NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Ceftriaxone is a third generation cephalosporin antibiotic which has been associated with development of biliary sludge and biliary colic when given parenterally and in high doses. Ceftriaxone is also associated with rare instances of immunoallergic, usually cholestatic hepatitis similar to the injury associated with other cephalosporins.
Background
Ceftriaxone (sef" trye ax' one) is a third generation cephalosporin was approved for use in the United States in 1984, and it continues to be widely used. Ceftriaxone is available in a parenteral formulation generically and under the brand name of Rocephin. It can be given either intravenously or intramuscularly and is indicated for therapy of moderate-to-severe bacterial infections caused by susceptible organisms. Typical dose regimens in adults are 1 to 2 grams given im or iv in one or two divided doses daily for 7 to 14 days. The parenteral cephalosporins are widely used in medicine for serious infections and can be safely given to patients with advanced liver disease, dose modifications being required mainly for renal insufficiency. Ceftriaxone is also approved for use in children. Ceftriaxone is generally well tolerated; adverse events can include diarrhea, nausea, abdominal pain, dyspepsia, headache, and rash. Rare but potentially severe adverse events include Clostridium difficile-associated diarrhea, hypersensitivity reactions, angioedema, anaphylaxis and Stevens Johnson syndrome/toxic epidermal necrolysis.
Hepatotoxicity
Parenteral administration of ceftriaxone has been associated with development of biliary sludge in 3% to 46% of patients. The incidence may be higher in children than adults and is associated with higher doses and longer courses of treatment and possibly with fasting or dehydration. The syndrome is referred to as “pseudolithiasis” as the sludge and stones consist largely of ceftriaxone and they resolve spontaneously when the drug is stopped, indicating that surgery can be avoided. Most cases occur with minimal or no symptoms. Frank symptoms of cholecystitis are reported in up to 5% of patients who develop pseudo-lithiasis. Typically, serum enzymes and bilirubin levels remain normal even with biliary colic, but in rare instances there is cholestatic jaundice or gallstone pancreatitis that can be severe and require surgical intervention. Sludge and symptoms of gallbladder disease can arise within a few days of starting therapy, but typically resolve rapidly once ceftriaxone is stopped, although sludge and gallstones may be detectable by ultrasound for several months.
Ceftriaxone can also lead to an immunoallergic form of cholestatic hepatitis similar to what has been described with other cephalosporins. This reaction is idiosyncratic and is very rare. Symptoms of abdominal pain, nausea, pruritis and jaundice arise within 1 to 4 weeks of initiation of therapy and may worsen for 1 to 2 weeks after stopping the antibiotic. A cholestatic pattern of serum enzyme elevations and immunoallergic features of fever, rash and eosinophilia are common. The injury is usually mild and self-limited.
Likelihood score: B (ceftriaxone is a very likely cause of clinically apparent liver injury and can also lead to biliary sludge and “pseudolithiasis” caused by crystallization of ceftriaxone in bile present in the gallbladder or biliary tree).
Mechanism of Injury
The mechanism of biliary sludge formation during ceftriaxone therapy appears to be the propensity of ceftriaxone to bind calcium and form insoluble crystals in bile in the gallbladder, resulting in biliary sludge or frank stones. This complication is not idiosyncratic, being demonstrable in animal models. In a similar manner, calcium salts of ceftriaxone can precipitate in urine and lead to sludge in ureters or bladder that are capable of causing urinary obstruction or symptomatic kidney and bladder stones. The rare, idiosyncratic acute cholestatic liver injury linked to ceftriaxone is probably due to hypersensitivity.
Outcome and Management
In most case reports, recovery has been rapid within 1 to 2 weeks, but some patients have required cholecystectomy during the acute illness and stones or sludge may persist for up to several months. The immunoallergic form of cholestatic hepatitis generally resolves rapidly and has not been associated with acute liver failure or chronic injury.
Drug Class: Antiinfective Agents, Cephalosporins
CASE REPORT
Case 1. Biliary sludge and cholestatic hepatitis during intravenous administration of ceftriaxone.(1)
A 73 year old woman with rheumatoid arthritis developed nausea and right upper quadrant pain on the twelfth day of therapy with iv ceftriaxone for Klebsiella sepsis. A DISIDA scan demonstrated nonvisualization of the gallbladder and no excretion after 4 hours, suggesting common bile duct obstruction. Serum enzymes and amylase levels, which had been normal on admission, were increased. Abdominal ultrasound showed multiple small calculi in the gallbladder, but no biliary dilatation. Ultrasound done early during the hospitalization (for evaluation of renal disease) had shown a normal gallbladder without stones. Ceftriaxone was stopped a few days later and her abdominal pain and nausea improved promptly. Four days later, amylase and ALT levels were normal. Four weeks later, ultrasound demonstrated disappearance of the gallstones and alkaline phosphatase levels were normal.
