NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Ethambutol is a first line but adjunctive antituberculosis medication which is used only in combination with other agents such as isoniazid and rifampin. Ethambutol therapy has been associated with minor, transient and asymptomatic elevations in serum aminotransferase levels, and is a reported but rare cause of clinically apparent acute liver injury.
Background
Ethambutol (eth am' bue tol) is a semisynthetic antibiotic which is bacteriostatic against Mycobacterium tuberculosis. Ethambutol interferes with the incorporation of mycolic acid into the mycobacterial cell wall, thus inhibiting its cell wall synthesis. Ethambutol has a broader spectrum of activity against mycobacterial species than isoniazid or rifampin and is therefore used largely in patients with suspected resistance or in atypical mycobacterial infections. Ethambutol is available in generic forms in tablets of 100 and 400 mg. The recommended dose is 15 mg/kg once daily in combination with other antituberculosis medications such as isoniazid, rifampin, pyrazinamide, and streptomycin. Higher doses are recommended for patients being retreated after a relapse. It is most typically given with pyrazinamide for the first two months of combination therapy of suspected resistant tuberculosis with isoniazid and rifampin, the latter two continuing for at least six months. Common side effects include gastrointestinal upset, nausea, dizziness, fever, and rash.
The management of drug-resistant tuberculosis is challenging and should be under the direction of physicians with expertise in tuberculosis therapy and management of its side effects. Optimal regimens of therapy for tuberculosis are complex and change frequently. Regularly updated recommendations on use of drugs for tuberculosis, including indications, contraindications, warnings, dosages and monitoring recommendations are available at the Centers for Disease Control and Prevention website: https://www.cdc.gov/tb/publications/guidelines/Treatment.htm.
Hepatotoxicity
Because ethambutol is almost always used in combination with isoniazid, rifampin or other antituberculosis agents, the frequency of serum aminotransferase elevations attributable to ethambutol alone cannot be estimated with any confidence. The addition of ethambutol to isoniazid, rifampin or pyrazinamide does not appear to increase the rate of transient ALT elevations during therapy. In addition, ethambutol is a rare cause of acute, symptomatic liver injury. Despite 50 years of use, ethambutol has been linked to clinically apparent liver injury in only a few case reports. In the best described instance (Case 1), the onset of symptoms was 2 months after starting combination antituberculosis therapy and, in contrast to liver injury due to isoniazid or pyrazinamide, the pattern of serum enzymes was distinctly cholestatic. The recurrence of liver injury upon rechallenge with ethambutol but not isoniazid made the attribution convincing. Other case reports have described liver injury occurring in the context of DRESS syndrome, arising within 2 to 6 weeks of starting antituberculosis therapy with fever, rash, eosinophilia and other organ involvement such as liver, kidney and lung. Several published instances have described recurrence of injury after rechallenge with ethambutol.
Likelihood score: C (probable cause of clinically apparent liver injury often in the context of DRESS syndrome).
Mechanism of Injury
The cause of liver injury due to ethambutol is not known, but is likely due to hypersensitivity.
Outcome and Management
Ethambutol is one of the few antituberculosis medications that is generally safe in the setting of liver disease. In the unlikely event of clinically apparent liver injury or allergic reaction to ethambutol, rechallenge, if necessary, should be done with caution. There does not appear to be cross sensitivity to liver injury with other antituberculosis medications.
[First line medications used in the therapy of tuberculosis in the US include ethambutol, isoniazid, pyrazinamide, rifabutin, rifampin, and rifapentine. Second line medications include streptomycin, capreomycin, cycloserine, ethionamide, bedaquiline, pretomanid, fluoroquinolones such as levofloxacin and moxifloxacin, aminoglycosides such as amikacin, and para-aminosalicylic acid (PAS).]
Drug Class: Antituberculosis Agents
Other Drugs in the Class: Bedaquiline, Capreomycin, Cycloserine, Ethionamide, Isoniazid, Pretomanid, Pyrazinamide, Rifabutin, Rifampin, Rifapentine, Streptomycin
CASE REPORT
Case 1. Cholestatic hepatitis caused by ethambutol.(1)
A 76 year old woman with pulmonary tuberculosis developed jaundice without abdominal pain or nausea two months after starting a course of isoniazid, streptomycin and ethambutol. She had no history of liver disease or excessive alcohol use. Liver tests were reported to be normal before starting therapy. Laboratory results showed a total serum bilirubin of 11.8 mg/dL with marked elevations in alkaline phosphatase [976 U/L] and gamma glutamyl transpeptidase [616 U/L] (Table). Tests for hepatitis A and B and for autoantibodies were negative. Ultrasound examination of the abdomen showed no evidence of biliary obstruction, and a liver biopsy showed intrahepatic cholestasis. On first presentation with jaundice, all medications were stopped and she began to improve. The initial diagnosis was isoniazid induced liver injury. Accordingly, therapy was reinstituted using ethambutol alone. Within 6 days, serum AST and alkaline phosphatase levels increased (Table). Ethambutol was stopped for 11 days and again restarted; however, serum alkaline phosphatase levels again rose, and ethambutol was permanently discontinued. After further improvements, isoniazid and streptomycin were restarted and subsequent treatment was well tolerated without recurrence of evidence of liver injury.
Key Points
| Medication: | Ethambutol (750 mg daily) |
|---|---|
| Pattern: | Cholestatic (R=0.9) |
| Severity: | 3+ (jaundice, hospitalization) |
| Latency: | 2 months |
| Recovery: | Interrupted by rechallenge, ultimately complete |
| Other medications: | Isoniazid, streptomycin |
Laboratory Values
| Time After Starting | Time After Stopping | AST* (U/L) | Alk P* (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|
| 8 weeks | 0 | 264 | 976 | 11.8 | Therapy stopped |
| 9 weeks | 1 week | 144 | 767 | ||
| Ethambutol restarted for 6 days | |||||
| 10 weeks | 0 | 356 | 1225 | ||
| 2 days | 280 | 1050 | |||
| 4 days | 300 | 1020 | |||
| 11 weeks | 7 days | 260 | 950 | ||
| 10 days | 76 | 812 | |||
| Ethambutol restarted for 4 days | |||||
| 12 weeks | 0 | 228 | 1128 | ||
| 3 days | 120 | 1020 | |||
| 6 days | 110 | 1000 | |||
| 13 weeks | 10 days | 105 | 700 | ||
| Normal Values | <40 | <130 | <1.2 | ||
- *
Some values estimated from Figure 1.
Comment
The role of ethambutol was defined by the response to rechallenge on two occasions during recovery and the later tolerance of isoniazid without worsening of blood test results. Also suggestive was the cholestatic pattern which is rare with isoniazid as well as the lack of viral hepatitis-like symptoms of nausea, abdominal pain and fatigue. Cholestatic hepatitis is marked by slow improvements in liver tests. The laboratory results were said to have “…slowly returned to normal…” but were not provided.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Ethambutol – Generic, Myambutol®
DRUG CLASS
Antituberculosis Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Ethambutol | 74-55-5 | C10-H24-N2-O2 |
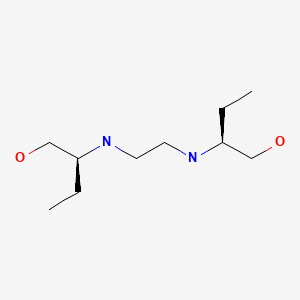
|
CITED REFERENCES
- 1.
