NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Didanosine is a purine nucleoside analogue and reverse transcriptase inhibitor that was previously widely used in combination with other agents in the therapy of human immunodeficiency virus (HIV) infection and the acquired immunodeficiency syndromes (AIDS). Didanosine therapy is associated with an appreciable rate of serum enzyme elevations during therapy, and it is a well established cause of clinically apparent acute liver injury as well as mitochondrial injury to many organs which can be associated with severe liver injury, lactic acidosis and hepatic failure. Finally, long term therapy with didanosine can cause noncirrhotic portal hypertension due to hepatoportal sclerosis and nodular regenerative hyperplasia. Because of its many, potentially severe adverse events, didanosine has been largely replaced by better tolerated agents and is now rarely used.
Background
Didanosine (dye dan' oh seen) is a synthetic nucleoside analogue of inosine (2’,3’-dideoxyinosine, ddI) that is converted intracellularly to dideoxyadenosine. Didanosine inhibits HIV replication by competing with naturally occurring adenosine for incorporation into the growing viral DNA chain, causing inhibition of the viral polymerase and chain termination. Didanosine is a first generation antiretroviral agent and was approved for use in the United States in 1991. It was widely used for many years, but has now been replaced by better tolerated agents. Because of its relatively low cost, didanosine continues to be used in developing nations. Didanosine is indicated for the treatment of HIV infection in combination with other antiretroviral agents. Didanosine is available in generic forms and under the trade names Videx in 100 mg tablets and as extended release capsules of 125, 200, 250, and 400 mg, as well as in an oral powder for suspension (10 mg/mL). The recommended dose in adults is 250 to 400 mg orally once daily. Common side effects include rash, abdominal pain, diarrhea, nausea and vomiting, asthenia, headache, and fever. Uncommon but potentially severe adverse events include pancreatitis, hepatotoxicity, peripheral neuropathy, immune reconstitution syndrome, lipodystrophy and severe hypersensitivity reactions.
Hepatotoxicity
Mild and transient elevations in liver enzymes occur in up to 9% of patients on didanosine, but these are generally asymptomatic and self-limited. Clinically apparent hepatotoxicity is uncommon, but well described. Several forms of liver injury have been associated with didanosine use: acute idiosyncratic liver injury, lactic acidosis with steatosis and hepatic dysfunction (LASH), and noncirrhotic portal hypertension due to nodular regenerative hyperplasia or hepatoportal sclerosis.
Rare instances of acute, seemingly idiosyncratic liver injury due to didanosine have been described, particularly in children. The injury arises within a few weeks of starting therapy and is associated with hepatocellular pattern of serum enzymes (Case 3). Immunoallergic features (rash, fever and eosinophilia) may occur, but are not prominent and autoantibodies are generally not present. Recovery is rapid with stopping didanosine, but the injury can be severe and lead to acute liver failure. This form of didanosine injury is rare and resembles the idiosyncratic acute hepatitis-like injury that occurs with many medications.
The second pattern of injury due to didanosine is more common and is characterized by development of marked lactic acidosis, microvesicular steatosis, and hepatic synthetic dysfunction (LASH). This form of liver injury typically arises after at least two months of therapy and is preceded by nonspecific prodromal symptoms of anorexia, nausea, vomiting, diarrhea, and weakness which is followed by dyspnea, jaundice and confusion. Lactic acidosis usually accompanies the hepatic injury and may be the predominant clinical feature. Jaundice arises late and serum enzymes are unusually only mildly or moderated elevated, the pattern being mixed or actually cholestatic. Pancreatitis, myopathy and neuropathy may also occur. This distinctive form of hepatotoxicity associated with didanosine can be rapidly fatal (Case 1), but is potentially reversible with intensive support and early withdrawal of therapy (Case 2). Preexisting liver injury, female sex, older age, obesity, alcohol use and concurrent therapy with stavudine, ribavirin or tenofovir appear to increase the risk of this syndrome. LASH is most commonly associated with stavudine therapy, but can also occur with zidovudine, fialuridine, intravenous tetracycline, linezolid and aspirin (Reye syndrome).
A final form of didanosine associated liver injury is noncirrhotic portal hypertension due to nodular regenerative hyperplasia or hepatoportal sclerosis (Case 4). This chronic form of liver injury due to didanosine generally arises after several years of therapy. Patients usually present with signs of portal hypertension and advanced liver disease, ascites, variceal hemorrhage, muscle wasting and weakness with no obvious cause (absence of hepatitis B or C and no history of alcoholism). Serum enzymes are only modestly elevated and bilirubin levels can be normal. Platelet counts tend to be low and a fall of platelet count can be a useful surrogate marker for the development of portal hypertension. These patients have typically been on multiple antiretroviral agents and the attribution of injury to didanosine cannot always be made. The clinical presentation can be after didanosine has been discontinued. Stavudine and zidovudine have also been implicated as have other nucleoside analogues such as azathioprine, mercaptopurine and thioguanine. Major risk factors associated with this complication include duration of didanosine use, low CD4 cell counts and concurrent use of stavudine. Liver biopsy shows the absence of cirrhosis and changes of nodular regenerative hyperplasia or hepatoportal sclerosis or both. Hepatic venous pressure gradients are usually elevated, but probably underestimate the degree of portal hypertension because of its presinusoidal nature. Similarly, noninvasive tests for hepatic fibrosis, such a transient elastography, are usually abnormal but not to the degree that is usually associated with the portal hypertension that occurs with cirrhosis.
Interestingly, a high proportion of patients who develop noncirrhotic portal hypertension have an underlying thrombophilic condition such as protein S or protein C deficiency and are prone to develop portal vein thrombosis which can be the cause of acute decompensation.
Likelihood score: A (well known cause of both acute and chronic forms of clinically apparent liver injury).
Mechanism of Injury
The clinically apparent hepatotoxicity from didanosine associated with lactic acidosis and hepatic steatosis is probably a direct effect of the nucleoside due to widespread injury or depletion of mitochondria. In vitro, didanosine has been found to inhibit the gamma polymerase responsible for replication of mitochondria and maintenance of mitochondrial function and numbers. After several months of therapy, mitochondrial numbers can fall to critical levels. The mitochondrial failure in hepatocytes leads to inability to metabolize lactic acid and free fatty acids and to support usual hepatic synthetic and excretory function. Mitochondrial injury to other tissue can lead to pancreatitis, myopathy and neuropathy.
The pathogenesis of the other forms of liver injury from didanosine are not well defined. Nodular regenerative hyperplasia and hepatoportal sclerosis are suspected to be caused by chronic vascular injury to small portal vein radicles and may be another manifestation of mitochondrial depletion and injury. The idiosyncratic hepatitis associated with didanosine may be immunologically mediated and is probably unrelated to mitochondrial injury.
Didanosine is chiral molecule that can exist in left (L or levorotatory) and right (D or dextrotatory) forms. Didanosine is a synthetic nucleoside analogue and is a racemic mixture of equal amounts of the D and L forms. In nature, however, only the D-forms of nucleosides are made and used to synthesize DNA. Mitochondrial injury appears to be more frequent with the D- rather than L- enantiomers of nucleoside analogues, and the greater safety of L-isoforms has been used to produce safer and better tolerated antiviral agents such as lamivudine (L-3TC), telbivudine (L-thymidine) and clevudine (L-FMAU).
Outcome and Management
The severity of liver injury due to didanosine ranges from mild and transient enzyme elevations to clinically apparent hepatitis and to severe hepatic steatosis with intractable lactic acidosis and hepatic failure. Cases of acute hepatic failure with centrolobular necrosis have also been reported with didanosine, largely in children. Long term therapy is associated with noncirrhotic portal hypertension, but there have also been isolated reports of cirrhosis after nucleoside analogue induced severe hepatic steatosis and lactic acidosis. Didanosine has not been associated with vanishing bile duct syndrome. Rechallenge can lead to recurrence of mitochondrial dysfunction and should be avoided. Persons who develop didanosine hepatotoxicity should avoid use of other dideoxynucleosides such as stavudine and zalcitabine, and caution should be used in use of zidovudine. Various interventions have been used in attempts to treat the severe lactic acidosis and hepatic steatosis induced by nucleoside analogues. These interventions have included bicarbonate infusions, thiamine, l-carnitidine as well as renal dialysis and mechanical ventilation. Intravenous 20% glucose decreases lactic acid levels in some patients. Liver transplantation has reversed lactic acidosis in the rare patient that has undergone emergency transplantation, but this option is rarely practical.
Nodular regenerative hyperplasia from long term therapy with didanosine, stavudine or zidovudine should be treated conservatively with management of the signs and symptoms of portal hypertension. Stavudine (and all first generation nucleoside analogues) should be discontinued and the patient switched to other antiretroviral agents. Typically, nodular regenerative hyperplasia improves after drug withdrawal, and symptoms, signs and abnormal liver tests may ultimately resolve. The long term consequences of nodular regenerative hyperplasia after drug withdrawal is uncertain and not well defined. Unrelated acute severe medical conditions such as pneumonia or sepsis can cause reappearance of signs and symptoms of portal hypertension (ascites, variceal hemorrhage) and evidence of hepatic decompensation (weakness, jaundice, and hepatic encephalopathy).
Drug Class: Antiviral Agents, Antiretroviral Agents
Other Drugs in the Subclass, Nucleoside Analogues: Abacavir, Adefovir, Emtricitabine, Entecavir, Lamivudine, Stavudine, Telbivudine, Tenofovir, Zidovudine
CASE REPORTS
Case 1. Lactic acidosis, marked hepatic steatosis and liver failure due to didanosine.(1)
A 45 year old man with HIV infection and AIDS on zidovudine for 7 months and didanosine for 4 months developed nausea, vomiting, diarrhea, abdominal pain and shortness of breath. Blood testing showed severe metabolic acidosis with a serum bicarbonate of 3 mmol/L and lactate 43 mmol/L. Serum bilirubin and enzymes were only modestly elevated (Table). The patient had no history of liver or biliary tract disease or alcohol abuse. Tests for hepatitis A, B, C, D and E were negative. Despite intensive support, mechanical ventilation, bicarbonate therapy, and renal dialysis, he developed progressive hepatic and multiorgan failure and died 7 days after admission. Transjugular liver biopsy showed diffuse microvesicular steatosis with moderate cholestasis, but little hepatocellular necrosis, parenchymal inflammation or fibrosis.
Key Points
| Medication: | Didanosine (7.5 mg/kg/day) for 13 weeks |
|---|---|
| Pattern: | Mixed; mild enzyme elevations (R=2.3) |
| Severity: | 5+ (fatal) |
| Latency: | 13 weeks |
| Recovery: | None |
| Other medications: | Zidovudine, pentamidine |
Laboratory Values
Comment
Typical course of lactic acidosis due to nucleoside analogue therapy with a 1 to 2 week prodromal period of nausea, diarrhea, and anorexia followed by progressive dyspnea and confusion. The injury is typically “delayed” requiring months of therapy before there is significant decline in mitochondrial function or numbers. Despite severe hepatic synthetic failure, serum enzymes are usually only modestly elevated and serum bilirubin elevations occur late. The lactic acidosis of nucleoside analogue therapy appears to respond transiently to infusions of 20% glucose and can be reversed by emergency liver transplantation, but this is rarely feasible.
Case 2. Lactic acidosis arising during therapy with didanosine after addition of tenofovir.(2)
A 45 year old woman with HIV infection and chronic hepatitis C had been treated with a combination of didanosine, stavudine, and tenofovir for 8 weeks when she developed vomiting, abdominal pain, and obtundation. Nevirapine had been stopped and tenofovir added to a long term regimen of stavudine and didanosine 8 weeks before admission because of minor serum enzymes elevations which then returned to initial values. On admission, she was jaundiced and disoriented and had tender hepatomegaly. Serum bilirubin was 12.6 mg/dL, ALT 157 U/L, and an INR was 2.1. She had lactic acidosis with blood pH of 7.24 and lactate levels of 16.4 mmol/L. Imaging of the liver suggested fatty infiltration of the liver. Antiretrovirals were discontinued, but the lactic acidosis and hepatic failure worsened, and she died two days after admission.
