NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Entacapone is a catechol-O-methyltransferase inhibitor used in the therapy of Parkinson disease as adjunctive therapy in combination with levodopa and carbidopa. Entacapone has been associated with a low rate of serum enzyme elevations during treatment, but has yet to be implicated in cases of clinically apparent acute liver injury with jaundice.
Background
Entacapone (en tak' a pone) is a specific inhibitor of cathechol-O-methyltransferase (COMT) which is a major enzyme in the pathway of levodopa metabolism. As a result, entacapone slows the metabolism of levodopa, causing an increase in its bioavailability and duration of action. Entacapone inhibits COMT activity only peripherally, unlike tolcapone which acts both peripherally and centrally. Entacapone was approved for use in the United States in 2003, the second COMT inhibitor approved for use in the therapy of symptomatic Parkinson disease as an adjunct to levodopa/carbidopa therapy in patients with motor complications. Entacapone is available in tablets of 200 mg generically and under the brand name of Comtan. It is also available in several fixed dose combinations with carbidopa and levodopa generically and under the brand name Stalevo. Entacapone is typically initiated in doses of 200 mg with each dose of levodopa/carbidopa to a maximum of 1600 mg daily. Common side effects include somnolence, dizziness, confusion, dyskinesia, vivid dreams, hallucinations, depression, fatigue, headache, diarrhea and gastrointestinal upset, side effects that are largely due to enhancement of the dopaminergic effects of levodopa. Uncommon, but potentially severe adverse events include hypotension, hallucinations, acute psychosis, impulsive behaviors, excessive daytime sleepiness, diarrhea and colitis and severe dyskinesia.
Hepatotoxicity
Entacapone therapy has been associated with serum aminotransferase elevations (above 3 times the upper limit of normal) in only 0.3% to 0.5% of patients, which is similar or minimally higher than the rate in subjects receiving placebo. The elevations were usually transient and asymptomatic and rarely required dose adjustment. In preliminary clinical trials, there were no reports of clinically apparent serious liver injury with jaundice. Subsequently, isolated instances of hepatotoxicity have been reported to the sponsor, injury arising 2 to 6 weeks after starting entacapone with mild jaundice and cholestatic pattern of liver enzyme elevations, and rapid recovery on stopping. Immunoallergic and autoimmune features were not present. The clinical phenotype of injury and associated features have not been reported in any detail. Thus, entacapone may rarely cause clinically apparent liver injury, but it has not been associated with the severe hepatitis and acute liver failure that characterized cases of tolcapone induced liver injury.
Likelihood score: D (possible, rare cause of clinically apparent liver injury).
Mechanism of Injury
Entacapone is extensively metabolized by the liver and eliminated though biotransformation, mostly by glucuronidation via UDP-glucuronosyl transferase. Polymorphisms of this enzyme have been linked to liver enzyme elevations during therapy, but the relationship between dose, metabolism and liver injury due to entacapone has not been defined.
Outcome and Management
The cases of hepatotoxicity attributed to entacapone have been mild and self-limiting. There have been no reports of acute liver failure, chronic liver injury or vanishing bile duct syndrome associated with entacapone therapy. In at least one case report, a patient who developed raised serum enzymes during tolcapone therapy, redeveloped enzyme elevations after being switched to entacapone. Otherwise, the liver injury associated with these two COMT inhibitors has been distinct, without evidence of a class effect.
Drug Class: Parkinson Disease Agents
Other Drugs in the Subclass, COMT Inhibitors: Opicapone, Tolcapone
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Entacapone – Generic, Comtan®
DRUG CLASS
Parkinson Disease Agents
Product labeling at DailyMed, National Library of Medicine, NIH
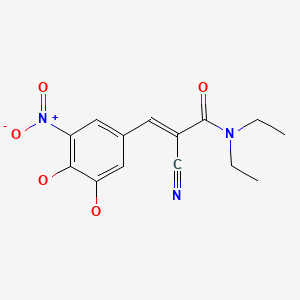
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Entacapone | 130929-57-6 | C14-H15-N3-O5 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 25 October 2021
Abbreviations used: COMT, catechol O-methyltransferase; MAO, monoamine oxidase.
