NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Lamotrigine is a widely used antiseizure medication that is a rare but well known cause of idiosyncratic liver injury, that can be severe and even fatal.
Background
Lamotrigine (la moe' tri jeen) is a phenyltriazine and belongs to an anticonvulsant class of its own. Lamotrigine is believed to inhibit the release of excitatory neurotransmitters, particularly glutamate and aspartate but may also act directly on sodium channels on neuronal cells. Lamotrigine was approved for use in the United States for epilepsy in 1994 and it is still in common use. Lamotrigine is indicated for prevention and management of partial and generalized seizures either alone or in combination with other anticonvulsant agents. Lamotrigine is also approved for use as a mood stabilizer in bipolar disorders. It is used off-label for several other conditions, including peripheral neuropathy, neuropathic pain, migraine headaches and trigeminal neuralgia. Lamotrigine is available tablets of 25, 100, 150 and 200 mg in generic formulations and under the brand name Lamictal. Pediatric formulations as chewable tablets are available in doses of 2, 5 and 25 mg. The recommended starting dose is 25 mg taken orally once daily, escalating to a maximum dose of 400 mg in two divided doses daily. Common side effects include somnolence, fatigue, nervousness, dizziness, and rash.
Hepatotoxicity
Prospective studies suggest that less than 1% of subjects develop elevations in serum aminotransferase levels during long term lamotrigine therapy. However, clinically apparent hepatotoxicity from lamotrigine is well known and is estimated to occur in one in 2,000 to 10,000 treated patients. The liver injury is usually part of a systemic immuno-allergic reaction (anticonvulsant hypersensitivity syndrome [AHS] or drug rash with eosinophilia and systemic symptoms [DRESS] syndrome). The latency is typically short, ranging from one to several weeks. Presenting symptoms are a diffuse maculopapular rash, followed in a few days by high fever, nausea and vomiting. The rash can develop into a systemic hypersensitivity reaction and multiorgan failure or be associated with jaundice and hepatitis. Eosinophilia is common, and facial edema, lymphadenopathy and atypical lymphocytosis can occur. The pattern of liver enzyme elevations is usually hepatocellular and severity ranges from mild-to-moderate ALT elevations accompanying the generalized hypersensitivity reaction, to an icteric hepatitis, to a severe hepatitis and acute liver failure. Liver biopsy shows portal inflammation, hepatocellular necrosis and bile duct proliferation. In some instances of severe hypersensitivity syndrome with acute multiorgan failure, the hepatic involvement may represent ischemic injury.
Lamotrigine has also been linked to rare instances of hemophagocytic lymphohistiocytosis, a rare and severe immune related reaction characterized by unremitting activation of CD8+ T cells and macrophages that causes multiorgan damage including liver injury, hepatitis and liver failure. Cases of HLH linked to lamotrigine arose within 1 to 4 weeks of starting the drug, usually in infants or children, marked clinically by fever, rash, cytopenias, hepatitis, high triglycerides and ferritin levels and bone marrow or liver histology demonstrating hemophagocytosis
Likelihood score: A (well known cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism of lamotrigine hepatotoxicity is thought to be hypersensitivity or an immunological response to a metabolically generated drug-protein complex. Women, children and patients taking valproic acid appear to be more susceptible. Higher doses and rapid dose escalations have also been linked with higher incidence of lamotrigine hepatotoxicity. Genetic studies have shown little or no association of lamotrigine hepatotoxicity with specific variants, such as those associated with carbamazepine related hypersensitivity reactions.
Outcome and Management
Lamotrigine hepatotoxicity is usually rapidly reversible within a few days of the drug being stopped. In cases of multiorgan failure, disseminated intravascular consumption (DIC), rhabdomyolysis and renal failure are common. If lamotrigine is stopped promptly, recovery is expected within one to two weeks. Prolonged exposure after the appearance of symptoms may escalate the injury to result in irreversible, rapidly progressive liver failure. Corticosteroids have been used to treat lamotrigine hepatoxicity but with uncertain effectiveness. Chronic injury from lamotrigine therapy has not been reported. Lamotrigine is not an aromatic convulsant and is unrelated structurally to phenytoin and carbamazepine. However, some degree of overlap in hypersensitivity response occurs, and patients with hepatotoxicity related to lamotrigine should best avoid exposure to the aromatic anticonvulsants such as phenytoin, phenobarbital and carbamazepine.
Hemophagocytolytic lymphohistiocytosis caused by lamotrigine can be life-threatening and is usually treated with immunosuppression including corticosteroids and etoposide and occasionally with monoclonal antibody to interferon gamma (emapalumab).
Drug Class: Anticonvulsants
CASE REPORT
Case 1. Acute hepatitis-like injury due to lamotrigine.(1)
A 27 year old female with a history of seizure disorder developed a rash with fever, itching, nausea, vomiting and fatigue 3 weeks after starting lamotrigine. The dose had been increased from 50 mg daily to 100 mg daily one week before onset of symptoms. She continued to take lamotrigine for another week before seeking medical attention, when she was found to have elevated serum aminotransferase levels and was admitted to for further evaluation. Physical examination revealed an extensive maculopapular rash and fever but no jaundice. Initially, serum ALT levels were ~25 fold elevated, but serum bilirubin levels were normal. Eosinophil counts were not available. Tests for viral hepatitis, autoimmune hepatitis and ultrasound were unremarkable. The patient had no history of alcohol or drug abuse or previous history of liver disease or drug allergy. After two days in the hospital she became jaundiced with serum bilirubin that eventually rose to 6.4 mg/dL. Over the following days, laboratory test results began to improve and were close to normal 2 weeks later.