Key Points
Comment
Acute microvesicular hepatic steatosis with liver failure and lactic acidosis is a syndrome associated with several medications including the nucleoside analogues, particularly didanosine, stavudine and zidovudine. A similar syndrome occurs with intravenous tetracycline, aspirin (Reyes syndrome) and valproate, but the timing and course is different for those agents (shorter latency period), probably because they directly affect function of mitochondria rather than by causing functional failure by inhibition of mitochondrial replication. Mitochondria have a half-life of several weeks, so that inhibition of mitochondrial replication would be expected to lead to severe dysfunction (mitochondrial failure) after 2 to 3 months. Both didanosine and stavudine have been linked to many cases of hepatic steatosis and lactic acidosis, and the addition of tenofovir appears to increase the risk of the complication. By itself, tenofovir has not been associated with this syndrome. Other risk factors for hepatic steatosis with lactic acidosis include presence of underlying liver disease (such as hepatitis C), female sex, older age, obesity and alcohol use.
Case 3. Severe liver toxicity attributed to didanosine therapy.(3)
A 36 year old man with long standing HIV infection who had been treated with multiple antiretroviral regimens and who was on long term stavudine therapy was started on didanosine, nelfinavir and nevirapine and developed nausea and liver test abnormalities 3 months later [bilirubin 1.5 mg/dL, ALT 217 U/L, Alk P normal]. Antiretroviral therapy was stopped and he recovered rapidly. Two months later, all liver tests were normal and he was restarted on didanosine, stavudine, and hydroxyurea. Three months later, he again developed nausea, right upper quadrant pain and jaundice. Serum bilirubin was 14.5 mg/dL, ALT 241 U/L, and alkaline phosphatase 121 U/L, with prolongation of the prothrombin time and decrease in serum albumin to 2.1 g/dL. Lactic acid levels were not provided, but serum bicarbonate was normal. Tests for hepatitis A, B and C were negative, and he had no history of alcohol abuse. The antiretroviral agents were stopped. A liver biopsy showed prominent ballooning degeneration, hepatocyte necrosis, chronic inflammatory infiltrates and cholestasis without steatosis. He improved slowly. Four months later the he was anicteric and asymptomatic, and eight months later serum enzyme levels were normal and an antiretroviral regimen of abacavir, efavirenz and nelfinavir was started without further evidence of hepatotoxicity.
Key Points
| Medication: | Didanosine |
|---|---|
| Pattern: | Mixed |
| Severity: | 4+ (jaundice and prothrombin time prolongation) |
| Latency: | 3 months |
| Recovery: | Several months |
| Other medications: | Stavudine, hydroxyurea, trimethoprim-sulfamethoxazole |
Comment
This patient developed a severe hepatitis 3 months after adding didanosine to an antiretroviral regimen that included stavudine. The clinical features were somewhat different from those typical of hepatic steatosis and lactic acidosis due to mitochondrial injury. Similar cases of severe acute hepatitis due to didanosine have been reported in children. The pathogenesis is unknown, but is likely to be idiosyncratic.
Case 4. Noncirrhotic portal hypertension caused by long-term didanosine therapy.(4)
A 10 year old girl with vertically acquired HIV infection developed progressive enlargement of the spleen of unknown cause. She had been on antiretroviral therapy for 8 years which had consisted of didanosine, tenofovir and lopinavir/ritonavir for the previous 7 years. The HIV infection was well controlled with a CD4+ lymphocyte count of 425 cells/µL and undetectable levels of HIV RNA (<37 copies/mL). The platelet count was 154,000/µL, INR 1.28, bilirubin 0.8 mg/dL, ALT 48 U/L, AST 52 U/L, Alk P 231 U/L, GGT 35 U/L and albumin 3.5 g/dL. Tests for hepatitis B and C were negative as were autoantibodies. There was mild decreases in protein C (49% of normal) and free protein S antigen (60%), but no other evidence of genetic hypercoagulability (such as Factor V Leiden or prothrombin genetic mutations). Imaging showed diffuse inhomogeneity of the liver texture and nodularity, splenomegaly, mild ascites and patency of the portal and hepatic veins, but presence of venous collaterals. Upper endoscopy revealed esophageal varices. Hepatic venous pressure gradient measurements were normal (3.8 mm Hg) and liver biopsy showed hepatoportal sclerosis with absence of portal vein branches in portal tracts, mild-to-moderate steatosis, focal sinusoidal fibrosis and central venosclerosis. Thus, she was believed to have noncirrhotic portal hypertension and hepatoportal sclerosis, possibly due to long term didanosine therapy. Her antiretroviral regimen was changed to darunavir/ritonavir and etravirine. Serum HIV RNA levels remained undetectable and liver tests were stable. However, within a year of diagnosis of noncirrhotic portal hypertension she had an esophageal variceal bleed for which she underwent band ligation and therapy with propranolol.
Key Points
| Medication: | Didanosine for 7 years |
|---|---|
| Pattern: | (R=2.3) |
| Severity: | 4+ (severe, portal hypertension) |
| Latency: | 7 years |
| Recovery: | None |
| Other medications: | Tenofovir, lopinavir, ritonavir, iron |
Comment
This case demonstrates a fairly typical presentation of noncirrhotic portal hypertension in a patient with HIV infection on long term didanosine therapy. Concurrent use of tenofovir may have predisposed to this complication. Routine liver tests were only mildly abnormal, but the platelet count was low. The unusual features were the patient's age and lack of other complications of HIV. These features, however, show that this complication is independent of serious comorbidities of HIV, HBV or HCV and occurs in patients with excellent control of the underlying HIV infection. As described, the liver histology was most compatible with hepatoportal sclerosis, but features of nodular regeneration were not commented upon and they are often subtle and easy to miss. The pathogenesis of the liver injury that causes these two histological patterns is not well understood, but appears to be related to portal venopathy with loss of small portal radicles with subsequent portal fibrosis or nodular regeneration or both. Venous thromboses in the portal system are also frequent as are mild-to-moderate decreases in protein C and S, which can result in portal vein thrombosis. Whether the underlying pathogenesis is an inflammatory venopathy, mitochondrial damage to portal endothelial cells or microthromboses in the portal system (or all three) is unclear. The prognosis of noncirrhotic portal hypertension is guarded, even with prompt discontinuation of didanosine and avoidance of other nucleoside analogues, slowly progressive portal hypertension and its complications can occur. Within a year, this child developed a variceal hemorrhage. Because of the multiplicity of complications of long term didanosine therapy, it is now rarely used in developed countries. Patients who are on didanosine should be monitored for complications and serial platelet counts may represent the most convenient surrogate means of early detection of portal hypertension. A decrease of more than 14,000 platelets/µL per year (even while in the normal range) is suggestive of the development of mild portal hypertension.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Didanosine – Generic, Videx®
DRUG CLASS
Antiviral Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Didanosine | 69655-05-6 | C10-H12-N4-O3 |
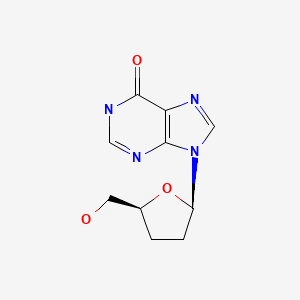
|
CITED REFERENCES
- 1.
- Bissuel F, Bruneel F, Habersetzer F, Chassard D, Cotte L, Chevallier M, Bernuau J, et al. Fulminant hepatitis with severe lactate acidosis in HIV-infected patients on didanosine therapy. J Intern Med. 1994;235:367–71. [PubMed: 8151270]
- 2.
- Rivas P, Polo J, de Górgolas M, Fernández-Guerrero ML. Drug points: Fatal lactic acidosis associated with tenofovir. BMJ. 2003;327:711. [PMC free article: PMC200801] [PubMed: 14512477]
- 3.
- Ware AJ, Berggren RA, Taylor WE. Didanosine-induced hepatitis. Am J Gastroenterol. 2000;95:2141–3. [PubMed: 10950090]
- 4.
- Giacomet V, Viganò A, Penagini F, Manfredini V, Maconi G, Camozzi M, Zuccotti GV. Splenomegaly and variceal bleeding in a ten-year-old HIV-infected girl with noncirrhotic portal hypertension. Pediatr Infect Dis J. 2012;31:1059–60. [PubMed: 22828640]
ANNOTATED BIBLIOGRAPHY
References updated: 25 June 2020
Abbreviations used: AIDS, acquired immune deficiency syndrome; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HPA, hepatoportal sclerosis; HVPG, hepatic venous pressure gradient; mt, mitochondrial; NASH, nonalcoholic steatohepatitis; NCPH, noncirrhotic portal hypertension; NRH, nodular regenerative hyperplasia; TE, transient elastography.
- Núñez M. Hepatic toxicity of antiviral agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 505-18.(Review of hepatotoxicity of antiviral agents including didanosine).
- Flexner C. Antiretroviral agents and treatment of HIV infection. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1137-58.(Textbook of pharmacology and therapeutics).
- National Institutes of Health. http://aidsinfo
.nih.gov/guidelines. (Clinical guidelines on the use of antiretroviral agents in HIV-1 infected adults, adolescents and children). - Cooley TP, Kunches LM, Saunders CA, Perkins CJ, Kelley SL, McLaren C, McCaffrey RP, et al. Treatment of AIDS and AIDS-related complex with 2',3'-dideoxyinosine given once daily. Rev Infect Dis. 1990;12:S552–S560. [PubMed: 1974727](Phase 1 trial of didanosine [6 dose regimens] in 36 patients with AIDS for up to 65 weeks; 6 [17%] had ALT elevations requiring dose reduction or interruption, not all attributable to drug).
- Lai KK, Gang DL, Zawacki JK, Cooley TP. Fulminant hepatic failure associated with 2',3'-dideoxyinosine (ddI). Ann Intern Med. 1991;115:283–4. [PubMed: 1906693](36 year old man with AIDS developed nausea and weakness after 15 weeks of didanosine therapy [bilirubin 5.3 mg/dL, ALT 263 U/L, Alk P 90 U/L], with progressive lactic acidosis and hepatic failure, autopsy showing large liver [2550 g], marked cholestasis and microvesicular fat).
- Kahn JO, Lagakos SW, Richman DD, Cross A, Pettinelli C, Liou SH, Brown M, et al. A controlled trial comparing continued zidovudine with didanosine in human immunodeficiency virus infection. The NIAID AIDS Clinical Trials Group. N Engl J Med. 1992;327:581–7. [PubMed: 1353607](913 patients with HIV infection on zidovudine were randomized to switch to didanosine [500 or 750 mg/day] or continue zidovudine; pancreatitis occurred in 7-13% of didanosine [2 fatalities], but only 3% of zidovudine treated patients; no mention of ALT levels of hepatotoxicity).
- Freiman JP, Helfert KE, Hamrell MR, Stein DS. Hepatomegaly with severe steatosis in HIV-seropositive patients. AIDS. 1993;7:379–85. [PubMed: 8471200](Reports of 6 fatal and 2 nonfatal cases of hepatomegaly and steatosis in patients with HIV on zidovudine for 3-12 months).
- Chattha G, Arieff AI, Cumings G, Tierney LM Jr. Lactic acidosis complicating the acquired immunodeficiency syndrome. Ann Intern Med. 1993;118:37–9. [PubMed: 8416156](Report of 7 patients with HIV infection who developed lactic acidosis of unknown cause, presenting with nausea, anorexia and weight loss followed by dyspnea, stupor and death [in 4]; 4 on zidovudine, 1 ganciclovir and 1 clofazimine; initial arterial pH 7.09-7.27, lactate 10.4-17.4 mmol/L).