Key Points
| Medication: | Ceftriaxone, 1.0 gram iv every 12 hours for 14 days |
|---|---|
| Pattern: | Cholestatic (R=1.7) |
| Severity: | 1+ (no jaundice) |
| Latency: | 12 days |
| Recovery: | Complete in 4 weeks |
| Other medications: | Piroxicam, ranitidine, furosemide, thyroxine, vitamin D, calcium |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|
| Pre | 51 | 103 | 0.2 | Admission for GI bleeding | |
| 0 | Ceftriaxone started (day 7 of hospitalization) | ||||
| 12 days | Abdominal pain and nausea: gallbladder sludge | ||||
| 13 days | 92 | 183 | 0.4 | Amylase 513 | |
| 14 days | 252 | 299 | Normal | Ceftriaxone stopped | |
| 18 days | 4 days | 52 | 245 | Ultrasound: single gallstone | |
| 6 weeks | 4 weeks | Normal | Normal | Normal | Ultrasound: no gallstones |
| Normal Values | Not given | <1.2 | |||
Comment
Only 5% to 10% of patients who develop biliary sludge (pseudolithiasis) during ceftriaxone therapy develop symptoms of biliary colic and, even with symptoms, patients rarely develop elevations of serum enzymes or bilirubin levels. The case described above developed what appeared to be a gallstone pancreatitis and partial biliary obstruction leading to mild elevations in serum aminotransferase and alkaline phosphatase levels, but without jaundice. The development of ceftriaxone biliary stones during therapy and dissolution afterwards was nicely documented in this case report. The biliary symptoms resolved with a day or two and the stones within 4 weeks of stopping therapy.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Ceftriaxone – Generic, Rocephin®
DRUG CLASS
Antiinfective Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
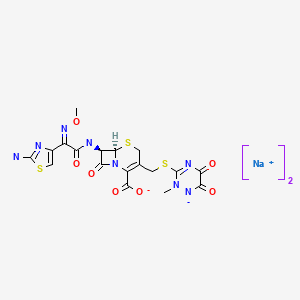
| Ceftriaxone Sodium | 74578-69-1 | C18-H18-N8-O7-S3.2Na |
|
CITED REFERENCES
- 1.
- Modified from. Zinberg J, Chernaik R, Coman E, Rosenblatt R, Brandt LJ. Reversible symptomatic biliary obstruction associated with ceftriaxone pseudolithiasis. Am J Gastroenterol. 1991;86(9):1251–4. [PubMed: 1882806]
ANNOTATED BIBLIOGRAPHY
References updated: 20 December 2021
- Zimmerman HJ. Cephalosporins. In, Hepatotoxicity: The Adverse Effects of Drugs and Other Chemicals on the Liver. 2nd Ed. Philadelphia: Lippincott, 1999. p. 589-92.(Expert review of cephalosporins and liver injury published in 1999).
- Moseley RH. Hepatotoxicity of antimicrobials and antifungal agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013. p. 463-83.(Review of cephalosporin induced liver injury mentions that ceftriaxone has been linked to biliary sludge and concretions and with occasional reports of cholestatic hepatitis).
- Petri WA Jr. Penicillins, cephalosporins, and other ß-lactam antibiotics. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's The pharmacological basis of therapeutics, 12th ed. New York: McGraw-Hill, 2011: 1477-1504.(Textbook of pharmacology and therapeutics).
- File TM Jr, Tan JS, Salstrom SJ. Clinical evaluation of ceftriaxone. Clin Ther. 1984;6:653–61. [PubMed: 6090021](Analysis of 77 patients receiving ceftriaxone for serious infections; 93% efficacy; ALT elevations in 8 [10%, peak levels 92 U/L] ).
- Oakes M, MacDonald H, Wilson D. Abnormal laboratory test values during ceftriaxone therapy. Am J Med. 1984;77:89–96. [PubMed: 6093527](Analysis of laboratory test abnormalities among 2640 patients receiving ceftriaxone for 1 day to 6 weeks at varying doses in prelicensure studies; ALT elevations occurred in 3.1%, Alk P 1.0% and bilirubin in 0.3%; rates that were similar to those of other iv cephalosporins; one case had ALT elevations with jaundice which resolved ultimately, but was not otherwise characterized).
- Moskovitz BL. Clinical adverse effects during ceftriaxone therapy. Am J Med. 1984;77(4C):84–8. [PubMed: 6093526](Review of adverse effects of ceftriaxone in prelicensure studies; 2640 patients in 153 studies, allergic reactions in 3%; jaundice in 2 patients, both of whom were septic and resolved with stopping therapy; no mention of biliary cholic).
- Parry MF. Toxic and adverse reactions encountered with new beta-lactam antibiotics. Bull N Y Acad Med. 1984;60:358–68. [PMC free article: PMC1911778] [PubMed: 6586251](Review suggesting that hepatitis occurs in "2-5%" of cephalosporin- and penicillin treated patients).
- Barson WJ, Miller MA, Brady MT, Powell DA. Prospective comparative trial of ceftriaxone vs. conventional therapy for treatment of bacterial meningitis in children. Pediatr Infect Dis. 1985;4:362–8. [PubMed: 3895175](Trial comparing ceftriaxone to ampicillin with chloramphenicol for meningitis in 50 children; similar efficacy, more diarrhea with ceftriaxone and 11% had minor ALT elevations, returning to normal during or after therapy).
- Schaad UB, Tschäppeler H, Lentze MJ. Transient formation of precipitations in the gallbladder associated with ceftriaxone therapy. Pediatr Infect Dis. 1986;5:708–10. [PubMed: 3540889](Initial report of transient biliary sludge in a 18 year old male with chronic granulomatous disease receiving iv ceftriaxone, who was found to have gallbladder abnormalities incidentally while being followed for a splenic abscess with repeat ultrasound examinations; the stones appeared during therapy and resolved soon after ceftriaxone was stopped).
- Norrby SR. Side effects of cephalosporins. Drugs. 1987;34 Suppl 2:105–20. [PubMed: 3319495](Clinical review of cephalosporins: ALT elevations occur in 1-8% of treated patients, but clinically apparent hepatitis is rare, usually occurring with allergic symptoms; little evidence for cross hypersensitivity with penicillins).