Key Points
| Medication: | Lamotrigine (50→100 mg/day) |
|---|---|
| Pattern: | Hepatocellular (R=56) |
| Severity: | 3+ (jaundice and hospitalization) |
| Latency: | 26 days |
| Recovery: | Complete after 19 days |
| Other medications: | None mentioned |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|
| 0 | 0 | Lamotrigine started, 50 mg daily | |||
| 2 weeks | 0 | Lamotrigine increased to 100 mg | |||
| 3 weeks | 0 | Rash, itching, nausea, vomiting | |||
| 4 weeks | 0 | 1576 | 79 | 0.4 | Lamotrigine stopped |
| 2 days | 5705 | 172 | 4.9 | ||
| 3 days | 4160 | 147 | 6.6 | ||
| 4 days | 3372 | 129 | 6.4 | ||
| 5 weeks | 5 days | 2460 | 127 | 5.5 | Discharged |
| 7 weeks | 19 days | 74 | 63 | 1.2 | |
| Normal Values | <42 | <115 | <1.2 | ||
Comment
A typical acute hepatitis-like injury arose after 3 weeks of lamotrigine therapy with somewhat delayed discontinuation. The timing of this reaction and the signs of hypersensitivity including fever and rash support the diagnosis and suggest an immuno-allergic etiology. Therapy with other anticonvulsants may be tolerated but monitoring for evidence of liver injury would be appropriate.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Lamotrigine – Generic, Lamictal®
DRUG CLASS
Anticonvulsants
COMPLETE LABELING
Product labeling at DailyMed, National Library of Medicine, NIH
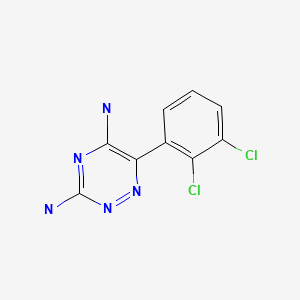
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Lamotrigine | 84057-84-1 | C9-H7-C12-N5 |
|
CITED REFERENCE
- 1.
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159]
ANNOTATED BIBLIOGRAPHY
References updated: 18 April 2019
- Zimmerman HJ. Anticonvulsants. In, Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999: pp. 498-516.(Expert review of anticonvulsants and liver injury published in 1999; lamotrigine is not discussed).
- Pirmohamed M, Leeder SJ. Anticonvulsant agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013: pp 423-41.(Review of anticonvulsant induced liver injury; liver injury due to lamotrigine can be a component of a generalized hypersensitivity reaction and result in a prominent and severe hepatitis which can be fatal).
- Smith MD, Metcalf CS, Wilcox KS. Pharmacotherapy of the epilepsies. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 303-26.(Textbook of pharmacology and therapeutics).
- Cohen AF, Land GS, Breimer DD, Yuen WC, Winton C, Peck AW. Lamotrigine, a new anticonvulsant: pharmacokinetics in normal humans. Clin Pharmacol Ther. 1987;42:535–41. [PubMed: 3677542](Study of healthy volunteers; a minor rise in serum bilirubin was found with iv lamotrigine; not reproduced in later studies; no increase in liver enzymes or GGT).
- Brodie MJ. Lamotrigine. Lancet. 1992;339:1397–1400. [PubMed: 1350815](Review article on lamotrigine; rare side effects mentioned were macropapular rash, angioedema, Stevens-Johnson syndrome and acute multiorgan failure).
- Yuen AW, Bihari DJ. Multiorgan failure and disseminated intravascular coagulation in severe convulsive seizures. Lancet. 1992;340:618. [PubMed: 1355200](Letter in response to Brodie review, summarizing 28 reported cases of acute organ failure attributed to lamotrigine, suggesting that these were due to severe convulsive seizures with rhabdomyolysis, disseminated intravascular coagulation and ischemic injury to multiple tissues including liver).
- Schaub JEM, Williamson PJ, Barnes EW, Trewby PN. Multisystem adverse reaction to lamotrigine. Lancet. 1994;344:481. [PubMed: 7914595](45 year old woman developed fever, rash and multisystem involvement 14 days after starting lamotrigine, with AST 1200 U/L, LDH 6830 U/L, CPK 7770 U/L, myoglobinemia, disseminated intravascular coagulation and renal insufficiency without preceding severe convulsive seizures; no mention of jaundice; resolved in 2 weeks).
- Makin AJ, Fitt S, Williams R, Duncan JS. Fulminant hepatic failure induced by lamotrigine. BMJ. 1995;311:292. [PMC free article: PMC2550348] [PubMed: 7633236](22 year old woman developed fever and rash 3 weeks after starting lamotrigine [2 days after increasing dose] with AST 913 U/L; despite stopping the drug, she went into coma and died; no bilirubin levels or other test results provided).
