NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Memantine is an oral N-methyl-D-aspartate glutamate receptor antagonist used in the therapy of Alzheimer disease and dementia. Memantine is associated with a minimal rate of serum enzyme elevations during therapy and has only rarely been implicated as a cause of clinically apparent acute liver injury.
Background
Memantine (mem' an teen) is an antagonist of the N-methyl-D-aspartate (NMDA) glutamate receptors and has been shown to reduce the rate of clinical deterioration in moderate-to-severe Alzheimer disease. The mechanism by which inhibition of NMDA receptors is beneficial in dementia is not known, but Alzheimer disease is characterized by persistent activation of these receptors and their antagonism may reduce excitotoxicity. Memantine was approved for use in the United States in 2003 as therapy for moderate-to-severe dementia due to Alzheimer disease. Memantine is available in tablets of 5 and 10 mg and in capsules of extended release capsules of 7, 14, 21 and 28 mg generically and under the brand name Namenda. It is also available as an oral solution (2 mg/mL) and combination forms with donepezil. The usual maintenance dose is 10 to 20 mg daily of the standard formulation or 14 to 28 mg daily of the extended release capsules. Side effects are not common but can include headache, dizziness, agitation, anxiety, fatigue, insomnia, diarrhea, nausea, vomiting and rash.
Hepatotoxicity
In large placebo controlled trials, the rate of serum enzyme elevations during memantine therapy was similar to that in patients on placebo and no instances of clinically apparent liver injury were reported. Nevertheless, since its introduction into clinical use, memantine has been implicated in at least one report of clinically apparent hepatotoxicity. The time to onset was 3 weeks and the clinical syndrome was that of an acute cholestatic hepatitis which was mild-to-moderate in severity and rapidly reversible upon drug discontinuation (Case 1). Immunoallergic and autoimmune features were not present.
Likelihood score: D (possible rare cause of clinically apparent liver injury).
Mechanism of Injury
Memantine is minimally metabolized by the liver and excreted mainly in the urine. The mechanism by which it might cause hepatotoxicity is not known.
Outcome and Management
Cases of hepatotoxicity from memantine have been too few to characterize clinically. There have been no published reports of acute liver failure, chronic hepatitis or vanishing bile duct syndrome attributed to memantine. There is no information on the possible cross sensitivity to liver injury of memantine with the acetylcholinesterase inhibitors or other NMDA antagonists such as amantadine.
References regarding the safety and potential hepatotoxicity of the drugs used for Alzheimer disease are provided together after the Overview section of Alzheimer Disease Agents.
Drug Class: Alzheimer Disease Agents
CASE REPORT
Case 1. Cholestatic hepatitis due to memantine.(1)
A 92 year old woman with dementia developed pruritus, dark urine and jaundice 16 days after starting memantine (5 mg daily for 8 days followed by 10 mg daily). She had no history of liver disease, risk factors for viral hepatitis or alcohol use. Her other medications included digoxin, lisinopril, tiapride, alprazolam and promazine which she had taken chronically. She had no history of drug allergies. She had no fever or rash. Liver tests showed serum bilirubin of 4.3 mg/dL (2.5 mg/dL direct), ALT 132 U/L, AST 74 U/L, alkaline phosphatase 912 U/L and GGT 155 U/L. Serum immunoglobulins were normal and AMA was negative as were tests for hepatitis A, B and C. Ultrasound of the abdomen showed no evidence of biliary obstruction. Memantine was discontinued and laboratory results began to improve (Table). Her other medications were continued. All liver tests were normal 20 days after stopping memantine.
Key Points
| Medication: | Memantine (10 mg daily) |
|---|---|
| Pattern: | Cholestatic (R=0.3) |
| Severity: | 2+ (jaundice, no mention of hospitalization) |
| Latency: | 16 days |
| Recovery: | Within 20 days |
| Other medications: | Digoxin, lisinopril, tiapride, alprazolam and promazine |
Laboratory Values
Comment
An elderly woman developed a mild cholestatic hepatitis within 2 to 3 weeks of starting memantine and recovered promptly with its discontinuation. The major differential diagnosis is biliary obstruction from gallstones or malignancy which was effectively excluded on the basis of the normal ultrasound, absence of fever and abdominal pain and full recovery on stopping the implicated medication. Memantine is generally well tolerated and liver injury from its use is very rare.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Memantine – Namenda®
DRUG CLASS
Alzheimer Disease Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
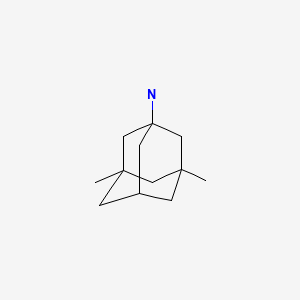
| Memantine | 19982-08-2 | C12-H21-N |
|
CITED REFERENCE
- 1.