- Zimmerman HJ. Antiparkinsonism drugs. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 715-7.(Expert review of hepatotoxicity published in 1999; among anticholinergic agents, "only trihexyphenidyl has been incriminated in hepatic injury"; other antiparkinsonism drugs discussed include levodopa, lergotrile [no longer available], pergolide and bromocriptine, but not entacapone).
- Larrey D, Ripault MP. Hepatotoxicity of psychotropic drugs and drugs of abuse. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier Inc, 2013, pp. 443-62.(Review of hepatotoxicity of agents acting on the central nervous system).
- Roberson ED. Parkinson Disease. Treatment of central nervous system degenerative disorders. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 328-333.(Textbook of pharmacology and therapeutics).
- Waters CH, Kurth M, Bailey P, Shulman LM, LeWitt P, Dorflinger E, Deptula D. S. Tolcapone in stable Parkinson's disease: efficacy and safety of long-term treatment. Tolcapone Stable Study Group. Neurology. 1998 May;50(5) Suppl 5:S39–45. [PubMed: 9591521](Among 298 patients with stable Parkinson disease treated with levodopa and either tolcapone [n=196] or placebo [n=102], ALT abnormalities occurred in 3-5% of tolcapone treated patients between month 1 and 6 of therapy, 4 were withdrawn and recovered; abnormalities resolved in another 4 despite continuing on therapy).
- Hauser RA, Molho E, Shale H, Pedder S, Dorflinger EE. A pilot evaluation of the tolerability, safety, and efficacy of tolcapone alone and in combination with oral selegiline in untreated Parkinson's disease patients. Tolcapone De Novo Study Group. Mov Disord. 1998;13:643–7. [PubMed: 9686768](Among 83 patients with Parkinson disease treated with tolcapone with or without selegiline for 8 weeks, ALT elevations occurred in 1 patient [2%] on tolcapone alone).
- Tolcapone for Parkinson's disease. Med Lett Drugs Ther. 1998;40(1028):60–1. [PubMed: 9629124](Concise summary of clinical efficacy and safety of tolcapone shortly after its approval in the US; common side effects were diarrhea, increase in levodopa related [dopaminergic] side effects and serum ALT elevations).
- Assal F, Spahr L, Hadengue A, Rubbia-Brandt L, Burkhard PR. Tolcapone and fulminant hepatitis. Lancet. 1998;352:958. [PubMed: 9752821](74 year old woman with Parkinson disease developed jaundice 9 weeks after starting tolcapone [bilirubin 17.1 mg/dL, ALT 2904 U/L, Alk P 177 U/L, protime 21 sec], progressing to hepatic failure and death 2 weeks later).
- Rivest J, Barclay CL, Suchowersky O. COMT inhibitors in Parkinson's disease. Can J Neurol Sci. 1999;26 Suppl 2:S34–8. [PubMed: 10451758](Review of efficacy and safety of tolcapone and entacapone in Parkinson disease; ALT elevations above 3 times the ULN occurred in 2-5% of tolcapone, but in no entacapone recipients; reports of 3 cases of acute liver failure due to tolcapone led to its withdrawal in several countries).
- Kaakkola S. Clinical pharmacology, therapeutic use and potential of COMT inhibitors in Parkinson's disease. Drugs. 2000;59:1233–50. [PubMed: 10882160](Review of the mechanism of action, pharmacology, efficacy and side effects of tolcapone and entacapone; both enhance dopaminergic effects of levodopa and diarrhea is a frequent dose modifying side effect; hepatotoxicity occurs with tolcapone, but has not been reported with entacapone).