- McKenzie R, Fried MW, Sallie R, Conjeevaram H, Di Bisceglie AM, Park Y, Savarese B, et al. Hepatic failure and lactic acidosis due to fialuridine (FIAU), an investigational nucleoside analogue for chronic hepatitis B. N Engl J Med. 1995;333:1099–105. [PubMed: 7565947](Description of syndrome of lactic acidosis, hepatic failure and pancreatitis arising after 8-11 weeks of fialuridine treatment in 15 patients with chronic hepatitis B; among 7 patients affected, 5 died of intractable lactic acidosis and 2 survived, but required emergency liver transplantation).
- Lewis W, Dalakas MC. Mitochondrial toxicity of antiviral drugs. Nat Med. 1995;1:417–22. [PubMed: 7585087](Review of mechanisms for mitochondrial injury by nucleoside analogues including inhibition of mitochondrial DNA polymerase gamma).
- Styrt B, Freiman JP. Hepatotoxicity of antiviral agents. Gastroenterol Clin North Am. 1995;24:839–52. [PubMed: 8749901](Early review of liver toxicity of antiviral agents; covering the first four nucleoside analogues used for HIV infection: zidovudine, didanosine, zalcitabine and stavudine).
- Bissuel F, Bruneel F, Habersetzer F, Chassard D, Cotte L, Chevallier M, Bernuau J, et al. Fulminant hepatitis with severe lactate acidosis in HIV-infected patients on didanosine therapy. J Intern Med. 1994;235:367–71. [PubMed: 8151270](Two cases of fatal acute liver failure with lactic acidosis and microvesicular fatty liver in HIV-positive patients treated with didanosine for 3-4 months with initial bilirubin 1.6 and 1.1 mg/dL, ALT 146 and 40 U/L, Alk P 146 and 151 U/L; liver histology showed diffuse microvesicular fat and mild cholestasis without fibrosis or hepatocellular necrosis: Case 1).
- Lacaille F, Ortigao MB. Debré, Rouzioux C, Brousse N, Blanche S. Hepatic toxicity associated with 2.3.-dideoxyinosine in children with AIDS. J Pediatr Gastroenterol Nutr. 1995;20:287–90. [PubMed: 7608823](Among 34 children in a trial of didanosine [120 or 270 mg/m2 daily] for HIV infection, 2 developed acute liver failure after 2 and 18 months of therapy with ALT 1320 and 1440 U/L and severe centrolobular necrosis; 4 others had marked Alk P elevations of 1113-6930 U/L 5-12 months after starting didanosine, improving with stopping).
- Brinkman K, ter Hofstede HJ, Burger DM, Smeitink JAM, Koopmans PP. Adverse effects of reverse transcriptase inhibitors: mitochondrial toxicity as a common pathway. AIDS. 1998;12:1735–44. [PubMed: 9792373](Review of mitochondrial function and role of mitochondrial toxicity or depletion in the adverse side effects of nucleoside analogues).
- Finkle HI. Hepatic mitochondrial toxicity from nucleoside analog therapy. Arch Pathol Lab Med. 1999;123:189. [PubMed: 10086505](42 year old man with HIV infection developed lactic acidosis on combination therapy with stavudine and didanosine [ALT 73 U/L], autopsy showing microvesicular steatosis).
- Allaouchiche B, Duflo F, Cotte L, Mathon L, Chassard D. Acute pancreatitis with severe lactic acidosis in an HIV-infected patient on didanosine therapy. J Antimicrob Chemother. 1999;44:137–8. [PubMed: 10459826](58 year old man with HIV infection developed abdominal pain and amylase elevations [1059 U/L] and jaundice after treatment with didanosine, stavudine and indinavir [bilirubin 7.3 mg/dL, ALT 75 U/L, Alk P 107 U/L, lactate 13 mmol/L], resolving within 3 weeks of stopping).
- Frippiat F, Derue G, Heller F, Honore P, Moreau M, Vandercam B. Acute pancreatitis associated with severe lactic acidosis in human immunodeficiency virus-infected patients receiving triple therapy. J Antimicrob Chemother. 2000;45:411–2. [PubMed: 10702573](Letter in response to Allaouchiche et al. suggesting that stavudine may have been the cause or contributed to the mitochondrial toxicity and pancreatitis).
- Bleeker-Rovers CP, Kadir S, van Leusen R, Richter C. Hepatic steatosis and lactic acidosis caused by stavudine in an HIV-infected patient. Neth J Med. 2000;57:190–3. [PubMed: 11063865](45 year old man with HIV developed nausea and abdominal pain after 2 years of didanosine and 3 months of stavudine therapy [bilirubin 14.0 mg/dL, ALT 300 U/L, Alk P 400 U/L, no obvious acidosis], resolving slowly stopping and having recurrent rise in lactate within one week of restarting [9.1 mmol/L]).
- ter Hofstede HJ, de Marie S, Foudraine NA, Danner SA, Brinkman K. Clinical features and risk factors of lactic acidosis following long-term antiretroviral therapy: 4 fatal cases. Int J STD AIDS. 2000;11:611–6. [PubMed: 10997508](Four patients with fatal lactic acidosis arising after long term therapy for HIV, all on stavudine and 3 on didanosine as well for 6-18 months, presenting with nausea, abdominal pain and weight loss with pH 7.04-7.17; autopsies in 2 showed severe hepatomegaly, micro- and macro-steatosis, and cholestasis).
- Gérard Y, Maulin L, Yazdanpanah Y, De La Tribonniè X, Amiel C, Maurage CA, Robin S, et al. Symptomatic hyperlactataemia: an emerging complication of antiretroviral therapy. AIDS. 2000;14:2723–30. [PubMed: 11125891](Identified 14 patients with symptoms of elevated lactate [levels 0.9 to 9.4 mmol/L] with fatigue, abdominal pain, weight loss, neuropathy and dyspnea on exertion, all on stavudine [9 also on didanosine] for 2-29 months).
- Brivet FG, Nion I, Mérbane B, Slama A, Brivet M, Rustin P, Munnich A. Fatal lactic acidosis and liver steatosis associated with didanosine and stavudine treatment: a respiratory chain dysfunction? J Hepatol. 2000;32:364–5. [PubMed: 10707883](Patient with HIV on didanosine and stavudine for 9 months developed nausea and anorexia, [bilirubin 1.2 mg/dL, ALT 206 U/L] and progressive lactic acidosis leading to death 5 days later; autopsy showed pancreatitis and hepatomegaly with severe macrovesicular steatosis).
- Claessens Y-E, Cariou A, Chiche J-D, Dauriat G, Dhainaut J-F. L-carnitine as a treatment of life-threatening lactic acidosis induced by nucleoside analogues. AIDS. 2000;14:472–3. [PubMed: 10770558](34 year old man developed abdominal pain and nausea 11 months after starting stavudine, didanosine and saquinavir with severe lactic acidosis, cholestasis and fatty liver; given intravenous L-carnitine and slowly recovered).
- Lonergan JT, Behling C, Pfander H, Hassanein TI, Mathews WC. Hyperlactatemia and hepatic abnormalities in 10 human immunodeficiency virus-infected patients receiving nucleoside analogue combination regimens. Clin Infect Dis. 2000;31:162–6. [PubMed: 10913415](Over a 6 month period, 10 HIV-positive patients presented with hyperlactatemia [2.9-6.2 mmol/L]; all were taking stavudine, 5 also on didanosine and 7 lamivudine for 4-20 months; 8 with symptoms of abdominal pain, nausea or distension; all with ALT elevations [2-10.7 times ULN], 3 with HBV or HCV; imaging showed fatty liver in 5; all resolved with stopping, lactate levels falling to normal after 16 to 111 days).
- Ware AJ, Berggren RA, Taylor WE. Didanosine-induced hepatitis. Am J Gastroenterol. 2000;95:2141–3. [PubMed: 10950090](36 year old man with HIV infection developed nausea 3 months after didanosine was added to antiretroviral regimen of stavudine nelfinavir, and nevirapine [bilirubin 1.5 mg/dL ALT 217 U/L, Alk P normal], resolving on stopping and recurring 3 months after didanosine and stavudine [without nelfinavir] were restarted [peak bilirubin 14.5 mg/dL, ALT 241 U/L Alk P 121 U/L], biopsy showing acute and chronic inflammatory changes, hepatocyte necrosis and fibrosis without fat: Case 3).
- Sulkowski MS, Thomas DL, Chaisson RE, Moore RD. Hepatotoxicity associated with antiretroviral therapy in adults infected with human immunodeficiency virus and the role of hepatitis C or B virus infection. JAMA. 2000;283:74–80. [PubMed: 10632283](Among 298 patients with HIV infection, ALT elevations above 5 times ULN occurred in 10.4% per year during antiretroviral treatment; factors associated with ALT elevations included ritonavir [27.3%] and coinfection with either HCV or HBV; ALT with bilirubin elevations occurred in 3 patients; 2 on indinavir and all 3 with coinfection).
- Carr A, Miller J, Law M, Cooper DA. A syndrome of lipoatrophy, lactic acidaemia and liver dysfunction associated with HIV nucleoside analogue therapy: contribution to protease inhibitor-related syndrome. AIDS. 2000;14:F25–32. [PubMed: 10716495](Description of 14 patients with lipodystrophy who never received protease inhibitors, but most had received stavudine [86%], didanosine [71%] for >6 months and most had hyperlactatemia, ALT elevations and weight loss, nausea and fatigue, most resolved slowly upon withdrawal).
- John M, Moore CB, James IR, Nolan D, Upton RP, McKinnon EJ, Mallal SA. Chronic hyperlactatemia in HIV-infected patients taking antiretroviral therapy. AIDS. 2001;15:717–23. [PubMed: 11371686](349 patients with HIV infection were screened for lactate levels on multiple occasions; 2 had symptomatic lactic acidosis and were both on stavudine: estimated incidence at 3.9 per 1000 person years).
- Coghlan ME, Sommadossi JP, Jhala NC, Many WJ, Saag MS, Johnson VA. Symptomatic lactic acidosis in hospitalized antiretroviral-treated patients with human immunodeficiency virus infection: a report of 12 cases. Clin Infect Dis. 2001;33:1914–21. [PubMed: 11692304](Experience with 12 cases of lactic acidosis in patients with HIV infection seen over 6 years: cases typically presented with anorexia, nausea and weight loss for several weeks, AST levels were variably elevated; 11 patients were on stavudine, 9 didanosine, 1 zidovudine alone; 6 had pancreatitis; 6 had liver biopsies, all of which showed macro- and micro-steatosis; five died).
- Boubaker K, Flepp M, Sudre P, Furrer H, Haensel A, Hirschel B, Boggian K, et al. Hyperlactatemia and antiretroviral therapy: the Swiss HIV Cohort Study. Clin Infect Dis. 2001;33:1931–7. [PubMed: 11692306](Cross sectional analysis of lactate testing on 880 Swiss patients with HIV receiving antiretroviral therapy; elevated levels were associated with stavudine and didanosine use and with lipodystrophy [and high glucose and triglycerides], but not age, sex, CD4 counts or HIV RNA levels).
- Lederman JC, Nawaz H. Toxic interaction of didanosine and acetaminophen leading to severe hepatitis and pancreatitis: a case report and review of the literature. Am J Gastroenterol. 2001;96:3474–5. [PubMed: 11774996](Patient on didanosine for 1 month took 4 g of acetaminophen over 3 days period and developed jaundice and ascites [bilirubin 15.1 mg/dL, ALT 280 IU/L, Alk P 280 IU/L], resolving completely after stopping didanosine).
- Carr A, Morey A, Mallon P, Williams D, Thorburn DR. Fatal portal hypertension, liver failure, and mitochondrial dysfunction after HIV-1 nucleoside analogue-induced hepatitis and lactic acidaemia. Lancet. 2001;357:1412–4. [PubMed: 11356442](65 year old developed hyperlactatemia and ascites 14 months after starting didanosine and stavudine [lactate 7 mmol/L, no acidosis, ALT 181 U/L], recovered slowly after stopping, but presented 1 year later with signs of portal hypertension, ascites, encephalopathy and varices; biopsy did not show cirrhosis; possibly nodular regenerative hyperplasia).