- Schaad UB, Wedgwood-Krucko J, Tschäppeler H. Reversible ceftriaxone-associated biliary pseudolithiasis in children. Lancet. 1988;2:1411–3. [PubMed: 2904533](Prospective ultrasound study of 37 children receiving ceftriaxone; 16 [43%] developed sludge and 1 urolithiasis in 4 to 22 days, 3 [8%] symptomatic, all resolved in 2-63 days; referred to syndrome as "biliary pseudolithiasis").
- Jacobs RF. Ceftriaxone-associated cholecystitis. Pediatr Infect Dis J. 1988;7:434–6. [PubMed: 3293002](16 year old girl given 2 g ceftriaxone iv twice daily, and 3 days later developed biliary colic with raised GGT [184 U/L], resolved in 8 weeks).
- Meyboom RH, Kuiper H, Jansen A. Ceftriaxone and reversible cholelithiasis. BMJ. 1988;297:858. [PMC free article: PMC1834624] [PubMed: 3140956](Two cases of biliary colic in adults during ceftriaxone therapy, both resolved; cholecystectomy later in one showed no stones and no abnormalities of gallbladder).
- Pigrau C, Pahissa A, Gropper S, Sureda D, Martinez Vazquez JM. Ceftriaxone-associated biliary pseudolithiasis in adults. Lancet. 1989;2:165. [PubMed: 2567936](Prospective ultrasound study in 20 adults receiving iv ceftriaxone; 5 [25%] developed stones by 4-17 days, all resolving in 7-26 days, serum enzymes normal and no symptoms; stones correlated with total ceftriaxone dose [5-80 g]).
- Cometta A, Gallot-Lavallée-Villars S, Iten A, Cantoni L, Anderegg A, Convers JJ, Glauser MP. Incidence of gallbladder lithiasis after ceftriaxone treatment. J Antimicrob Chemother. 1990;25:689–95. [PubMed: 2190975](Prospective study of 34 patients given amoxicillin/clavulanate vs 40 given ceftriaxone; follow up 6 and 12 months later showed only one gallstone - in an amoxicillin/clavulanate treated patient; ultrasound not done during therapy).
- Heim-Duthoy KL, Caperton EM, Pollock R, Matzke GR, Enthoven D, Peterson PK. Apparent biliary pseudolithiasis during ceftriaxone therapy. Antimicrob Agents Chemother. 1990;34:1146–9. [PMC free article: PMC171774] [PubMed: 2203305](Prospective study in 28 adults on iv ceftriaxone and 8 controls for 14 days found abnormalities in 6 [21%] treated and 1 [12%] controls after 14 days; 2 were symptomatic, all resolved spontaneously within 9 to 26 days).
- Shiffman ML, Keith FB, Moore EW. Pathogenesis of ceftriaxone-associated biliary sludge. In vitro studies of calcium-ceftriaxone binding and solubility. Gastroenterology. 1990;99:1772–8. [PubMed: 2227290](Ceftriaxone binds calcium in vitro and forms precipitations when added at high concentrations to human bile).
- Schaad UB, Suter S, Gianella-Borradori A, Pfenninger J, Auckenthaler R, Bernath O, Cheseaux JJ, et al. A comparison of ceftriaxone and cefuroxime for treatment of bacterial meningitis in children. N Engl J Med. 1990;322:141–7. [PubMed: 2403654](Prospective study comparing ceftriaxone and cefuroxime in 106 children with meningitis; all were cured; ultrasound identified sludge in 16 of 35 treated with ceftriaxone, but none in 35 cefuroxime treated children after 3-10 days; sludge resolved completely in 11 to 63 days after therapy; 3 [9%] had colic).
- Fekety FR. Safety of parenteral third-generation cephalosporins. Am J Med. 1990;88 Suppl 4A:38S–44S. [PubMed: 2183609](Review article stating that aminotransferase elevations can occur on cephalosporin therapy, but clinically apparent liver disease is rare).
- Lopez AJ, O'Keefe P, Morrissey M, Pickleman J. Ceftriaxone-induced cholelithiasis. Ann Intern Med. 1991;115:712–14. [PubMed: 1929040](Patient on iv ceftriaxone for endocarditis for unclear period of time developed pancreatitis and later underwent cholecystectomy demonstrating five soft, green ceftriaxone stones).
- Zinberg J, Chernaik R, Coman E, Rosenblatt R, Brandt LJ. Reversible symptomatic biliary obstruction associated with ceftriaxone pseudolithiasis. Am J Gastroenterol. 1991;86:1251–4. [PubMed: 1882806](73 year old woman with multiple medical problems developed biliary colic and pancreatitis [peak amylase 513 U/L] after 12 days of iv ceftriaxone for Klebsiella sepsis, with mild elevations in Alk P [299 U/L] and ALT [252 U/L], but no jaundice, resolving once ceftriaxone was stopped: Case 1).
- Park HZ, Lee SP, Schy AL. Ceftriaxone-associated gallbladder sludge. Identification of calcium-ceftriaxone salt as a major component of gallbladder precipitate. Gastroenterology. 1991;100:1665–70. [PubMed: 2019372](Surgical specimens from 4 patients with biliary sludge after ceftriaxone therapy showed no stones, but rather granular-crystalline material of cholesterol [2%], bilirubin [14%] and calcium-ceftriaxone).
- Kim YS, Kestell MR, Lee SP. Gall-bladder sludge: lessons from ceftriaxone. J Gastroenterol Hepatol. 1992;7:618–21. [PubMed: 1486190](Review and discussion of pathogenesis of ceftriaxone associated biliary sludge and stone formation).