- Lambert D, Waters CH. Comparative tolerability of the newer generation antiparkinsonian agents. Drugs Aging. 2000;16:55–65. [PubMed: 10733264](Review of mechanism of action, tolerability and safety of selegiline, pramipexole, ropinirole, tolcapone and entacapone in Parkinson disease).
- Olanow CW. Tolcapone and hepatotoxic effects. Tasmar Advisory Panel. Arch Neurol. 2000;57:263–7. [PubMed: 10681087](Consensus recommendations for monitoring patients on tolcapone after 4 reports of acute liver failure; among 1535 patients treated in phase III studies, ALT or AST elevations [>3 times ULN] occurred in 1.3-3.7% of patients, returning to normal when discontinued and one woman developed jaundice and died; postmarketing reports included 4 patients, ages 66-74, with onset of symptoms and jaundice after 2-4 months, [bilirubin 6.9-26.1 mg/dL, ALT 1245-5020 U/L, Alk P 66-347 U/L], 3 died within 1-2 weeks of presentation).
- Spahr L, Rubbia-Brandt L, Burkhard PR, Assal F, Hadengue A. Tolcapone-related fulminant hepatitis: electron microscopy shows mitochondrial alterations. Dig Dis Sci. 2000;45:1881–4. [PubMed: 11052337](Histologic analysis of patient with acute liver failure due to tolcapone [Assal 1988], showed multilobular collapse, inflammatory infiltrates including eosinophils, cholestasis, and focal microvesicular steatosis; electron microscopy suggested mitochondrial injury: Case 1).
- Watkins P. COMT inhibitors and liver toxicity. Neurology. 2000;55(11) Suppl 4:S51–2. [PubMed: 11147510](Review of hepatotoxicity of tolcapone and entacapone suggesting that liver injury is not a class effect and that there have been no reports of jaundice attributed to entacapone).
- Entacapone for Parkinson's disease. Med Lett Drugs Ther. 2000;42:7–8. [PubMed: 10696231](Concise summary of clinical efficacy and safety of entacapone shortly after its approval in the US; common side effects were diarrhea, increase in levodopa related side effects, but no reported hepatotoxicity as occurs with tolcapone).
- Blum MW, Siegel AM, Meier R, Hess K. Neuroleptic malignant-like syndrome and acute hepatitis during tolcapone and clozapine medication. Eur Neurol. 2001;46:158–60. [PubMed: 11598337](70 year old woman developed stupor, rigidity and hyperthermia with increases in CPK [3132 U/L] and ALT [988 U/L], but not bilirubin or alkaline phosphatase while on the combination of tolcapone and clozapine, resolving rapidly on stopping therapy; compatible with neuroleptic malignant-like syndrome).
- Myllylä VV, Kultalahti ER, Haapaniemi H, Leinonen M., FILOMEN Study Group. Twelve-month safety of entacapone in patients with Parkinson's disease. Eur J Neurol. 2001;8:53–60. [PubMed: 11509081](Among 326 patients with Parkinson disease treated with entacapone or placebo, ALT elevations occurred in 6.9% on drug vs 4.6% on placebo and were above 3 times ULN in 0.9% vs 0.0%; no patient developed clinically apparent liver injury that could be attributed to entacapone).
- Acuña G, Foernzler D, Leong D, Rabbia M, Smit R, Dorflinger E, Gasser R, et al. Pharmacogenetic analysis of adverse drug effect reveals genetic variant for susceptibility to liver toxicity. Pharmacogenomics J. 2002;2:327–34. [PubMed: 12439739](DNA genotyping of 30 single nucleotide polymorphisms in 135 patients who had liver enzyme elevations during tolcapone therapy and controls found variants within the UDP-glucuronsyl transferase gene that were associated with liver injury).