- Gulliford M, Mackay AD, Prowse K. Cholestatic jaundice caused by ethambutol. Br Med J (Clin Res Ed). 1986;292:866. [PMC free article: PMC1339973] [PubMed: 3083914]
ANNOTATED BIBLIOGRAPHY
References updated: 24 December 2020
Abbreviations: HIV, human immunodeficiency virus; DRESS, drug rash with eosinophilia and systemic symptoms; MAC, Mycobacterium avium complex; PAS, para-aminosalicylic acid.
- Zimmerman HJ. Antituberculosis agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 611-21.(Extensive review of hepatotoxicity of antituberculosis medications including ethambutol published in 1999; mentions a single case report of hepatotoxicity).
- Verma S, Kaplowitz N. Hepatotoxicity of antituberculosis drugs. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 483-504.(Review of hepatotoxicity of antituberculosis drugs).
- Gumbo T. Chemotherapy of tuberculosis, mycobacterium avium complex disease and leprosy. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1067-86.(Textbook of pharmacology and therapeutics).
- Lederman RJ, Davis FB, Davis PJ. Exchange transfusion as treatment of acute hepatic failure due to antituberculosis drugs. Ann Intern Med. 1968;68:830–8. [PubMed: 5642965](23 year old woman developed acute fever, rash and malaise 41 days after starting isoniazid, ethambutol and PAS for tuberculosis [bilirubin 3.0 rising to 30 mg/dL, ALT 850, Alk P 2 times ULN], accompanied by confusion and coma; treated with exchange transfusions and recovered; PAS was stopped promptly, but isoniazid continued for a period).
- Hellström PE, Repo UK. Capreomycin, ethambutol and rifampicin in apparently incurable pulmonary tuberculosis. Scand J Respir Dis Suppl. 1969;69:69–74. [PubMed: 4906377](Retrospective analysis of 35 patients with severe, chronic or relapsing tuberculosis who were treated with capreomycin, ethambutol and rifampin; “liver damage” occurred in 49%, but was reversible in all; details not given).
- Repo UK, Hellström PE. Capreomycin and ethambutol in pulmonary tuberculosis. A preliminary report. Scand J Respir Dis Suppl. 1970;72:72–5. [PubMed: 5273730](Retrospective analysis of 29 patients with severe tuberculosis treated with capreomycin and ethambutol with a third agent; “liver damage” occurred in 10%, but was reversible in all and consisted of mild elevations in ALT, AST and BSP retention).
- Lees AW, Allan GW, Smith J, Tyrrell WF, Fallon RJ. Toxicity from rifampicin plus isoniazid and rifampicin plus ethambutol therapy. Tubercle. 1971;52:182–90. [PubMed: 4255439](Among 105 patients with active tuberculosis treated with isoniazid and rifampin, 22% had ALT and 13% bilirubin elevations, 8% skin rash and 3 a hypersensitivity reaction, two with jaundice [bilirubin 3.8 and 1.8 mg/dL; ALT 330 and 235 U/L], resolving on stopping).
- Zierski M. A trial of intermittent rifampin and ethambutol in retreatment regimens. Scand J Respir Dis Suppl. 1973;84:132–5. [PubMed: 4522067](122 patients with tuberculosis were treated with rifampin and ethambutol daily for 2 months and then once or twice weekly for 1-2 years; abnormal liver tests occurred in 10% during daily regimen, 5% during long term treatment, but all changes were transient and mild not requiring discontinuation and attributed to rifampin).
- Austerhoff A, Kindler U, Knop P, Knieriem HJ. Dtsch Med Wochenschr. 1974;99:1182. [Liver toxicity of combined rifampicin-isoniazid-ethambutol medication] German. [PubMed: 4835559]
- Năstase M, Năstase R, Brener E. Rev Ig Bacteriol Virusol Parazitol Epidemiol Pneumoftiziol Pneumoftiziol. 1975;24:241–3. [Side effects in strictly supervised treatment with rifampicin and ethambutol] Romanian. [PubMed: 174182]
- Rossouw JE, Saunders SJ. Hepatic complications of antituberculous therapy. Q J Med. 1975;44:1–16. [PubMed: 50605](Retrospective review identified 38 cases of hepatitis [~0.32%] due to antituberculosis therapy [Capetown, SA] 16 due to PAS, 12 to PAS with isoniazid, 3 isoniazid alone, 1 each of others including ethambutol; details of case attributed to ethambutol were not provided).
- Casteels-Van Daele M, Igodt-Ameye L, Corbeel L, Eeckels R. Hepatotoxicity of rifampicin and isoniazid in children. J Pediatr. 1975;86:739–41. [PubMed: 1079531](13 year old boy developed symptoms 7 days and coma 11 days after starting isoniazid, rifampin and ethambutol for active tuberculosis [bilirubin 5.2 mg/dL, ALT 1500, Alk P 302, prothrombin index <10%], but recovering spontaneously and later treated with isoniazid without recurrence).
- Girling DJ. The hepatic toxicity of antituberculosis regimens containing isoniazid, rifampicin and pyrazinamide. Tubercle. 1978;59:13–32. [PubMed: 345572](History and review of hepatotoxicity of first line antituberculosis medications).
- Chapoy P, Ferracci JP, Mattei JF, Granjon B, Louchet E. Pediatrie. 1978;3:637–45. [Severe hepatitis induced by chemotherapy with antitubercular agents in childhood. 2 cases] French. [PubMed: 740454](Two children, ages 4 and 12 years with onset of severe hepatitis 3 and 8 months after starting rifampin, isoniazid and PAS/prothionamide; one case was fatal and one resulted in cirrhosis; authors attributed injury to isoniazid).
- Addington WW. The side effects and interactions of antituberculosis drugs. Chest. 1979;76(6) Suppl:782–4. [PubMed: 510025](Review of side effects of antituberculosis medications states that isoniazid, rifampin and pyrazinamide are major causes of hepatotoxicity, whereas ethambutol rarely causes liver injury).
- Zierski M, Bek E. Side-effects of drug regimens used in short-course chemotherapy for pulmonary tuberculosis. A controlled clinical study. Tubercle. 1980;61:41–9. [PubMed: 6989067](Among 530 patients treated with 5 regimens [which included isoniazid, rifampin, streptomycin, pyrazinamide and ethambutol] for active tuberculosis and monitored monthly, 9% developed hepatic injury but usually without symptoms, ALT >250 U/L in 6 [1.1%], bilirubin rise in 7 [1.3%]).
