NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Metaxalone is a centrally acting skeletal muscle relaxant that has been in use for more than 40 years. Metaxalone has not been associated with serum aminotransferase elevations during therapy or with clinically apparent hepatic injury.
Background
Metaxalone (me tax' a lone) acts centrally as a skeletal muscle relaxant, but its efficacy and precise mechanism of action are not well documented. Metaxalone was approved for use in the United States in 1962 and has been a widely used muscle relaxant, but its use recently has declined. Current indications include the treatment of pain from acute musculoskeletal conditions and muscle spasms. The recommended dosage is 800 mg orally three to four times daily. Metaxalone is available by prescription only in 400 and 800 mg tablets in generic forms as well as under the commercial name Skelaxin. Sparse data are available regarding metaxalone safety. Side effects are not common, but can include drowsiness, dizziness, headache, nausea, and dry mouth. When combined with other serotonergic medications (such as serotonin reuptake inhibitors, tricyclic antidepressants, triptans, opiates and others), metaxalone can cause acute serotonin syndrome marked by agitation, confusion, hallucinations, tachycardia, hyperthermia, incoordination, neuromuscular rigidity, nausea and abdominal pain.
Hepatotoxicity
According to the product brochure, metaxalone may cause jaundice, although there are no specific case reports of hepatotoxicity from metaxalone in the literature and no prospective trials with routine monitoring of aminotransferase levels. Given its long history, metaxalone appears to be without significant hepatotoxicity.
Likelihood score: E (Unlikely cause of clinically apparent liver injury).
Drug Class: Muscle Relaxants
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Metaxalone – Generic, Skelaxin®
DRUG CLASS
Muscle Relaxants
Product Labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
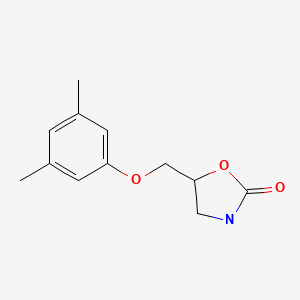
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Metaxalone | 1665-48-1 | C12-H15-N-O3 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 13 September 2021
- Zimmerman HJ. Muscle spasmolytics. In, Hepatotoxicity: The Adverse Effects of Drugs and Other Chemicals on the Liver. 2nd Ed. Philadelphia: Lippincott, 1999. p. 544-45.(Expert review of hepatotoxicity published in 1999; dantrolene, chlorzoxazone and baclofen are discussed; mentions that metaxalone has been cited as causing jaundice but that no such case reports have appeared in the literature).
- Hibbs RE, Zambon AC. Agents acting at the neuromuscular junction and autonomic ganglia. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s The pharmacological basis of therapeutics, 12th ed. New York: McGraw-Hill, 2011. p. 255-76.(Textbook of pharmacology and therapeutics)
- Toth PP, Urtis J. Commonly used muscle relaxant therapies for acute low back pain: a review of carisoprodol, cyclobenzaprine hydrochloride, and metaxalone. Clin Ther. 2004;26:1355–67. [PubMed: 15530999](A review of safety and efficacy of muscle relaxants which states "Although rare instances of hepatic enzyme elevation and anemia have been reported [with metaxalone], this association appears to be based on a false-positive hepatic assay using the cephalin flocculation test.").
- Chou R, Peterson K, Helfand M. Comparative efficacy and safety of skeletal muscle relaxants for spasticity and musculoskeletal conditions: a systematic review. J Pain Symptom Manage. 2004;28:140–75. [PubMed: 15276195](Thorough review of the pharmacology, efficacy and side effects of the muscle relaxants).
- Moore KA, Levine B, Fowler D. A fatality involving metaxalone. Forensic Sci Int. 2005;149:249–51. [PubMed: 15749367](54 year old woman found dead in whom postmortem analysis indicated metaxalone overdose; liver reported as being normal).
- Poklis JL, Ropero-Miller JD, Garside D, Winecker RE. Metaxalone (Skelaxin)-related death. J Anal Toxicol. 2004;28:537–41. [PubMed: 15516312](21 year old woman found dead in whom postmortem analysis indicated metaxalone overdose; no mention of liver abnormalities, although highest levels of drug were present in liver).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl. 2004;10:1018–23. [PubMed: 15390328](Among ~50,000 liver transplants done in the US between 1990 and 2002, 270 [0.5%] were done for drug induced acute liver failure, but none were attributed to muscle relaxants).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, one was attributed to chlorzoxazone, but none to metaxalone).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were due to metaxalone or other muscle relaxants).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the General population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none were attributed to metaxalone or other muscle relaxants).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–1352. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 5 [0.7%] were attributed to muscle relaxants, one of which was possibly related to metaxalone).
- Li Y, Delcher C, Reisfield GM, Wei YJ, Brown JD, Winterstein AG. Utilization patterns of skeletal muscle relaxants among commercially insured adults in the United States from 2006 to 2018. Pain Med. 2021:pnab088. Epub ahead of print. [PubMed: 33690860](Analysis of utilization of skeletal muscle relaxants in the US over a 12 year period demonstrated an increasing use of cyclobenzaprine [65% of prescriptions], baclofen and tizanidine but decreasing use of metaxalone and carisoprodol).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Serotonin syndrome following metaxalone overdose and therapeutic use of a selective serotonin reuptake inhibitor.[Clin Toxicol (Phila). 2015]Serotonin syndrome following metaxalone overdose and therapeutic use of a selective serotonin reuptake inhibitor.Martini DI, Nacca N, Haswell D, Cobb T, Hodgman M. Clin Toxicol (Phila). 2015 Mar; 53(3):185-7. Epub 2015 Feb 11.
- Metaxalone Suppresses Production of Inflammatory Cytokines Associated with Painful Conditions in Mouse Macrophages RAW264.7 Cells in Vitro: Synergistic Effect with β-caryophyllene.[Curr Mol Med. 2020]Metaxalone Suppresses Production of Inflammatory Cytokines Associated with Painful Conditions in Mouse Macrophages RAW264.7 Cells in Vitro: Synergistic Effect with β-caryophyllene.Yamaguchi M, Levy RM. Curr Mol Med. 2020; 20(8):643-652.
- Polydrug fatality involving metaxalone.[J Forensic Sci. 2003]Polydrug fatality involving metaxalone.Gruszecki AC, Kloda S, Simmons GT, Daly TM, Hardy RW, Robinson CA. J Forensic Sci. 2003 Mar; 48(2):432-4.
- Review Commonly used muscle relaxant therapies for acute low back pain: a review of carisoprodol, cyclobenzaprine hydrochloride, and metaxalone.[Clin Ther. 2004]Review Commonly used muscle relaxant therapies for acute low back pain: a review of carisoprodol, cyclobenzaprine hydrochloride, and metaxalone.Toth PP, Urtis J. Clin Ther. 2004 Sep; 26(9):1355-67.
- Review Review of oral skeletal muscle relaxants for the craniomandibular disorder (CMD) practitioner.[Cranio. 1990]Review Review of oral skeletal muscle relaxants for the craniomandibular disorder (CMD) practitioner.Stanko JR. Cranio. 1990 Jul; 8(3):234-43.
- Metaxalone - LiverToxMetaxalone - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...