- Fisher A, Croft-Baker J, Davis M, Purcell P, McLean AJ. Entacapone-induced hepatotoxicity and hepatic dysfunction. Mov Disord. 2002;17:1362–5. [PubMed: 12465084](Three cases of clinically apparent liver injury attributed to entacapone use; 74 year old woman developed nausea and fatigue 2 weeks after adding entacapone to a regimen of levodopa/benserazide for Parkinson disease [bilirubin 2.4 mg/dL, ALT 104 U/L, Alk P 238 U/L], with rapid improvement on stopping; 2 other cases were reported to Australian Drug Reaction Database with only partial documentation, arising 5 and 6 weeks after starting entacapone, with cholestatic liver enzyme elevations and mild jaundice).
- Benabou R, Waters C. Hepatotoxic profile of catechol-O-methyltransferase inhibitors in Parkinson's disease. Expert Opin Drug Saf. 2003;2:263–7. [PubMed: 12904105](Review of hepatotoxicity of tolcapone and entacapone).
- Borges N. Tolcapone-related liver dysfunction: implications for use in Parkinson's disease therapy. Drug Saf. 2003;26:743–7. [PubMed: 12908845](Review of hepatotoxicity of tolcapone and possible mechanisms).
- Brooks DJ. Safety and tolerability of COMT inhibitors. Neurology. 2004;62(1) Suppl 1:S39–46. [PubMed: 14718679](Review of safety and side effects of entacapone; in phase III trials ALT elevations >3 times ULN occurred in 0.3-0.5% of patients taking entacapone and 0.4% on placebo, and there were no cases of jaundice or clinically apparent liver injury among ~1600 entacapone treated patients).
- Deane KH, Spieker S, Clarke CE. Catechol-O-methyltransferase inhibitors for levodopa-induced complications in Parkinson's disease. Cochrane Database Syst Rev. 2004;(4):CD004554. [PMC free article: PMC8830033] [PubMed: 15495119](Systematic review of efficacy of tolcapone and entacapone; ALT elevations reported in variable proportions of patients on tolcapone).
- Levodopa + carbidopa + entacapone. Entacapone: a second look: new preparations. Parkinson's disease: a modest effect. Prescrire Int. 2005;14:51–4. [PubMed: 15875340](Review of risks and benefits of a fixed dose combination of levodopa, carbidopa and entacapone mentions that entacapone may cause cholestatic hepatitis and that is has not been shown to be more effective than bromocriptine).
- Borges N. Tolcapone in Parkinson's disease: liver toxicity and clinical efficacy. Expert Opin Drug Saf. 2005;4:69–73. [PubMed: 15709899](Review of hepatotoxicity of tolcapone and its possible mechanisms).
- Korri H, Awada A. Rev Neurol (Paris). 2005;161:1113–5. [Serious tolcapone-induced hepatitis 17 months after commencing treatment] French. [PubMed: 16288178](61 year old man with Parkinson disease developed jaundice and fever 17 months after starting tolcapone [bilirubin 3.1 mg/dL, ALT 399 U/L, Alk P 115 U/L], resolving upon stopping).
- Martignoni E, Cosentino M, Ferrari M, Porta G, Mattarucchi E, Marino F, Lecchini S, et al. Two patients with COMT inhibitor-induced hepatic dysfunction and UGT1A9 genetic polymorphism. Neurology. 2005;65:1820–2. [PubMed: 16344532](Two patients who had ALT elevations [78 and 284 U/L] during tolcapone therapy, one of whom had similar elevations during entacapone treatment; both had the A(T)9AT sequence [1A9*1] in the promoter of the UGT1A9 gene).
- Tolcapone: new drug. In Parkinson's disease: unacceptable risk of severe hepatitis. Prescrire Int. 2006;15:54–7. [PubMed: 16604736](Review of tolcapone as adjunctive therapy in Parkinson disease suggests that the hepatotoxicity risk makes it an unacceptable option).