- Sterker M, Berrouschot J, Schneider D. Fatal course of toxic epidermal necrolysis under treatment with lamotrigine. Int J Clin Pharmacol Ther. 1995;33:595–7. [PubMed: 8688983](56 year old man developed rash one week after starting lamotrigine; 2 weeks later, found unconscious with severe necrotizing rash involving 80% of his body [bilirubin 2.1 mg/dL, ALT 529 U/L, LDH 1260 U/L], with disseminated intravascular coagulation, myoglobulinemia, respiratory failure and subsequent death).
- Sullivan JR, Watson A. Lamotrigine-induced toxic epidermal necrolysis treated with intravenous cyclosporin: a discussion of pathogenesis and immunosuppressive management. Australas J Dermatol. 1996;37:208–12. [PubMed: 8961591](Review of toxic epidermal necrolysis related to lamotrigine).
- Chattergoon DS, McGuigan MA, Koren G, Hwang P, Ito S. Multiorgan dysfunction and disseminated intravascular coagulation in children receiving lamotrigine and valproic acid. Neurology. 1997;49:1442–4. [PubMed: 9371937](3 year old boy and 11 year old girl developed multiorgan failure while on valproate, arising 9 days after starting lamotrigine, presenting with fever, rash and progressing to disseminated intravascular coagulation, rhabdomyolysis and mild hepatitis [bilirubin normal, ALT 128 and 279 U/L, Alk P normal and 145 U/L], ultimate recovery; concise review of literature).
- Jones D, Chhiap V, Resor S, Appel G, Grossman ME. Phenytoin-like hypersensitivity associated with lamotrigine. J Am Acad Dermatol. 1997;36:1016–8. [PubMed: 9204073](Adult who developed rash after 1 and high fever after 3 weeks of lamotrigine with bilirubin 1.7 mg/dL, ALT 130 U/L; treated with corticosteroids with slow recovery).
- Messenheimer JA. Rash in adult and pediatric patients treated with lamotrigine. Can J Neurol Sci. 1998;25:S14–8. [PubMed: 9827240](24 year old man developed rash after 1 week and fever after 3 weeks of lamotrigine therapy [bilirubin 1.7 mg/dL, ALT 130 U/L, Alk P 274 U/L], resolving within 3 weeks on intravenous methylprednisolone; review of rates of rash in clinical trials of lamotrigine found reports of rash in 0.3% of adults and 1% of children; Stevens-Johnson syndrome in 0.1% of adults and 0.5% of children).
- Schlienger RG, Knowles SR, Shear NH. Lamotrigine associated anticonvulsant hypersensitivity syndrome. Neurology. 1998;51:1172–5. [PubMed: 9781550](Review of lamotrigine hypersensitivity cases in the literature up to 1998).
- Gómez Caturla A, Arteta Jiménez M, Fernández Planelles C, Portillo J. Med Clin (Barc). 1998;111:675–6. [Acute hepatitis associated with lamotrigine administration] Spanish. [PubMed: 9881354](35 year old man with Down syndrome treated with valproate and carbamazepine for many years, developed fatigue and abdominal pain 6 weeks after starting lamotrigine [bilirubin 10.0 mg/dL, ALT 732 U/L, Alk P 418 U/L, 16% eosinophils], with signs of hepatic failure but ultimate full recovery)
- Knowles SR, Shapiro LE, Shear NH. Anticonvulsant hypersensitivity syndrome: incidence, prevention and management. Drug Saf. 1999;21:489–501. [PubMed: 10612272](Review of anticonvulsant hypersensitivity syndrome: triad of fever, rash and internal organ injury occurring 1-8 weeks after starting anticonvulsant; liver being most common internal organ involved; occurs in 1:1000-1:10,000 initial exposures to phenytoin, carbamazepine, phenobarbital or lamotrigine, unrelated to dose, perhaps predisposed by valproate; liver injury arises 1-4 weeks after onset of rash and ranges in severity from asymptomatic ALT elevations to icteric hepatitis to acute liver failure. High mortality rate with jaundice; other organs that can be involved include muscle, kidney, brain, heart and lung. Pseudolymphoma syndrome and serum sickness like syndrome are separate complications of anticonvulsants. Role of corticosteroids uncertain; cross reactivity among the agents should be assumed).
- Arnon R, DeVivo D, Defelice AR, Kazlow PG. Acute hepatic failure in a child treated with lamotrigine. Pediatr Neurol. 1998;18:251–2. [PubMed: 9568923](8 year old boy developed fever after 2-3 weeks of lamotrigine, followed by jaundice [bilirubin 14.3 mg/dL, ALT 5218 U/L, Alk P 234 U/L, elevated INR, ammonia, and high lamotrigine levels], rapid improvement with stopping).
- Fayad M, Choueiri R, Mikati M. Potential hepatotoxicity of lamotrigine. Pediatr Neurol. 2000;22:49–52. [PubMed: 10669206](3 children, ages 3, 4 and 10 years, developed liver injury after 1, 2 and 6 weeks of lamotrigine therapy [bilirubin levels not reported, ALT 612, 257 and 291 U/L; Alk P 152, 344 and 254 U/L], resolving rapidly with stopping; one child also had a rash).