- Church JA, Mitchell WG, Gonzalez-Gomez I, Christensen J, Vu TH, Dimauro S, Boles RG. Mitochondrial DNA depletion, near-fatal metabolic acidosis, and liver failure in an HIV-infected child treated with combination antiretroviral therapy. J Pediatr. 2001;138:748–51. [PubMed: 11343055](2 year old treated for 18 months with didanosine, zidovudine and nelfinavir developed lactic acidosis and hepatic failure [bilirubin 30.6 mg/dL, AST 224 U/L], resolving slowly after stopping drugs).
- Clark SJ, Creighton S, Portmann B, Taylor C, Wendon JA, Cramp ME. Acute liver failure associated with antiretroviral treatment for HIV: a report of six cases. J Hepatol. 2002;36:295–301. [PubMed: 11830344](6 patients with HIV infection who developed acute liver failure on stavudine [n=5], lamivudine [n=3], didanosine [n=2], saquinavir [n=2], efavirenz [n=2], nevirapine [n=2], and nelfinavir, delavirdine or zidovudine [n=1] for 1-3 months [peak bilirubin 2.7-32 mg/dL, AST 240-8650 U/L, Alk P 122-191 U/L]; 2 with signs of hypersensitivity; 2 with hepatitis B; 5 died, autopsies showing massive necrosis; one with massive steatosis; likely multiple causes).
- Lemberg DA, Palasanthiran P, Goode M, Ziegler JB. Tolerabilities of antiretrovirals in paediatric HIV infection. Drug Saf. 2002;25:973–91. [PubMed: 12408730](Review of adverse events to antiretroviral agents in children; rates of hepatotoxicity appear to be similar in children as adults, mitochondrial toxicity is rare, but deaths due to pancreatitis and liver failure have been reported in children on didanosine).
- Falcó V, Rodríguez D, Ribera E, Martínez E, Miró JM, Domingo P, Diazaraque R, et al. Severe nucleoside-associated lactic acidosis in human immunodeficiency virus-infected patients: report of 12 cases and review of the literature. Clin Infect Dis. 2002;34:838–46. [PubMed: 11850865](Between 1997-2000, 12 cases of lactic acidosis were identified in HIV-infected patients at 4 hospitals in Spain; ~1:1000 patient-years of treatment; all receiving nucleoside analogue for 1-36 months, 1 attributed to zidovudine, 11 to stavudine [1 also on didanosine] with ALT 30-524 U/L; 33% fatality rate).
- Moyle GJ, Datta D, Mandalia S, Morlese J, Asboe D, Gazzard BG. Hyperlactataemia and lactic acidosis during antiretroviral therapy: relevance, reproducibility and possible risk factors. AIDS. 2002;16:1341–9. [PubMed: 12131210](Retrospective analysis of results of testing for lactate levels in 1239 patients with HIV infection, 108 [9%] had elevated levels >2.5 mmol/L and 9 [1%] >5 mmol/L, of whom 4 had lactic acidosis and 2 died; in multivariate analysis, elevations were associated with didanosine use and female sex).
- Spengler U, Lichterfeld M, Rockstroh JK. Antiretroviral drug toxicity - a challenge for the hepatologist? J Hepatol. 2002;36:283–94. [PubMed: 11830343](Review of the diagnosis of drug induced liver disease in patients with HIV on antiretroviral agents, with discussion of mechanisms including mitochondrial toxicity and hypersensitivity reactions).
- Hillaire S, Bonte E, Denninger MH, Casadevall N, Cadranel JF, Lebrec D, Valla D, et al. Idiopathic non-cirrhotic intrahepatic portal hypertension in the West: a re-evaluation in 28 patients. Gut. 2002;51:275–80. [PMC free article: PMC1773310] [PubMed: 12117894](Among 28 cases of NCPH diagnosed between 1994 and 1998, none were diagnosed with HIV infection and no discussion of drug relatedness or didanosine; 12 had a prothrombotic disorder).
- Thio CL, Seaberg EC, Skolasky R Jr, Phair J, Visscher B, Muñoz A, Thomas DL., Multicenter AIDS Cohort Study. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet. 2002;360(9349):1921–6. [PubMed: 12493258](Cohort study of 5293 men who have sex with men followed for an average of 10.5 years found liver related mortality highest in HIV/HBV coinfected [1.4% yearly] than in HIV monoinfected [0.2%] or those with HBsAg alone [0.08%]).
- Koch RO, Graziadel IW, Zangerle R, Romani N, Maier H, Vogel W. Acute hepatic failure and lactic acidosis associated with antiretroviral treatment for HIV. Wien Klin Wochenschr. 2003;115:135–40. [PubMed: 12674693](36 year old woman with HIV developed nausea and abdominal pain 18 months after starting didanosine and stavudine [bilirubin 1.2.12.0 mg/dL, ALT 40.177 U/L], with pancreatitis, lactic acidosis, hepatic encephalopathy and ascites, recovered slowly upon stopping drugs; liver biopsy showed microvesicular fat and cholestasis, with little inflammation).
- Cornejo-Juárez P, Sierra-Madero J, Volkow-Fernández P. Metabolic acidosis and hepatic steatosis in two HIV-infected patients of stavudine (d4T) treatment. Arch Med Res. 2003;34:64–9. [PubMed: 12604378](1 woman and 1 man developed fatigue and jaundice 5 and 1 months after switching to an antiretroviral regimen that included stavudine [bilirubin 14.3 and 27.0 mg/dL, ALT 45 and 62U/L, Alk P 835 and 296 U/L], with lactic acidosis, hepatic steatosis and fatal outcome).
- Murphy MD, O., Hearn M, Chou S. Fatal lactic acidosis and acute renal failure after addition of tenofovir to an antiretroviral regimen containing didanosine. Clin Infect Dis. 2003;36:1082–5. [PubMed: 12684925](49 year old man with HIV infection and renal insufficiency on long term didanosine developed fatal lactic acidosis 6 weeks after starting tenofovir [lactate 5.5 rising to 16.7 mmol/L]; no mention of liver injury).
- Bonnet F, Bonarek M, Morlat P, Mercie P, Dupon M, Gemain MC, Malvy D, et al. Risk factors for lactic acidosis in HIV-1-infected patients treated with nucleoside reverse-transcriptase inhibitors: a case-control study. Clin Infect Dis. 2003;36:1324–8. [PubMed: 12746780](Case control study of 9 patients [5 women] with HIV infection and lactic acidosis, 6 with hepatomegaly and 5 with jaundice, 8 on stavudine, 7 on didanosine, 6 on zidovudine; 6 died; risk factors were renal insufficiency and low CD4 counts but numbers of cases were few).
- Arenas-Pinto A, Grant AD, Edwards S, Weller IVD. Lactic acidosis in HIV-1 infected patients: a systematic review of published cases. Sex Transm Infect. 2003;79:340–3. [PMC free article: PMC1744718] [PubMed: 12902594](Review of 217 published cases of lactic acidosis; 53% female, all taking at least one nucleoside for 1-36 months, 61% on stavudine, 33% didanosine, 31% zidovudine, 30% lamivudine; 92% had hepatic steatosis on biopsy or autopsy; 48% died).
- Kontorinis N, Dieterich D. Hepatotoxicity of antiretroviral therapy. AIDS Rev. 2003;5:36–43. [PubMed: 12875106](Review of hepatotoxicity of antiretroviral drugs; definition of hepatotoxicity in antiretroviral studies; grade 1=1.25-2.5 times, grade 2=2.6-5 times, grade 3=5.1-10 times and grade 4=>10 times ULN or baseline ALT values; abacavir and lamivudine are least likely to cause hepatotoxicity).
- Ogedegbe AE, Thomas DL, Diehl AM. Hyperlactataemia syndromes associated with HIV therapy. Lancet Infect Dis. 2003;3:329–37. [PubMed: 12781504](Review of mechanisms of hyperlactatemia with antiretroviral therapy, occurs mostly with use of nucleoside analogues, stavudine, didanosine and zidovudine, attributed to mitochondrial depletion, but other mechanisms may be involved).
- Ofotokun I, Pomeroy C. Sex differences in adverse reactions to antiretroviral drugs. Top HIV Med. 2003;11:55–9. [PubMed: 12717043](Review of sex differences in adverse events; higher frequency of mitochondrial toxicity and hypersensitivity in women than men).
- Ogedegbe AO, Sulkowski MS. Antiretroviral-associated liver injury. Clin Liver Dis. 2003;7:475–99. [PubMed: 12879995](Review of hepatotoxicity of antiretrovirals; ALT elevations above 5 times ULN reported in 7% with zidovudine, 16% didanosine, 9-13% stavudine, <1% lamivudine, tenofovir and abacavir, 3-10% protease inhibitors, 10% nevirapine and 8% efavirenz; recommend monitoring at 4 weeks and then every 12 weeks, stopping if ALT levels are >10 times ULN or if symptoms of liver injury are present, monitoring more closely if ALT levels are elevated).
- Pecora Fulco P, Kirian MA. Effect of tenofovir on didanosine absorption in patients with HIV. Ann Pharmacother. 2003;37:1325–8. [PubMed: 12921517](When given together, tenofovir increases plasma concentrations of didanosine).
- Blanchard JN, Wohlfeiler M, Canas A, King K, Lonergan JT. Pancreatitis with didanosine and tenofovir disoproxil fumarate. Clin Infect Dis. 2003;37:e57–e62. [PubMed: 12942419](4 patients developed pancreatitis and lactic acidosis arising 2-6 months after adding tenofovir to HIV regimen including didanosine; 1 died, 3 who survived were able to restart tenofovir without didanosine; liver tests mentioned only in fatal case [bilirubin 5.3 mg/dL, ALT 89 U/L).
- Rivas P, Polo J, de Gólas M, Fernáez-Guerrero ML. Drug Points: Fatal lactic acidosis associated with tenofovir. BMJ. 2003;327:711. [PMC free article: PMC200801] [PubMed: 14512477](45 year old woman with HIV-HCV co-infection on long term didanosine and stavudine developed jaundice 8 weeks after switching from nevirapine to tenofovir [bilirubin 12.6 mg/dL, ALT 157 U/L], CT showed fatty liver; despite stopping antivirals, lactic acidosis worsened and she died 36 hours later: Case 2).
- Callens S, De Schacht C, Huyst V, Colebunders R. Pancreatitis in an HIV-infected person on a tenofovir, didanosine and stavudine containing highly active antiretroviral treatment. J Infect. 2003;47:188–9. [PubMed: 12860159](33 year old woman developed pancreatitis 5 months after starting didanosine and 1 month after addition of tenofovir to HIV regimen [bilirubin not given, ALT 386 U/L, Alk P 208 U/L, amylase 11395 U/L], resolving rapidly when antivirals were stopped).
- Walker UA, Bärle J, Laguno M, Murillas J, Mauss S, Schmutz G, Setzer B, et al. Depletion of mitochondrial DNA in liver under antiretroviral therapy with didanosine, stavudine, or zalcitabine. Hepatology. 2004;39:311–7. [PubMed: 14767983](Liver biopsies from 94 patients with chronic hepatitis C [80 with concurrent HIV] were assessed for mitochondrial DNA content, which was on average 50% lower in patients on zalcitabine, didanosine, or stavudine compared to other nucleoside analogues; usually required 6 months or more of therapy).
- Guo Y, Fung HB. Fatal lactic acidosis associated with coadministration of didanosine and tenofovir disoproxil fumarate. Pharmacotherapy. 2004;24:1089–94. [PubMed: 15338857](63 year old man with HIV and HCV developed fatal lactic acidosis 1.5 years after starting didanosine-tenofovir-lopinavir-ritonavir regimen, with pancreatitis, multiorgan failure and death; liver injury not mentioned).
- Fleischer R, Boxwell D, Sherman KE. Nucleoside analogues and mitochondrial toxicity. Clin Infect Dis. 2004;38:e79–80. [PubMed: 15095236](Among 31 HIV-HCV coinfected patients reported to the FDA with severe mitochondrial toxicity, 90% had received didanosine, 71% stavudine and combination with ribavirin seemed to increase the risk).
