NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Acalabrutinib is an oral inhibitor of Bruton’s tyrosine kinase that is used in the therapy of B cell malignancies including refractory mantle cell lymphoma and chronic lymphocytic leukemia. Acalabrutinib has been associated with mild-to-moderate serum enzyme elevations during therapy but has not been linked to instances of idiosyncratic acute liver injury, although it has been associated with cases of reactivation of hepatitis B which can be severe and even fatal.
Background
Acalabrutinib (a kal" a broo' ti nib) is an orally available, small molecule inhibitor of Bruton’s tyrosine kinase (BTK), which is an essential component in the B cell receptor signaling pathway. Inhibition of this pathway prevents B cell activation, differentiation and proliferation. Deficiency of BTK is the cause of X linked (Bruton’s) agammaglobulinemia, and B cell receptor signaling through BTK has been shown to be critical for proliferation and survival of malignant B lymphocytes in mantle cell lymphoma and chronic lymphocytic leukemia (CLL). Unlike ibrutinib, another BTK inhibitor, acalabrutinib has a high degree of specificity for BTK and has little or no activity against other tyrosine kinases. Acalabrutinib was approved for use in the United States as therapy for refractory mantle cell lymphoma in 2017 and for CLL and small lymphocytic lymphoma in 2019. It is under evaluation in other malignancies such as Waldenstrӧm’s macroglobulinemia, pancreatic and non-small cell lung cancer. Acalabrutinib is available in capsules of 100 mg under the brand name Calquence. The recommended dose is 100 mg twice daily. Side effects are common, but usually mild-to-moderate in severity; they include myelosuppression, fatigue, diarrhea, nausea, headache, arthralgia, myalgia, bruising and rash. Uncommon, but potentially serious side effects include severe bone marrow suppression, severe or opportunistic infections, bleeding episodes, hypertension, cardiac arrhythmias and secondary malignancies.
Hepatotoxicity
In open label clinical trials of acalabrutinib in patients with CLL and mantle cell lymphoma, serum aminotransferase elevations occurred in 19% to 23% of patients during therapy and rose to above 5 times ULN in 2% to 3%. These elevations were transient and resolved spontaneously but occasionally led to early drug discontinuation. Among the 610 patients treated with acalabrutinib in pre-registration trials, there were no instances of clinically apparent liver injury attributed to its use, but there was a single instance of acute liver failure and death due to reactivation of hepatitis B. Similar cases of reactivation have been reported with ibrutinib, another small molecule inhibitor of Bruton's tyrosine kinase. Experience with acalabrutinib has been limited and the frequency of clinically apparent liver injury and reactivation of hepatitis B are not known. The majority of cases have occurred in patients taking multiple immunosuppressive agents and not just acalabrutinib alone.
Likelihood score: D (possible rare cause of reactivation of hepatitis B).
Mechanism of Injury
The mechanism by which acalabrutinib might cause liver injury is unknown but may be due to off-target inhibition of tyrosine kinases. Acalabrutinib is metabolized in the liver largely by the CYP 3A4 and is susceptible to drug-drug interactions with inhibitors or inducers of this enzyme reactivity. Reactivation of hepatitis B from acalabrutinib is probably the result of profound B cell suppression, which can lead to increases in viral replication, which can result in severe hepatitis upon immune reconstitution.
Outcome and Management
Liver injury due to acalabrutinib is generally mild and asymptomatic. Reactivation of hepatitis B, however, can result in severe hepatitis and even acute hepatic failure. Patients who are to receive B cell inhibitors such as acalabrutinib, ibrutinib, rituximab and usteokinumab should be screened for serologic markers of hepatitis B infection, including HBsAg and anti-HBc before starting chemotherapy, and those who are positive given prophylaxis against reactivation using oral antiviral agent with activity against HBV such as tenofovir or entecavir. Alternatively, patients can be monitored carefully for changes in HBV DNA levels during therapy. If HBV DNA levels appear de novo or increase significantly (by 10-fold or greater; at least one log increase in HBV DNA), initiation of antiviral therapy is appropriate. Therapy should be continued for at least six months after immunosuppressive therapy has been completed.