- Sauvé G, Bresson-Hadni S, Prost P, Le Calvez S, Becker M-C, Galmiche J, Carbillet J-P, Miguet J-P. Acute hepatitis after lamotrigine administration. Dig Dis Sci. 2000;45:1874–7. [PubMed: 11052335](28 year old woman developed fever and rash 14 days after starting lamotrigine [bilirubin 1.7 mg/dL, ALT 503 U/L and Alk P 287 U/L], with drowsiness, fever and abnormal protime, but rapid recovery after stopping).
- Wong IC, Lhatoo SD. Adverse reactions to new anticonvulsant drugs. Drug Saf. 2000;23:35–56. [PubMed: 10915031](Review of adverse events associated with lamotrigine reported in the literature).
- Schaub N, Bircher AJ. Severe hypersensitivity syndrome to lamotrigine confirmed by lymphocyte stimulation in vitro. Allergy. 2000;55:191–3. [PubMed: 10726736](36 year old man developed rash and fever after 4 weeks of lamotrigine and valproate therapy, but no mention of hepatic involvement; positive lymphocyte stimulation).
- Overstreet K, Costaanza C, Behling C, Hassanin T, Masliah E. Fatal progressive hepatic necrosis associated with lamotrigine treatment: a case report and literature review. Dig Dis Sci. 2002;47:1921–5. [PubMed: 12353830](35 year old woman developed fever, rash and abdominal pain after 5 weeks of lamotrigine therapy with ALT 560 U/L on admission, drug continued for a few days, and liver tests worsened [peak bilirubin 10.3 mg/dL, ALT 2485 U/L, Alk P 815 U/L], followed by progressive hepatic failure and death, autopsy showed massive necrosis).
- Bin-Nakhi HA, Sadeq S, Pinto RG, Habeeb Y. Anticonvulsant hypersensitivity syndrome: report of 2 cases from Kuwait. Med Princ Pract. 2003;12:197–9. [PubMed: 12766341](Child with rash, fever and lymphadenopathy 2 weeks after starting lamotrigine with pancytopenia, eosinophilia, atypical lymphocytes [bilirubin 1.1, ALT 104 U/L, Alk P 104 U/L], resolving within 3 weeks of switching to clonazepam).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl. 2004;10:1018–23. [PubMed: 15390328](Among ~50,000 liver transplants done in the United States between 1990 and 2002, 137 [0.2%] were done for idiosyncratic drug induced acute liver failure, of which 10 were attributed to phenytoin, 10 to valproate and 1 to carbamazepine but none to lamotrigine or other anticonvulsants).
- Björnsson E, Jerlstad P, Bergqvist A, Olsson R. Fulminant drug-induced hepatic failure leading to death or liver transplantation in Sweden. Scand J Gastroenterol. 2005;40:1095–101. [PubMed: 16165719](36 years of reporting to Swedish registry identified 103 cases of acute liver failure due to drugs, of which 1 was attributed to phenytoin, 1 to valproate and 1 to carbamazepine, but none to lamotrigine or other anticonvulsants).
- Mecarelli O, Pulitano P, Mingoia M, Ferretti G, Rossi M, Berloco PB, Muda AO. Acute hepatitis associated with lamotrigine and managed with the molecular adsorbents recirculating system (MARS). Epilepsia. 2005;46:1687–9. [PubMed: 16190944](30 year old woman developed fever, rash and jaundice 20 days after starting lamotrigine [bilirubin 14 mg/dL, ALT 7268 U/L, INR 2.4 and hepatic encephalopathy], resolving within 3-4 weeks of stopping lamotrigine despite therapy with MARS).
- Chang CC, Shiah IS, Yeh CB, Wang TS, Chang HA. Lamotrigine associated anticonvulsant hypersensitivity syndrome in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:741–4. [PubMed: 16442685](48 year old woman developed fever and rash ~10 days after starting lamotrigine for bipolar illness and subsequently had pneumonitis, hepatitis [ALT 174 U/L, but no other results given] and bone marrow suppression, resolving rapidly once lamotrigine was stopped; valproate and venlafaxine later restarted without difficulty).
- Fix OK, Peters MG, Davern TJ. Eosinophilic hepatitis caused by lamotrigine. Clin Gastroenterol Hepatol. 2006;4:xxvi. [PMC free article: PMC4113389] [PubMed: 16616339](20 year old woman developed pneumonitis, jaundice and rash 6 weeks after starting lamotrigine [bilirubin 10.4 mg/dL, ALT 233 U/L, Alk P 171 U/L, eosinophils 15%], no follow up provided).
- Björnsson E, Olsson R. Suspected drug-induced liver fatalities reported to the WHO database. Dig Liver Dis. 2006;38:33–8. [PubMed: 16054882](In WHO database of fatal adverse drug reactions from 1968-2003, there were 4690 reports of fatal drug induced liver injury: phenytoin was the only anticonvulsant within the 19 most common causes, ranking 12th [57 cases]).