- Riccabona M, Kerbl R, Schwinger W, Spork D, Millner M, Grubbauer HM. Klin Padiatr. 1993;205:421–3. [Ceftriaxone-induced cholestasis - a harmless side-effect?] German. [PubMed: 8309205](Among 43 children receiving iv ceftriaxone, 20 developed sludge by ultrasound within 10 days, 2 developing serum enzyme abnormalities, another 3 had severe pain, but all resolved within 2 months).
- Kirejczyk WM, Crowe HM, Mackay IM, Quintiliani R, Cronin EB. Disappearing “gallstones”: biliary pseudolithiasis complicating ceftriaxone therapy. AJR Am J Roentgenol. 1992;159:329–30. [PubMed: 1632349](19 year old woman with Lyme disease developed biliary colic 1 week after starting a 14 day course of iv ceftriaxone; had symptoms of fever and colic with gallstones on ultrasound that resolved in the next 3 weeks without therapy).
- Michielsen PP, Fierens H, Van Maercke YM. Drug-induced gallbladder disease. Incidence, aetiology and management. Drug Saf. 1992;7:32–45. [PubMed: 1536697](Review article on drug induced gallbladder disease focusing upon estrogens, clofibrate, ceftriaxone, octreotide, and hepatic artery chemotherapy).
- Thompson JW, Jacobs RF. Adverse effects of newer cephalosporins. An update. Drug Saf. 1993;9:132–142. [PubMed: 8397890](Extensive review; transient increases in ALT, AST or Alk P have been reported in 0.7%, 6%, 11% and 28% of prospectively followed patients treated with various cephalosporins; clinically significant biliary sludging with ceftriaxone, particularly in children, not found with other cephalosporins).
- Blais C, Duperval R. Biliary pseudolithiasis in a child associated with 2 days of ceftriaxone therapy. Pediatr Radiol. 1994;24:218–9. [PubMed: 7936805](18 month old boy given iv ceftriaxone for 2 days, developed biliary colic on day 5, normal gallbladder and no stones found on cholecystectomy on day 11).
- Barzilai M. Harefuah. 1994;127:163–5. [Sonographic demonstration of pseudo-cholelithiasis after ceftriaxone] Hebrew. [PubMed: 7995584](Case series of 2 children who developed symptoms and gallbladder sludge on ceftriaxone therapy; resolved with discontinuation).
- Di Martino V, Cadranel JF, Attali P. Gastroenterol Clin Biol. 1994;18:839–46. [Hepatobiliary complications induced by cephalosporins] French. [PubMed: 7875391](Review of hepatobiliary complications of cephalosporins mentioning rare case reports of hepatotoxicity and issue of pseudolithiasis with ceftriaxone).
- Ettestad PJ, Campbell GL, Welbel SF, et al. Biliary complications in the treatment of unsubstantiated Lyme disease. J Infect Dis. 1995;171:356–61. [PubMed: 7844372](Case control study in a hospital that reported 1730 admissions for therapy of Lyme disease; among 1352 patients, 24 developed gallstone disease and 14 had cholecystectomy; all had received iv ceftriaxone).
- Stabile A, Ferrara P, Marietti G, Maresca G. Ceftriaxone-associated gallbladder lithiasis in children. Eur J Pediatr. 1995;154:590. [PubMed: 7556332](Prospective study of 41 children receiving iv ceftriaxone; 5 [12%] developed stones/sludge, one with symptoms, all resolved in 10-30 days).
- Benedetti M, Zanchetta S, Bagnani A, Praderio R, Perbellini S, Melo C. Pediatr Med Chir. 1995;17:369–71. [Pseudolithiasis caused by ceftriaxone in children: a case report] Italian. [PubMed: 7491336](1 year old child developed symptomatic pseudolithiasis during iv ceftriaxone therapy, which resolved within 4 weeks of stopping).
- George DK, Crawford DH. Antibacterial-induced hepatotoxicity. Incidence, prevention and management. Drug Saf. 1996;15:79–85. [PubMed: 8862966](Review of hepatotoxicity from antibiotics with one sentence on cephalosporins, describing liver injury from this class as being extremely rare, although elevations in aminotransferases occurred in 0.7-11% of treated patients).
- Robertson FM, Crombleholme TM, Barlow SE, Verhave M, Brown D. Ceftriaxone choledocholithiasis. Pediatrics. 1996;98:133–5. [PubMed: 8668387](9 year old boy given 7 day course of iv ceftriaxone, developed biliary colic 5 days later, abnormal Alk P [335 U/L] and biliary dilatation on ultrasound; surgery found ceftriaxone stone in common bile duct).
- Kong MS, Chen CY. Risk factors leading to ceftriaxone-associated biliary pseudolithiasis in children. Changgeng Yi Xue Za Zhi. 1996;19:50–4. [PubMed: 8935375](Prospective ultrasound study in 151 children receiving iv ceftriaxone; 5 [3%] developed asymptomatic pseudolithiasis after 3-7 days; risk factors were fasting and older age).
- Longo F, Hastier P, Buckley MJ, Chichmanian RM, Delmont JP. Acute hepatitis, autoimmune hemolytic anemia, and erythroblastocytopenia induced by ceftriaxone. Am J Gastroenterol. 1998;93:836–7. [PubMed: 9625142](Elderly man developed jaundice with mixed enzyme elevations 3 days after a 12 day course of oral ceftriaxone; bilirubin 22 times, ALT 11 times and Alk P 6 times ULN, later developed severe hemolytic anemia requiring prednisone and resolving only by six months).