- Ferrara N, Corbi G, Capuano A, Filippelli A, Rossi F. Memantine-induced hepatitis with cholestasis in a very elderly patient. Ann Intern Med. 2008;148:631–2. [PubMed: 18413635]
ANNOTATED BIBLIOGRAPHY
References updated: 28 January 2020
- Zimmerman HJ. Miscellaneous drugs and diagnostic chemicals. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 709-42.(Expert review of hepatotoxicity published in 1999; tacrine, the first cholinesterase inhibitor approved for use in Alzheimer disease, was associated with a very high rate of serum ALT elevations [~50%], but rarely caused clinically apparent liver injury; the other Alzheimer disease agents are not discussed).
- Larrey D, Ripault MP. Hepatotoxicity of psychotropic drugs and drugs of abuse. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 518.(Review of hepatotoxicity of psychotropic agents ; drugs for Alzheimer disease are not specifically discussed).
- Roberson ED. Alzheimer's disease. Treatment of central nervous system degenerative disorders. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 333-5.(Textbook of pharmacology and therapeutics).
- Reisberg B, Doody R, Stöer A, Schmitt F, Ferris S, Mös HJ., Memantine Study Group. Memantine in moderate-to-severe Alzheimer's disease. N Engl J Med. 2003;348:1333–41. [PubMed: 12672860](Controlled trial of 28 weeks of memantine vs placebo in 252 patients with Alzheimer disease; no differences in rates of any adverse event; serum ALT levels and hepatotoxicity not mentioned).
- Tariot PN, Farlow MR, Grossberg GT, Graham SM, McDonald S, Gergel I., Memantine Study Group. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004;291:317–24. [PubMed: 14734594](Controlled trial of 24 weeks of memantine vs placebo in 404 patients with Alzheimer disease receiving donepezil: side effects that were more common with memantine were confusion and headache; "No clinically significant differences were detected between treatment groups...in laboratory tests").
- Bullock R. Efficacy and safety of memantine in moderate-to-severe Alzheimer disease: the evidence to date. Alzheimer Dis Assoc Disord. 2006;20:23–9. [PubMed: 16493232](Review of the safety of memantine from 3 pivotal controlled trials and more than 100,000 patient-years of use: overall rates of side effects were not different from placebo, were mild-to-moderate, and often considered unrelated; no mention of hepatotoxicity).
- Farlow MR, Cummings JL. Effective pharmacologic management of Alzheimer's disease. Am J Med. 2007;120:388–97. [PubMed: 17466645](Review of safety and efficacy of medications for Alzheimer disease; no discussion of hepatotoxicity).
- Bakchine S, Loft H. Memantine treatment in patients with mild to moderate Alzheimer's disease: results of a randomised, double-blind, placebo-controlled 6-month study. J Alzheimers Dis. 2008;13:97–107. [PubMed: 18334761](Controlled trial of 24 weeks of memantine vs placebo in 470 patients with Alzheimer disease: adverse events occurred in similar rates with memantine and placebo, were all mild-to-moderate, and there "were no clinically meaningful differences between treatment groups" in laboratory tests).
- Ferrara N, Corbi G, Capuano A, Filippelli A, Rossi F. Memantine-induced hepatitis with cholestasis in a very elderly patient. Ann Intern Med. 2008;148:631–2. [PubMed: 18413635](92 year old woman developed jaundice and pruritus 16 days after starting memantine for dementia [bilirubin 4.3 mg/dL, ALT 132 U/L, Alk P 912 U/L], resolving within 3 weeks of stopping).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008; none were attributed to a drug used to treat Alzheimer disease).