- Leegwater-Kim J, Waters C. Tolcapone in the management of Parkinson's disease. Expert Opin Pharmacother. 2006;7:2263–70. [PubMed: 17059382](Review on use of tolcapone in Parkinson disease suggesting that with proper monitoring, the potential for hepatotoxicity is "negligibly small").
- Stocchi F, De Pandis MF. Utility of tolcapone in fluctuating Parkinson's disease. Clin Interv Aging. 2006;1:317–25. [PMC free article: PMC2699640] [PubMed: 18046910](Review of role of tolcapone in treatment of Parkinson disease, indicating its efficacy in patients with fluctuating symptoms and its safety with proper monitoring of serum enzymes).
- Entacapone to Tolcapone Switch Study Investigators. Entacapone to tolcapone switch: Multicenter double-blind, randomized, active-controlled trial in advanced Parkinson's disease. Mov Disord. 2007;22:14–9. [PubMed: 17089403](Randomized controlled trial of replacing entacapone with tolcapone in patients with Parkinson disease and motor fluctuations on long term levodopa therapy; ALT elevations occurred in 3% on entacapone and 9% on tolcapone, but were mild and self-limiting).
- Leegwater-Kim J, Waters C. Role of tolcapone in the treatment of Parkinson's disease. Expert Rev Neurother. 2007;7:1649–57. [PubMed: 18052761](Review of the pharmacology, metabolism, clinical efficacy and safety of tolcapone, indicating that the risk of hepatotoxicity "is very small if proper hepatic monitoring guidelines are followed").
- Olanow CW, Watkins PB. Tolcapone: an efficacy and safety review (2007). Clin Neuropharmacol. 2007;30:287–94. [PubMed: 17909307](Review of the efficacy and safety of tolcapone focusing upon the hepatotoxicity concludes that tolcapone can be used safety if there is strict adherence to routine liver test monitoring requirements of every 2-4 weeks for 6 months and at 3-6 month intervals thereafter).
- Lees AJ, Ratziu V, Tolosa E, Oertel WH. Safety and tolerability of adjunctive tolcapone treatment in patients with early Parkinson's disease. J Neurol Neurosurg Psychiatry. 2007;78:944–8. [PMC free article: PMC2117861] [PubMed: 17098835](Controlled trial of tolcapone vs placebo combined with levodopa and carbidopa in 677 patients with early Parkinson disease; ALT or AST elevations occurred in 20% of placebo- vs 27% of tolcapone treated patients and were >3 times ULN in 1.2% [placebo] vs 1.8% [tolcapone], almost all during first 6 months; 1% of tolcapone treated patients stopped because of ALT elevations, but none developed jaundice or clinically apparent liver injury).
- Lew MF, Kricorian G. Results from a 2-year centralized tolcapone liver enzyme monitoring program. Clin Neuropharmacol. 2007;30:281–6. [PubMed: 17909306](Centralized testing for ALT and AST in 1725 patients with Parkinson disease treated with tolcapone for up to 2 years; 69 [3.9%] had at least one elevation, but <1% had an elevation above 2 times the ULN and most returned to normal despite continuing therapy).
- Entacapone: hepatitis (continued). The risk of liver damage is being confirmed. It is better not to expose parkinsonian patients to this drug. Prescrire Int. 2008;17:113–4. [PubMed: 18630358](Commentary mentions that the European Medicines Agency has reported 29 cases of hepatic disorders linked to entacapone).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver injury in the US collected between 2004 and 2008, none were due to drugs used for Parkinson disease).
- Canesi M, Zecchinelli AL, Pezzoli G, Antonini A. Clinical experience of tolcapone in advanced Parkinson's disease. Neurol Sci. 2008;29 Suppl 5:S380–2. [PubMed: 19381768](Among 66 patients with advanced Parkinson disease treated with tolcapone, 2 [3%] were withdrawn from therapy because of ALT elevations).