- Maranan MC, Gerber SI, Miller GG. Gallstone pancreatitis caused by ceftriaxone. Pediatr Infect Dis J. 1998;17:662–3. [PubMed: 9686742](13 year old developed pancreatitis 4 days after stopping 5 weeks of ceftriaxone with several other antibiotics; amylase 1133 U/L but normal ALT. Cholecystectomy showed stones and chronic cholecystitis; analysis of stone showed "100%"ceftriaxone).
- Herek O, Sarioğlu A, Koçer N, Tiryaki A, Akkemik B. Biliary pseudolithiasis in childhood: a case report. Eur J Pediatr Surg. 1999;9:337–9. [PubMed: 10584197](Case report of symptomatic biliary sludge arising after 6 days of intravenous ceftriaxone, resolving within 11 days of stopping).
- Vega C, Quinby PM, Aspy CB. Hepato-biliary abnormalities secondary to ceftriaxone use: a case report. J Okla State Med Assoc. 1999;92:432–4. [PubMed: 10461415](10 month old with rotavirus infection given ceftriaxone developed elevated Alk P [3580 U/L] and AST [84 U/L] after ~7 days, normal gallbladder ultrasound, rapid recovery).
- Papadopoulou F, Efremidis S, Karyda S, Badouraki M, Karatza E, Panteliadis C, Malaka K. Incidence of ceftriaxone-associated gallbladder pseudolithiasis. Acta Paediatr. 1999;88(12):1352–5. [PubMed: 10626521](Prospective study in 44 children given iv ceftriaxone for 3 to 14 days; 11 [25%] developed pseudolithiasis after 2-9 days, 2 with symptoms [5%]; all resolved completely in 8-23 days).
- de Moor RA, Egberts AC, Schröder CH. Ceftriaxone-associated nephrolithiasis and biliary pseudolithiasis. Eur J Pediatr. 1999;158:975–7. [PubMed: 10592073](7 year old boy developed urinary stone and biliary sludge after 4 days of high dose iv ceftriaxone and passed a ceftriaxone containing urinary stone after 10 days).
- Bonnet JP, Abid L, Dabhar A, Lévy A, Soulier Y, Blangy S. Early biliary pseudolithiasis during ceftriaxone therapy for acute pyelonephritis in children: a prospective study in 34 children. Eur J Pediatr Surg. 2000;10:368–71. [PubMed: 11215777](Prospective study in 34 children given iv ceftriaxone with ultrasound before and at end of therapy; 5 [15%] developed asymptomatic gallstones, all resolved within 5 months).
- Tuckuviene R, Myrtue GS. Ugeskr Laeger. 2000;162:4271–2. [Symptomatic pseudolithiasis caused by Rocephalin] Danish. [PubMed: 10962947](5 year old girl developed symptomatic pseudolithiasis on ceftriaxone, resolving rapidly with stopping).
- Palanduz A, Yalçin I, Tonguç E, Güler N, Oneş U, Salman N, Somer A. Sonographic assessment of ceftriaxone-associated biliary pseudolithiasis in children. J Clin Ultrasound. 2000;28:166–8. [PubMed: 10751736](Prospective study of 118 children receiving 1-3 weeks of iv ceftriaxone found 17% developed sludge; all were asymptomatic and all resolved in 2 weeks; no risk factors observed).
- Grasberger H, Otto B, Loeschke K. Ceftriaxone-associated nephrolithiasis. Ann Pharmacother. 2000;34:1076–7. [PubMed: 10981257](31 year old man developed symptomatic kidney stones 8 days after starting iv ceftriaxone, resolving spontaneously).
- Prince JS, Senac MO Jr. Ceftriaxone-associated nephrolithiasis and biliary pseudolithiasis in a child. Pediatr Radiol. 2003;33:648–51. [PubMed: 12830336](14 year old boy developed nephrolithiasis with pain and hematuria and increased creatinine; both biliary and urinary sludge found, referred to as "toothpaste"; resolution in 3 weeks).
- Ravisha MS, Godambe SV. Ceftriaxone induced cholestasis in a neonate: a case report. Indian J Med Sci. 2004;58:73–4. [PubMed: 14993721](17 day old male developed cholestasis after 7 days of iv ceftriaxone; sludge on ultrasound, bilirubin 2.6 mg/dL, ALT 98 U/L, Alk P 1194 U/L, resolution within 3-7 days).
- Wen HH, Huang YK, Zheng GL. Ceftriaxone-associated gallbladder pseudolithiasis: report of one case. Acta Paediatr Taiwan. 2004;45:290–2. [PubMed: 15868813](5 year old boy developed cholelithiasis 5 days after starting ceftriaxone, resolving after 1 month).
- Acun C, Erdem LO, Sogut A, Erdem CZ, Tomac N, Gundogdu S. Ceftriaxone-induced biliary pseudolithiasis and urinary bladder sludge. Pediatr Int. 2004;46(3):368–70. [PubMed: 15151561](5 year old girl developed biliary colic after 3 days of ceftriaxone; laboratory tests were normal, but ultrasound showed both gallbladder and urinary bladder sludge, resolving spontaneous within 12 days of stopping).
- Acun C, Erdem LO, Söğüt A, Erdem CZ, Tomaç N, Gündoğdu S, Cavuldak S. Gallbladder and urinary tract precipitations associated with ceftriaxone therapy in children: a prospective study. Ann Trop Paediatr. 2004;24:25–31. [PubMed: 15005963](Prospective ultrasound study of 36 children given iv ceftriaxone for up to 14 days; 5 [14%] developed gallbladder and one [3%] urinary bladder stones, most were symptomatic, all resolved spontaneously).