- Bobrowitz ID. Ethambutol compared to rifampin in original treatment of pulmonary tuberculosis. Lung. 1980;157:117–25. [PubMed: 7382540](218 patients treated with 3 regimens for tuberculosis; isoniazid with rifampin or ethambutol or both for 4 months followed by isoniazid for 20 months; 39 [18%] developed abnormal liver tests; 5 cases of hepatitis attributed to rifampin [2-8 weeks: overall 3.4%] and 3 to isoniazid [1-16 months: 1.3%], none to ethambutol).
- Snider D Jr, Long M, Zierski M, Rogowski J, Bek E. Preliminary results of six-month regimens studied in the United States and in Poland. Chest. 1981;80:727–9. [PubMed: 7307596](Comparison of efficacy of 6-month vs 15-month regimens [the latter including ethambutol] for active tuberculosis in 672 patients, 9 relapses after short but none after long duration regimen; 21 [3.1%] had hepatotoxicity).
- Døssing M, Andreasen PB. Drug-induced liver disease in Denmark. An analysis of 572 cases of hepatotoxicity reported to the Danish Board of Adverse Reactions to Drugs. Scand J Gastroenterol. 1982;17:205–11. [PubMed: 6982502](Among 572 reports of drug induced liver injury from Denmark between 1968 and 1978, the most common causes were halothane [25%], chlorpromazine [9%], sulfonamides [9%], antituberculosis agents [7%], oxyphenisatin[4%], and methyldopa [2%]).
- Fiala W, Häcki MA, Brändli O. Schweiz Med Wochenschr. 1983;113:1956–9. [Pyrazinamide versus ethambutol in short-term therapy of lung tuberculosis. A randomized study] German. [PubMed: 6658432](Controlled trial comparing pyrazinamide [n=52: <2 g/day] vs ethambutol [n=61] for first 2-3 months with isoniazid and rifampin for 9 months to treat active tuberculosis; hepatitis occurred in 5 on pyrazinamide and 2 on ethambutol, all in first month, 3 symptomatic, no deaths, most were >70 years of age).
- Cohen CD, Sayed AR, Kirsch RE. Hepatic complications of antituberculosis therapy revisited. S Afr Med J. 1983;63:960–3. [PubMed: 6857425](Among 5565 patients treated for tuberculosis in Capetown SA, 17 [0.3%] developed hepatitis, rate similar to that when PAS was used. Among 28 cases seen, 13 attributed to isoniazid, 16 pyrazinamide and 8 rifampin, mostly in combination; 2 deaths).
- Homberg JC, Abuaf N, Helmy-Khalil S, Biour M, Poupon R, Islam S, Darnis F, et al. Drug-induced hepatitis associated with anticytoplasmic organelle autoantibodies. Hepatology. 1985;5:722–7. [PubMed: 4029887](Analysis of autoantibodies in 157 cases of drug induced liver injury; 3 categories–drugs that are associated with and without antibodies to cytoplasmic organelles and those with specific antibodies; 4 cases of isoniazid hepatitis had no antibodies to nuclei, mitochondria, liver microsomes or smooth muscle).
- Gulliford M, Mackay AD, Prowse K. Cholestatic jaundice caused by ethambutol. Br Med J (Clin Res Ed). 1986;292:866. [PMC free article: PMC1339973] [PubMed: 3083914](78 year old woman developed jaundice 2 months after starting isoniazid, streptomycin and ethambutol [bilirubin 11.8 mg/dL, AST 264 U/L, Alk P 976 U/L] with negative rechallenge to isoniazid and positive rechallenge twice to ethambutol [Alk P rising from 767 to 1225 U/L, AST from 144 to 356 U/L], later tolerating isoniazid and streptomycin long term: Case 1).
- van Aalderen WMC, Knoester H, Knol K. Fulminant hepatitis during treatment with rifampicin, pyrazinamide and ethambutol. Eur J Pediatr. 1987;146:290–1. [PubMed: 3595648](10 year old girl developed nausea and ALT elevations 2 weeks after starting isoniazid, rifampin, pyrazinamide and ethambutol resolving with stopping all drugs, but severe recurrence with fever, rash and fatal acute liver failure 6 weeks after adding pyrazinamide back to rifampin, ethambutol and kanamycin).
- Donald PR, Schoeman JF, O’Kennedy A. Hepatic toxicity during chemotherapy for severe tuberculosis meningitis. Am J Dis Child. 1987;141:741–3. [PubMed: 2884866](Among 33 children with tuberculous meningitis treated with 3-4 agents, liver test abnormalities were common [85%], but usually mild and transient, only one child developing jaundice who was also IgM anti-HAV positive and who later tolerated therapy without recurrence).
- Kasantikul V. Isoniazid-rifampicin-induced submassive hepatic necrosis. J Med Assoc Thai. 1989;72:56–8. [PubMed: 2723568](58 year old woman developed nausea 3 weeks after starting isoniazid, rifampin and ethambutol and jaundice 3 weeks later [bilirubin 3.7 mg/dL, ALT 1590 U/L, Alk P 54 U/L, protime 72 sec], dying 5 days later).
- Wu JC, Lee SD, Yeh PF, Chan CY, Wang YJ, Huang YS, Tsai YT, et al. Isoniazid-rifampin-induced hepatitis in hepatitis B carriers. Gastroenterology. 1990;98:502–4. [PubMed: 2295408](Among 1783 patients with active tuberculosis treated with combination therapy [isoniazid, rifampin and ethambutol], 42 [2.3%] developed clinical hepatitis of whom 15 were HBsAg positive, fatality rate being 47% vs 4%, but no information on background features in the treated cohort or exclusion of reactivation of hepatitis B).
- Taneja DP, Kaur D. Study on hepatotoxicity and other side-effects of antituberculosis drugs. J Indian Med Assoc. 1990;88:278–80. [PubMed: 2090682]
- Chiu J, Nussbaum J, Bozzette S, Tilles JG, Young LS, Leedom J, Heseltine PN, et al. California Collaborative Treatment Group. Treatment of disseminated Mycobacterium avium complex infection in AIDS with amikacin, ethambutol, rifampin, and ciprofloxacin. Ann Intern Med. 1990;113:358–61. [PubMed: 2382918](17 patients with AIDs and mycobacterium avium complex infection were treated with amikacin for 4 weeks and then 12 weeks of ciprofloxacin, ethambutol and rifampin; therapy stopped early in 2 patients for hepatitis, but no details given).
- Aziz S, Agha F, Hassan R, Fairoz SA, Hassan K. Hepatotoxicity to different antituberculosis drug combinations. J Pak Med Assoc. 1990;40:290–4. [PubMed: 2126569]
- Altman C, Biour M, Grangé JD. Presse Med. 1993;22:1212–6. [Hepatic toxicity of antitubercular agents. Role of different drugs. 199 cases] French. [PubMed: 8248040](Analysis of 199 cases of hepatotoxicity from antituberculosis medications from literature [n=169] and French pharmacovigilance system [n=30]; overall mortality rate was 23%, rifampin cases had short latency [average 2 weeks] compared to isoniazid [11 weeks] and pyrazinamide [7 weeks]; one case attributed to ethambutol in the literature, none in their own series).