- Petkov T, Pehlivanov G, Grozdev I, Kavaklieva S, Tsankov N. Toxic epidermal necrolysis as a dermatological manifestation of drug hypersensitivity syndrome. Eur J Dermatol. 2007;17:422–7. [PubMed: 17673387](3 cases of toxic epidermal necrolysis, one in a 28 year old man who developed facial edema and rash progressing to severe exfoliation 1 week after adding lamotrigine to long term valproate therapy [bilirubin 0.6 mg/dL, ALT 50 U/L, GGT 48 U/L, eosinophils 10%], resolving with corticosteroid therapy).
- Gerstner T, Bauer MO, Longin E, Bell N, Koenig SA. Reversible hepatotoxicity, pancreatitis, coagulation disorder and simultaneous bone marrow suppression with valproate in a 2-year-old girl. Seizure. 2007;16:554–6. [PubMed: 17493839](2 year old girl with suspected valproate hepatotoxicity, liver abnormalities improved on withdrawal of valproate, despite continuation of lamotrigine and topiramate).
- Moeller KE, Wei L, Jewell AD, Carver LA. Acute hepatotoxicity associated with lamotrigine. Am J Psychiatry. 2008;165:539–40. [PubMed: 18381923](21 year old man being treated with lamotrigine for bipolar disorder and schizophrenia was found to have ALT 347 U/L without fever or jaundice and recovered within 7 days of stopping).
- Delima SI, Walsh LE, Golomb MR. Simultaneous toxicities in a child on multiple anticonvulsants. J Child Neurol. 2008;23:1054–7. [PubMed: 18344455](3 year old girl with epilepsy on valproate, lamotrigine and phenytoin for unstated period developed fever, cough and liver test abnormalities [bilirubin not given, ALT 7390 U/L, Alk P 381, INR 1.26], and mild bone marrow insufficiency; all 3 drugs were stopped and liver abnormalities resolved, evidently able to tolerate lamotrigine without problems later).
- Björnsson E. Hepatotoxicity associated with antiepileptic drugs. Acta Neurol Scand. 2008;118:281–90. [PubMed: 18341684](Review of liver injury due to anticonvulsants including lamotrigine: fatal cases of lamotrigine injury have been reported, usually arising within 3 weeks of starting therapy, often while receiving other anticonvulsants and in context of hypersensitivity syndrome).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, valproate accounted for 6 cases, lamotrigine 5, phenytoin 5, and gabapentin and topiramate 1 each).
- Su-Yin AN, Tai WW, Olson KR. Lamotrigine-associated reversible severe hepatitis: a case report. J Med Toxicol. 2008;4:258–60. [PMC free article: PMC3550118] [PubMed: 19031378](43 year old woman developed rash and serum enzyme elevations 2 weeks after starting lamotrigine [bilirubin 0.4 rising to 3.9 mg/dL, ALT 2490 U/L, Alk P 93 U/L], which worsened for several days but then improved and resolved completely within 1 month of stopping).
- Aouam K, Ben Romdhane F, Loussaief C, Salem R, Toumi A, Belhadjali H, Chaabane A, et al. Hypersensitivity syndrome induced by anticonvulsants: possible cross-reactivity between carbamazepine and lamotrigine. J Clin Pharmacol. 2009;49:1488–91. [PubMed: 19723672](14 year old boy developed facial edema and fever 6 weeks after starting carbamazepine [bilirubin not given, ALT 193 U/L, eosinophils 15%], which resolved with stopping, and months later he developed fever, rash and pneumonitis 7 weeks after starting lamotrigine [ALT 154 U/L, eosinophils 12%], resolving upon stopping).
- Amante MF, Filippini AV, Cejas N, Lendoire J, Imventarza O, Parisi C. Dress syndrome and fulminant hepatic failure induced by lamotrigine. Ann Hepatol. 2009;8:75–7. [PubMed: 19221540](21 year old woman developed fever and sore throat within 18 days of starting lamotrigine which progressed to desquamation, jaundice and pruritus with subsequent hepatic failure and emergency liver transplant, the explant showing submassive, confluent necrosis).
- Ouellet G, Tremblay L, Marleau D. Fulminant hepatitis induced by lamotrigine. South Med J. 2009;102:82–4. [PubMed: 19077780](40 year old woman developed fever 12 days after starting lamotrigine followed by rash and jaundice [bilirubin 1.6 rising to 12.2 mg/dL, ALT 9600 U/L, Alk P not available, INR 5.7], but eventual spontaneous resolution without corticosteroids within 10 weeks).
- Ferrajolo C, Capuano A, Verhamme KM, Schuemie M, Rossi F, Stricker BH, Sturkenboom MC. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol. 2010;70:721–8. [PMC free article: PMC2997312] [PubMed: 21039766](Worldwide pharmacovigilance database contains 9036 hepatic adverse drug reactions in children; lamotrigine accounted for 112 cases [1.2%: ranking 7th], yielding an adjusted relative risk odds ratio of 2.2).