- Avci Z, Koktener A, Uras N, et al. Nephrolithiasis associated with ceftriaxone therapy: a prospective study in 51 children. Arch Dis Child. 2004;89:1069–72. [PMC free article: PMC1719698] [PubMed: 15499067](Prospective study of 51 children with various infections treated with ceftriaxone iv or im for 5-10 days; nephrolithiasis found by ultrasound in 4 children [8%], all asymptomatic, 3 resolved in follow up; no risk factors found).
- Bor O, Dinleyici EC, Kebapci M, Aydogdu SD. Ceftriaxone-associated biliary sludge and pseudocholelithiasis during childhood: a prospective study. Pediatr Int. 2004;46:322–4. [PubMed: 15151550](Prospective study of 38 children receiving iv ceftriaxone; ultrasound at 10 days showed gallstones in 29% and sludge in 8%; one child had symptoms; resolution usually occurred within 1 month, but sometimes required 3).
- Evliyaoglu C, Kizartici T, Bademci G, Unal B, Keskil S. Ceftriaxone-induced symptomatic pseudolithiasis mimicking ICP elevation. Zentralbl Neurochir. 2005;66:92–4. [PubMed: 15846537](Patient developed pseudolithiasis after neurosurgery and iv ceftriaxone; made differential diagnosis difficult).
- Ceran C, Oztoprak I, Cankorkmaz L, Gumuç C, Yildiz T, Koyluoglu G. Ceftriaxone-associated biliary pseudolithiasis in paediatric surgical patients. Int J Antimicrob Agents. 2005;25:256–9. [PubMed: 15737522](Prospective ultrasound study of 50 children treated with ceftriaxone after surgery; 13 [26%] developed sludge or stones within 4-22 days; resolved within 3-63 days, no enzyme elevations, no risk factors found).
- Bell MJ, Stockwell DC, Luban NL, Shirey S, Shaak L, Ness PM, Wong ECC. Ceftriaxone-induced hemolytic anemia and hepatitis in an adolescent with hemoglobin SC disease. Pediatr Crit Care Med. 2005;6:363–6. [PubMed: 15857541](17 year old boy with sickle cell disease and severe hemolytic anemia given ceftriaxone developed progressive renal and hepatic failure and death; liver failure likely due to shock).
- Bickford CL, Spencer AP. Biliary sludge and hyperbilirubinemia associated with ceftriaxone in an adult: case report and review of the literature. Pharmacotherapy. 2005;25:1389–95. [PubMed: 16185184](Review of literature on ceftriaxone associated pseudolithiasis; case report of 53 year old man with chronic hepatitis C who developed jaundice after 7 days of iv ceftriaxone with little change in ALT or AST [bilirubin 5.8 mg/dL, ALT 41 U/L, Alk P 88 U/L], which resolved only once ceftriaxone was stopped, but role of sepsis could not be excluded).
- Rivkin AM. Hepatocellular enzyme elevations in a patient receiving ceftriaxone. Am J Health Syst Pharm. 2005;62:2006–10. [PubMed: 16174837](Case report and literature review; seriously ill 31 year old man in ICU had increase in ALT from 9 to 56 to 442 U/L, but normal Alk P and bilirubin and no symptoms after 7 days of ceftriaxone therapy, with resolution within 2 weeks of switching antibiotics).
- Biner B, Oner N, Celtik C, Bostancioğlu M, Tunçbilek N, Güzel A, Karasalihoğlu S. Ceftriaxone-associated biliary pseudolithiasis in children. J Clin Ultrasound. 2006;34:217–222. [PubMed: 16673364](Among 156 children given iv ceftriaxone who were followed prospectively with serial abdominal ultrasounds, 27 [17%] developed biliary stones or sludge and 5 [3%] became symptomatic; resolving within 10-30 days of stopping; risk factors were older age and higher doses of ceftriaxone).
- Araz N, Okan V, Demirci M, Araz M. Pseudolithiasis due to ceftriaxone treatment for meningitis in children: report of 8 cases. Tohoku J Exp Med. 2007;211:285–90. [PubMed: 17347554](Case series of 8 children with ceftriaxone induced pseudolithiasis; all resolved within 30 days).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, one case was attributed to ceftriaxone alone [85 year old man developed cholestatic hepatitis 3 weeks after a four day course of intravenous ceftriaxone]).
- Peker E, Cagan E, Dogan M. Ceftriaxone-induced toxic hepatitis. World J Gastroenterol. 2009;15:2669–71. [PMC free article: PMC2691501] [PubMed: 19496200](12 year old boy developed fatigue after 3 days of ceftriaxone therapy followed by jaundice [bilirubin 4.2 mg/dL, ALT 871 U/L, Alk P 143 U/L, 8% eosinophils], resolving within 10 weeks of stopping).
- Ferrajolo C, Capuano A, Verhamme KM, Schuemie M, Rossi F, Stricker BH, Sturkenboom MC. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol. 2010;70:721–8. [PMC free article: PMC2997312] [PubMed: 21039766](Worldwide pharmacovigilance database contained 9036 hepatic adverse drug reactions in children, 104 of which were attributed to ceftriaxone, ranking 10th in frequency and being the only cephalosporin listed).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, one of which was attributed to cefepime, but none to ceftriaxone or other cephalosporins).
- Kaur I, Singh J. Cholestatic hepatitis with intravenous ceftriaxone. Indian J Pharmacol. 2011;43:474–5. [PMC free article: PMC3153719] [PubMed: 21845011](24 year old woman developed dark urine 24 hours after starting ceftriaxone and piroxicam and 3 days later was jaundiced [bilirubin 6.5 mg/dL, ALT 164 U/L, Alk P 580 U/L], resolving within 3 weeks of stopping both drugs).