- Türktaş H, Unsal M, Tülek N, Orüç O. Hepatotoxicity of antituberculosis therapy (rifampicin, isoniazid and pyrazinamide) or viral hepatitis. Tuber Lung Dis. 1994;75:58–60. [PubMed: 8161767](Among 705 Turkish adults with tuberculosis, 57 [8%] developed hepatitis with jaundice during therapy with isoniazid and rifampin; serologic testing showed hepatitis A in none, B in 6 and C in 4).
- Singh J, Arora A, Garg PK, Thakur VS, Pande JN, Tandon RK. Antituberculosis treatment-induced hepatotoxicity: role of predictive factors. Postgrad Med J. 1995;71:359–62. [PMC free article: PMC2398146] [PubMed: 7644398](Case control study of 60 patients with liver injury due to antituberculosis medications and 60 controls from India identified lower BMI and pyrazinamide, but not age, sex, or acetylator status as risk factors).
- Singh J, Garg PK, Tandon RK. Hepatotoxicity due to antituberculosis therapy. Clinical profile and reintroduction of therapy. J Clin Gastroenterol. 1996;22:211–4. [PubMed: 8724260](Among 72 patients with symptomatic liver injury due to antituberculosis medications, 12 had acute or subacute liver failure; among those who recovered, reinstitution of therapy was possible in 93%).
- Ormerod LP, Horsfield N. Frequency and type of reactions to antituberculosis drugs: observations in routine treatment. Tuber Lung Dis. 1996;77:37–42. [PubMed: 8733412](Among 1317 patients treated for active tuberculosis, hepatitis was attributed to rifampin in 1.4%, pyrazinamide in 1.2% and isoniazid in 0.3%, but none to ethambutol or streptomycin).
- Ormerod LP, Skinner C, Wales J. Hepatotoxicity of antituberculosis drugs. Thorax. 1996;51:111–3. [PMC free article: PMC473008] [PubMed: 8711637](Review of hepatotoxicity of antituberculosis medications and recommendations on monitoring, with biochemical monitoring recommended only for patients with preexisting liver disease; in the UK between 1965-86, there were 243 reports of liver injury due to antituberculosis therapy and 45 fatalities).
- Pande JN, Singh SP, Khilnani GC, Khilnani S, Tandom RK. Risk factors for hepatotoxicity from antituberculosis drugs: a case-control study. Thorax. 1996;51:132–6. [PMC free article: PMC473016] [PubMed: 8711642](Comparison of 86 patients with hepatitis due to antituberculosis therapy with 406 patients who tolerated therapy; risk factors were older age, history of high alcohol intake [20% vs 5%], more extensive disease [14% vs 3.5%], slow acetylator status [83% vs 64%] and use of pyrazinamide [63% vs 25%]).
- Døssing M, Wilcke JT, Askgaard DS, Nybo B. Liver injury during antituberculosis treatment: an 11-year study. Tuber Lung Dis. 1996;77:335–40. [PubMed: 8796249](Retrospective chart review on 765 Danish patients treated for tuberculosis with 3 or 4 drugs for 6-9 months; 16% had AST elevations >2 times ULN usually in first month, but only 2% required modification of the regimen; 7 with jaundice, no fatalities; risk factors for hepatotoxicity were female sex, age and severe tuberculosis).
- Durand F, Jebrak G, Pessayre D, Fournier M, Bernuau J. Hepatotoxicity of antitubercular treatments. Rationale for monitoring liver status. Drug Saf. 1996;15:394–405. [PubMed: 8968694](Review and recommendations from France regarding monitoring of serum enzymes during therapy of tuberculosis; isoniazid may have direct hepatotoxicity because of dose relatedness and usual absence of recurrence on rechallenge; rifampin is rare cause of liver injury, usually with short latency period; pyrazinamide is clearly hepatotoxic at higher doses which should be kept to a minimum and for only 2 months).
- García Rodríguez LA, Ruigómez A, Jick H. A review of epidemiologic research on drug-induced acute liver injury using the general practice research data base in the United Kingdom. Pharmacotherapy. 1997;17:721–8. [PubMed: 9250549](In epidemiological studies, antituberculosis medications have the highest relative risk for liver injury, hepatitis occurring in 0.4% of recipients).
- Tahaoğlu K, Ataç G, Sevim T, Tärün T, Yazicioğlu O, Horzum G, Gemci I, et al. The management of anti-tuberculosis drug-induced hepatotoxicity. Int J Tuberc Lung Dis. 2001;5:65–9. [PubMed: 11263519](Description of 45 patients with hepatotoxicity from antituberculosis therapy, ages 15-76 years, ALT 42-897 U/L, bilirubin 0.2-7.0 mg/dL, arising in 6-102 days, with resolution in 4-58 days. No recurrence in those with gradual reintroduction of regimen without pyrazinamide compared to 6 cases [24%] in those with abrupt reintroduction).
- Ohkawa K, Hashiguchi M, Ohno K, Kiuchi C, Takahashi S, Kondo S, Echizen H, et al. Risk factors for antituberculous chemotherapy-induced hepatotoxicity in Japanese pediatric patients. Clin Pharmacol Ther. 2002;72:220–6. [PubMed: 12189369](Retrospective analysis of 99 children who received therapy for tuberculosis, 8 developed hepatotoxicity; risk factors identified were young age and pyrazinamide exposure).
- Hussain Z, Kar P, Husain SA. Antituberculosis drug-induced hepatitis: risk factors, prevention and management. Indian J Exp Biol. 2003;41:1226–32. [PubMed: 15332488](Review article on role of genetic polymorphisms of NAT2, CYP 2E1 and glutathione-S-transferase in hepatotoxicity of antituberculosis therapies).
- el-Agroudy AE, Refaie AF, Moussa OM, Ghoneim MA. Tuberculosis in Egyptian kidney transplant recipients: study of clinical course and outcome. J Nephrol. 2003;16:404–11. [PubMed: 12832742](Among 1200 kidney transplant recipients, 45 [4%] developed tuberculosis usually after several years, all treated with isoniazid, rifampin and ethambutol; hepatotoxicity in 11 [25%], but severe in only 3).
- Kurokawa I, Nakahigashi Y, Teramachi M. Erythema multiforme-type drug eruption due to ethambutol with eosinophilia and liver dysfunction. Int J Antimicrob Agents. 2003;21:596–7. [PubMed: 12791479](42 year old woman developed erythema multiforme 10 weeks after starting isoniazid, rifampin, pyrazinamide and ethambutol for pulmonary tuberculosis with 34% eosinophils, ALT 312 U/L and normal bilirubin resolving in 2 weeks on prednisone, positive rechallenge to ethambutol with rash; tolerated other agents).