- Brooks DJ, Leinonen M, Kuoppamäki M, Nissinen H. Five-year efficacy and safety of levodopa/DDCI and entacapone in patients with Parkinson's disease. J Neural Transm. 2008;115:843–9. [PubMed: 18259682](Retrospective, pooled analysis of 5 controlled trials with 5 year extension phases that included 806 patients with Parkinson disease treated with entacapone added to levodopa/carbodopa; there were "few clinically significant changes in liver function tests" and none of the 478 serious adverse events that were reported were due to hepatotoxicity).
- Ebersbach G, Storch A. Tolcapone in elderly patients with Parkinson's disease: a prospective open-label multicenter non-interventional trial. Arch Gerontol Geriatr. 2009;49:e40–4. [PubMed: 18835049](Among 237 patients with advanced Parkinson disease treated with tolcapone, diarrhea was the most common side effect [3.4%], ALT or AST elevations occurred in 18%, but were mostly mild and "not clinically significant").
- McBurney RN, Hines WM, Von Tungeln LS, Schnackenberg LK, Beger RD, Moland CL, Han T, et al. The liver toxicity biomarker study: phase I design and preliminary results. Toxicol Pathol. 2009;37:52–64. [PubMed: 19171931](Design and early results of a comprehensive study of rats given 28 days of entacapone or tolcapone as examples of two related agents, one of which causes liver injury in man and one which does not, assessing liver enzymes, histology, gene transcription, proteomics, metabolomics and possible biomarkers to identify predictors of idiosyncratic liver injury in humans).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury but none were attributed to agents used for Parkinson disease).
- Fischer JJ, Michaelis S, Schrey AK, Graebner OG, Glinski M, Dreger M, Kroll F, et al. Capture compound mass spectrometry sheds light on the molecular mechanisms of liver toxicity of two Parkinson drugs. Toxicol Sci. 2010;113:243–53. [PubMed: 19783845](In vitro study of binding of tolcapone and entacapone to other proteins; unlike entacapone, tolcapone interacted with a number of non-COMT intracellular proteins which are involved in the respiratory chain actions, fatty acid beta-oxidation and bile acid synthesis, perhaps accounting for its potential for hepatotoxicity).
- Haasio K. Toxicology and safety of COMT inhibitors. Int Rev Neurobiol. 2010;95:163–89. [PubMed: 21095462](Extensive review of the mechanism of hepatic injury from tolcapone; "at the moment there is no explanation to the hepatotoxicity appeared in clinical use").
- Marsala SZ, Gioulis M, Ceravolo R, Tinazzi M. A systematic review of catechol-0-methyltransferase inhibitors: efficacy and safety in clinical practice. Clin Neuropharmacol. 2012;35:185–90. [PubMed: 22805229](Systematic review of literature on safety and efficacy of tolcapone and entacapone recommends use of tolcapone only if entacapone treatment fails and liver tests are normal).
- McBurney RN, Hines WM, VonTungeln LS, Schnackenberg LK, Beger RD, Moland CL, Han T, et al. The liver toxicity biomarker study phase I: markers for the effects of tolcapone or entacapone. Toxicol Pathol. 2012;40:951–64. [PubMed: 22573522](Comparison of the molecular effects of tolcapone vs entacapone on rat liver and plasma biomarkers found that changes from the two drugs only partially overlapped and different effects were present at 3 and 28 days, suggesting that some of these "off-target" and specific effects of tolcapone may account for its occasional hepatotoxicity).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–1425. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none of the 96 were attributed to an agent used to treat Parkinson disease).
- Drugs for Parkinson's disease. Treat Guidel Med Lett. 2013;11(135):101–6. [PubMed: 24165688](Concise review of recommendations for therapy of Parkinson disease with description of mechanisms of action, efficacy and adverse events).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, none were attributed to an agent to treat Parkinson disease).
