NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Acamprosate is a synthetic amino acid and a neurotransmitter analogue that is used as an alcohol deterrent in management of alcohol dependence and abuse. Acamprosate has not been linked to serum enzyme elevations during therapy and has not been linked to cases of clinically apparent liver injury.
Background
Acamprosate (a kam' proe sate) is N-acetylhomotaurine, a synthetic amino acid analogue similar to gamma aminobutyric acid (GABA) and taurine that has been shown to decrease alcohol craving in animal models. Acamprosate appears to function as a neurotransmitter which acts as a GABA agonist and partial glutamate (N-methyl-D-aspartate [NMDA]) antagonist, but its precise mechanism of action in decreasing alcohol craving is unknown. When used in a comprehensive alcohol treatment program, acamprosate has been shown to decrease relapse to alcohol use, at least over the short term. Acamprosate was approved for use in the therapy of alcohol dependence and abuse in the United States in 2004. Current indications are for maintenance of alcohol abstinence in patients with alcohol dependence who are abstinent at the time of drug initiation. Acamprosate is available in delayed release tablets of 333 mg generically and under the brand name Campral. The typical maintenance dose is 666 mg three times daily and it is recommended to be used only as a part of a comprehensive alcohol treatment program. The most common side effects are fatigue, anorexia, diarrhea, flatulence, nausea, headache, dizziness, insomnia, paresthesia, itching and sweating. Rare but potentially serious adverse events include suicidal ideation and behavior and hypersensitivity reactions.
Hepatotoxicity
Acamprosate therapy has not been associated with serum enzyme elevations over and above rates that occur with placebo therapy. Despite widescale use in alcohol treatment programs, there have yet to be published reports of clinically apparent acute liver injury attributed to acamprosate therapy.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
Acamprosate is a synthetic amino acid, is minimally metabolized in the liver, and is excreted in the urine largely unchanged perhaps accounting for its lack of hepatotoxicity.
Drug Class: Substance Abuse Treatment Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Acamprosate – Generic, Campral®
DRUG CLASS
Substance Abuse Treatment Agents
Product labeling at DailyMed, National Library of Medicine, NIH
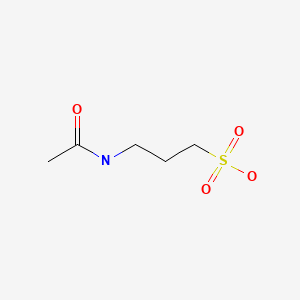
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Acamprosate | 77337-76-9 | C5-H11-N-O4-S |
|
ANNOTATED BIBLIOGRAPHY
References updated: 07 September 2021
- Zimmerman HJ. Drugs used in aversion therapy. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 724-6.(Expert review of hepatotoxicity published in 1999: discussion of hepatotoxicity of alcohol aversion pharmacotherapy is limited to disulfiram).
- Mihic SJ, Koob GF, Mayfield J, Harris RA. Ethanol. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 421-31.(Textbook of pharmacology and therapeutics).
- Paille FM, Guelfi JD, Perkins AC, Royer RJ, Steru L, Parot P. Double-blind, randomized multicentre trial of acamprosate in maintaining abstinence from alcohol. Alcohol Alcohol. 1995;30:239–47. [PubMed: 7662044](Controlled trial of 2 doses of acamprosate vs placebo in 538 patients with alcohol abuse; only side effect that was more frequent with drug compared to placebo was diarrhea [8-12% vs 3%]; no mention of ALT levels and no cases of jaundice or hepatitis).
- Whitworth AB, Fischer F, Lesch OM, Nimmerrichter A, Oberbauer H, Platz T, Potgieter A, et al. Comparison of acamprosate and placebo in long-term treatment of alcohol dependence. Lancet. 1996;347:1438–42. [PubMed: 8676626](Randomized controlled trial of 1 year course of acamprosate vs placebo in 448 recently abstinent alcoholic patients; only 40% of patients finished 1 year of therapy, abstinent rates were higher with acamprosate than placebo [18% vs 7%]; "Acamprosate had no effect on haematology or serum biochemistry").
- Chick J, Howlett H, Morgan MY, Ritson B. United Kingdom Multicentre Acamprosate Study (UKMAS): a 6-month prospective study of acamprosate versus placebo in preventing relapse after withdrawal from alcohol. Alcohol Alcohol. 2000;35:176–87. [PubMed: 10787394](Randomized controlled trial of 6 month course of acamprosate vs placebo in 581 recently abstinent alcoholic patients, found decrease in alcohol craving with acamprosate but no differences in rates of relapse; "No changes which could be attributed to the drug occurred in ... biochemistry test results").
- Bouza C, Angeles M, Muñoz A, Amate JM. Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: a systematic review. Addiction. 2004;99:811–28. [PubMed: 15200577](Systematic review of 33 studies of acamprosate and naltrexone; compliance rates were low, but acamprosate was associated with increased abstinence rates and had a "good safety pattern"; no mention of hepatotoxicity).
- Verheul R, Lehert P, Geerlings P, Koeter MW, van den Brink W. Predictors of acamprosate efficacy: results from a pooled analysis of seven European trials including 1485 alcohol-dependent patients. Psychopharmacology. 2005;178:167–73. [PubMed: 15322728](Combined analysis of 7 controlled trials of acamprosate in 1485 patients; no discussion of side effects).