- Sulkowski MS, Mehta SH, Torbenson M, Afdhal NH, Mirel L, Moore RD, Thomas DL. Hepatic steatosis and antiretroviral drug use among adults coinfected with HIV and hepatitis C virus. AIDS. 2005;19:585–92. [PubMed: 15802977](Among 112 patients with HIV-HCV coinfection undergoing liver biopsy, 40% had steatosis [which affected >5% of hepatocytes in 18%]; presence of fat correlated with white race, higher BMI, hyperglycemia, and stavudine use).
- Abrescia N, D'Abbraccio M, Figoni M, Busto A, Maddaloni A, De Marco M. Hepatotoxicity of antiretroviral drugs. Curr Pharm Des. 2005;11:3697–710. [PubMed: 16305505](Review of hepatotoxicity of antiretrovirals; major syndrome with nucleoside analogues is mitochondrial injury with lactic acidosis, hepatomegaly and steatosis).
- Masiá M, Gutiérrez F, Padilla S, Ramos JM, Pascual J. Severe toxicity associated with the combination of tenofovir and didanosine: case report and review. Int J STD AIDS. 2005;16:646–8. [PubMed: 16176639](45 year old man with HIV-HCV coinfection developed lactic acidosis and "mild cholestasis" 3 months after adding tenofovir to didanosine, resolving slowly after stopping therapy).
- Weber R, Sabin CA, Friis-Møller N, Reiss P, El-Sadr WM, Kirk O, Dabis F, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166:1632–41. [PubMed: 16908797](Cohort study of 23,441 HIV infected persons followed for a median of 3.5 years identified 1246 deaths [1.6% per year] caused by AIDS [31%], liver disease [14.5%], cardiovascular [11%], cancer [9.4%]; 70% of liver related were due to HCV or HBV and associated with low CD4 counts).
- Maida I, Núñez M, Ríos MJ, Martín-Carbonero L, Sotgiu G, Toro C, Rivas P, et al. Severe liver disease associated with prolonged exposure to antiretroviral drugs. J Acquir Immune Defic Syndr. 2006;42:177–82. [PubMed: 16688096](Among 3200 HIV-infected persons followed in 2 large Spanish cohorts, 17 [0.5%] had severe chronic liver disease of unknown cause [bilirubin normal in all except one, ALT 25-131 U/L, Alk P 114-430 U/L], 4 with cirrhosis, all had long term exposure to didanosine [mean = 4 years]).
- Podevin P, Spiridon G, Terris B, Chauvelot-Moachon L, Guillevin L, Chaussade S, Sogni P, Salmon-Ceron D. Nodular regenerative hyperplasia of the liver after IL-2 therapy in an HIV-infected patient. AIDS. 2006;20:313–5. [PubMed: 16511439](65 year old man with HIV infection on antiretroviral therapy including didanosine for 7 years presented with ascites and esophageal varices 2 months after starting IL2 therapy [bilirubin and ALT normal, Alk P ~525 U/L], liver biopsy showing nodular regenerative hyperplasia).
- Mallet V, Blanchard P, Verkarre V, Vallet-Pichard A, Fontaine H, Lascoux-Combe C, Pol S. Nodular regenerative hyperplasia is a new cause of chronic liver disease in HIV-infected patients. AIDS. 2007;21:187–92. [PubMed: 17197809](Description of 8 patients with HIV infection presenting between 2003 and 2006 with cryptogenic liver disease, ages 33-59 years, 4 men and 4 women, with evidence of portal hypertension, 3 with ascites, all with esophageal varices and thrombocytopenia [71-149,000/uL], bilirubin 0.4-2.0 mg/dL, minimal ALT, AST and Alk P elevations, often with low CD4 counts, all receiving didanosine, liver biopsies showing nodular regenerative hyperplasia).
- Arey B, Markov M, Ravi J, Prevette E, Batts K, Nadir A. Nodular regenerative hyperplasia of liver as a consequence of ART. AIDS. 2007;21:1066–8. [PubMed: 17457112](Letter in response to Mallet [2007] describing a 27 year old man who presented with splenomegaly and varices [bilirubin 0.6 mg/dL, ALT 109 U/L, Alk P 165 U/L] 4 weeks after switching from didanosine [given for 4 years] to nevirapine).
- Sandrine PF, Sylvie A, André E, Abdoulaye D, Bernard L, André C. Nodular regenerative hyperplasia: a new serious antiretroviral drugs side effect? AIDS. 2007;21:1498–9. [PubMed: 17589205](Letter in response to Mallet [2007] describing a patient with noncirrhotic portal hypertension from Martinque; 49 year old woman treated with didanosine for 9 years had chronic enzyme elevations and developed thrombocytopenia, ascites and varices, showing nodular regeneration).
- Garvey LJ, Thomson EC, Lloyd J, Cooke GS, Goldin RD, Main J. Response to Mallet et al., 'Nodular regenerative hyperplasia is a new cause of chronic liver disease in HIV-infected patients'. AIDS. 2007;21:1494–5. [PubMed: 17589202](Letter in response to Mallet [2007] describing 6 HIV infected patients with unexplained portal hypertension, ages 26-48 years, 5 had received didanosine for >2 years, 1 died of variceal hemorrhage).
- Schiano TD, Kotler DP, Ferran E, Fiel MI. Hepatoportal sclerosis as a cause of noncirrhotic portal hypertension in patients with HIV. Am J Gastroenterol. 2007;102:2536–40. [PubMed: 17640321](Description of 4 men with HIV infection, ages 35-60 years, who presented with esophageal varices [bilirubin 0.5-1.6 mg/dL, ALT 37-95 U/L, Alk P 103-316 U/L, INR 1.0-1.3, platelets 85-135,000/μL], biopsy showing hepatoportal sclerosis with cirrhosis; no mention of didanosine exposure).
- Lapadula G, Izzo I, Costarelli S, Cologni G, Bercich L, Casari S, Gambarotti M, et al. Dideoxynucleoside HIV reverse transcriptase inhibitors and drug-related hepatotoxicity: a case report. J Med Case Rep. 2007;1:19. [PMC free article: PMC1868747] [PubMed: 17488516](43 year old woman with HIV infection on stavudine for 3 years and tenofovir for 6 months developed rising ALT levels [222 to 392 U/L], which persisted despite stopping indinavir and resolved slowly on stopping stavudine; liver biopsy showed inflammation, fat and Mallory bodies).
- Jain MK. Drug-induced liver injury associated with HIV medications. Clin Liver Dis. 2007;11:615–39. vii-viii. [PubMed: 17723923](Review of hepatotoxicity of antiretroviral medications; ALT elevations occur in 2-18% of patients, but often resolve spontaneously even without dose modification; classes of injury include hypersensitivity [nevirapine, efavirenz, abacavir], mitochondrial injury [stavudine, didanosine, zidovudine], flares of hepatitis B [lamivudine, emtricitabine, tenofovir], flares of hepatitis C [any potent regimen], idiosyncratic injury [ritonavir, nevirapine, efavirenz], and cholestatic hepatitis [many agents]).
- Mallet V, Blanchard P, Verkarre V, Vallet-Pichard A, Fontaine H, Lascoux-Combe C, Pol S. Nodular regenerative hyperplasia is a new cause of chronic liver disease in HIV-infected patients. AIDS. 2007;21:187–92. [PubMed: 17197809](Among 8 patients with HIV infection referred for evaluation of liver disease of unknown cause, all had nodular regenerative hyperplasia and had received didanosine [and many received stavudine or zidovudine] for 1-2 years [bilirubin 0.2-2.0 mg/dL, ALT 0.4-2.0 times ULN, Alk P 0.9-19.1 times ULN, platelets 71-149,000/μL], all had varices and 5 had ascites).
- Wester CW, Okezie OA, Thomas AM, Bussmann H, Moyo S, Muzenda T, Makhema J, et al. Higher-than-expected rates of lactic acidosis among highly active antiretroviral therapy-treated women in Botswana: preliminary results from a large randomized clinical trial. J Acquir Immune Defic Syndr. 2007;46:318–22. [PubMed: 18090299](Among 650 patients starting antiretroviral therapy, 2% developed hyperlactatemia [>4.4 mmol/L] and 1% lactic acidosis [all female, trend towards older age and higher body mass index], all on stavudine, didanosine, and/or zidovudine; 6 died, 4 of pancreatitis).
- Bolhaar MG, Karstaedt AS. A high incidence of lactic acidosis and symptomatic hyperlactatemia in women receiving highly active antiretroviral therapy in Soweto, South Africa. Clin Infect Dis. 2007;45:254–60. [PubMed: 17578788](Among 1735 adults with HIV infection started on antiretroviral therapy, 3% developed hyperlactatemia; 23 [1%] with lactic acidosis [22 were women and 22 were on stavudine, 1 on didanosine and zidovudine], 30% mortality; 44 had symptomatic hyperlactatemia [37 women, all 44 on stavudine, 3 also with didanosine], able to switch to zidovudine without recurrence, 2 of 3 relapsed on restarting stavudine).
- Lactic Acidosis International Study Group. Risk factors for lactic acidosis and severe hyperlactataemia in HIV-1-infected adults exposed to antiretroviral therapy. AIDS. 2007;21:2455–64. [PubMed: 18025882](Retrospective case control study of 110 cases of hyperlactataemia and 220 controls, identified risk factors of older age, female gender, lower CD4 counts, and use of stavudine, didanosine or both).
- Thoden J, Lebrecht D, Venhoff N, Neumann J, Muller K, Walker UA. Highly active antiretroviral HIV therapy-associated fatal lactic acidosis: quantitative and qualitative mitochondrial DNA lesions with mitochondrial dysfunction in multiple organs. AIDS. 2008;22:1093–4. [PubMed: 18520357](62 year old man developed high lactate levels [3.7 mmol/L] without acidosis or symptoms 16 months after starting didanosine-stavudine-efavirenz, and 2 months later developed fatal lactic acidosis and multiorgan failure; mitochondrial copy numbers assessed in multiple organs being reduced to 7% of normal levels in liver, 20% in kidney, 28% in muscle and 72% in heart).
- Maida I, Garcia-Gasco P, Sotgiu G, Rios MJ, Vispo ME, Martin-Carbonero L, Barreiro P, et al. Antiretroviral-associated portal hypertension: a new clinical condition? Prevalence, predictors and outcome. Antivir Ther. 2008;13:103–7. [PubMed: 18389904](Among 3200 HIV-infected persons in 2 large Spanish cohorts, 17 [0.5%] had severe chronic liver disease of unknown cause [bilirubin normal in 16, ALT 25-131 U/L, Alk P 114-430 U/L], 4 with cirrhosis, all had long term exposure to didanosine [mean=4 years]).
- Saifee S, Joelson D, Braude J, Shrestha R, Johnson M, Sellers M, Galambos MR, Rubin RA. Noncirrhotic portal hypertension in patients with human immunodeficiency virus-1 infection. Clin Gastroenterol Hepatol. 2008;6:1167–9. [PubMed: 18639498](Description of 11 patients with HIV infection and noncirrhotic portal hypertension [bilirubin 0.4-3.2 mg/dL, ALT 13-99 U/L, Alk P 112-419 U/L, platelets 77-581,000/μL], often with protein S or C deficiency; all had received didanosine and had nodular regenerative hyperplasia; 5 had ascites and 1 died of esophageal variceal bleeding).
- Tateo M, Sebagh M, Bralet MP, Teicher E, Azoulay D, Mallet V, Pol S, et al. A new indication for liver transplantation: nodular regenerative hyperplasia in human immunodeficiency virus-infected patients. Liver Transpl. 2008;14:1194–8. [PubMed: 18668652](Description of 3 patients with HIV infection and nodular regenerative hyperplasia who underwent liver transplantation, 2 women and 1 man, ages 38 to 43 years, having received didanosine for 3-8 years, 2 with portal vein thrombosis, all with varices [bilirubin 1.3-3.6 mg/dL, INR 1.2-1.6, creatinine 0.6-1.1 mg/dL], all were alive without liver abnormalities 3-7 months after deceased donor liver transplantation).