Drug Class: Antineoplastic Agents, Protein Kinase Inhibitors
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Acalabrutinib – Calquence®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Acalabrutinib | 1420477-60-6 | C26-H23-N7-O2 |
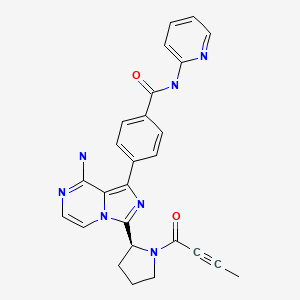
|
| Ibrutinib | 936563-96-1 | C25-H24-N6-O2 |
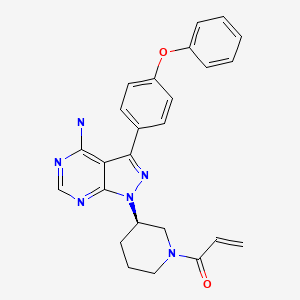
|
ANNOTATED BIBLIOGRAPHY
References updated: 21 March 2021
Abbreviations: BTK, Bruton’s tyrosine kinase; CLL, chronic lymphocytic leukemia; EGFR, epidermal growth factor receptor.
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Review of hepatotoxicity published in 1999 before the availability of tyrosine kinase receptor inhibitors).
- DeLeve LD. Kinase inhibitors. Gefitinib. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, p. 556.(Review of hepatotoxicity of cancer chemotherapeutic agents, does not discuss acalabrutinib or ibrutinib).
- Chabner BA, Barnes J, Neal J, Olson E, Mujagic H, Sequist L, Wilson W, et al. Targeted therapies: tyrosine kinase inhibitors, monoclonal antibodies, and cytokines. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1731-54.(Textbook of pharmacology and therapeutics).
- FDA. https://www
.accessdata .fda.gov/drugsatfda_docs /nda/2017/210259Orig1s000MultidisciplineR.pdf. (FDA scientific review of the NDA for safety and efficacy of acalabrutinib, October 26, 2017). - Ponader S, Burger JA. Bruton's tyrosine kinase: from X-linked agammaglobulinemia toward targeted therapy for B-cell malignancies. J Clin Oncol. 2014;32:1830–9. [PMC free article: PMC5073382] [PubMed: 24778403](History of discovery of X-linked agammaglobulinemia, identification of Bruton’s tyrosine kinase [BTK] as its cause, elucidation of role of BTK in the pathway of B cell activation, and development of BTK inhibitors including ibrutinib).
- de Jésus Ngoma P, Kabamba B, Dahlqvist G, Sempoux C, Lanthier N, Shindano T, Van Den Neste E, et al. Occult HBV reactivation induced by ibrutinib treatment: a case report. Acta Gastroenterol Belg. 2015;78:424–6. [PubMed: 26712054](80 year old man with chronic lymphocytic leukemia [CLL] and anti-HBc without HBsAg in serum [HBV DNA 420 IU/mL] developed reactivation of hepatitis B 5 months after starting ibrutinib [HBsAg positive, HBV DNA 23 million IU/mL, ALT rising to 103 U/L], improving on entecavir therapy with decline in HBV DNA and ALT levels, but HBsAg remained positive).
- Byrd JC, Harrington B, O'Brien S, Jones JA, Schuh A, Devereux S, Chaves J, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374:323–32. [PMC free article: PMC4862586] [PubMed: 26641137](Among 61 patients with relapsed CLL treated with acalabrutinib [100 to 400 mg daily], the overall response rate was 95% and common adverse events included headache [43%], diarrhea [39%], fever [23%], fatigue [21%], hypertension [20%], nausea [20%] and weight loss [26%]; no mention of ALT elevations or hepatotoxicity).
