24117908]
- 107.
- Press VG, Arora VM, Shah LM, et al. Teaching the use of respiratory inhalers to hospitalized patients with asthma or COPD: a randomized trial. J Gen Intern Med. 2012;27(10):1317-1325. [PMC free article: PMC3445679] [PubMed: 22592354]
- 108.
- Dantic D. A critical review of the effectiveness of “teach-back” technique in teaching COPD patients self-management using respiratory inhalers. Health Educ J. 2014;73(1):41-50.
- 109.
- Health Resources & Service Administration (HRSA). Health center program look-alikes. Updated November 2018. Accessed June 17, 2019. https://bphc
.hrsa.gov /programopportunities/lookalike/index .html
Related Publications
- •.
- Huang B, Willard-Grace R, De Vore D, et al. Health coaching to improve self-management and quality of life for patients with chronic obstructive pulmonary disease (COPD): protocol for a randomized controlled trial. BMC Pulm Med. 2017;17:90. [PMC free article: PMC5466738] [PubMed: 28599636]
- •.
- Huang B, e Vore D, Chirinos C, et al. Strategies for recruitment and retention of underrepresented populations with chronic obstructive pulmonary disease for a clinical trial. BMC Med. Res. Methodol. 2019;19:39. doi:10.1186/s12874-019-0679-y [PMC free article: PMC6385381] [PubMed: 30791871] [CrossRef]
- •.
- Tsao S, Willard-Grace R, Wolf J, et al. Pulmonary nurse practitioner specialist-health coach consultation model to improve guideline-based care for COPD patients from an urban, underserved population. Int J Chron Obstruct Pulm Dis. Submitted November 2017.
Published
Submitted
Acknowledgments
We thank the clinicians, leaders, and staff at our clinic partners, Maxine Hall, Silver Avenue Health Center, Southeast Health Center, Family Health Center, Richard Fine People's Clinic, Potrero Hill Health Center, and Castro Mission Health Center, for their participation and active support of the AIR study. We also thank Eula Lewis, RT, and the Community Spirometry Program at Zuckerberg San Francisco General Hospital for all the time and effort spent training our team and for sharing their wealth of knowledge. Members of our study advisory board provided invaluable advice, insights, and new ideas that substantially improved the study. Specifically, we would like to thank our patient representatives. We also thank other advisory board members, including Jennifer Coffey, NP; Hali Hammer, MD; DorAnn Donesky, RN, PhD; Chris Garvey, MS, FNP; Eula Lewis, RT; Camille Prado; Lydia Leung, MD; and Rosaly Ferrer, RN; and our health coach experts Adrianna Najmabadi, Amireh Ghorob, and Tom Bodenheimer, MD. We owe a particular debt to Devon Low, our patient research partner, for his many insights and contributions to the AIR study.
Research reported in this report was [partially] funded through a Patient-Centered Outcomes Research Institute® (PCORI®) Award (#AD-1306-03900) Further information available at: https://www.pcori.org/research-results/2013/does-working-health-coach-help-patients-copd-improve-their-quality-life-and
Suggested citation:
Thom DH, Su G, Hessler D, Willard-Grace R. (2019). Does Working with a Health Coach Help Patients with COPD Improve Their Quality of Life and Breathe Easier? Patient-Centered Outcomes Research Institute (PCORI). https://doi.org/10.25302/9.2019.AD.130603900
Disclaimer
The [views, statements, opinions] presented in this report are solely the responsibility of the author(s) and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute® (PCORI®), its Board of Governors or Methodology Committee.
- Does Working with a Health Coach Help Patients with COPD Improve Their Quality o...Does Working with a Health Coach Help Patients with COPD Improve Their Quality of Life and Breathe Easier?
Your browsing activity is empty.
Activity recording is turned off.
See more...