- Yee D, Valiquette C, Pelletier M, Parisien I, Rocher I, Menzies D. Incidence of serious side effects from first-line antituberculosis drugs among patients treated for active tuberculosis. Am J Respir Crit Care Med. 2003;167:1472–7. [PubMed: 12569078](Among 408 adult patients treated for tuberculosis, 37 [9%] had 46 serious adverse events including 12 instances of hepatitis [3%: 11 symptomatic, ALT >5 times ULN]; risk factors were age [hazard ratio 4.8-7.7], female sex [2.2] and Asian birthplace [2.2]; hepatitis arose in 2% on pyrazinamide and 1% on isoniazid).
- Fernández-Villar A, Sopeña B, Fernández-Villar J, Vázquez-Gallardo R, Ulloa F, Leiro V, Mosteiro M, et al. The influence of risk factors on the severity of anti-tuberculosis drug-induced hepatotoxicity. Int J Tuberc Lung Dis. 2004;8:1499–505. [PubMed: 15636498](Among 471 patients receiving antituberculosis therapy, 56 [12%] developed ALT elevations above 3 times ULN, 16 [3.4%] and symptoms and 5 [1%] were jaundiced; no deaths. Rates of hepatotoxicity higher in patients with risk factors than without [18.2% vs 5.6%]).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl. 2004;10:1018–23. [PubMed: 15390328](Among ~50,000 liver transplants reported to UNOS between 1990 and 2002, 270 [0.5%] were done for drug induced acute liver failure, 124 for acetaminophen and 137 for other toxins, the most common being isoniazid [24], propylthiouracil [13], phenytoin [10], valproate [10], amanita [9], nitrofurantoin [7], herbals [7], ketoconazole [6], disulfiram [6], troglitazone [4] and 28 others).
- Marra F, Cox VC, FitzGerald JM, Moadebi S, Elwood RK. Successful treatment of multidrug-resistant tuberculosis following drug-induced hepatic necrosis requiring liver transplant. Int J Tuberc Lung Dis. 2004;8:905–9. [PubMed: 15260286](28 year old woman with tuberculous lymphadenitis treated with isoniazid, rifampin, ethambutol and pyrazinamide, switching to ciprofloxacin with pyrazinamide and ethambutol when resistance testing was done; four days later she developed fever, rash and fatigue [bilirubin normal, ALT 285 U/L, Alk P normal], but then worsened [bilirubin 15.2 mg/dL, ALT 1165 U/L, Alk P 141 U/L] and ultimately required liver transplant, yet later was treated successfully with levofloxacin, amikacin and streptomycin).
- Younossian AB, Rochat T, Ketterer JP, Wacker J, Janssens JP. High hepatotoxicity of pyrazinamide and ethambutol for treatment of latent tuberculosis. Eur Respir J. 2005;26:462–4. [PubMed: 16135729](Among 12 persons treated with pyrazinamide and ethambutol for latent tuberculosis and contact with a patient with multidrug resistant disease, 7 developed liver injury [ALT 82-1338 U/L], after 87 to 247 days, 3 with symptoms, no mention of bilirubin levels).
- Andrade RJ, Lucena MI, Fernandez MC, Pelaez G, Pachkoria K, Garcia-Ruiz E, Garcia-Munoz B, et al. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish Registry over a 10-year period. Gastroenterology. 2005;129:512–21. [PubMed: 16083708](Among 446 cases of drug induced liver injury collected in Spain between 1984-2004, isoniazid [with or without rifampin and pyrazinamide] was implicated in 22 cases [5%: ranking 3rd] and was fatal or required liver transplant in 5 [ranking first]).
- Lee BH, Koh WJ, Choi MS, Suh GY, Chung MP, Kim H, Kwon OJ. Inactive hepatitis B surface antigen carrier state and hepatotoxicity during antituberculosis chemotherapy. Chest. 2005;127:1304–11. [PubMed: 15821209](Retrospective case control study of 110 HBsAg carriers and 97 controls from Korea who received 3-4 drug antituberculosis therapy; any ALT elevations occurred in 34% of carriers vs 20% of controls and were >3 times ULN in 8% vs 4%; no risk factors identified, most tolerated reintroduction of therapy without pyrazinamide).
- Yew WW, Leung CC. Antituberculosis drugs and hepatotoxicity. Respirology. 2006;11:699–707. [PubMed: 17052297](Review of incidence, causes, risk factors and management of hepatotoxicity of antituberculosis medications).
- Saukkonen JJ, Cohn DL, Jasmer RM, Schenker S, Jereb JA, Nolan CM, Peloquin CA, et al. ATS (American Thoracic Society) Hepatotoxicity of Antituberculosis Therapy Subcommittee. An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med. 2006;174:935–52. [PubMed: 17021358](American Thoracic Society recommendations regarding hepatotoxicity of antituberculosis therapy; for latent infection, 9 months of isoniazid is first choice and 4 months of rifampin second; clinical monitoring is recommend for all patients and biochemical monitoring for those at high risk and possibly the elderly [ALT values at 1, 3 and 6 months or every 1-2 months]; hold therapy if ALT above 5 times ULN or if symptoms are present and ALT is above 3 times ULN; mentions that there has been only one report of ethambutol hepatoxicity).
- Forget EJ, Menzies D. Adverse reactions to first-line antituberculosis drugs. Expert Opin Drug Saf. 2006;5:231–49. [PubMed: 16503745](Review of side effects including hepatotoxicity of isoniazid, asymptomatic elevations in ALT levels occur in 10-22% of patients, but 80% of these resolve even with continuing therapy; overall rate of hepatotoxicity is 0.9%, mortality 0.04%).
- Shigeto E., Committee for Treatment Japanese Society for Tuberculosis. Kekkaku. 2007;82:467–73. [Survey of anti-tuberculosis drug-induced severe liver injury in Japan] Japanese. [PubMed: 17564126](Abstract: Survey questionnaire to 114 Japanese hospitals identified 70 cases of severe liver injury and 8 deaths due to antituberculosis therapy between 1994-2003).
- Idilman R, Ersoz S, Coban S, Kumbasar O, Bozkaya H. Antituberculous therapy-induced fulminant hepatic failure: successful treatment with liver transplantation and nonstandard antituberculous therapy. Liver Transpl. 2006;12:1427–30. [PubMed: 16933231](19 year old woman with peritoneal tuberculosis developed jaundice 4 days after starting isoniazid, rifampin, ethambutol and pyrazinamide [bilirubin 10.5 mg/dL, ALT 1332 U/L, protime 71 sec], undergoing living donor liver transplantation within 2 days and afterwards treated with streptomycin, ethambutol and cycloserine with no recurrence).
- Björnsson E, Kalaitzakis E, Olsson R. The impact of eosinophilia and hepatic necrosis on prognosis in patients with drug-induced liver injury. Aliment Pharmacol Ther. 2007;25:1411–21. [PubMed: 17539980](Review of 570 case reports of drug induced liver injury suggested that eosinophilia was associated with a favorable prognosis, lower peak bilirubin and lower fatality rate).
