NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Opicapone is a catechol-O-methyltransferase inhibitor that is used as adjunctive therapy to levodopa/carbidopa in patients with Parkinson disease experiencing difficulty with “off” episodes when motor symptoms breakthrough on treatment. Opicapone has been associated with a minimal rate of serum enzyme elevations during therapy and has not been linked to cases of clinically apparent liver injury with jaundice.
Background
Opicapone (oh pik’ a pone) is a catechol O-methyltransferase (COMT) inhibitor that blocks the peripheral metabolism of levodopa and prolongs its serum half-life and effectiveness in providing dopamine to the brain. Levodopa/carbidopa is the mainstay of therapy of Parkinson disease, increasing brain dopamine levels and typically alleviating the motor symptoms of Parkinson disease for 5 to 6 hours. After the first 2 to 5 years of therapy, however, the duration of benefit becomes shorter (wearing off effect), and patients develop fluctuations between mobility and immobility (on-off effect). Opicapone, by slowing the metabolism of levodopa increases its duration of action. In several prospective randomized, placebo controlled trials, opicapone was found to decrease the duration of wake “off” time episodes and increase the amount of “on” time. Opicapone was approved for use as adjunctive therapy to levodopa and carbidopa for adults with Parkinson disease who experienced off episodes in 2020. Opicapone is currently available in capsules of 25 and 50 mg under the brand name Ongentys. The recommended dose is 50 mg once daily. While opicapone may improve control of symptoms, there is no evidence that it slows the progression of Parkinson disease. Common side effects include dyskinesia, dizziness, constipation, nausea, daytime sleepiness, and insomnia. Less common but potentially severe adverse events include hallucinations, psychotic and compulsive behaviors, hypersexuality, and severe dyskinesia. Abrupt discontinuation of opicapone can be followed by a withdrawal symptoms of hyperpyrexia and confusion.
Hepatotoxicity
In prelicensure controlled trials, serum ALT elevations occurred uncommonly in opicapone-treated subjects and in rates similar to that of placebo controls. In studies of more than 1000 patients treated with opicapone there were no instances of serious hepatic events and no relevant changes in serum enzymes. After its approval and more widespread use, there have been no reports clinically apparent liver injury attributable to opicapone. There has, however, been limited clinical experience with its use.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
Opicapone was the third COMT inhibitor approved as adjuvant therapy with levodopa in patients with Parkinson disease who were experiencing motor complications (“off” episodes). The initial COMT inhibitor approved for use was tolcapone, which was associated with a high rate of marked serum aminotransferase elevations during therapy and subsequently with several reports of clinically apparent liver injury. For that reason, tolcapone is subjected to a strict monitoring program in which regular testing of serum aminotransferase levels is strongly advised. Because opicapone has not been found to cause aminotransferase elevations, regular monitoring is not required or even recommended. The reasons for the relative safety of opicapone in comparison to tolcapone are unclear. Opicapone is metabolized by sulfonylation and glucuronidation rather than by the cytochrome P450 microsomal enzymes (CYP).
Outcome and Management
Opicapone has been not been linked to serum enzyme elevations during therapy. Discontinuation for serum enzyme elevations is rarely necessary, but should be done if the elevations are accompanied by symptoms or jaundice or for ALT elevations of more than 5 times the upper limit of normal (ULN). There is no information on cross sensitivity to liver injury between opicapone and other agents used in the therapy of Parkinson disease.
Drug Class: Parkinson Disease Agents
Other Drugs in the Subclass, COMT Inhibitors: Entacapone, Tolcapone
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Opicapone – Ongentys®
DRUG CLASS
Parkinson Disease Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Opicapone | 923287-50-7 | C15-H10-Cl2-N4-O6 |
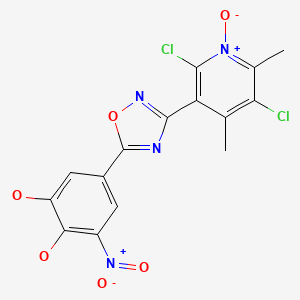
|
ANNOTATED BIBLIOGRAPHY
References updated: 25 October 2021
Abbreviations used: COMT, catechol O-methyltransferase; CPK, creatine phosphokinase; MAO, monoamine oxidase.