- Choi YY, Jung YH, Choi SM, Lee CS, Kim D, Hur KY. Gallbladder pseudolithiasis caused by ceftriaxone in young adult. J Korean Surg Soc. 2011;81:423–6. [PMC free article: PMC3243861] [PubMed: 22200045](Two men, ages 21 and 22 years, developed gallstones found by CT scan 5 and 17 days after starting ceftriaxone without symptoms or laboratory abnormalities, resolving within 1 month of stopping).
- Hanata N, Imamura T, Koyama R, Koizumi Y, Tamura T, Ito J, Takeuch K. Nihon Naika Gakkai Zasshi. 2012;101:2955–7. [Case report; a case of biliary pseudolithiasis associated with ceftriaxone] Japanese. [PubMed: 23214106]
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, of which 2 were attributed to cephalosporins, one with jaundice to cephalexin and one with enzyme elevations only to ceftazidime).
- Rodríguez Rangel DA, Pinilla Orejarena AP, Bustacara Diaz M, Henao García L, López Cadena A, Montoya Camargo R, Moreno LA. An Pediatr (Barc). 2014;80:77–80. [Gallstones in association with the use of ceftriaxone in children.] Spanish. [PubMed: 23759541](Prospective study in 73 children receiving ceftriaxone, identified sludge or gallstones [4-14 mm in diameter] by ultrasound in 31 [43%], of whom 7 had symptoms, all resolving within 2 months of stopping, but one requiring surgery).
- Zheng FY, Xing Y, Lu S, Liu DM. Zhongguo Dang Dai Er Ke Za Zhi. 2013;15:696–7. [Ceftriaxone-associated biliary pseudolithiasis in a child with nephrotic syndrome] Chinese. [PubMed: 23965889]
- Tomoda T, Ueki T, Saito S, Tatsukawa M, Nawa T, Hamamoto H, Endo H, et al. Nihon Shokakibyo Gakkai Zasshi. 2013;110:1481–6. [A case of ceftriaxone-associated pseudolithiasis in an adult patient that disappeared after the discontinuation of ceftriaxone] Japanese. [PubMed: 23912008](47 year old woman found to have developed biliary stones and sludge on CT scan after 8 days of intravenous ceftriaxone, which disappeared within 6 days).
- von Martels JZ, Van de Meeberg EK, Holman M, Ligtenberg JJ, Ter Maaten JC. Pseudolithiasis after recent use of ceftriaxone: an unexpected diagnosis in a child with abdominal pain. Am J Emerg Med 2013; 31: 1294. e5-6. [PubMed: 23809091](14 year old boy treated for Lyme disease with a 2 week course of iv ceftriaxone presented 4 days later with abdominal pain and biliary stones by ultrasound and ERCP [bilirubin 4.2 mg/dL, ALT 187 U/L, Alk P 398, GGT 291 U/L], resolving with conservative management within a few days and no stones found on follow-up ultrasound 4 weeks later).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, but none were attributed to ceftriaxone or a cephalosporin).
- Alemayehu H, Desai AA, Thomas P, Sharp SW, St Peter SD. Ceftriaxone-induced pseudolithiasis in children treated for perforated appendicitis. Pediatr Surg Int. 2014;30:323–6. [PubMed: 24464035](Among 71 children treated with iv ceftriaxone for perforated appendicitis, 10 [14%] developed gallbladder stones or sludge, one of whom developed symptoms and underwent cholecystectomy).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: the DILIN prospective study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury seen over a ten year period at 8 U.S. medical centers, 323 [36%] were attributed to antibiotics of which 36 [4%] were cephalosporins including cefazolin [21], ceftriaxone [4], cefalexin [3], cefadroxil [2] and cefuroxime [2] and four others [1 case each]).
- Alqahtani SA, Kleiner DE, Ghabril M, Gu J, Hoofnagle JH, Rockey DC., Drug-Induced Liver Injury Network (DILIN) Study Investigators. Identification and characterization of cefazolin-induced liver injury. Clin Gastroenterol Hepatol. 2015;13:1328–36.e2. [PMC free article: PMC4472636] [PubMed: 25528012](Among 1019 patients enrolled in a US database of drug induced liver injury between 2004 and 2012, 33 [3%] were due to cephalosporins, of which 19 presented 6-29 days after a single iv injection of cefazolin exhibiting a cholestatic pattern of injury, mild immunoallergic features, moderate severity and invariably a self-limited course).
- Nakaharai K, Sakamoto Y, Yaita K, Yoshimura Y, Igarashi S, Tachikawa N. Drug-induced liver injury associated with high-dose ceftriaxone: a retrospective cohort study adjusted for the propensity score. Eur J Clin Pharmacol. 2016;72(8):1003–11. [PubMed: 27126206](Retrospective analysis of 471 patients treated with ceftriaxone found that 15 [3.2%] developed abnormal liver tests within two weeks of treatment, including 16% receiving high dose [4 g daily] vs 2.1% receiving standard dose [2 g daily]).
- Bonkovsky HL, Kleiner DE, Gu J, Odin JA, Russo MW, Navarro VM, Fontana RJ, et al. U.S. Drug Induced Liver Injury Network Investigators. Clinical presentations and outcomes of bile duct loss caused by drugs and herbal and dietary supplements. Hepatology. 2017;65:1267–1277. [PMC free article: PMC5360519] [PubMed: 27981596](Among 26 patients with drug induced liver injury and bile duct loss on liver biopsy, most developed severe cholestatic hepatitis an evidence of chronic residual liver injury suggestive of varying degrees of vanishing bile duct syndrome, 1 case was attributed to oral cephalexin and one to parenteral cefazolin but none to ceftriaxone).