- Vispo E, Maida I, Barreiro P, Moreno V, Soriano V. Upper gastrointestinal bleeding may unmask didanosine-associated portal hepatopathy in HIV/HCV co-infected patients. HIV Clin Trials. 2008;9:440–4. [PubMed: 19203910](3 patients with HIV-HCV coinfection presented with signs of portal hypertension, but had little evidence of advanced fibrosis and only mild liver test abnormalities; all 3 had received didanosine [for 30-64 months] and HIV RNA levels were undetectable; liver biopsies not done).
- Hammer SM, Eron JJ Jr, Reiss P, Schooley RT, Thompson MA, Walmsley S, Cahn P, et al. International AIDS Society-USA. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA. 2008;300:555–70. [PubMed: 18677028](Updated recommendations on use of antiviral therapy in adults with HIV infection including use of recently approved agents raltegravir, maraviroc and etravirine).
- Inductivo-Yu I, Bonacini M. Highly active antiretroviral therapy-induced liver injury. Current Drug Safety. 2008;3:4–13. [PubMed: 18690975](Review of drug induced liver injury due to antiretroviral agents).
- Soriano V, Puoti M, Garcia-Gascó Rockstroh JK, Benhamou Y, Barreiro P, McGovern B. Antiretroviral drugs and liver injury. AIDS. 2008;22:1–13. [PubMed: 18090386](Review of hepatotoxicity of antiretroviral drugs with recommendations on management, stopping therapy if symptoms arise, with overt jaundice [direct bilirubin], evidence of mitochondrial toxicity, ALT >10 times ULN, ALT at lower levels if newly marketed agent; important to rule out other causes: problematic agents include didanosine, stavudine and zidovudine; nevirapine and efavirenz, full dose ritonavir and tipranavir).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, 7 were attributed to antiretroviral agents, 2 nevirapine, 1 efavirenz and 4 miscellaneous combinations, but none with didanosine).
- Panos G, Farouk L, Stebbing J, Holmes P, Valero S, Randell P, Bower M, et al. Cryptogenic pseudocirrhosis: a new clinical syndrome of noncirrhotic portal hypertension (unassociated with advanced fibrosis) that can be detected by transient elastography in patients with HIV. J Acquir Immune Defic Syndr. 2009;52:525–7. [PubMed: 19901620](Among 5 patients with noncirrhotic portal hypertension [NCPH] identified by elastography rather than symptoms or signs, all had received didanosine and biopsies showed portal fibrosis only).
- Chang PE, Miquel R, Blanco JL, Laguno M, Bruguera M, Abraldes JG, Bosch J, Garcia-Pagan JC. Idiopathic portal hypertension in patients with HIV infection treated with highly active antiretroviral therapy. Am J Gastroenterol. 2009;104:1707–14. [PubMed: 19471257](Among 8 patients with HIV infection and NCPH, all had received didanosine, hepatic venous pressure gradients were 3.5 to 14.5 mm Hg, underestimating degree of portal pressure, and elastography measurements were unreliable; 6 patients developed portal vein thrombosis).
- Mendizabal M, Craviotto S, Chen T, Silva MO, Reddy KR. Noncirrhotic portal hypertension: another cause of liver disease in HIV patients. Ann Hepatol. 2009;8:390–5. [PubMed: 20009143](Description of 6 patients with HIV infection and NCPH, ages 37 to 47 years presenting with varices, 2 with ascites and 2 with portal vein thrombosis, biopsies showing nodular regenerative hyperplasia in 2, hepatoportal sclerosis in 1 and both in 3 [bilirubin 1.1-1.4 mg/dL, ALT 13-73 U/L, platelets 65-273,000/μL], 4 having been exposed to didanosine).
- Ingiliz P, Valantin MA, Duvivier C, Medja F, Dominguez S, Charlotte F, Tubiana R, et al. Liver damage underlying unexplained transaminase elevation in human immunodeficiency virus-1 mono-infected patients on antiretroviral therapy. Hepatology. 2009;49:436–42. [PubMed: 19085967](Among 30 patients with HIV infection and unexplained ALT or AST elevations, liver biopsies showed NASH in 16, fibrosis in 19 and cirrhosis in 3; abnormalities did not correlate with any one antiretroviral agent).
- Kovari H, Ledergerber B, Peter U, Flepp M, Jost J, Schmid P, Calmy A, et al. Swiss HIV Cohort Study. Association of noncirrhotic portal hypertension in HIV-infected persons and antiretroviral therapy with didanosine: a nested case-control study. Clin Infect Dis. 2009;49:626–35. [PubMed: 19589079](15 patients with noncirrhotic portal hypertension [NCPH] were identified between 2000-2007 in a large Swiss cohort of HIV-infected persons and compared to 75 matched controls; those with NCPH were older [52 vs 43 years], with lower CD4 counts, higher ALT and Alk P, lower platelet counts; all had received didanosine and 4 died of liver disease).
- Dinh MH, Stosor V, Rao SM, Miller FH, Green RM. Cryptogenic liver disease in HIV-seropositive men. HIV Med. 2009;10:447–53. [PubMed: 19459992](Among 9 patients with HIV infection referred for evaluation of liver disease of unknown cause, 3 had nodular regenerative hyperplasia and several others had portal hypertension without cirrhosis; no mention of antiretroviral regimens).
- Mendizabal M, Craviotto S, Chen T, Silva MO, Reddy KR. Noncirrhotic portal hypertension: another cause of liver disease in HIV patients. Ann Hepatol. 2009;8:390–5. [PubMed: 20009143](Description of 6 patients with HIV infection and noncirrhotic portal hypertension; ages 37 to 47 years, on long term [8-18 years] antiretroviral therapy including didanosine in 4; all had esophageal varices, 5 with bleeding and 2 with ascites; liver biopsies showed NRH in 2, HPS in 1 and both patterns in 3).
- Lafeuillade A, Hittinger G, Cheret A, Poggi C. Didanosine withdrawal is associated with improved liver function tests. HIV Clin Trials. 2009;10:341. [PubMed: 19906627](Letter describing 38 patients with HIV infection on didanosine for 3-8 years who were switched to a protease inhibitor regimen and followed; all remained HIV RNA negative and had decreases in means of ALT [47 to 29 U/L] and AST [54 to 30 U/L]).
- Ferrajolo C, Capuano A, Verhamme KM, Schuemie M, Rossi F, Stricker BH, Sturkenboom MC. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol. 2010;70:721–8. [PMC free article: PMC2997312] [PubMed: 21039766](Worldwide pharmacovigilance database contained 9036 hepatic adverse drug reactions in children, 3 antiretroviral agents were among the top 41 causes, including zidovudine [8th, 106 cases], lamivudine [26th, 45 cases] and nevirapine [36th, 37 cases]).
- Merchante N, Pérez-Camacho I, Mira JA, Rivero A, Macías J, Camacho A, Gómez-Mateos J, et al. Grupo Andaluz para el Estudio de las Hepatitis Víricas de la Sociedad Andaluza de Enfermedades Infecciosas. Prevalence and risk factors for abnormal liver stiffness in HIV-infected patients without viral hepatitis coinfection: role of didanosine. Antivir Ther. 2010;15(5):753–63. [PubMed: 20710057](Among 258 patients with HIV infection without hepatitis B or C who underwent ultrasound elastography, elevated values [>7.2 kPa] were found in 29 patients; these abnormalities were associated independently with time on didanosine).
- Palacios R, Rivero A, Santos I, Ríos MJ, Castaño M, del Arco A, Santos González J. VITOX Group. Rapid improvement in fasting lipids and hepatic toxicity after switching from didanosine/lamivudine to tenofovir/emtricitabine in patients with toxicity attributable to didanosine. HIV Clin Trials. 2010;11:118–20. [PubMed: 20542848](Among 147 patients with HIV infection switched from didanosine to tenofovir, fasting lipids and liver test abnormalities were said to be improved in all, although mean ALT and AST values did not change).
- Vispo E, Moreno A, Maida I, Barreiro P, Cuevas A, Albertos S, Soriano V. Noncirrhotic portal hypertension in HIV-infected patients: unique clinical and pathological findings. AIDS. 2010;24:1171–6. [PubMed: 20299955](Clinical description of 12 patients with HIV infection on long term antiretroviral therapy with didanosine presenting with portal hypertension without cirrhosis, 11 biopsies showing nodular regenerative hyperplasia [NRH =3] or hepatoportal sclerosis [HPS = 8]; patient ages 39-67 years, 2 women and 10 men, ALT 23-196 U/L, 5 with variceal bleeding and 2 with ascites).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury and 4 to antiretroviral agents, including 2 to the combination of didanosine and stavudine).
- Hartleb M, Gutkowski K, Milkiewicz P. Nodular regenerative hyperplasia: evolving concepts on underdiagnosed cause of portal hypertension. World J Gastroenterol. 2011;17:1400–9. [PMC free article: PMC3070012] [PubMed: 21472097](Review of etiology, course and management of nodular regenerative hyperplasia; mentions its association with azathioprine, mercaptopurine, thioguanine, oxaliplatin and antiretroviral agents).
- Scourfield A, Jackson A, Waters L, Gazzard B, Nelson M. The value of screening HIV-infected individuals for didanosine-related liver disease? Antivir Ther. 2011;16(6):941–2. [PubMed: 21900728](Among 2070 patients with HIV infection, 26 presented with idiopathic portal hypertension, all having received didanosine [mean of 66 months], with mild ALT and Alk P elevations only).
- Dlamini J, Ledwaba L, Mokwena N, Mokhathi T, Orsega S, Tsoku M, Kowo H, et al. Lactic acidosis and symptomatic hyperlactataemia in a randomized trial of first-line therapy in HIV-infected adults in South Africa. Antivir Ther. 2011;16:605–9. [PubMed: 21685549](Among 1771 patients with HIV infection treated with one of 4 antiviral regimens with didanosine or stavudine, 13 developed lactic acidosis [3.5/1000 patient-years]; lactic acidosis cases were more likely female, with higher BMI and exposure to stavudine).
- Feeney ER, Chazallon C, O'Brien N, Meiffrédy V, Goodall RL, Aboulker JP, Cooper DA, Yeni P, Mallon PW., INITIO Trial International Co-ordinating Committee. Hyperlactataemia in HIV-infected subjects initiating antiretroviral therapy in a large randomized study (a substudy of the INITIO trial). HIV Med. 2011;12:602–9. [PubMed: 21599820](Among 911 patients with HIV infection started on antiretroviral therapy with didanosine and stavudine, 24 developed elevated lactate levels; abnormalities were more frequent in women than men and those with higher BMI; not predicted by or accompanied by peripheral lymphocyte mitochondrial DNA levels).
- Cachay ER, Peterson MR, Goicoechea M, Mathews WC. Didanosine exposure and noncirrhotic portal hypertension in a HIV clinic in North America: a follow-up study. Br J Med Med Res. 2011;1:346–55. [PMC free article: PMC3261794] [PubMed: 22268001](Among 8 men with HIV infection diagnosed with NCPH between 1990 and 2010 at a single University HIV clinic, all had been exposed to didanosine for a median of 37 [range 24-66] months, [bilirubin 0.5-2.0 mg/dL, ALT 24-86 U/L, Alk P 48-253 U/L, INR 1.0-1.2, platelet counts 47,000-136,000]; most were stable once didanosine was stopped with medical management of portal hypertension).
- Alvarez Díaz H, Mariño Callejo A, García Rodríguez JF. Non-cirrhotic portal hypertension in human immunodeficiency virus-infected patients: a new challenge in antiretroviral therapy era. Open AIDS J. 2011;5:59–61. [PMC free article: PMC3134955] [PubMed: 21760875](Two patients with HIV infection developed portal hypertension on didanosine therapy; 58 year old man and 41 year old woman presented with esophageal varices after didanosine therapy for 1 and 5 years and remained stable after stopping).