- Roberson ED. Parkinson Disease. Treatment of central nervous system degenerative disorders. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 328-333.(Textbook of pharmacology and therapeutics; COMT inhibitors are discussed).
- FDA. https://www
.accessdata .fda.gov/drugsatfda_docs /nda/2020/212489Orig1s000MedR.pdf. (FDA website, including product label and clinical review of data submitted in support of approval of opicapone, mentions that therapy is associated with a minimal rate of ALT elevations which was comparable to that of placebo [<1%], that elevations above 3 times ULN were rare, and there were no cases with concurrent bilirubin elevations). - Ferreira JJ, Lees A, Rocha JF, Poewe W, Rascol O, Soares-da-Silva P. Bi-Park 1 investigators. Opicapone as an adjunct to levodopa in patients with Parkinson's disease and end-of-dose motor fluctuations: a randomised, double-blind, controlled trial. Lancet Neurol. 2016;15:154–165. [PubMed: 26725544](Among 600 adults with Parkinson disease with motor fluctuations while on levodopa who were treated with opicapone [5, 25, or 50 mg], entacapone [200 mg], or placebo once daily for 14-15 weeks, “off” time decreased by 60 minutes in the 50 mg dose group in comparison to placebo, which was non-inferior to entacapone, and total adverse event rates were similar in the three groups [54% vs 57% and 50%], dyskinesia was more frequent with opicapone [16% vs 8% and 4%] and “changes in laboratory tests…differed by <2% across visits for any treatment groups”).
- Lees AJ, Ferreira J, Rascol O, Poewe W, Rocha JF, McCrory M, Soares-da-Silva P., BIPARK-2 Study Investigators. Opicapone as adjunct to levodopa therapy in patients With Parkinson disease and motor fluctuations: a randomized clinical trial. JAMA Neurol. 2017;74:197–206. [PubMed: 28027332](Among 427 adults with Parkinson disease and motor fluctuations while on levodopa treated with opicapone [25 or 50 mg] vs placebo for 14-15 weeks and then in an open phase with opicapone for one year, “off” time decreased 102 to 118 minutes with opicapone vs 65 minutes with placebo, and effects were maintained for the full year, while total adverse event rates were 71% vs 64%, dyskinesia rates 24% vs 8% and “no relevant liver function finding occurred in either phase”).
- Lees A, Ferreira JJ, Rocha JF, Rascol O, Poewe W, Gama H, Soares-da-Silva P. Safety profile of opicapone in the management of Parkinson's disease. J Parkinsons Dis. 2019;9:733–740. [PubMed: 31498127](Analysis of pooled results of controlled trials and open label studies of opicapone as an adjunct for Parkinson disease [1614 patients] identified total adverse event rates for opicapone [25 and 50 mg daily combined] vs placebo to be 63.5% vs 57.5%, serious adverse events 3.5% vs 4.3%, dyskinesia 18.3% vs 6.2%, constipation 5.7% vs 1.9%, insomnia 5.1% vs 1.6%, CPK elevations 3.9% vs 1.9%, dizziness 3.7% vs 1.2%, somnolence 2.9% vs 1.9%, hallucinations 1.8% vs 0.4%, and that there were no relevant changes in liver enzymes and no serious hepatic adverse events).
- Ferreira JJ, Lees A, Rocha JF, Poewe W, Rascol O, Soares-da-Silva P. Long-term efficacy of opicapone in fluctuating Parkinson's disease patients: a pooled analysis of data from two phase 3 clinical trials and their open-label extensions. Eur J Neurol. 2019;26:953–960. [PMC free article: PMC6593852] [PubMed: 30681754](Pooled results of two placebo-controlled trials of opicapone in adults with Parkinson disease and motor complications yielded an overall average decrease in “off” time compared to placebo of -35 minutes for the 25 mg dose and -58 minutes for the 50 mg dose with an accompanying increase in “on” time without worsening of dyskinesia; no information provided on safety results).