- Rosenthal RN, Gage A, Perhach JL, Goodman AM. Acamprosate; safety and tolerability in the treatment of alcohol dependence. J Addict Med. 2008;2:40–50. [PubMed: 21768971](Analysis of 13 controlled trials of acamprosate in 4234 patients [1962 on placebo]; no clinically meaningful differences in clinical chemistry results were reported).
- Kranzler HR, Gage A. Acamprosate efficacy in alcohol-dependent patients: summary of results from three pivotal trials. Am J Addict. 2008;17:70–6. [PubMed: 18214726](Reanalysis of 3 pivotal trials conducted in Europe in support of the US approval of acamprosate including 998 patients treated for 13-52 weeks; on reanalysis, complete abstinence rates were higher with acamprosate in all 3 studies [risk ratios: 1.7-2.9]; no discussion of side effects or hepatotoxicity).
- Kennedy WK, Leloux M, Kutscher EC, Price PL, Morstad AE, Carnahan RM. Acamprosate. Expert Opin Drug Metab Toxicol. 2010;6:363–80. [PubMed: 20163323](Review of biochemistry, pharmacology, metabolism, efficacy and safety of acamprosate; no evidence of hepatotoxicity; indeed, abstinence is usually associated with decreases in liver enzymes).
- Rösner S, Hackl-Herrwerth A, Leucht S, Lehert P, Vecchi S, Soyka M. Acamprosate for alcohol dependence. Cochrane Database Syst Rev. 2010;(9):CD004332. [PubMed: 20824837](Systematic analysis of efficacy and safety of acamprosate for alcohol dependence, states that diarrhea is the only side effect occurring more frequently with acamprosate therapy; does not mention ALT elevations or hepatotoxicity).
- Witkiewitz K, Saville K, Hamreus K. Acamprosate for treatment of alcohol dependence: mechanisms, efficacy, and clinical utility. Ther Clin Risk Manag. 2012;8:45–53. [PMC free article: PMC3277871] [PubMed: 22346357](Review of safety and efficacy of acamprosate; "pharmavigilance data from over 1.5 million patients has revealed no serious health risks").
- Yahn SL, Watterson LR, Olive MF. Safety and efficacy of acamprosate for the treatment of alcohol dependence. Subst Abuse. 2013;6:1–12. [PMC free article: PMC3565569] [PubMed: 23399877](Review of safety and efficacy of acamprosate; no mention of hepatotoxicity or ALT elevations).
- Jonas DE, Amick HR, Feltner C, Bobashev G, Thomas K, Wines R, Kim MM, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311:1889–900. [PubMed: 24825644](Systematic review of the safety and efficacy of pharmacotherapies of alcohol dependence and abuse concludes that acamprosate helps to decrease alcohol relapse; no mention of hepatototixicity or ALT elevations).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–1352. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to acamprosate).
- Goh ET, Morgan MY. Review article: pharmacotherapy for alcohol dependence – the why, the what and the wherefore. Aliment Pharmacol Ther. 2017;45:865–882. [PubMed: 28220511](Review of medical therapies for alcohol dependence mentions that acamprosate is not metabolized in the liver and is generally safe in persons with impaired hepatic function, and that despite 30 years of clinical use it has not been reported to have serious adverse effects).
- Antonelli M, Ferrulli A, Sestito L, Vassallo GA, Tarli C, Mosoni C, Rando MM, et al. Alcohol addiction – the safety of available approved treatment options. Expert Opin Drug Saf. 2018;17:169–177. [PubMed: 29120249](Review of current pharmacologic treatments of alcohol addiction mentions that acamprosate has not been reported to be hepatotoxic and can be used safely in persons with mild liver disease).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Deterrent Action of Acamprosate: A Case Report.[Addict Health. 2019]Deterrent Action of Acamprosate: A Case Report.Spoorthy MS, Godi S, Singh LK. Addict Health. 2019 Oct; 11(4):276-280.
- Review Acamprosate. A review of its pharmacology and clinical potential in the management of alcohol dependence after detoxification.[Drugs. 1997]Review Acamprosate. A review of its pharmacology and clinical potential in the management of alcohol dependence after detoxification.Wilde MI, Wagstaff AJ. Drugs. 1997 Jun; 53(6):1038-53.
- Acamprosate for treatment of alcohol dependence: mechanisms, efficacy, and clinical utility.[Ther Clin Risk Manag. 2012]Acamprosate for treatment of alcohol dependence: mechanisms, efficacy, and clinical utility.Witkiewitz K, Saville K, Hamreus K. Ther Clin Risk Manag. 2012; 8:45-53. Epub 2012 Feb 1.
- Review Acamprosate in the treatment of alcohol dependence.[Expert Opin Pharmacother. 2005]Review Acamprosate in the treatment of alcohol dependence.Mason BJ. Expert Opin Pharmacother. 2005 Oct; 6(12):2103-15.
- Review Acamprosate in alcohol dependence: how does it work?[Addiction. 1995]Review Acamprosate in alcohol dependence: how does it work?Littleton J. Addiction. 1995 Sep; 90(9):1179-88.
- Acamprosate - LiverToxAcamprosate - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...