- Eggert K, Oertel WH, Lees AJ. German Competence Network on Parkinson’s disease. Safety and efficacy of tolcapone in the long-term use in Parkinson disease: an observational study. Clin Neuropharmacol. 2014;37:1–5. [PubMed: 24434524](Among 391 patients with Parkinson disease treated with tolcapone in an observation study conducted at 48 neurologic centers and followed for one year, 34 [8.7%] developed liver enzyme elevation, usually within the first 3 months, which were above twice ULN in only 5 [1.3%] and resolved spontaneously in most; no patient developed clinically apparent liver injury).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury from the US enrolled in a prospective database between 2004 and 2012, none were attributed to an agent used to treat Parkinson disease).
- Longo DM, Yang Y, Watkins PB, Howell BA, Siler SQ. Elucidating differences in the hepatotoxic potential of tolcapone and entacapone with DILIsym(®), a mechanistic model of drug-induced liver injury. CPT Pharmacometrics Syst Pharmacol. 2016;5(1):31–9. [PMC free article: PMC4728295] [PubMed: 26844013](Description of mechanistic simulation models of the metabolism and toxicity of tolcapone and entacapone which predicted their differential hepatotoxicity).
- Lv X, Wang XX, Hou J, Fang ZZ, Wu JJ, Cao YF, Liu SW, et al. Comparison of the inhibitory effects of tolcapone and entacapone against human UDP-glucuronosyl-transferases. Toxicol Appl Pharmacol. 2016;301:42–9. [PubMed: 27089846](Comparison of the inhibitory effects of tolcapone and entacapone against recombinant human UGTs showed more potent inhibition by tolcapone for most isoforms).
- Drugs for Parkinson's disease. Med Lett Drugs Ther. 2017;59(1534):187–194. [PubMed: 29136401](Concise review of medications approved for use in Parkinson disease including levodopa/carbidopa, dopamine agonists, MAO-B inhibitors, anticholinergics, and COMT inhibitors, mentions hepatotoxicity of tolcapone but not of levodopa or any of the other adjunctive therapies: “Use of tolcapone requires written informed consent and monitoring of liver function every 2-4 weeks for the first 6 months of treatment and periodically thereafter. Serious hepatoxicity has not been reported with entacapone”).
- Margolesky J, Singer C. Extended-release oral capsule of carbidopa-levodopa in Parkinson disease. Ther Adv Neurol Disord. 2017;11:1756285617737728. [PMC free article: PMC5784558] [PubMed: 29399046](Review of the pharmacology, efficacy and safety of extended release carbidopa/levodopa in listing of reported adverse reactions; there is no mention of serious hepatic events or ALT elevations).
- Mak A, Kato R, Weston K, Hayes A, Uetrecht J. Editor's highlight: An impaired immune tolerance animal model distinguishes the potential of troglitazone/pioglitazone and tolcapone/entacapone to cause IDILI. Toxicol Sci. 2018;161:412–420. [PMC free article: PMC5837423] [PubMed: 29087505](In an animal model of idiosyncratic liver injury using PD-1 knock out mice treated with anti-CTLA-4 to decrease the natural inhibition of T cell responses [immune check point], treatment with tolcapone caused greater injury than treatment with entacapone, while neither agent caused liver injury in wild type mice or PD-1 knock out mice without anti-CTLA-4 treatment, suggesting that idiosyncratic liver injury is immune mediated, caused by an unregulated CD-8+ response and failure of immune tolerance).
- Fremont R, Manoochehri M, Armstrong NM, Mattay VS, Apud JA, Tierney MC, Devanand DP, et al. Tolcapone treatment for cognitive and behavioral symptoms in behavioral variant frontotemporal dementia: a placebo-controlled crossover study. J Alzheimers Dis. 2020;75:1391–1403. [PMC free article: PMC10131251] [PubMed: 32444540](Among 28 adults with frontotemporal dementia with behavioral symptoms who were treated with tolcapone [200 mg] or placebo three times daily for 9 days in a cross-over design, tolcapone therapy was associated with slight improvements in some cognitive and behavioral scores and was well tolerated, although 21% of patients developed mild liver enzyme elevations [less than twice ULN] that were asymptomatic and resolved rapidly).