- Wu J, Zhang M, Liu D. Acalabrutinib (ACP-196): a selective second-generation BTK inhibitor. J Hematol Oncol. 2016;9:21. [PMC free article: PMC4784459] [PubMed: 26957112](Review of the mechanism of action, preclinical evaluation and status of clinical studies of acalabrutinib mentions its specificity for BTK and lack of effect on epidermal growth factor receptor [EGFR] and other tyrosine kinases).
- Herishanu Y, Katchman H, Polliack A. Severe hepatitis B virus reactivation related to ibrutinib monotherapy. Ann Hematol. 2017;96:689–90. [PubMed: 28058492](79 year old man with CLL and anti-HBc without HBsAg in serum developed reactivation of hepatitis B 12 months after starting ibrutinib [HBsAg positive, HBV DNA 1.9 million IU/mL, ALT 987 U/L, direct bilirubin 14.2 mg/dL], resolving with tenofovir therapy and later tolerated restarting ibrutinib while continuing tenofovir to prevent reactivation).
- Wang M, Rule S, Zinzani PL, Goy A, Casasnovas O, Smith SD, Damaj G, et al. Acalabrutinib in relapsed or refractory mantle cell lymphoma (ACE-LY-004): a single-arm, multicentre, phase 2 trial. Lancet. 2018;391(10121):659–67. [PMC free article: PMC7864374] [PubMed: 29241979](Among 124 patients with relapsed or refractory mantle cell lymphoma treated with acalabrutinib, 81% had an objective response which was complete in 40%, and the most common side effects were headache, diarrhea, fatigue and myalgia; no mention of ALT elevations or hepatotoxicity).
- Markham A, Dhillon S. Acalabrutinib: first global approval. Drugs. 2018;78:139–45. [PubMed: 29209955](Review of the history of development, mechanism of action, clinical efficacy and safety of acalabrutinib, mentions that reactivation of hepatitis B occurred in one of 610 subjects treated with acalabrutinib, but does not mention ALT elevations or hepatotoxicity).
- In brief: Acalabrutinib (Calquence) for mantle cell lymphoma. Med Lett Drugs Ther. 2018;60(1559):e184. [PubMed: 30681658](Concise review of the mechanism of action, clinical efficacy and safety of acalabrutinib shortly after its approval for use in the US mentions serious adverse events of neutropenia, anemia, pneumonia and hemorrhage; no mention of ALT elevations or hepatotoxicity).
- Awan FT, Schuh A, Brown JR, Furman RR, Pagel JM, Hillmen P, Stephens DM, et al. Acalabrutinib monotherapy in patients with chronic lymphocytic leukemia who are intolerant to ibrutinib. Blood Adv. 2019;3:1553–62. [PMC free article: PMC6517672] [PubMed: 31088809](Among 33 patients with CLL who were intolerant to ibrutinib therapy and were then treated with acalabrutinib, recurrence of adverse events was uncommon and usually mild; no mention of hepatic adverse events).
- Owen RG, McCarthy H, Rule S, D'Sa S, Thomas SK, Tournilhac O, Forconi F, et al. Acalabrutinib monotherapy in patients with Waldenström macroglobulinemia: a single-arm, multicentre, phase 2 study. Lancet Haematol. 2020;7:e112–e121. [PubMed: 31866281](Among 106 patients with Waldenström macroglobulinemia treated with acalabrutinib, overall response rates were 93% while adverse events were frequent including grade 3 neutropenia [16%], pneumonia [7%], atrial fibrillation [1%], bleeding [7%], ALT elevations above 5 times ULN [5%] which led to early drug discontinuation in one patient but was not associated with clinically apparent liver injury).