- An DM, Wu XT, Hu FY, Yan B, Stefan H, Zhou D. Association study of lamotrigine-induced cutaneous adverse reactions and HLA-B*1502 in a Han Chinese population. Epilepsy Res. 2010;92:226–30. [PubMed: 21071176](Among 25 Chinese patients with cutaneous adverse reactions to lamotrigine, 4 [16%] had HLA-B*1502 compared to 8.5% of lamotrigine tolerant controls).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, 11 of which were due to anticonvulsants, but none to lamotrigine).
- Shi YW, Min FL, Liu XR, Zan LX, Gao MM, Yu MJ, Liao WP. HLA-B alleles and lamotrigine-induced cutaneous adverse drug reactions in the Han Chinese population. Basic Clin Pharmacol Toxicol. 2011;109:42–6. [PubMed: 21306565](Among 2 Chinese patients with lamotrigine associated Stevens Johnson syndrome, neither had HLA-B*1502 and rates in those with less severe skin hypersensitivity reactions were not elevated).
- Molleston JP, Fontana RJ, Lopez MJ, Kleiner DE, Gu J, Chalasani N., Drug-Induced Liver Injury Network. Characteristics of idiosyncratic drug-induced liver injury in children: results from the DILIN Prospective Study. J Pediatr Gastroenterol Nutr. 2011;53:182–9. [PMC free article: PMC3634369] [PubMed: 21788760](Among 30 children with drug induced liver injury enrolled in a prospective US database between 2004 and 2008, 8 were due to anticonvulsants [lamotrigine in 3 and valproate in 3], none of which were fatal or led to chronic injury).
- Bakker CV, Hegt VN, Praag MC. Lamotrigine hypersensitivity syndrome and spiking Fever. Indian J Dermatol. 2012;57:504. [PMC free article: PMC3519273] [PubMed: 23248384](24 year old woman developed fever and rash 4 weeks after starting lamotrigine [bilirubin not given, ALT 236 U/L, Alk P 215 U/L], fever and liver injury resolving with prednisone therapy).
- Knowles SR, Dewhurst N, Shear NH. Anticonvulsant hypersensitivity syndrome: an update. Expert Opin Drug Saf. 2012;11:767–78. [PubMed: 22794330](DRESS syndrome is marked by triad of fever, rash and internal organ involvement, usually liver; while lamotrigine is not an aromatic anticonvulsant, it has been linked to cases of DRESS and Stevens Johnson syndrome).
- Neuman MG, Cohen L, Nanau RM, Hwang PA. Genetic and immune predictors for hypersensitivity syndrome to antiepileptic drugs. Transl Res. 2012;159:397–406. [PubMed: 22500513](Review of hypersensitivity reactions to antiepileptic agents including lamotrigine and lymphocyte toxicity assays).
- Sato T, Kuniba H, Matsuo M, Matsuzaka T, Moriuchi H. No To Hattatsu. 2012;44:69–72. [Case of drug-induced hypersensitivity syndrome due to lamotrigine: demonstration of sequential reactivation of herpesviruses] Japanese. [PubMed: 22352035](Abstract: 14 year old girl developed fever, rash, eosinophilia and "liver dysfunction" during lamotrigine therapy, with relapsing course and hypothyroidism).
- Bhayana H, Appasani S, Thapa BR, Das A, Singh K. Lamotrigine-induced vanishing bile duct syndrome in a child. J Pediatr Gastroenterol Nutr. 2012;55:e147–8. [PubMed: 22008955](12 year old boy developed rash 2 weeks after starting lamotrigine followed by jaundice 2 weeks later [bilirubin 14.8 mg/dL, ALT 321 U/L, Alk P 123 U/L], which progressed to vanishing bile duct syndrome and referral for liver transplant within a year of onset [had history of rash after carbamazepine]).
- Hassan I. HLA-B*1502 screening in carbamazepine and lamotrigine candidates of Asian background. Aust N Z J Psychiatry. 2012;46:1106–7. [PubMed: 22833576](Letter expressing opinion that HLA-B*1502 screening should also be used for Asian patients planning to take lamotrigine).
- McCormack M, Urban TJ, Shianna KV, Walley N, Pandolfo M, Depondt C, Chaila E, et al. Genome-wide mapping for clinically relevant predictors of lamotrigine- and phenytoin-induced hypersensitivity reactions. Pharmacogenomics. 2012;13:399–405. [PMC free article: PMC3428903] [PubMed: 22379998](Genome wide association studies failed to identify any significant variation [including HLA-A*3101, previously associated with carbamazepine reactions in Europeans] associated with hypersensitivity to lamotrigine [n=46] or phenytoin [n=44]).
- Patel PP, Gandhi AM, Desai CK, Desai MK, Dikshit RK. An analysis of drug induced Stevens-Johnson syndrome. Indian J Med Res. 2012;136:1051–3. [PMC free article: PMC3612312] [PubMed: 23391805](59 cases of drug induced Stevens Johnson syndrome were seen at a single referral center in India between 2005-2010, 25% in children, arising 1-39 days after starting drug, major causes being phenytoin [18] and nevirapine [13], 2 were attributed to lamotrigine).