- Scourfield A, Waters L, Holmes P, Panos G, Randell P, Jackson A, Mandalia S, Gazzard B, Nelson M. Non-cirrhotic portal hypertension in HIV-infected individuals. Int J STD AIDS. 2011;22:324–8. [PubMed: 21680667](Among 17 patients with HIV infection and noncirrhotic portal hypertension, risk factors were prolonged didanosine exposure [59 vs 21 months]).
- Hofmaenner D, Kovari H, Weber A, Weishaupt D, Speck RF. Nodular regenerative hyperplasia of the liver associated with didanosine persists for years even after its interruption. BMJ Case Rep. 2011;2011:bcr0320113928. [PMC free article: PMC3089926] [PubMed: 22696691](47 year old man with HIV infection developed noncirrhotic portal hypertension 6-7 years after starting didanosine which was stopped; he remained stable, but still had varices and evidence of portal hypertension 10 years later).
- Vispo E, Morello J, Rodriguez-Novoa S, Soriano V. Noncirrhotic portal hypertension in HIV infection. Curr Opin Infect Dis. 2011;24:12–8. [PubMed: 21157331](Review of the problem of noncirrhotic portal hypertension in patients with HIV infection, stressing the role of didanosine and possible role of bacterial translocation causing endothelial damage, thromboses and portal venopathy).
- Schiano TD, Uriel A, Dieterich DT, Fiel MI. The development of hepatoportal sclerosis and portal hypertension due to didanosine use in HIV. Virchows Arch. 2011;458:231–5. [PubMed: 21057809](45 year old man with HIV infection developed nodular regenerative hyperplasia 5 years after starting a regimen of didanosine, stavudine and protease inhibitors that progressed despite switching to zidovudine, lamivudine, abacavir and tenofovir, with biopsy 5 years later showing hepatoportal sclerosis).
- Cotte L, Bénet T, Billioud C, Miailhes P, Scoazec JY, Ferry T, Brochier C, et al. The role of nucleoside and nucleotide analogues in nodular regenerative hyperplasia in HIV-infected patients: a case control study. J Hepatol. 2011;54:489–96. [PubMed: 21056493](Case controlled study of 13 patients with HIV infection and nodular regenerative hyperplasia [NRH] and 78 HIV infected controls found that age, duration of didanosine and stavudine therapy were independently associated with NRH).
- Blanco F, Barreiro P, Ryan P, Vispo E, Martín-Carbonero L, Tuma P, Labarga P, et al. Risk factors for advanced liver fibrosis in HIV-infected individuals: role of antiretroviral drugs and insulin resistance. J Viral Hepat. 2011;18:11–6. [PubMed: 20088890](Among 681 unselected patients with HIV infection undergoing ultrasound elastography, 215 had elevated values [>9.5 kPa], which were independently associated with HCV coinfection, ALT elevations, history of alcohol abuse, abnormal HOMA values and exposure to didanosine or stavudine).
- Young J, Klein MB, Ledergerber B. Noncirrhotic portal hypertension and didanosine: a re-analysis. Clin Infect Dis. 2011;52:154–5. [PubMed: 21148537](Statistical reanalysis of the results from Kovari from "2020" concludes that the findings do "not appear to be an artifact").
- Schouten JN, Van der Ende ME, Koëter T, Rossing HH, Komuta M, Verheij J, van der Valk M, et al. Risk factors and outcome of HIV-associated idiopathic noncirrhotic portal hypertension. Aliment Pharmacol Ther. 2012;36:875–85. [PubMed: 22971050](Comparison of 16 patients with HIV infection and NCPH vs 64 controls identified in the Netherlands, cases were younger [44 vs 52 years], had lower CD4 counts, higher ALT and Alk P, and more likely to have received didanosine [16] or stavudine [11] or both).
- Suárez-Zarracina T, Valle-Garay E, Collazos J, Montes AH, Cárcaba V, Carton JA, Asensi V. Didanosine (ddI) associates with increased liver fibrosis in adult HIV-HCV coinfected patients. J Viral Hepat. 2012;19:685–93. [PubMed: 22967099](Among 111 patients with HIV/HCV coinfection, didanosine was associated with higher scores by transient elastography, a noninvasive means of assessing hepatic stiffness).
- Giacomet V, Viganò A, Penagini F, Manfredini V, Maconi G, Camozzi M, Zuccotti GV. Splenomegaly and variceal bleeding in a ten-year-old HIV-infected girl with noncirrhotic portal hypertension. Pediatr Infect Dis J. 2012;31:1059–60. [PubMed: 22828640](10 year old girl with HIV infection developed splenomegaly, ascites and varices having been on didanosine for 7 years [bilirubin 0.8 mg/dL, ALT 48 U/L, Alk P 231 U/L, platelets 154,000/μL], biopsy showing hepatoportal sclerosis).
- Saison J, Cotte L, Chidiac C, Ferry T. Fatal cumulative toxicities of HAART in a stable, AIDS-free, HIV-infected patient. BMJ Case Rep. 2012;2012:bcr1020114905. [PMC free article: PMC3316843] [PubMed: 22605589](57 year old man with HIV infection and severe lipodystrophy developed ascites 5 years after starting didanosine, biopsy showing nodular generative hyperplasia).
- Jackson BD, Doyle JS, Hoy JF, Roberts SK, Colman J, Hellard ME, Sasadeusz JJ, et al. Non-cirrhotic portal hypertension in HIV mono-infected patients. J Gastroenterol Hepatol. 2012;27:1512–9. [PubMed: 22497527](Among 1000 patients with HIV infection without HCV or HBV coinfection followed in Melbourne, Australia, 5 men, ages 40-611 years, had unexplained portal hypertension [bilirubin 0.4-0.7 mg/dL, ALT 25-82 U/L, Alk P 73-889 U/L, fibroscan 6.0-13 kPa], biopsy in 4 showing no cirrhosis).
- Chang HM, Tsai HC, Lee SS, Wann SR, Chen YS. Noncirrhotic portal hypertension associated with didanosine: a case report and literature review. Jpn J Infect Dis. 2012;65:61–5. [PubMed: 22274160](80 year old Chinese man with HIV infected presented with ascites and esophageal varices 34 months after starting didanosine, lamivudine and nevirapine [bilirubin normal, ALT 36 U/L, Alk P 188 U/L, platelets 140,000/μL], with portal vein thrombosis and dying 3 months later of variceal bleed).
- Dragovic G, Jevtovic D. The role of nucleoside reverse transcriptase inhibitors usage in the incidence of hyperlactatemia and lactic acidosis in HIV/AIDS patients. Biomed Pharmacother. 2012;66:308–11. [PubMed: 22658063](Among 396 patients with HIV infection started on antiretroviral therapy, hyperlactemia developed in 19 [none died] and lactic acidosis arose in 15 [4 died]; risk factors for lactic acidosis were exposure to didanosine [n=3], stavudine [n=4] or both [n=8]).
- Arenas-Pinto A, Weller I, Ekong R, Grant A, Karstaedt A, Reiss P, Telisinghe L, et al. Common inherited mitochondrial DNA mutations and nucleoside reverse transcriptase inhibitor-induced severe hyperlactataemia in HIV-infected adults: an exploratory study. Antivir Ther. 2012;17:275–82. [PubMed: 22293466](Among 40 South African patients with HIV infection who developed hyperlactemia on stavudine based therapy, distribution of mitochondrial DNA haplotypes was the same as in controls).
- Barlow-Mosha L, Eckard AR, McComsey GA, Musoke PM. Metabolic complications and treatment of perinatally HIV-infected children and adolescents. J Int AIDS Soc. 2013;16:18600. [PMC free article: PMC3691550] [PubMed: 23782481](Review of metabolic complications [such as lipodystrophy and lactic acidosis] of long term antiretroviral therapy in children and adolescents).
- Wagner TA, Lin CH, Tobin NH, Côté HC, Sloan DD, Jerome KR, Frenkel LM. Quantification of mitochondrial toxicity in HIV-infected individuals by quantitative PCR compared to flow cytometry. Cytometry B Clin Cytom. 2013;84:55–8. [PMC free article: PMC3966180] [PubMed: 23044657](Ratios of concentrations of mitochondrial vs nuclear DNA and proteins in peripheral blood mononuclear cells were similar in patients, with or without clinical evidence of mitochondrial injury due to antiretroviral therapy).
- Hui YT, Lam WY, Lee MP, Lam TW, Li P. An unusual cause of oesophageal variceal bleeding in a Chinese human immunodeficiency virus-infected patient. Hong Kong Med J. 2013;19:77–9. [PubMed: 23378360](49 year old Chinese man with HIV infection developed variceal hemorrhage and was found to have hepatoportal sclerosis 5 years after stopping a 6 year course of didanosine).
- Verheij J, Schouten JN, Komuta M, Nevens F, Hansen BE, Janssen HL, Roskams T. Histological features in western patients with idiopathic non-cirrhotic portal hypertension. Histopathology. 2013;62:1083–91. [PubMed: 23600724](Histological analysis of 70 patients with NCPH, 13 with HIV infection who were more likely to have NRH [93% vs 46%] and who had a similar risk for underlying thrombophilic condition [31% vs 40%]).
- Vispo E, Cevik M, Rockstroh JK, Barreiro P, Nelson M, Scourfield A, Boesecke C, et al. European Network of Clinical Trials (NEAT). Genetic determinants of idiopathic noncirrhotic portal hypertension in HIV-infected patients. Clin Infect Dis. 2013;56:1117–22. [PubMed: 23315321](Case control study of 22 HIV infected patients with NCPH and 58 controls for single nucleotide polymorphisms in genes for enzymes involved in purine metabolism identified 2 single nucleotide polymorphisms in the 5-nucleotidase and 2 in the xanthine oxidase genes associated with presence of NCPH).
- Sood A, Castrejón M, Saab S. Human immunodeficiency virus and nodular regenerative hyperplasia of liver: A systematic review. World J Hepatol. 2014;6:55–63. [PMC free article: PMC3953810] [PubMed: 24653794](A review of the literature on nodular regenerative hyperplasia in HIV infected patients).
- Parikh ND, Martel-Laferriere V, Kushner T, Childs K, Vachon ML, Dronamraju D, Taylor C, et al. Clinical factors that predict noncirrhotic portal hypertension in HIV-infected patients: a proposed diagnostic algorithm. J Infect Dis. 2014;209:734–8. [PubMed: 23911709](Comparison of 35 HIV infected persons with NCPH to 68 controls identified multiple clinical differences; authors propose a diagnostic algorithm that includes exposure to didanosine, splenomegaly, low platelet count or serum enzyme elevations as suggestive of diagnosis and warranting further evaluation).
- Yajima K, Uehira T, Otera H, Koizumi Y, Watanabe D, Kodama Y, Kuzushita N, et al. A case of non-cirrhotic portal hypertension associated with anti-retroviral therapy in a Japanese patient with human immunodeficiency virus infection. J Infect Chemother. 2014;20:582–5. [PubMed: 25034388](35 year old Japanese woman with HIV infection presented with ascites and gastrointestinal bleeding 4 years after stopping a 5 year course of didanosine [bilirubin 0.2 mg/dL, ALT 82 U/L, AST 83 U/L, Alk P 268 U/L, platelets 207,000 /µL, TE=9.3 kPa, biopsy showing no cirrhosis], with improvement and resolution of ascites and abnormal liver tests after control of bleeding and avoidance of nucleoside analogs).
- Brescini L, Orsetti E, Gesuita R, Piraccini F, Marchionni E, Staffolani S, Castelli P, et al. Evaluating liver fibrosis by transient elastometry in patients with HIV-HCV coinfection and monoinfection. Hepat Mon. 2014;14:e15426. [PMC free article: PMC4199183] [PubMed: 25337140](Among 354 adults undergoing transient elastography (TE), abnormal stiffness was found in 13% with HIV infection alone, 39% with HCV infection alone and 51% with both; treatment with stavudine and didanosine being associated with higher TE scores).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, 5 of which [3%] were attributed to antiretroviral agents [nevirapine, zidovudine and lamivudine], but none to didanosine).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 12 cases [1.3%] were attributed to antiretroviral agents, including one to didanosine: a 44 year old woman with HIV infection who presented with ascites and was found to have noncirrhotic portal hypertension having been on didanosine and tenofovir for 6 years [bilirubin 0.5 mg/dL, ALT 94 U/L, Alk P 310 U/L]).