- Sabaté M, Ibáñez L, Pérez E, Vidal X, Buti M, Xiol X, Mas A, et al. Risk of acute liver injury associated with the use of drugs: a multicentre population survey. Aliment Pharmacol Ther. 2007;25:1401–9. [PubMed: 17539979](Population based survey of 126 cases of acute liver injury due to drugs between 1993-1999 in Spain calculated relative risk of injury compared to the general population to be 1300 with use of triple therapy [isoniazid, rifampin and pyrazinamide] and 154 for isoniazid alone, ranking first and third).
- Kwon YS, Koh WJ, Suh GY, Chung MP, Kim H, Kwon OJ. Hepatitis C virus infection and hepatotoxicity during antituberculosis chemotherapy. Chest. 2007;131:803–8. [PubMed: 17356096](Retrospective analysis of 54 patients with HCV infection and 97 controls receiving therapy for active tuberculosis; ALT >3 times ULN occurred in 13% of HCV infected vs 4% of controls; none died or required hospitalization).
- Aouam K, Chaabane A, Loussaïef C, Ben Romdhane F, Boughattas NA, Chakroun M. Med Mal Infect. 2007;37:253–61. [Adverse effects of antitubercular drugs: epidemiology, mechanisms, and patient management] French. [PubMed: 17336011](Review of toxicities of antituberculosis agents; ethambutol can cause optic neuritis, allergic reactions, and rash; liver injury is rare except for mild hyperbilirubinemia not requiring dose adjustment).
- Huang YS. Genetic polymorphisms of drug-metabolizing enzymes and the susceptibility to antituberculosis drug-induced liver injury. Expert Opin Drug Metab Toxicol. 2007;3:1–8. [PubMed: 17269890](Two gene variants have been linked to an increased risk of hepatotoxicity of antituberculosis medications; NAT2 and CYP 2E1, but the associations require further confirmation).
- Marzuki OA, Fauzi AR, Ayoub S, Kamarul Imran M. Prevalence and risk factors of anti-tuberculosis drug-induced hepatitis in Malaysia. Singapore Med J. 2008;49:688–93. [PubMed: 18830542](Among 473 patients treated for tuberculosis, 46 [9.7%] developed ALT elevations above 3 times ULN; in a case control analysis, concurrent HIV infection was a risk factor).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, isoniazid was implicated in 15 cases [5%: ranking 3rd] being the only agent implicated in 13 cases and implicated with other agents in two cases; but no case was linked to ethambutol).
- Kaneko Y, Nagayama N, Kawabe Y, Shimada M, Suzuki J, Kunogi M, Matsui Y, et al. Kekkaku. 2008;83:13–9. [Drug-induced hepatotoxicity caused by anti-tuberculosis drugs in tuberculosis patients complicated with chronic hepatitis] Japanese. [PubMed: 18283910](Abstract: reports on substantial higher frequency of ALT elevations in patients with chronic hepatitis C associated with pyrazinamide therapy).
- Tostmann A, Boeree MJ, Aarnoutse RE, de Lange WC, van der Ven AJ, Dekhuijzen R. Antituberculosis drug-induced hepatotoxicity: concise up-to-date review. J Gastroenterol Hepatol. 2008;23:192–202. [PubMed: 17995946](Review of incidence, pathogenesis, clinical course, risk factors and management of drug induced liver disease due to antituberculosis medications).
- Lee JH, Park HK, Heo J, Kim TO, Kim GH, Kang DH, Song GA, et al. Drug Rash with Eosinophilia and Systemic Symptoms (DRESS) syndrome induced by celecoxib and anti-tuberculosis drugs. J Korean Med Sci. 2008;23:521–5. [PMC free article: PMC2526540] [PubMed: 18583892](29 year old woman developed fever, rash, eosinophilia and jaundice 6 weeks after starting celecoxib and 5 weeks after starting antituberculosis therapy with isoniazid, rifampin, pyrazinamide and ethambutol [bilirubin 3.6 mg/dL, ALT 327 U/L, Alk P 788 U/L, eosinophils 12%], improving upon stopping and recurring upon restarting with severe myositis and pneumonitis, treated with prednisone and ultimately resolved, but had positive patch tests to celecoxib and ethambutol).
- Ferrajolo C, Capuano A, Verhamme KM, Schuemie M, Rossi F, Stricker BH, Sturkenboom MC. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol. 2010;70:721–8. [PMC free article: PMC2997312] [PubMed: 21039766](Worldwide pharmacovigilance database contained 9036 hepatic adverse drug reactions in children, 2 antituberculosis agents were among the top 40 cases, including isoniazid [24th, 47 cases] and rifampin [35th, 37 cases], but not ethambutol).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury and 25 to antituberculosis agents, including 15 to isoniazid alone [ranking first], 6 to isoniazid combined with other agents, 3 to rifampin and pyrazinamide and 1 to dapsone, but no cases were attributed even secondarily to ethambutol).
- Devarbhavi H, Dierkhising R, Kremers WK, Sandeep MS, Karanth D, Adarsh CK. Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol. 2010;105:2396–404. [PubMed: 20648003](Among 313 cases of drug induced liver injury seen between 1997 and 2008 at a large hospital in Bangalore, India, 181 [58%] were attributed to antituberculosis agents which accounted for 39 of 54 [72%] fatal cases; ethambutol not specifically mentioned).
- Palmero D, Castagnino J, Musella RM, Mosca C, González Montaner P, de Casado GC. Difficult clinical management of anti-tuberculosis DRESS syndrome. Int J Tuberc Lung Dis. 2013;17:76–8. [PubMed: 23114284](Analysis of 11 patients with DRESS syndrome attributed to antituberculosis medications, one was attributed to ethambutol and isoniazid and one to ethambutol with isoniazid, rifampin and pyrazinamide).
- Li C, Long J, Hu X, Zhou Y. GSTM1 and GSTT1 genetic polymorphisms and risk of anti-tuberculosis drug-induced hepatotoxicity: an updated meta-analysis. Eur J Clin Microbiol Infect Dis. 2013;32:859–68. [PubMed: 23377313](Metaanalysis of studies on the association of tuberculosis drug associated liver injury and polymorphisms of the glutathione S-transferase genes found weak associations only).
- Shin HJ, Lee HS, Kim YI, Lim SC, Jung JP, Ko YC, Kwon YS. Hepatotoxicity of anti-tuberculosis chemotherapy in patients with liver cirrhosis. Int J Tuberc Lung Dis. 2014;18:347–51. [PubMed: 24670574](Retrospective analysis of 197 patients treated for tuberculosis found serum enzyme elevations [72% vs 46%] and drug induced liver injury [8% vs 2.7%] more common in 50 patients who had cirrhosis than in 147 without, but all patients recovered; specific agents responsible for injury were not identified).