- Mayeux R. Early Alzheimer's disease. N Engl J Med. 2010;362:2194–201. [PubMed: 20558370](Case discussion and review of current understanding of Alzheimer disease including role of therapy; common side effects of cholinesterase inhibitors include nausea, vomiting, anorexia, diarrhea, dizziness, muscle cramps, insomnia and vivid dreams; memantine can cause constipation, dizziness, headache and body pains; no mention of hepatotoxicity).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were attributed to drugs used to treat Alzheimer disease).
- Tan CC, Yu JT, Wang HF, Tan MS, Meng XF, Wang C, Jiang T, et al. Efficacy and Safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer's disease: a systematic review and meta-analysis. J Alzheimers Dis. 2014;41:615–31. [PubMed: 24662102](Systematic review of safety and efficacy of 4 Alzheimer drugs does not mention ALT elevations or hepatotoxicity).
- Tricco AC, Soobiah C, Berliner S, Ho JM, Ng CH, Ashoor HM, Chen MH, Hemmelgarn B, Straus SE. Efficacy and safety of cognitive enhancers for patients with mild cognitive impairment: a systematic review and meta-analysis. CMAJ. 2013;185(16):1393–401. [PMC free article: PMC3826344] [PubMed: 24043661](Systematic review of 8 clinical trials and 3 reports on the safety and efficacy of Alzheimer drugs mentions that side effects of nausea, diarrhea, vomiting and headaches were usually more frequent with the active drugs compared to placebo; no mention of ALT elevations or clinically apparent liver injury).
- Wang HF, Yu JT, Tang SW, Jiang T, Tan CC, Meng XF, Wang C, et al. Efficacy and safety of cholinesterase inhibitors and memantine in cognitive impairment in Parkinson's disease, Parkinson's disease dementia, and dementia with Lewy bodies: systematic review with meta-analysis and trial sequential analysis. J Neurol Neurosurg Psychiatry. 2015;86:135–43. [PubMed: 24828899](Systematic review of 10 trials of Alzheimer disease drugs in Parkinson disease and other forms of dementia reported that the common adverse events were cholinergic in nature [anorexia, nausea, diarrhea] and were generally mild-to-moderate in severity; serious adverse events were similar to rates with placebo; no mention of ALT elevations or hepatotoxicity).
- Nakamura Y, Kitamura S, Homma A, Shiosakai K, Matsui D. Efficacy and safety of memantine in patients with moderate-to-severe Alzheimer's disease: results of a pooled analysis of two randomized, double-blind, placebo-controlled trials in Japan. Expert Opin Pharmacother. 2014;15:913–25. [PMC free article: PMC4025599] [PubMed: 24673497](Among 633 Japanese patients with Alzheimer disease treated with either memantine or placebo, the rate of adverse events was similar in the two groups; no mention of ALT elevations or clinically apparent liver injury).
- Dysken MW, Sano M, Asthana S, Vertrees JE, Pallaki M, Llorente M, Love S, et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: the TEAM-AD VA cooperative randomized trial. JAMA. 2014;311:33–44. [PMC free article: PMC4109898] [PubMed: 24381967](Among 561 patients with Alzheimer disease treated with vitamin E, memantine, the combination or placebo for an average of 2.3 years, overall adverse events were similar in the 4 groups, but serious infections were more common with memantine than placebo (15% vs 7%); no mention of ALT elevations or hepatotoxicity).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none of the cases were attributed to a drug used to treat Alzheimer disease).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, none were attributed to a drug for Alzheimer disease).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were due to a drug for Alzheimer disease).
- Dou KX, Tan MS, Tan CC, Cao XP, Hou XH, Guo QH, Tan L, et al. Comparative safety and effectiveness of cholinesterase inhibitors and memantine for Alzheimer's disease: a network meta-analysis of 41 randomized controlled trials. Alzheimers Res Ther. 2018;10:126. [PMC free article: PMC6309083] [PubMed: 30591071](Meta-analysis of 41 published randomized controlled trials of drugs for Alzheimer disease concluded that all had beneficial effects on cognition and function but not on neuropsychiatric symptoms, and all had adverse effects but memantine showed “the best profile of acceptability”; no mention of ALT elevations or hepatotoxicity).