- Biton V, Shneker BF, Naritoku D, Hammer AE, Vuong A, Caldwell PT, Messenheimer JA. Long-term tolerability and safety of lamotrigine extended-release: pooled analysis of three clinical trials. Clin Drug Investig. 2013;33:359–64. [PubMed: 23475541](Pooled analysis of adverse events in 662 patients treated with extended release lamotrigine in 3 controlled trials, found no "clinically important" differences in clinical chemistry results between lamotrigine and placebo recipients).
- Cheung YK, Cheng SH, Chan EJ, Lo SV, Ng MH, Kwan P. HLA-B alleles associated with severe cutaneous reactions to antiepileptic drugs in Han Chinese. Epilepsia. 2013;54(7):1307–14. [PubMed: 23692434](HLA-B*15:02 was found in 24 of 26 [92%] Chinese patients with carbamazepine related, but only 2 of 6 [33%] with lamotrigine related Stevens Johnson syndrome versus 12% of controls).
- Drugs for epilepsy. Treat Guidel Med Lett. 2013;11:9–18. [PubMed: 23348233](Drugs of choice for epilepsy; mentions that the adverse effects of lamotrigine include acute hepatitis and Stevens Johnson syndrome; erratum in Treat Guidel Med Lett. 2013;11:112, dosage error in article text).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, including 3 attributed to anticonvulsants: phenytoin 2 and gabapentin 1, but none attributed to lamotrigine).
- Kaur S, Dogra A. Toxic epidermal necrolysis due to concomitant use of lamotrigine and valproic Acid. Indian J Dermatol. 2013;58:406. [PMC free article: PMC3778793] [PubMed: 24082198](Two Indian patients with epilepsy on long term valproate developed toxic epidermal necrolysis 2 and 4 weeks after starting lamotrigine, with fever and skin rash involving 30% and 60% of the body surface area [no mention of liver test abnormalities], resolving ultimately with prednisolone therapy).
- Parveen S, Javed MA. Stevens Johnson Syndrome associated with Lamotrigine. Pak J Med Sci. 2013;29:1450–2. [PMC free article: PMC3905385] [PubMed: 24550973](58 year old Pakistani woman developed conjunctivitis, facial swelling and rash 4-6 weeks after starting lamotrigine for depression, resolving within 2 weeks of stopping; no mention of liver test abnormalities).
- Błaszczyk B, Szpringer M, Czuczwar SJ, Lasoń W. Single centre 20 year survey of antiepileptic drug-induced hypersensitivity reactions. Pharmacol Rep. 2013;65:399–409. [PubMed: 23744424](Among 300 patients with epilepsy seen over a 10 year period at a single center in Poland, 30 developed skin rash on anticonvulsant therapy, most commonly with carbamazepine [20 of 130], phenytoin [13 of 40], lamotrigine [13 of 53] and oxcarbazepine [4 of 26]; a single case of Stevens Johnson syndrome was attributed to carbamazepine).
- Ginory A, Chaney-Catchpole M, Demetree JM, Mayol Sabatier LM, Nguyen M. Drug reaction with eosinophilia and systemic symptoms (DRESS) in an adolescent treated with lamotrigine. J Pediatr Pharmacol Ther. 2013;18:236–40. [PMC free article: PMC3775558] [PubMed: 24052787](17 year old female developed rash, fever, fatigue and then jaundice within 3 weeks of starting lamotrigine for bipolar II disorder [bilirubin 6.5 mg/dL, ALT 2076 U/L, Alk P 455 U/L, mild eosinophilia], improving with stopping and high-dose corticosteroid therapy).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, 7 [4%] of which were attributed to anticonvulsants but none to lamotrigine).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 40 [4.5%] were due to anticonvulsants including 9 [1%] due to lamotrigine, the second most common anticonvulsant cause [after phenytoin]).
- Sultan SJ, Sameem F, Ashraf M. Drug reaction with eosinophilia and systemic symptoms: manifestations, treatment, and outcome in 17 patients. Int J Dermatol. 2015;54:537–42. [PubMed: 24738653](Among 17 patients with DRESS or drug-hypersensitivity syndrome seen at a single Indian medical center over a 4 year period, 11 were associated with anticonvulsant therapy including 6 attributed to phenytoin, 2 to phenobarbital, and 1 each to carbamazepine, oxcarbazepine and lamotrigine; all had ALT elevations and 65% were jaundiced).
- Mehrholz D, Urban AE, Herstowska M, Nowicki R, Cubała W, Barańska-Rybak W. A retrospective study of DRESS - drug reaction with eosinophilia and systemic symptoms. Psychiatr Pol. 2017;51:1079–93. [PubMed: 29432504](Among 261 adults with cutaneous drug reactions seen at a single Polish medical center between 2004 and 2017, 10 met criteria for DRESS syndrome, including 6 attributed to carbamazepine, 1 oxycarbamazepine and 2 lamotrigine).
- Han SH, Hur MS, Youn HJ, Roh NK, Lee YW, Choe YB, Ahn KJ. Drug reaction with eosinophilia and systemic symptom syndrome induced by lamotrigine. Ann Dermatol. 2017;29:206–9. [PMC free article: PMC5383747] [PubMed: 28392649](47 year old Korean man developed rash followed by fever 3 weeks after starting lamotrigine [bilirubin 1.4 mg/dL, ALT 66 U/L, Alk P 148 U/L, eosinophils 28%], responding to methylprednisolone therapy).