- Kaswala DH. Drug rash with eosinophilia and systemic symptoms syndrome due to anti-TB medication. J Family Med Prim Care. 2013;2:83–5. [PMC free article: PMC3894017] [PubMed: 24479051](68 year old man with tuberculosis developed rash, eosinophilia and serum aminotransferase elevations [details not given] 8 weeks after starting isoniazid, rifampin, pyrazinamide and ethambutol, resolving within a month of stopping and with prednisone therapy).
- Li C, Long J, Hu X, Zhou Y. GSTM1 and GSTT1 genetic polymorphisms and risk of anti-tuberculosis drug-induced hepatotoxicity: an updated meta-analysis. Eur J Clin Microbiol Infect Dis. 2013;32:859–68. [PubMed: 23377313](Metaanalysis of studies on the association of tuberculosis drug associated liver injury and polymorphisms of the glutathione S-transferase genes found weak associations only).
- Kumar N, Kedarisetty CK, Kumar S, Khillan V, Sarin SK. Antitubercular therapy in patients with cirrhosis: challenges and options. World J Gastroenterol. 2014;20:5760–72. [PMC free article: PMC4024786] [PubMed: 24914337](Review of the frequency, pathogenesis and management of hepatotoxicity of antituberculosis therapy in patients with preexisting cirrhosis, who appear to have an increased risk of liver injury from these agents as well as an increased risk of severe outcomes).
- Huang YS. Recent progress in genetic variation and risk of antituberculosis drug-induced liver injury. J Chin Med Assoc. 2014;77:169–73. [PubMed: 24593909](Critical review of studies on genetic variations associated with antituberculosis drug induced liver injury focusing on N-acetyltransferase (NAT), CYP 2E1, glutathione S transferase and manganese superoxide dismutase, the strongest association being with NAT and slow acetylator status).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, 13 [7%] due to antituberculosis agents including 6 to isoniazid, 1 to pyrazinamide, 1 to rifampin and 5 to combinations; ethambutol not specifically mentioned).
- Jeong I, Park JS, Cho YJ, Yoon HI, Song J, Lee CT, Lee JH. Drug-induced hepatotoxicity of anti-tuberculosis drugs and their serum levels. J Korean Med Sci. 2015;30:167–72. [PMC free article: PMC4310943] [PubMed: 25653488](Among 195 Korean patients with tuberculosis treated with a 4-drug regimen of isoniazid, rifampin, ethambutol and pyrazinamide, drug levels taken within the first week of treatment did not correlate with subsequent rate or severity of antituberculosis drug induced liver injury [n=12: 6%]).
- Horita N, Miyazawa N, Yoshiyama T, Kojima R, Ishigatsubo Y, Kaneko T. Currently used low-dose pyrazinamide does not increase liver-injury in the first two months of tuberculosis treatment. Intern Med. 2015;54:2315–20. [PubMed: 26370854](Among 383 Chinese patients with tuberculosis treated with isoniazid, rifampin and ethambutol with or without pyrazinamide analyzed in a retrospective study, liver injury arising within the first two months of therapy was less frequent with four drug [8%] than 3-drug regimen [24%]).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 54 [6%] were attributed to antituberculosis agents including 48 to isoniazid, 2 to rifampin, 2 to pyrazinamide, 1 to ethambutol and 1 to isoniazid and rifampin).
- Sharma P, Tyagi P, Singla V, Bansal N, Kumar A, Arora A. Clinical and biochemical profile of tuberculosis in patients with liver cirrhosis. J Clin Exp Hepatol. 2015;5:8–13. [PMC free article: PMC4415191] [PubMed: 25941429](Among 67 patients with cirrhosis treated for tuberculosis with isoniazid, rifampin, ethambutol and either ofloxacin or pyrazinamide, evidence of drug induced liver injury arose in 35% of cases, but none were attributed to ethambutol or pyrazinamide and there were no deaths attributed to drug toxicity).
- Golemba AS, Ferreyra FG, Martearena RE, Achinelli FR, Rovai GB. Drug-induced hepatotoxicity and tuberculosis in a hospital from the Argentinian northeast: cross-sectional study. Medwave. 2015;15:e6135. [PubMed: 26035138](Among 118 patients with tuberculosis treated with isoniazid, rifampin and ethambutol between 2011 and 2014, 9 (8%) developed hepatotoxicity, but only two had jaundice and none died or underwent transplantation).
- Blair PW, Herrin D, Abaalkhail N, Fiser W. DRESS syndrome presenting after initiation of mycobacterium avium complex osteomyelitis treatment. BMJ Case Rep. 2015;2015:bcr2015210907. [PMC free article: PMC4600809] [PubMed: 26438676](79 year old man developed rash, fever and eosinophilia 4 weeks after starting isoniazid, rifampin, ethambutol and clarithromycin for MAC osteomyelitis [ALT 56 rising to 86 U/L, bilirubin and Alk P not provided] which did not improve upon stopping the medications but did once high dose corticosteroids were begun; no follow up on rechallenge or outcome of osteomyelitis).
- Choi JH, Heo NY, Park SH, Park CS, Jo KM, Kim WG, Nam KH. Korean J Gastroenterol. 2016;67:267–71. [Concomitant drug reaction with eosinophilia and systemic symptom syndrome from ethambutol and autoimmune hepatitis from isoniazid] [PubMed: 27206439](Woman with tuberculosis developed fever, rash and eosinophilia within 2 months of starting combination therapy with isoniazid, rifampin, ethambutol and pyrazinamide [ALT rising to 650 U/L], responding to prednisone therapy and fever and rash recurring with reintroduction of ethambutol).
- Usui T, Whitaker P, Meng X, Watson J, Antoine DJ, French NS, Park BK, et al. Detection of drug-responsive T-lymphocytes in a case of fatal antituberculosis drug-related liver injury. Chem Res Toxicol. 2016;29:1793–5. [PubMed: 27933861](50 year old man developed liver injury 3 weeks after starting combination therapy for tuberculosis [peak bilirubin 32 mg/dL, ALT 2373 U/L, INR 4.4], dying within a month of onset; cloning of T cells taken early in the course revealed reactivity to ethambutol and rifampin but not isoniazid and pyrazinamide).
- Wang FJ, Wang Y, Niu T, Lu WX, Sandford AJ, He JQ. Update meta-analysis of the CYP2E1 RsaI/PstI and DraI polymorphisms and risk of antituberculosis drug-induced hepatotoxicity: evidence from 26 studies. J Clin Pharm Ther. 2016;41:334–40. [PubMed: 27062377](Metaanalysis of 26 studies involving 7423 patients found that the CYP2E1 RsaI/PstI c1/c1 polymorphism was associated with an increased risk for liver injury caused by antituberculosis therapy with an odds ratio of 1.32).
- Allouchery M, Logerot S, Cottin J, Pralong P, Villier C, Ben Saïd B. French Pharmacovigilance Centers Network and the French Investigators for skin adverse reactions to drugs. Antituberculosis drug-associated DRESS: a case series. J Allergy Clin Immunol Pract. 2018;6:1373–80. [PubMed: 29274824](Among 67 cases of DRESS syndrome attributed to antituberculosis medications reported to a French pharmacovigilance registry between 2005 and 2015, there were 40 women and 27 men, median age of 61 years and the most commonly implicated agents were isoniazid and rifampin).