- Khoury R, Rajamanickam J, Grossberg GT. An update on the safety of current therapies for Alzheimer's disease: focus on rivastigmine. Ther Adv Drug Saf. 2018;9:171–8. [PMC free article: PMC5810854] [PubMed: 29492246](Review of the safety of Alzheimer disease agents discusses gastrointestinal adverse events, cardiac side effects, skin reactions [to transdermal formulations] and neuropsychiatric effects, but not hepatic adverse events).
- Matsunaga S, Kishi T, Nomura I, Sakuma K, Okuya M, Ikuta T, Iwata N. The efficacy and safety of memantine for the treatment of Alzheimer's disease. Expert Opin Drug Saf. 2018;17:1053–61. [PubMed: 30222469](Review of the literature on efficacy and safety of memantine concluded that it improves cognitive function and behavioral disturbances and is well tolerated, most common adverse events being somnolence and weight gain; does not discuss ALT elevations or hepatotoxicity, but mentions that hepatitis is listed as an adverse event in the product label).
- Bhattacharjee S, Patanwala AE, Lo-Ciganic WH, Malone DC, Lee JK, Knapp SM, Warholak T, Burke WJ. Alzheimer's disease medication and risk of all-cause mortality and all-cause hospitalization: A retrospective cohort study. Alzheimers Dement (N Y). 2019;5:294–302. [PMC free article: PMC6626065] [PubMed: 31338414](Among more than 20,000 Medicare beneficiaries receiving Alzheimer disease drugs, overall survival was better for those on donepezil than memantine or rivastigmine; no mention of serious hepatic adverse events or liver related deaths).
- Shumar J, Ordway S, Junga Z, Sadowski B, Torres D. Memantine-induced liver injury with probable causality as assessed using the Roussel Uclaf Causality Assessment Method (RUCAM). ACG Case Rep J. 2019;6:e00184. [PMC free article: PMC6791641] [PubMed: 31737715](An 86 year old man with Alzheimer disease was found to have abnormal liver tests without symptoms or jaundice, 2 months after adding memantine to his usual medications [ALT 439 U/L, Alk P 169 U/L, bilirubin 0.9 mg/dL], resolving within 6 months of stopping memantine only).
- Li DD, Zhang YH, Zhang W, Zhao P. Meta-analysis of randomized controlled trials on the efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer's disease. Front Neurosci. 2019;13:472. [PMC free article: PMC6529534] [PubMed: 31156366](Meta-analysis of 36 controlled trials of drugs for Alzheimer disease focusing upon relative efficacy and rates of discontinuation in comparison to placebo).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review [Glutamate-related excitotoxicity neuroprotection with memantine, an uncompetitive antagonist of NMDA-glutamate receptor, in Alzheimer's disease and vascular dementia].[Rev Neurol. 2006]Review [Glutamate-related excitotoxicity neuroprotection with memantine, an uncompetitive antagonist of NMDA-glutamate receptor, in Alzheimer's disease and vascular dementia].Tanović A, Alfaro V. Rev Neurol. 2006 May 16-31; 42(10):607-16.
- Paradigm shift in NMDA receptor antagonist drug development: molecular mechanism of uncompetitive inhibition by memantine in the treatment of Alzheimer's disease and other neurologic disorders.[J Alzheimers Dis. 2004]Paradigm shift in NMDA receptor antagonist drug development: molecular mechanism of uncompetitive inhibition by memantine in the treatment of Alzheimer's disease and other neurologic disorders.Lipton SA. J Alzheimers Dis. 2004 Dec; 6(6 Suppl):S61-74.
- Review Pathologically-activated therapeutics for neuroprotection: mechanism of NMDA receptor block by memantine and S-nitrosylation.[Curr Drug Targets. 2007]Review Pathologically-activated therapeutics for neuroprotection: mechanism of NMDA receptor block by memantine and S-nitrosylation.Lipton SA. Curr Drug Targets. 2007 May; 8(5):621-32.
- Review Alzheimer Disease Agents.[LiverTox: Clinical and Researc...]Review Alzheimer Disease Agents.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Cerebrospinal fluid and serum concentrations of the N-methyl-D-aspartate (NMDA) receptor antagonist memantine in man.[Neurosci Lett. 1995]Cerebrospinal fluid and serum concentrations of the N-methyl-D-aspartate (NMDA) receptor antagonist memantine in man.Kornhuber J, Quack G. Neurosci Lett. 1995 Aug 4; 195(2):137-9.
- Memantine - LiverToxMemantine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...