- Zhao Z, Bao L, Yu X, Zhu C, Xu J, Wang Y, Yin M, et al. Acute vanishing bile duct syndrome after therapy with cephalosporin, metronidazole, and clotrimazole: A case report. Medicine (Baltimore). 2017;96:e8009. [PMC free article: PMC6392967] [PubMed: 28885366](27 year old woman was treated with “cephalosporin”, metronidazole and clotrimazole after removal of an intrauterine device and developed evidence of liver injury several weeks later [bilirubin 1.7 mg/dL, ALT 335 U/L, Alk P 655 U/L], with persistence of jaundice for several months and liver biopsy showing paucity of bile ducts, but ultimate resolution of jaundice but persistence of mild Alk P elevations).
- Low EXS, Zheng Q, Chan E, Lim SG. Drug induced liver injury: East versus West – a systematic review and meta-analysis. Clin Mol Hepatol. 2020;26:142–154. [PMC free article: PMC7160354] [PubMed: 31816676](Analysis of literature on causes of drug induced liver injury found that the most common causes from Asian reports were antituberculosis drugs [25%], phenytoin [3.5%], and cephalosporins [3%] while causes from European and American reports were most commonly amoxicillin/clavulanate [11%], nimesulide [6%], and ibuprofen [6%]).
- Park JH, Hong S, Jun DW, Yoon JH, Lee KN, Lee HL, Lee OY, et al. Prevalence and clinical characteristics of antibiotics associated drug induced liver injury. Ann Transl Med. 2021;9:642. [PMC free article: PMC8106034] [PubMed: 33987340](Among 166 patients seen at a single Korean medical center over a 12 month period [2017-18] for serum enzyme elevations, 113 were attributed to drugs, including 78 [64%] to antibiotics, most frequently flomoxef [a cephalomycin, n=24], cetrazole [9], ceftriaxone [6], vancomycin [5], piperacillin-tazobactam [5], and amoxicillin/clavulanate [4], all of whom recovered upon stopping; unclear whether any were symptomatic or jaundiced).
- Ferrajolo C, Verhamme KM, Trifirò G, 't Jong GW, Picelli G, Giaquinto C, Mazzaglia G, et al. Antibiotic-induced liver injury in paediatric outpatients: a case-control study in primary care databases. Drug Saf. 2017;40:305–315. [PMC free article: PMC5362651] [PubMed: 28025733](Among 938 cases of drug-associated liver injury in children identified in Italian and Dutch databases [with 93,665 controls] between 2000 and 2008, 138 arose after recent use of antibiotics including 26 linked to cephalosporins; cefaclor [n=8], cefixime [8], ceftriaxone [3], ceftibuten [3], cefpodoxime [2] and cefuroxime [1], the highest risk odds ratio being for ceftriaxone [14.7] and cefixime [6.1]).
- Khurram D, Shamban L, Kornas R, Paul M. Marked direct hyperbilirubinemia due to ceftriaxone in an adult with sickle cell disease. Case Rep Gastrointest Med. 2015;2015:462165. [PMC free article: PMC4458556] [PubMed: 26101675](32 year old African American man with sickle cell disease during admission for an acute crisis and ceftriaxone and azithromycin therapy for suspected pneumonitis, had rise in total bilirubin levels from 3.3 to a peak of 17 mg/dL, which fell to baseline on switching antibiotics and resolution of the acute crisis; no direct bilirubin levels provided but increase was likely due to hemolysis and sepsis).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Ceftriaxone-induced cholestatic hepatitis in a child: A case report and a review of the literature.[Front Pediatr. 2022]Ceftriaxone-induced cholestatic hepatitis in a child: A case report and a review of the literature.Castellazzi ML, Agostoni CV, Palella J, Civeriati D, Marchisio P, Nebbia G. Front Pediatr. 2022; 10:1051887. Epub 2022 Dec 5.
- Review Biliary sludge and hyperbilirubinemia associated with ceftriaxone in an adult: case report and review of the literature.[Pharmacotherapy. 2005]Review Biliary sludge and hyperbilirubinemia associated with ceftriaxone in an adult: case report and review of the literature.Bickford CL, Spencer AP. Pharmacotherapy. 2005 Oct; 25(10):1389-95.
- A case of ceftriaxone-induced liver injury and literature review.[Infez Med. 2022]A case of ceftriaxone-induced liver injury and literature review.Guarino M, Perna B, Pastorelli A, Bertolazzi P, Caio G, Maritati M, De Giorgio R, Contini C. Infez Med. 2022; 30(2):293-297. Epub 2022 Jun 1.
- Implications of current recommendations for third-generation cephalosporin use in the WHO Western Pacific Region following the emergence of multiresistant gonococci.[Sex Transm Infect. 2009]Implications of current recommendations for third-generation cephalosporin use in the WHO Western Pacific Region following the emergence of multiresistant gonococci.Tapsall JW. Sex Transm Infect. 2009 Aug; 85(4):256-8. Epub 2009 Mar 3.
- Review Acute cholecystitis and pancreatitis in a patient with biliary sludge associated with the use of ceftriaxone: a rare but potentially severe complication.[Ann Ital Med Int. 1999]Review Acute cholecystitis and pancreatitis in a patient with biliary sludge associated with the use of ceftriaxone: a rare but potentially severe complication.Famularo G, Polchi S, De Simone C. Ann Ital Med Int. 1999 Jul-Sep; 14(3):202-4.
- Ceftriaxone - LiverToxCeftriaxone - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...