- Gómez-Rubio J, Bárcena-Atalaya AB, Macías-García L, Lozano de León-Naranjo F. Med Clin (Barc). 2015;145:45. [Non-cirrhotic portal hypertension associated with didanosine: An unusual cause of gastrointestinal bleeding] Spanish. [PubMed: 25510631](42 year old woman with HIV infection presented with bleeding esophageal varices having been on didanosine for 6 years [bilirubin normal, ALT and Alk P elevated], liver biopsy showing nodular regenerative hyperplasia and liver tests improving after withdrawal of didanosine).
- Turon F, Silva-Junior G, Hernandez-Gea V, Garcia-Pagan JC. Hipertensión portal idiopática no cirrótica. Gastroenterol Hepatol. 2015;38:556–62. [Idiopathic non-cirrhotic portal hypertension] Spanish. [PubMed: 26321321](Review of noncirrhotic portal hypertension including epidemiology, pathogenesis, clinical presentation and management).
- Arora A, Sarin SK. Multimodality imaging of obliterative portal venopathy: what every radiologist should know. Br J Radiol. 2015;88:20140653. [PMC free article: PMC4614245] [PubMed: 25514699](Review of the radiologic findings of noncirrhotic portal hypertension that accompanies obliterative portal venopathy; typical findings being increased spleen size and stiffness with normal or shrunken liver, portal vein enlargement and varices).
- Stotts M, Chisholm P, Nurutdinova D, Maddur H. A case of non-cirrhotic portal hypertension associated with chronic didanosine therapy. ACG Case Rep J. 2015;2:119–20. [PMC free article: PMC4435383] [PubMed: 26157933](66 year old man with HIV on tenofovir, didanosine and nevirapine for many years developed weakness and abdominal distension and was found to have ascites and noncirrhotic portal hypertension [bilirubin 0.4 mg/dL, ALT 24 U/L, Alk P 157 U/L, HVPG 14]).
- Gouvêa Ade F, Machado DM, Beltrão SC, Carmo FB, Mattar RH, Succi RC. Hipertensão portal não cirrótica em adolescente infectado pelo vírus da imunodeficiência humana (HIV). Rev Paul Pediatr. 2015 Apr-Jun;33(2):246–50. [Noncirrhotic portal hypertension in a human immunodeficiency virus (HIV) infected adolescent] [PMC free article: PMC4516380] [PubMed: 25913495](13 year old boy with perinatally acquired HIV infection on long term tenofovir and didanosine was found to have enlargement of the liver and spleen [bilirubin not provided, ALT 36 U/L, Alk P 382 U/L, GGT 68 U/L, platelets 63,000/µL, TE 10 kPA, endoscopy small varices]; didanosine was stopped but no follow up given).
- Ioannou GN, Bryson CL, Weiss NS, Boyko EJ. Associations between lipodystrophy or antiretroviral medications and cirrhosis in patients with HIV infection or HIV/HCV coinfection. Eur J Gastroenterol Hepatol. 2015;27:577–84. [PubMed: 25769096](In an analysis of the Veterans Administration Healthcare system Clinical Care Registry, long term use of didanosine but not other antiretroviral agents was found to be a risk factor for cirrhosis in patients with HIV monotherapy in contrast to patients with HIV-HCV coinfection, in whom all long term antiretroviral therapies were risk factors for cirrhosis).
- Kovari H, Sabin CA, Ledergerber B, Ryom L, Reiss P, Law M, Pradier C, et al. Antiretroviral drugs and risk of chronic alanine aminotransferase elevation in human immunodeficiency virus (HIV)-monoinfected persons: the data collection on adverse events of anti-HIV drugs study. Open Forum Infect Dis. 2016;3:ofw009. [PMC free article: PMC4767274] [PubMed: 26925429](Among 21,495 persons observed for an average of 4-5 years in a prospective observation study of adverse events among HIV positive patients without HBV or HCV on antiretroviral therapy, 6368 [30%] developed chronically elevated liver tests [6 per 100 patient years] and risk factors included didanosine, stavudine and tenofovir therapy but not lamivudine, abacavir or most protease inhibitors).
- Vilarinho S, Sari S, Yilmaz G, Stiegler AL, Boggon TJ, Jain D, Akyol G, et al. Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension. Hepatology. 2016;63:1977–86. [PMC free article: PMC4874872] [PubMed: 26874653](Complete exome sequencing of DNA from 8 children with idiopathic noncirrhotic portal hypertension identified an extremely rare gene variant which was homozygous in all 8 children, the gene being deoxyguanosine kinase, an enzyme required for mitochondrial DNA replication that is also inhibited by didanosine perhaps explaining its hepatotoxicity).
- Ryom L, Lundgren JD, De Wit S, Kovari H, Reiss P, Law M, El-Sadr W, et al. D:A:D Study Group. Use of antiretroviral therapy and risk of end-stage liver disease and hepatocellular carcinoma in HIV-positive persons. AIDS. 2016;30:1731–43. [PubMed: 26752282](Among 45,544 individuals followed for a median of 8.4 years in a prospective observation study of adverse events in patients with HIV infection on antiretroviral drugs, 209 developed end stage liver disease and 110 hepatocellular carcinoma, the combined rate being 1 per 1000 patient years; increased risk for these outcomes occurred in those with HCV and HBV infection, but was also linked to long term therapy with stavudine, didanosine, and tenofovir).
- Scherpbier HJ, Terpstra V, Pajkrt D, Puthakanit T, Ananworanich J, Foster C, van den Bergh Weerman M, et al. Noncirrhotic portal hypertension in perinatally HIV-infected adolescents treated with didanosine-containing antiretroviral regimens in childhood. Pediatr Infect Dis J. 2016;35:e248–52. [PubMed: 27167116](6 adolescents, ages 13 to 19 years, with neonatally acquired HIV infection presented with noncirrhotic portal hypertension, all six were female and all had received long term didanosine therapy [4-10 years; 4 also received stavudine 5-10 years]; in follow up of 1-6 years not receiving didanosine, all were stable, one required shunt to control variceal hemorrhage).
- Logan S, Rodger A, Maynard-Smith L, O'Beirne J, Fernandez T, Ferro F, Smith C, et al. Prevalence of significant liver disease in human immunodeficiency virus-infected patients exposed to didanosine: a cross sectional study. World J Hepatol. 2016;8:1623–8. [PMC free article: PMC5192554] [PubMed: 28083085](Among 99 patients with HIV infection who had received didanosine for at least 6 months who were screened for evidence of liver disease, 37% had abnormal ALT levels, 12% had abnormal TE scores [>7.65 kPa], and liver biopsy in 17 patients showed NRH in 2 [ages 43 and 59 years, ALT 77 and 36 U/L, Alk P 83 and 93 U/L, platelets 175,000/µL, HVPG 5 and 4 mm Hg]).
- Gómez Alonso M, Goñi Esarte S, Bolado Concejo F, Urman Fernández JM, Basterra Ederra M, Kutz Leoz M, Zozaya Urmeneta JM. Idiopathic non-cirrhotic portal hypertension and portal vein thrombosis in a patient with HIV infection. Gastroenterol Hepatol. 2017;40:353–4. English, Spanish. [PubMed: 27084670](39 year old man with HIV infection presented with variceal hemorrhage having been treated with didanosine for 3.5 years [bilirubin 0.8 mg/dL, ALT 25 U/L, Alk P 98 U/L, INR 1.0], with large varices on endoscopy, HVPG 10 mm Hg, TE 13.8 kPa, and biopsy showing nonspecific changes and scant fibrosis).
- Gamero MT, Gallardo MS, Aguilar V, Bravo E, Guevara J, Mejia F. Hipertensión portal no cirrótica por didanosina. Un caso infrecuente. Rev Gastroenterol Peru. 2017;37:87–90. [Non-cirrhotic portal hypertension due to didanosina. A rare case] Spanish. [PubMed: 28489843](39 year old HIV positive woman treated with didanosine, lamivudine and efavirenz for 5 years presented with ascites and portal hypertension, biopsy showing nodular regeneration and subsequently suffered with intractable ascites, recurrent upper gastrointestinal bleeding and died within the next year).
- Figueiredo M, Nkuize M, Mulkay JP, De Wit S, Gomez M, Sersté T. Nodular Regenerative Hyperplasia in HIV-positive patients : a case series and review of the literature. Acta Gastroenterol Belg. 2017;80:15–9. [PubMed: 29364092](Clinical description of 3 HIV-positive patients [2 men, 1 woman, ages 33 to 52 years] who presented with noncirrhotic portal hypertension having been treated with didanosine for 4-11 years, liver histology demonstrating nodular regenerative hyperplasia).
- Lee M, Izzy M, Akki A, Tanaka K, Kalia H. Nodular regenerative hyperplasia: a case of rare prognosis. J Investig Med High Impact Case Rep. 2017;5:2324709617690742. [PMC free article: PMC5405903] [PubMed: 28491877](26 year old woman with perinatally acquired HIV infection developed splenomegaly and abnormal liver tests while on tenofovir, emtricitabine and atazanavir/ritonavir [bilirubin 0.7 mg/dL, ALT 83 U/L, AST 80 U/L, Alk P 260 U/L, platelets 72,000, INR 1.2, biopsy showing minimal fibrosis and nodular regeneration] who subsequently was found to have a hepatic mass on imaging suspected to be HCC; no mention of previous therapy with didanosine or stavudine).
- Ahmad AK, Atzori S, Taylor-Robinson SD, Maurice JB, Cooke GS, Garvey L. Spleen stiffness measurements using point shear wave elastography detects noncirrhotic portal hypertension in human immunodeficiency virus. Medicine (Baltimore). 2019;98:e17961. [PMC free article: PMC6882591] [PubMed: 31764798](Among 25 patients with HIV infection undergoing shearwave elastography, spleen stiffness was more reliable than liver stiffness in assessing noncirrhotic portal hypertension, being elevated in 6 of 11 cases of NCPH but in none of 14 controls).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- The development of hepatoportal sclerosis and portal hypertension due to didanosine use in HIV.[Virchows Arch. 2011]The development of hepatoportal sclerosis and portal hypertension due to didanosine use in HIV.Schiano TD, Uriel A, Dieterich DT, Fiel MI. Virchows Arch. 2011 Feb; 458(2):231-5. Epub 2010 Nov 6.
- Risk factors and outcome of HIV-associated idiopathic noncirrhotic portal hypertension.[Aliment Pharmacol Ther. 2012]Risk factors and outcome of HIV-associated idiopathic noncirrhotic portal hypertension.Schouten JN, Van der Ende ME, Koëter T, Rossing HH, Komuta M, Verheij J, van der Valk M, Hansen BE, Janssen HL. Aliment Pharmacol Ther. 2012 Nov; 36(9):875-85.
- Portal Hypertension Due to Hepatoportal Sclerosis in an HIV-Positive Patient Secondary to Didanosine Use.[Cureus. 2023]Portal Hypertension Due to Hepatoportal Sclerosis in an HIV-Positive Patient Secondary to Didanosine Use.Phrathep DD, Anthony S, Healey KD, Khan H, Herman M. Cureus. 2023 Mar; 15(3):e36364. Epub 2023 Mar 19.
- Review Nucleoside Analogues.[LiverTox: Clinical and Researc...]Review Nucleoside Analogues.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review [Pharmacological and clinical properties of didanosine (VIDEX), a nucleoside reverse transcriptase inhibitor].[Nihon Yakurigaku Zasshi. 2002]Review [Pharmacological and clinical properties of didanosine (VIDEX), a nucleoside reverse transcriptase inhibitor].Okiyama M, Kawashima H, Fukunishi S. Nihon Yakurigaku Zasshi. 2002 Aug; 120(2):115-22.
- Didanosine - LiverToxDidanosine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...