- Tweed CD, Wills GH, Crook AM, Dawson R, Diacon AH, Louw CE, McHugh TD, et al. Liver toxicity associated with tuberculosis chemotherapy in the REMoxTB study. BMC Med. 2018;16:46. [PMC free article: PMC5875008] [PubMed: 29592805](Among 1928 patients with pulmonary tuberculosis treated with ethambutol, isoniazid, rifampin and pyrazinamide or regimens in which moxifloxacin was substituted for either ethambutol or isoniazid, liver injury arose in 58 patients [3%] overall, and was less with regimen in which moxifloxacin replaced isoniazid [2.2%], but there was nevertheless a liver related death on this regimen).
- Kapur A, Rehan HS. Drug reaction with eosinophilia and systemic symptoms syndrome associated with ethambutol use: a case report. Curr Drug Saf. 2019;14:249–51. [PMC free article: PMC6864591] [PubMed: 30848209](34 year old woman developed fever, rash and eosinophilia 35 days after starting antituberculosis therapy with isoniazid, rifampin, pyrazinamide, ethambutol and streptomycin that responded to stopping therapy and systemic corticosteroids, but rapidly recurred upon restarting ethambutol alone and she later tolerated the other agents).
- Hagiwara E, Suido Y, Asaoka M, Katano T, Okuda R, Sekine A, Kitamura H, et al. Safety of pyrazinamide-including regimen in late elderly patients with pulmonary tuberculosis: A prospective randomized open-label study. J Infect Chemother. 2019;25:1026–30. [PubMed: 31229376](Among 89 elderly patients [80 years or older] with tuberculosis treated with isoniazid, rifampin and ethambutol with or without pyrazinamide, rates of discontinuation were similar between the two groups [33% vs 35%] as well as rates of ALT or AST elevations above 2.5 times ULN [18% vs 23%]).
- Wang Y, Xiang X, Huang WW, Sandford AJ, Wu SQ, Zhang MM, Wang MG, et al. Association of PXR and CAR polymorphisms and antituberculosis drug-induced hepatotoxicity. Sci Rep. 2019;9:2217. [PMC free article: PMC6379441] [PubMed: 30778091](Among 502 patients with tuberculosis treated with isoniazid, rifampin, ethambutol and pyrazinamide 19% developed ALT elevations above 10 times ULN, several polymorphisms of PXR [but not CAR] were associated with a lower incidence of liver injury).
- Dheda K, Gumbo T, Maartens G, Dooley KE, Murray M, Furin J, Nardell EA, Warren RM. Lancet Respiratory Medicine drug-resistant tuberculosis Commission group. The Lancet Respiratory Medicine Commission: 2019 update: epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant and incurable tuberculosis. Lancet Respir Med. 2019;7:820–6. [PubMed: 31486393](Review of the epidemiology, pathogenesis, diagnosis and management of multidrug resistant tuberculosis; does not discuss hepatotoxicity of therapeutic drug regimens).
- Beauglehole D, Nolan D, Italiano CM. Acute liver failure likely due to ethambutol in the treatment of AIDS-associated disseminated Mycobacterium avium complex infection. Intern Med J. 2020;50:1431–2. [PubMed: 33215828](34 year old man with HIV infection and Mycobacterium avium complex [MAC] infection developed cholestatic liver injury with jaundice [bilirubin ~12 mg/dL] within 4 weeks of starting isoniazid, pyrazinamide, clarithromycin, rifabutin and ethambutol and had recurrence 3 times with reinstitution of MAC therapy, eventually resolving only when switching to an ethambutol-free regimen).
- Zhao H, Wang Y, Zhang T, Wang Q, Xie W. Drug-induced liver injury from anti-tuberculosis treatment: a retrospective cohort study. Med Sci Monit. 2020;26:e920350. [PMC free article: PMC7077058] [PubMed: 32145061](Among 140 patients with liver injury due to antituberculosis therapy seen between 2009 and 2015, the median age was 40, time to onset ranged from 7-90 days [mean=24], 35% had fever, 16% rash, 68% were hepatocellular, 21% were severe and 7% died; most patients had received isoniazid, rifampin and pyrazinamide).
- Soni H, Kumar-M P, Mishra S, Bellam BL, Singh H, Mandavdhare HS, Medhi B, et al. Risk of hepatitis with various reintroduction regimens of anti-tubercular therapy: a systematic review and network meta-analysis. Expert Rev Anti Infect Ther. 2020;18:171–9. [PubMed: 31923369](Systematic review of the risk of recurrence of hepatotoxicity on reintroduction of antituberculosis agents after occurrence of drug induced liver injury indicated that sequential and incremental introduction is preferable to rechallenge with full doses of both agents concurrently).
- Gezahegn LK, Argaw E, Assefa B, Geberesilassie A, Hagazi M. Magnitude, outcome, and associated factors of anti-tuberculosis drug-induced hepatitis among tuberculosis patients in a tertiary hospital in North Ethiopia: A cross-sectional study. PLoS One. 2020;15:e0241346. [PMC free article: PMC7654771] [PubMed: 33170847](Among 188 patients treated for tuberculosis with isoniazid, rifampin, pyrazinamide and ethambutol in a single referral center in Ethiopia over a 3 year period, 26 [14%] developed evidence of drug induced liver injury including 3 who died).
- CDC. https://www
.cdc.gov/tb/ (CDC website with up-to-date recommendations on therapy of tuberculosis).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Pyrazinamide.[LiverTox: Clinical and Researc...]Review Pyrazinamide.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Inactive hepatitis B surface antigen carrier state and hepatotoxicity during antituberculosis chemotherapy.[Chest. 2005]Inactive hepatitis B surface antigen carrier state and hepatotoxicity during antituberculosis chemotherapy.Lee BH, Koh WJ, Choi MS, Suh GY, Chung MP, Kim H, Kwon OJ. Chest. 2005 Apr; 127(4):1304-11.
- Determinants of rifampin, isoniazid, pyrazinamide, and ethambutol pharmacokinetics in a cohort of tuberculosis patients.[Antimicrob Agents Chemother. 2...]Determinants of rifampin, isoniazid, pyrazinamide, and ethambutol pharmacokinetics in a cohort of tuberculosis patients.McIlleron H, Wash P, Burger A, Norman J, Folb PI, Smith P. Antimicrob Agents Chemother. 2006 Apr; 50(4):1170-7.
- Antituberculosis agents: isoniazid, rifampin, streptomycin, ethambutol.[Mayo Clin Proc. 1977]Antituberculosis agents: isoniazid, rifampin, streptomycin, ethambutol.Van Scoy RE. Mayo Clin Proc. 1977 Nov; 52(11):694-700.
- Review Isoniazid.[LiverTox: Clinical and Researc...]Review Isoniazid.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Ethambutol - LiverToxEthambutol - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...