- Bunchorntavakul C, Reddy KR. Drug hepatotoxicity: newer agents. Clin Liver Dis. 2017;21:115–34. [PubMed: 27842767](Review of the hepatotoxicity of newly approved agents including lamotrigine, which causes hepatocellular injury in 1-5 of 10,000 treated patients, typically with features of hypersensitivity and cutaneous signs and which can lead to fatal acute liver failure).
- Vidaurre J, Gedela S, Yarosz S. Antiepileptic drugs and liver disease. Pediatr Neurol. 2017;77:23–36. [PubMed: 29097018](Review of the use of anticonvulsants in patients with liver disease recommends use of agents that have little hepatic metabolism such as levetiracetam, lacosamide, topiramate, gabapentin and pregabalin; lamotrigine is problematic in that it is metabolized largely in the liver, has a high potential of drug interactions and requires dose adjustments for patients with moderate or severe cirrhosis).
- Drugs for epilepsy. Med Lett Drugs Ther. 2017;59(1526):121–130. [PubMed: 28746301](Concise review of drugs available for therapy of epilepsy; mentions that lamotrigine is a drug of choice for partial and generalized seizures and atypical, absence, myoclonic and atonic seizures and that adverse events include hepatitis, acute liver failure and Stevens Johnson syndrome).
- Comparison table: some oral antiepileptic drugs. Med Lett Drugs Ther. 2017;59(1521):e130–e136. [PubMed: 28746302](Table of the major oral antiepileptic drugs in current use and listing of oral formulations, usual dosages, adverse effects, drug interactions, current indications and costs).
- Iriki H, Ouchi T, Ito H, Sawada M, Mukai M, Nomura H, Baba Y, et al. Case of lamotrigine-induced drug adverse reaction under tocilizumab treatment with clinical and virological features of drug-induced hypersensitivity syndrome. J Dermatol. 2018;45:738–41. [PubMed: 29569382](29 year old woman with rheumatoid arthritis on tocilizumab [anti-IL6] developed rash, fever and lymphadenopathy 3 weeks after starting lamotrigine, responding to corticosteroid therapy but with mild transient ALT elevations [~120 U/L] and recurrence of rash on three occasions).
- Tawhari I, Tawhari F, Aljuaid M. Lamotrigine-induced drug reaction with eosinophilia and systemic symptoms (DRESS) during primary Epstein-Barr virus (EBV) infection. BMJ Case Rep. 2018;2018:bcr-2017-222416. pii. [PMC free article: PMC5786957] [PubMed: 29367368](21 year old man developed fever, sore throat, rash and abdominal pain 2 weeks after starting lamotrigine [bilirubin not given, ALT 506 U/L, Alk P 246 U/L, 10% atypical lymphocytes and positive heterophile antibody], resolving within 3 weeks on methylprednisolone therapy).
- In brief: A potentially fatal immune reaction to lamotrigine. Med Lett Drugs Ther. 2018;60(1549):105. [PubMed: 29913474](Brief summary of FDA warning of possible complication of lamotrigine therapy of hemophagocytic lymphohistiocytosis [HLH], based upon 8 cases reported to the agency arising within 24 days of starting lamotrigine).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Acute liver failure associated with lamotrigine in children with epilepsy: A report of two cases and thoughts on pharmacogenomics.[Epilepsy Behav Rep. 2022]Acute liver failure associated with lamotrigine in children with epilepsy: A report of two cases and thoughts on pharmacogenomics.Deng J, Fu ZR, Wang L, Liu J, Chen CH, Fang F, Wang XL. Epilepsy Behav Rep. 2022; 20:100568. Epub 2022 Oct 19.
- Incidence of Antiseizure Medication-Induced Severe Cutaneous Adverse Reactions in Malaysia.[J Clin Pharmacol. 2022]Incidence of Antiseizure Medication-Induced Severe Cutaneous Adverse Reactions in Malaysia.Fong SL, Lim KS, Hariraj V, Lee SC, Wo WK, Ramli A, Ho JH, Lai PSM, Ng WL. J Clin Pharmacol. 2022 Aug; 62(8):983-991. Epub 2022 Mar 29.
- Review Phenobarbital.[LiverTox: Clinical and Researc...]Review Phenobarbital.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Lamotrigine-associated reversible severe hepatitis: a case report.[J Med Toxicol. 2008]Lamotrigine-associated reversible severe hepatitis: a case report.Su-Yin AN, Tai WW, Olson KR. J Med Toxicol. 2008 Dec; 4(4):258-60.
- Review Chorea Associated with Lamotrigine Use.[Tremor Other Hyperkinet Mov (N...]Review Chorea Associated with Lamotrigine Use.Rael S, Badihian N, Smith KM, Coon EA. Tremor Other Hyperkinet Mov (N Y). 2023; 13:5. Epub 2023 Feb 20.
- Lamotrigine - LiverToxLamotrigine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...