- Artusi CA, Sarro L, Imbalzano G, Fabbri M, Lopiano L. Safety and efficacy of tolcapone in Parkinson's disease: systematic review. Eur J Clin Pharmacol. 2021;77:817–829. [PMC free article: PMC8128808] [PubMed: 33415500](Systematic review of the literature on the efficacy and safety of tolcapone identified 32 studies with 4780 patients, of whom 0.9% developed liver enzyme elevations above 2 times ULN; and, while there were 3 case reports of fatal acute liver failure all reported in 1998, since then there have been no deaths and only 3 non-fatal cases).
- Drugs for Parkinson's disease. Med Lett Drugs Ther. 2021;63(1618):25–32. [PubMed: 33647001](Concise review of current medications approved for use in Parkinson disease including levodopa/carbidopa, dopamine agonists, COMT inhibitors, MAO-B inhibitors, anticholinergics, and istradefylline, mentions hepatotoxicity of tolcapone but not of levodopa or any of the adjunctive therapies).
- Hauser RA, Hattori N, Fernandez H, Isaacson SH, Mochizuki H, Rascol O, Stocchi F, et al. Efficacy of istradefylline, an adenosine A2A receptor antagonist, as adjunctive therapy to levodopa in Parkinson's disease: a pooled analysis of 8 phase 2b/3 trials. J Parkinsons Dis. 2021;11:1663–75. [PMC free article: PMC8609697] [PubMed: 34486986](In a pooled analysis of 8 randomized placebo-controlled trials of istradefylline in 2719 patients with Parkinson disease and motor complications, while adverse event rates were similar in the 3 groups [71% and 70% vs 65%] except for dyskinesia [16% and 18% vs 10%], and “no clinically meaningful changes in laboratory parameters…were observed” in any group including those on levodopa/carbidopa alone).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Levodopa + carbidopa + entacapone. Entacapone: a second look: new preparations. Parkinson's disease: a modest effect.[Prescrire Int. 2005]Levodopa + carbidopa + entacapone. Entacapone: a second look: new preparations. Parkinson's disease: a modest effect.. Prescrire Int. 2005 Apr; 14(76):51-4.
- Effects of One-Day Application of Levodopa/Carbidopa/Entacapone versus Levodopa/Carbidopa/Opicapone in Parkinson's Disease Patients.[Cells. 2022]Effects of One-Day Application of Levodopa/Carbidopa/Entacapone versus Levodopa/Carbidopa/Opicapone in Parkinson's Disease Patients.Müller T, Schlegel E, Zingler S, Thiede HM. Cells. 2022 Apr 30; 11(9). Epub 2022 Apr 30.
- Review Entacapone: a catechol-O-methyltransferase inhibitor for the adjunctive treatment of Parkinson's disease.[Clin Ther. 2001]Review Entacapone: a catechol-O-methyltransferase inhibitor for the adjunctive treatment of Parkinson's disease.Najib J. Clin Ther. 2001 Jun; 23(6):802-32; discussion 771.
- The catechol-O-methyltransferase (COMT) inhibitor entacapone enhances the pharmacokinetic and clinical response to Sinemet CR in Parkinson's disease.[J Neurol Neurosurg Psychiatry....]The catechol-O-methyltransferase (COMT) inhibitor entacapone enhances the pharmacokinetic and clinical response to Sinemet CR in Parkinson's disease.Piccini P, Brooks DJ, Korpela K, Pavese N, Karlsson M, Gordin A. J Neurol Neurosurg Psychiatry. 2000 May; 68(5):589-94.
- Review Entacapone.[Ann Pharmacother. 2000]Review Entacapone.Chong BS, Mersfelder TL. Ann Pharmacother. 2000 Sep; 34(9):1056-65.
- Entacapone - LiverToxEntacapone - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...