- Reichmann H, Lees A, Rocha JF, Magalhães D, Soares-da-Silva P., OPTIPARK investigators. Effectiveness and safety of opicapone in Parkinson's disease patients with motor fluctuations: the OPTIPARK open-label study. Transl Neurodegener. 2020;9:9. [PMC free article: PMC7055125] [PubMed: 32345378](Among 495 adults with Parkinson disease and motor fluctuations on levodopa therapy who were treated in an open label trial of opicapone [50 mg daily] in Germany for 3 months and the UK for 6 months, subjective improvement was reported in 71-85% of patients while the overall adverse event rate was 75%, serious adverse event rate 6.9%, and discontinuation rate for treatment relate adverse events 13%; no mention of ALT elevations or serious hepatic adverse events).
- Scott LJ. Opicapone: a review in Parkinson's disease. CNS Drugs. 2021;35:121–131. [PubMed: 33428178](Review of the mechanism of action, pharmacology, clinical efficacy and tolerance of opicapone, the third COMT inhibitor approved in the US [having been approved in Europe in 2016], specifically comments that in clinical trials, it has no clinically relevant hepatobiliary adverse events).
- Opicapone (Ongentys) - a COMT inhibitor for Parkinson's disease. Med Lett Drugs Ther. 2021;63(1615):3–5. [PubMed: 33646998](Concise review of mechanism of action, clinical efficacy, safety and costs of opicapone shortly after its approval for use in Parkinson disease, mentions adverse events of dyskinesia, constipation, creatine phosphokinase [CPK] elevations, hypotension, syncope, weight loss, drowsiness, hallucinations, delusions, agitation and withdrawal symptoms; mentions specifically that it has not been associated with hepatotoxicity).
- Drugs for Parkinson's disease. Med Lett Drugs Ther. 2021;63(1618):25–32. [PubMed: 33647001](Concise review of current medications approved for use in Parkinson disease including levodopa/carbidopa, dopamine agonists, COMT inhibitors, monoamine oxidase [MAO]-B inhibitors, anticholinergics, and istradefylline, mentions hepatotoxicity of tolcapone but not of opicapone or any other of the adjunctive therapies).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Opicapone Pharmacokinetics and Effects on Catechol- O -Methyltransferase Activity and Levodopa Pharmacokinetics in Patients With Parkinson Disease Receiving Carbidopa/Levodopa.[Clin Neuropharmacol. 2023]Opicapone Pharmacokinetics and Effects on Catechol- O -Methyltransferase Activity and Levodopa Pharmacokinetics in Patients With Parkinson Disease Receiving Carbidopa/Levodopa.LeWitt P, Liang GS, Olanow CW, Kieburtz KD, Jimenez R, Olson K, Klepitskaya O, Loewen G. Clin Neuropharmacol. 2023 Mar-Apr 01; 46(2):43-50. Epub 2023 Jan 21.
- Effects of One-Day Application of Levodopa/Carbidopa/Entacapone versus Levodopa/Carbidopa/Opicapone in Parkinson's Disease Patients.[Cells. 2022]Effects of One-Day Application of Levodopa/Carbidopa/Entacapone versus Levodopa/Carbidopa/Opicapone in Parkinson's Disease Patients.Müller T, Schlegel E, Zingler S, Thiede HM. Cells. 2022 Apr 30; 11(9). Epub 2022 Apr 30.
- Effect of Opicapone on Levodopa Pharmacokinetics in Patients with Fluctuating Parkinson's Disease.[Mov Disord. 2022]Effect of Opicapone on Levodopa Pharmacokinetics in Patients with Fluctuating Parkinson's Disease.Ferreira JJ, Poewe W, Rascol O, Stocchi F, Antonini A, Moreira J, Guimarães B, Rocha JF, Soares-da-Silva P. Mov Disord. 2022 Nov; 37(11):2272-2283. Epub 2022 Aug 31.
- Review Istradefylline.[LiverTox: Clinical and Researc...]Review Istradefylline.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Opicapone for the Treatment of Parkinson's Disease "Off" Episodes: Pharmacology and Clinical Considerations.[Clin Drug Investig. 2022]Review Opicapone for the Treatment of Parkinson's Disease "Off" Episodes: Pharmacology and Clinical Considerations.Berger AA, Robinson C, Winnick A, Izygon J, Jacob BM, Noonan MJ, Kaye AD, Kaye JS, Kaye AM, Cornett EM, et al. Clin Drug Investig. 2022 Feb; 42(2):127-135. Epub 2021 Dec 21.
- Opicapone - LiverToxOpicapone - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...