- Sharman JP, Egyed M, Jurczak W, Skarbnik A, Pagel JM, Flinn IW, Kamdar M, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzumab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet. 2020;395(10232):1278–91. [PMC free article: PMC8151619] [PubMed: 32305093](Among 535 patients with previously untreated CLL treated with acalabrutinib alone (A), acalabrutinib and obinutuzumab (AO) or obinutuzumab with chlorambucil (OC), 24 month progression-free survival rates were higher with AO [93%] and A [87%] than with OC [47%], while adverse events with acalabrutinib included neutropenia [10%], atrial fibrillation [4%], hypertension [2%], serious bleeding [2%] and secondary malignancies [9%], while reactivation of hepatitis B arose in 3 patients on AO but none on A alone or OC; rates of ALT elevations were not reported).
- Ghia P, Pluta A, Wach M, Lysak D, Kozak T, Simkovic M, et al. ASCEND: phase III, randomized trial of acalabrutinib versus idelalisib plus rituximab or bendamustine plus rituximab in relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2020;38:2849–61. [PubMed: 32459600](Among 310 patients with refractory or relapsed CLL treated with acalabrutinib or standard regimens, the 12 month progression-free survival rate was greater with acalabrutinib [88% vs 68%] and adverse event rates were somewhat less [94% vs 99%] including severe adverse events [45% v 86%] and ALT elevations [1.9% vs 12%]).
- Lipsky A, Lamanna N. Managing toxicities of Bruton tyrosine kinase inhibitors. Hematology Am Soc Hematol Educ Program. 2020;2020:336–45. [PMC free article: PMC7727553] [PubMed: 33275698](Review of the frequency, severity, cause and management of adverse side effects of Bruton tyrosine kinase inhibitors including acalabrutinib focusing upon cardiac arrhythmias, bleeding risk, infections, hypertension, diarrhea, fatigue, arthralgias, myalgias, cytopenias, skin reactions and headache; no specific discussion of hepatotoxicity).
- Delgado J, Josephson F, Camarero J, Garcia-Ochoa B, Lopez-Anglada L, Prieto-Fernandez C, van Hennik PB, et al. EMA review of acalabrutinib for the treatment of adult patients with chronic lymphocytic leukemia. Oncologist. 2021;26:242–9. [PMC free article: PMC7930415] [PubMed: 33486852](Summary of the European Medicines Administration review of acalabrutinib which led to its approval in Europe for the therapy for CLL, adverse events included headache, diarrhea, neutropenia, nausea and infections and “hepatotoxicity” in 11% of cases in one study).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Ibrutinib.[LiverTox: Clinical and Researc...]Review Ibrutinib.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Safety and antitumor activity of acalabrutinib for relapsed/refractory B-cell malignancies: A Japanese phase I study.[Cancer Sci. 2021]Safety and antitumor activity of acalabrutinib for relapsed/refractory B-cell malignancies: A Japanese phase I study.Izutsu K, Ando K, Ennishi D, Shibayama H, Suzumiya J, Yamamoto K, Ichikawa S, Kato K, Kumagai K, Patel P, et al. Cancer Sci. 2021 Jun; 112(6):2405-2415. Epub 2021 May 7.
- Review A review of a novel, Bruton's tyrosine kinase inhibitor, ibrutinib.[J Oncol Pharm Pract. 2016]Review A review of a novel, Bruton's tyrosine kinase inhibitor, ibrutinib.Lee CS, Rattu MA, Kim SS. J Oncol Pharm Pract. 2016 Feb; 22(1):92-104. Epub 2014 Nov 25.
- Acalabrutinib, A Second-Generation Bruton's Tyrosine Kinase Inhibitor.[Recent Results Cancer Res. 2018]Acalabrutinib, A Second-Generation Bruton's Tyrosine Kinase Inhibitor.Kriegsmann K, Kriegsmann M, Witzens-Harig M. Recent Results Cancer Res. 2018; 212:285-294.
- Review Zanubrutinib.[LiverTox: Clinical and Researc...]Review Zanubrutinib.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Acalabrutinib - LiverToxAcalabrutinib - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...